Epidemiological and Clinical Characteristics of Acute Stroke in a Multi-Ethnic South Asian Population
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Data Analysis and Statistics
3. Results
3.1. Patient Characteristics
3.2. Diagnosis
3.3. Outcome
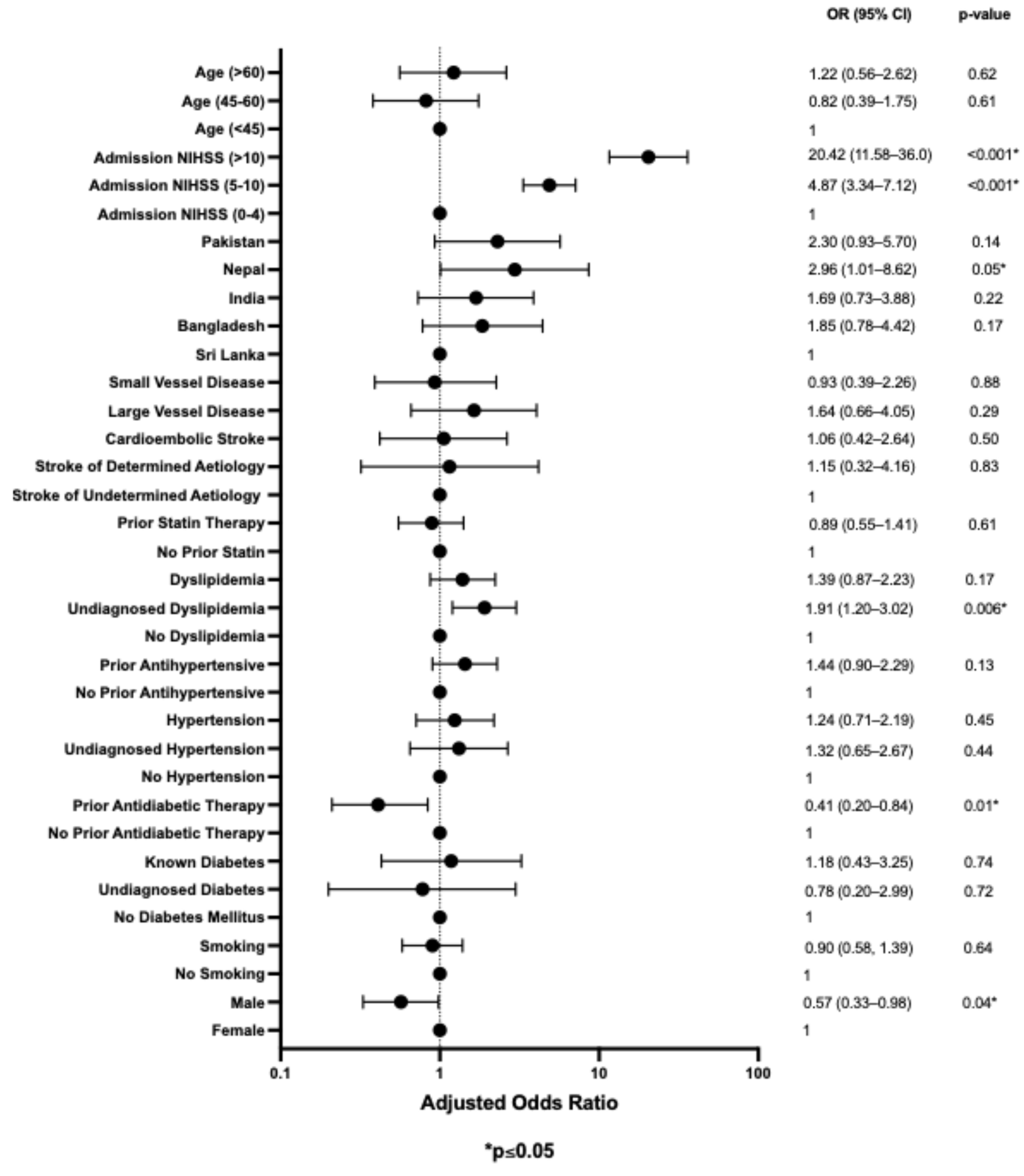
3.4. Multivariable Analysis for Risk Factors Associated with Stroke Outcome
3.5. Multivariable Analysis for Risk Factors Associated with Stroke Outcome by Ethnicity Stratification
3.6. Excluding Stroke Mimics
4. Discussion
4.1. Stroke Outcomes and Prognostic Factors
4.2. Altitude, Hypoxia, and Stroke Subtypes
4.3. Genetic, Occupational, and Environmental Influences
4.4. Mortality and TIA Patterns Across South Asian Subgroups
4.5. Cultural, Dietary, and Lifestyle Variations Among South Asians
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.O.; Rogers, J.L.; Reardon, T.; Shlobin, N.A.; Ballatori, A.M.; Brown, N.J.; Gendreau, J.; Shahrestani, S. Stroke management and outcomes in low-income and lower-middle-income countries: A meta-analysis of 8535 patients. J. Neurosurg. 2023, 139, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Salam, A.; Kamran, S.; Bourke, P.; Joseph, S.; Santos, M.; Khan, R.; Irfan, F.; Deleu, D.; Malik, R.A.; et al. Ethnic variation in acute cerebrovascular disease: Analysis from the Qatar stroke registry. Eur. Stroke J. 2016, 1, 231–241. [Google Scholar] [CrossRef]
- Tran, K.H.; Akhtar, N.; Joseph, S.; Morgan, D.; Uy, R.; Babu, B.; Shuaib, A. Undiagnosed major risk factors in acute ischaemic stroke patients in Qatar: Analysis from the Qatar stroke registry. BMJ Neurol. Open 2024, 6, e000819. [Google Scholar] [CrossRef]
- Abujaber, A.A.; Imam, Y.; Nashwan, A.; Own, A.M.; Akhtar, N. Stroke in Qatar: A decade of insights from a national registry. Neurol. Res. 2024, 46, 893–906. [Google Scholar] [CrossRef]
- Aurelius, T.; Maheshwari, A.; Ken-Dror, G.; Sharma, S.D.; Amlani, S.; Gunathilagan, G.; Cohen, D.L.; Rajkumar, C.; Maguire, S.; Ispoglou, S.; et al. Ischaemic stroke in South Asians: The BRAINS study. Eur. J. Neurol. 2023, 30, 353–361. [Google Scholar] [CrossRef]
- Ken-Dror, G.; Sureshkumar, P.; Han, T.S.; Sharma, S.D.; Sylaja, P.N.; Khan, F.Y.; Prasad, K.; Sharma, P.; Sami, E.A.; Hail, H.A.; et al. Ischemic heart disease among South Asians with ischaemic stroke in three countries across two continents: The BRAINS study. Ann. Epidemiol. 2025, 103, 48–54. [Google Scholar] [CrossRef]
- Gunarathne, A.; Patel, J.V.; Gammon, B.; Gill, P.S.; Hughes, E.A.; Lip, G.Y.H. Ischemic stroke in South Asians: A review of the epidemiology, pathophysiology, and ethnicity-related clinical features. Stroke 2009, 40, e415–423. [Google Scholar] [CrossRef]
- Singh, V.; Prabhakaran, S.; Chaturvedi, S.; Singhal, A.; Pandian, J. An Examination of Stroke Risk and Burden in South Asians. J. Stroke Cerebrovasc. Dis. 2017, 26, 2145–2153. [Google Scholar] [CrossRef]
- Gezmu, T.; Schneider, D.; Demissie, K.; Lin, Y.; Gizzi, M.S. Risk Factors for Acute Stroke among South Asians Compared to Other Racial/Ethnic Groups. PLoS ONE 2014, 9, e108901. [Google Scholar] [CrossRef]
- Scott, S.; Patwardhan, S.; Ruel, M.; Chakrabarti, S.; Neupane, S.; Manohar, S.; Moursi, M.; Menon, P. What Adults in Rural South Asia Eat and When They Eat It: Evidence from Bangladesh, India, and Nepal. J. Nutr. 2025, 155, 2406–2415. [Google Scholar] [CrossRef]
- Neupane, D.; McLachlan, C.S.; Sharma, R.; Gyawali, B.; Khanal, V.; Mishra, S.R.; Christensen, B.; Kallestrup, P. Prevalence of Hypertension in Member Countries of South Asian Association for Regional Cooperation (SAARC): Systematic Review and Meta-Analysis. Medicine 2014, 93, e74. [Google Scholar] [CrossRef]
- Patel, A.P.; Wang, M.; Kartoun, U.; Ng, K.; Khera, A.V. Quantifying and Understanding the Higher Risk of Atherosclerotic Cardiovascular Disease Among South Asian Individuals: Results from the UK Biobank Prospective Cohort Study. Circulation 2021, 144, 410–422. [Google Scholar] [CrossRef]
- Tu, J.V.; Chu, A.; Rezai, M.R.; Guo, H.; Maclagan, L.C.; Austin, P.C.; Booth, G.L.; Manuel, D.G.; Chiu, M.; Ko, D.T.; et al. The Incidence of Major Cardiovascular Events in Immigrants to Ontario, Canada: The CANHEART Immigrant Study. Circulation 2015, 132, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Brindle, P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: Cohort study using QResearch database. BMJ 2010, 341, c6624. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, R.; Unwin, N.; White, M.; Yallop, J.; Walker, L.; Alberti, K.G.; Harland, J.; Patel, S.; Ahmad, N.; Turner, C.; et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: Cross sectional study. BMJ 1999, 319, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Satish, P.; Vela, E.; Bilal, U.; Cleries, M.; Kanaya, A.M.; Kandula, N.; Virani, S.S.; Islam, N.; Valero-Elizondo, J.; Yahya, T.; et al. Burden of cardiovascular risk factors and disease in five Asian groups in Catalonia: A disaggregated, population-based analysis of 121 000 first-generation Asian immigrants. Eur. J. Prev. Cardiol. 2022, 29, 916–924. [Google Scholar] [CrossRef]
- Reddy, N.K.; Kaushal, V.; Kanaya, A.M.; Kandula, N.R.; Gujral, U.P.; Shah, N.S. Cardiovascular risk factor profiles in North and South Indian and Pakistani Americans: The MASALA Study. Am. Heart J. 2022, 244, 14–18. [Google Scholar] [CrossRef]
- Venketasubramanian, N. Stroke Epidemiology in Asia. Cerebrovasc. Dis. Extra 2025, 15, 81–92. [Google Scholar] [CrossRef]
- Venketasubramanian, N.; Yoon, B.W.; Pandian, J.; Navarro, J.C. Stroke Epidemiology in South, East, and South-East Asia: A Review. J. Stroke 2017, 19, 286–294. [Google Scholar] [CrossRef]
- Pandian, J.D.; Srivastava, M.V.P.; Aaron, S.; Ranawaka, U.K.; Venketasubramanian, N.; Sebastian, I.A.; Injety, R.J.; Gandhi, D.B.C.; Chawla, N.S.; Vijayanand, P.J.; et al. The burden, risk factors and unique etiologies of stroke in South-East Asia Region (SEAR). Lancet Reg. Health—Southeast Asia 2023, 17, 100290. [Google Scholar] [CrossRef]
- Vyas, M.V.; Austin, P.C.; Fang, J.; Laupacis, A.; Silver, F.L.; Kapral, M.K. Immigration Status, Ethnicity, and Long-term Outcomes Following Ischemic Stroke. Neurology 2021, 96, e1145–e1155. [Google Scholar] [CrossRef]
- Patel, M.; Abatcha, S.; Uthman, O. Ethnic differences between South Asians and White Caucasians in cardiovascular disease-related mortality in developed countries: A systematic literature review. Syst. Rev. 2022, 11, 207. [Google Scholar] [CrossRef]
- Hatano, S. Experience from a multicentre stroke register: A preliminary report. Bull. World Health Organ. 1976, 54, 541–553. [Google Scholar]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Touma, L.; Filion, K.B.; Sterling, L.H.; Atallah, R.; Windle, S.B.; Eisenberg, M.J. Stent Retrievers for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Neurol. 2016, 73, 275–281. [Google Scholar] [CrossRef] [PubMed]
- d’Emden, M.C.; Shaw, J.E.; Jones, G.R.; Cheung, N.W. Guidance concerning the use of glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus. Med. J. Aust. 2015, 203, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Fink, L.; Tolomelli, G.; Lee, J.C.H.; Stein, B.L.; Hickman, K.; Swierczek, S.; Kelley, T.W.; Berno, T.; Moliterno, A.R.; et al. Could hypoxia increase the prevalence of thrombotic complications in polycythemia vera? Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2013, 24, 311–316. [Google Scholar] [CrossRef]
- Gupta, N.; Ashraf, M.Z. Exposure to high altitude: A risk factor for venous thromboembolism? Semin. Thromb. Hemost. 2012, 38, 156–163. [Google Scholar] [CrossRef]
- Kotwal, J.; Apte, C.V.; Kotwal, A.; Mukherjee, B.; Jayaram, J. High altitude: A hypercoagulable state: Results of a prospective cohort study. Thromb. Res. 2007, 120, 391–397. [Google Scholar] [CrossRef]
- Jha, S.K.; Anand, A.C.; Sharma, V.; Kumar, N.; Adya, C.M. Stroke at high altitude: Indian experience. High Alt. Med. Biol. 2002, 3, 21–27. [Google Scholar] [CrossRef]
- Niaz, A.; Nayyar, S. Cerebrovascular stroke at high altitude. J. Coll. Physicians Surg. Pak. 2003, 13, 446–448. [Google Scholar] [PubMed]
- Jaillard, A.S.; Hommel, M.; Mazetti, P. Prevalence of stroke at high altitude (3380 m) in Cuzco, a town of Peru. A population-based study. Stroke 1995, 26, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mehata, S.; Shrestha, N.; Ghimire, S.; Atkins, E.; Karki, D.K.; Mishra, S.R. Association of altitude and urbanization with hypertension and obesity: Analysis of the Nepal Demographic and Health Survey 2016. Int. Health 2020, 13, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Cordovez, S.P.; Vasconez, E.; Viscor, G.; Roderick, P. Chronic high-altitude exposure and the epidemiology of ischaemic stroke: A systematic review. BMJ Open 2022, 12, e051777. [Google Scholar] [CrossRef]
- Liu, M.; Yan, M.; Guo, Y.; Xie, Z.; Li, R.; Li, J.; Ren, C.; Ji, X.; Guo, X. Acute Ischemic Stroke at High Altitudes in China: Early Onset and Severe Manifestations. Cells 2021, 10, 809. [Google Scholar] [CrossRef]
- Fang, J.; Zhuo-Ga, C.; Zhao, Y.; Kong, F.; Si, Y.; Liu, M.; Zhou, D. Characteristics of stroke in tibet autonomous region in china: A hospital-based study of acute stroke. Eur. Neurol. 2011, 66, 151–158. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, Z.; D’Souza, W.; Zhu, C.; Chun, H.; Zhuoga, C.; Zhang, Q.; Hu, X.; Zhou, D. An epidemiological survey of stroke in Lhasa, Tibet, China. Stroke 2010, 41, 2739–2743. [Google Scholar] [CrossRef]
- Zhu, H.; Li, F.; Zou, M.; Xue, X.; Yuan, J.; Feng, H.; Lin, J. Experimental high-altitude intracerebral hemorrhage in minipigs: Histology, behavior, and intracranial pressure in a double-injection model. Acta Neurochir. 2013, 155, 655–661. [Google Scholar] [CrossRef]
- Grotta, J.; Ackerman, R.; Correia, J.; Fallick, G.; Chang, J. Whole blood viscosity parameters and cerebral blood flow. Stroke 1982, 13, 296–301. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Geng, J.; Lin, C.; Zhang, Y.; Zhang, B.; Tan, Q.; Tao, Y.; Ye, D.; Chen, Z.; et al. Hemoglobin Concentration Affects Hypertensive Basal Ganglia Hemorrhage After Surgery: Correlation Analysis in a High-Altitude Region. World Neurosurg. 2019, 127, e835–e842. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Merchant, A.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Willett, W.C.; Ascherio, A. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ 2003, 327, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Akhavanzanjani, M.; Maghsoudi, Z.; Ghiasvand, R.; Khorvash, F.; Askari, G. Stroke and Nutrition: A Review of Studies. Int. J. Prev. Med. 2013, 4, S165–S179. [Google Scholar] [PubMed]
- Sha, Y.; Zhang, J.; Ci, Y.; Zhuoga, C.; Zhao, Y.; Zhou, L.; Ni, J. Cerebral venous thrombosis at high altitude: More severe symptoms and specific predisposing factors than plain areas. Thromb. J. 2024, 22, 73. [Google Scholar] [CrossRef]
- Toubasi, A.; Al-Sayegh, T.N. Short-term Exposure to Air Pollution and Ischemic Stroke: A Systematic Review and Meta-analysis. Neurology 2023, 101, e1922–e1932. [Google Scholar] [CrossRef]
- Fan, Y.-X.; Zhang, W.; Li, W.; Ma, Y.-J.; Zhang, H.-Q. Global, regional, and national impact of air pollution on stroke burden: Changing landscape from 1990 to 2021. BMC Public Health 2024, 24, 2786. [Google Scholar] [CrossRef]
- Bai, R.; Li, M.; Bhurtyal, A.; Zhu, W.; Dong, W.; Dong, D.; Sun, J.; Su, Y.; Li, Y. Temporal Mortality Trends Attributable to Stroke in South Asia: An Age–Period–Cohort Analysis. Healthcare 2024, 12, 1809. [Google Scholar] [CrossRef]
- Aly, Z.; Abbas, K.; Kazim, S.F.; Taj, F.; Aziz, F.; Irfan, A.; Sheikh, R.; Shakir, M.; Javed, S.M.; Fatmi, Z. Awareness of stroke risk factors, signs and treatment in a Pakistani population. JPMA J. Pak. Med. Assoc. 2009, 59, 495–499. [Google Scholar]
- Ovbiagele, B.; Saver, J.L. The smoking-thrombolysis paradox and acute ischemic stroke. Neurology 2005, 65, 293–295. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Liu, J.-F.; Wang, L.; Li, B.-Z.; Yan, X.-J.; Liu, W.; Wu, K.; Xiang, R.-L. “Smoking paradox” is not true in patients with ischemic stroke: A systematic review and meta-analysis. J. Neurol. 2021, 268, 2042–2054. [Google Scholar] [CrossRef]
- Irie, F.; Matsuo, R.; Mezuki, S.; Wakisaka, Y.; Kamouchi, M.; Kitazono, T.; Ago, T. Fukuoka Stroke Registry Investigators Effect of smoking status on clinical outcomes after reperfusion therapy for acute ischemic stroke. Sci. Rep. 2024, 14, 9290. [Google Scholar] [CrossRef] [PubMed]
- Aldayel, A.Y.; Alharbi, M.M.; Shadid, A.M.; Zevallos, J.C. The association between race/ethnicity and the prevalence of stroke among United States adults in 2015: A secondary analysis study using Behavioural Risk Factor Surveillance System (BRFSS). Electron. Physician 2017, 9, 5871–5876. [Google Scholar] [CrossRef] [PubMed]
- Hajat, C.; Heuschmann, P.U.; Coshall, C.; Padayachee, S.; Chambers, J.; Rudd, A.G.; Wolfe, C.D.A. Incidence of aetiological subtypes of stroke in a multi-ethnic population based study: The South London Stroke Register. J. Neurol. Neurosurg. Psychiatry 2011, 82, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Hyacinth, H.I.; Beckett, J.; Feng, W.; Chimowitz, M.; Ovbiagele, B.; Lackland, D.; Adams, R. Racial/Ethnic differences in poststroke rehabilitation outcomes. Stroke Res. Treat. 2014, 2014, 950746. [Google Scholar] [CrossRef]
- Emmett, E.S.; O’Connell, M.D.L.; Pei, R.; Douiri, A.; Wyatt, D.; Bhalla, A.; Wolfe, C.D.A.; Marshall, I.J. Trends in Ethnic Disparities in Stroke Care and Long-Term Outcomes. JAMA Netw. Open 2025, 8, e2453252. [Google Scholar] [CrossRef]
- Shariful Islam, M.; Rashid, M.; Sizear, M.I.; Hassan, R.; Rahman, M.; Parvez, S.M.; Hore, S.C.; Haque, R.; Jahan, F.; Chowdhury, S.; et al. Cigarette smoking and associated factors among men in five South Asian countries: A pooled analysis of nationally representative surveys. PLoS ONE 2022, 17, e0277758. [Google Scholar] [CrossRef]
- Shankar, S.R.; Bijlani, R.L.; Baveja, T.; Jauhar, N.; Vashisht, S.; Mahapatra, S.C.; Mehta, N.; Manchanda, S.C. Effect of partial replacement of visible fat by ghee (clarified butter) on serum lipid profile. Indian J. Physiol. Pharmacol. 2002, 46, 355–360. [Google Scholar]
- Jacobson, M.S. Cholesterol oxides in Indian ghee: Possible cause of unexplained high risk of atherosclerosis in Indian immigrant populations. Lancet 1987, 2, 656–658. [Google Scholar] [CrossRef]
- Kuchi Bhotla, H.; Meyyazhagan, A.; Pushparaj, K.; Pappuswamy, M.; Chaudhary, A.; Arumugam, V.A.; Balasubramanian, B.; Ragu Varman, D.; Orlacchio, A.; Rengasamy, K.R.R. Prevalence of Cardiovascular Diseases in South Asians: Scrutinizing Traditional Risk Factors and Newly Recognized Risk Factors Sarcopenia and Osteopenia/Osteoporosis. Curr. Probl. Cardiol. 2024, 49, 102071. [Google Scholar] [CrossRef]
- Tariq, M. Pakistan meat industry: Prospects and challenges for the future meat supply chain potential. Pak. J. Sci. 2022, 74. [Google Scholar] [CrossRef]
- Tran, K.H.; Akhtar, N.; Ali, A.; Joseph, S.; Morgan, D.; Babu, B.; Uy, R.T.; Shuaib, A. Impact of stroke severity on aspiration pneumonia risks in the medical ward versus the stroke unit: A 10-year retrospective cohort study. BMJ Open 2025, 15, e093328. [Google Scholar] [CrossRef]

| Overall | Bangladesh | India | Nepal | Pakistan | Sri Lanka | Overall Significance | |
|---|---|---|---|---|---|---|---|
| Total patients | 8825 | 1948 | 3951 | 1126 | 1205 | 595 | |
| Age | 55.0 (47.0–63.0) | 54.0 (47.0–61.0) | 56.0 (48.0–63.0) | 49.0 (44.0–55.0) | 63.0 (52.0–72.0) | 56.0 (48.0–62.0) | <0.001 * |
| <45 | 1565 (17.7) | 364 (18.7) | 631 (16.0) | 325 (28.9) | 163 (13.5) | 82 (13.8) | <0.001 |
| 45–60 | 4481 (50.8) | 1035 (53.1) | 2035 (51.5) | 737 (65.5) | 337 (28.0) | 337 (56.6) | |
| >60 | 2779 (31.5) | 549 (28.2) | 1285 (32.5) | 64 (5.7) | 705 (58.5) | 176 (29.6) | |
| Sex | <0.001 | ||||||
| Male | 8002 (90.7) | 1889 (97.0) | 3529 (89.3) | 1101 (97.8) | 251 (20.8) | 529 (88.9) | |
| Female | 823 (9.3) | 59 (3.0) | 422 (10.7) | 25 (2.2) | 954 (79.2) | 66 (11.1) | |
| Stroke Type | <0.001 | ||||||
| Central Venous Sinus Thrombosis | 127 (1.4) | 23 (1.2) | 54 (1.4) | 24 (2.1) | 19 (1.6) | 7 (1.2) | |
| Intracerebral Hemorrhage | 1101 (12.5) | 295 (15.1) | 392 (9.9) | 256 (22.7) | 90 (7.5) | 68 (11.4) | |
| Ischemic Stroke | 5070 (57.5) | 1168 (60.0) | 2273 (57.5) | 642 (57.0) | 629 (52.2) | 358 (60.2) | |
| Transient Ischemic Attack | 650 (7.4) | 98 (5.0) | 361 (9.1) | 54 (4.8) | 104 (8.6) | 33 (5.5) | |
| Stroke Mimic | 1877 (21.3) | 364 (18.7) | 871 (22.0) | 150 (13.3) | 363 (30.1) | 129 (21.7) | |
| TOAST | 0.04 | ||||||
| Small Vessel Disease | 2400 (46.3) | 573 (48.4) | 1070 (46.1) | 321 (48.3) | 265 (40.9) | 171 (47.0) | |
| Large Vessel Disease | 1128 (21.8) | 256 (21.6) | 505 (21.8) | 138 (20.8) | 146 (22.5) | 83 (22.8) | |
| Cardioembolic | 952 (18.4) | 219 (18.5) | 425 (18.3) | 105 (15.8) | 142 (21.9) | 61 (16.8) | |
| Determined Etiology | 448 (8.6) | 77 (6.5) | 216 (9.3) | 58 (8.7) | 63 (9.7) | 34 (9.3) | |
| Undetermined Etiology | 255 (4.9) | 60 (5.1) | 105 (4.5) | 43 (6.5) | 32 (4.9) | 15 (2.5) | |
| Medical Comorbidities | |||||||
| Diabetes | 3241 (36.7) | 784 (40.2) | 1481 (37.5) | 194 (17.2) | 576 (47.8) | 206 (34.6) | <0.001 |
| Hypertension | 4605 (52.2) | 1040 (53.4) | 2042 (51.7) | 494 (43.9) | 725 (60.2) | 304 (51.1) | <0.001 |
| Dyslipidemia | 1146 (13.0) | 243 (12.5) | 517 (13.1) | 50 (4.5) | 266 (22.1) | 70 (11.8) | <0.001 |
| Smoking | 1849 (21.0) | 506 (26.1) | 837 (21.3) | 202 (18.0) | 168 (14.0) | 136 (23.0) | <0.001 |
| Obesity (BMI ≥ 30) | 1639 (18.8) | 233 (12.1) | 727 (18.6) | 188 (16.8) | 396 (33.4) | 95 (16.1) | <0.001 |
| Management | |||||||
| Thrombolysis | 649 (7.4) | 130 (6.7) | 307 (7.8) | 86 (7.6) | 87 (7.2) | 39 (6.6) | 0.55 |
| Thrombectomy | 240 (2.7) | 46 (2.4) | 110 (2.8) | 44 (3.9) | 22 (1.8) | 18 (3.0) | 0.03 |
| NIHSS Admission | <0.001 | ||||||
| Mild Stroke (0–4) | 5718 (65.1) | 1206 (62.3) | 2676 (68.0) | 600 (53.4) | 848 (70.8) | 388 (65.8) | |
| Moderate Stroke (5–10) | 1659 (18.9) | 377 (19.5) | 732 (18.6) | 245 (21.8) | 197 (16.4) | 108 (18.3) | |
| Severe Stroke (≥11) | 1403 (16.0) | 353 (18.2) | 525 (13.3) | 278 (24.8) | 153 (12.8) | 94 (15.9) | |
| mRS at Admission | <0.001 | ||||||
| 0–2 | 8617 (97.7) | 1917 (98.4) | 3877 (98.2) | 1125 (99.9) | 1108 (92.0) | 590 (99.2) | |
| 3–6 | 207 (2.3) | 31 (1.6) | 73 (1.8) | 1 (0.1) | 97 (8.0) | 5 (0.8) | |
| NIHSS at Discharge | <0.001 | ||||||
| Mild Stroke (0–4) | 5341 (73.5) | 1125 (68.9) | 2497 (76.0) | 579 (63.2) | 785 (80.8) | 355 (76.0) | |
| Moderate Stroke (5–10) | 1143 (15.7) | 309 (18.9) | 472 (14.4) | 188 (20.5) | 104 (10.7) | 70 (15.0) | |
| Severe Stroke (≥11) | 786 (10.8) | 198 (12.1) | 315 (9.6) | 149 (16.3) | 82 (8.4) | 42 (9.0) | |
| mRS at 90 Days | <0.001 | ||||||
| 0–2 | 3794 (71.3) | 821 (69.2) | 1766 (74.4) | 454 (64.5) | 488 (70.2) | 265 (73.2) | |
| 3–6 | 1527 (28.7) | 365 (30.8) | 608 (25.6) | 250 (35.5) | 207 (29.8) | 97 (26.8) | |
| Mortality at 90 Days | 0.02 | ||||||
| No | 5080 (95.5) | 1119 (94.4) | 2291 (96.5) | 671 (95.3) | 657 (94.5) | 342 (94.5) | |
| Yes | 241 (4.5) | 67 (5.6) | 83 (3.5) | 33 (4.7) | 38 (5.5) | 20 (5.5) |
| Bangladesh | India | Nepal | Pakistan | Sri Lanka | |
|---|---|---|---|---|---|
| Age | |||||
| <45 | 1.25 | −3.90 * | 10.47 * | −4.11 * | −2.61 |
| 45–60 | 2.36 | 1.23 | 10.55 * | −17.04 * | 2.96 * |
| >60 | −3.56 * | 1.88 | −19.96 * | 21.73 * | −1.04 |
| Gender | |||||
| Male | 10.83 * | −3.94 * | 8.78 * | −14.78 * | −1.53 |
| Female | −10.83 * | 3.94 * | −8.78 * | 14.78 * | 1.53 |
| TOAST | |||||
| Small Vessel Disease | 1.61 | −0.27 | 1.09 | −2.95 | 0.27 |
| Large Vessel Disease | −0.15 | −0.01 | −0.68 | 0.51 | 0.50 |
| Cardioembolic | 0.11 | −0.09 | −1.84 | 2.49 | −0.82 |
| Determined Etiology | −2.99 | 1.53 | 0.08 | 1.04 | 0.49 |
| Undetermined Etiology | 0.26 | −1.19 | 1.97 | 0.02 | −0.73 |
| Stroke Type | |||||
| Central Venous Sinus Thrombosis | −1.08 | −0.51 | 2.09 | 0.42 | −0.56 |
| Intracerebral Hemorrhage | 4.04 * | −6.54 * | 11.15 * | −5.66 * | −0.80 |
| Ischemic Stroke | 2.54 | 0.14 | −0.32 | −3.97 * | 1.39 |
| Transient Ischemic Attack | −4.47 * | 5.74 * | −3.53 * | 1.81 | −1.76 |
| Stroke Mimic | −3.16* | 1.60 | −6.98 * | 8.08 * | 0.25 |
| Medical Comorbidities | |||||
| Diabetes | |||||
| Known | 3.65 * | 1.33 | −14.53 * | 8.58 * | −1.10 |
| Undiagnosed | 2.15 | 0.16 | 1.33 | −4.75 * | 0.85 |
| No | −4.84 * | −1.38 | 13.23 * | −5.41 * | 0.55 |
| Hypertension | |||||
| Known | 1.23 | −0.85 | −5.98 * | 5.97 * | −0.55 |
| Undiagnosed | −0.52 | 0.03 | 6.78 * | −5.01 * | −1.37 |
| No | −0.92 | 0.87 | 1.42 | −2.68 | 1.57 |
| Dyslipidemia | |||||
| Known | −0.78 | 0.24 | −9.11 * | 10.09 * | −0.90 |
| Undiagnosed | 0.88 | 2.08 | 0.93 | −7.82 * | 3.90 * |
| No | −0.24 | −2.02 | 5.48 * | −0.01 | −2.85 |
| Smoking | |||||
| Yes | 6.15 * | −0.48 | 2.63 | −6.45 * | −1.20 |
| No | −6.15 * | 0.48 | −2.63 | 6.45 * | 1.20 |
| BMI | |||||
| <30 | 8.48 * | 0.38 | 1.81 | −13.86 * | 1.74 |
| ≥30 | −8.48 * | −0.38 | −1.81 | 13.86 * | −1.74 |
| mRS at Admission | |||||
| 0–2 | −2.49 | −2.78 | −5.36 * | 14.08 * | −2.51 |
| 3–6 | 2.49 | 2.78 | 5.36 * | −14.08 * | 2.51 |
| NIHSS Admission | |||||
| 0–4 | −2.96 * | 5.16 * | −8.81 * | 4.42 * | 0.34 |
| 5–10 | 0.74 | −0.61 | 2.68 | −2.33 | −0.38 |
| ≥11 | 3.07 * | −6.06 * | 8.59 * | −3.26 * | −0.03 |
| Mortality at 90 Days | |||||
| Yes | 2.10 | −3.25 * | 0.22 | 1.28 | 0.94 |
| No | −2.10 | 3.25* | −0.22 | −1.28 | −0.94 |
| mRS at 90 Days | |||||
| 0–2 | 1.79 | 4.47 * | −4.29 * | −0.68 | 0.83 |
| 3–6 | −1.79 | −4.47 * | 4.29 * | 0.68 | −0.83 |
| NIHSS at Discharge | |||||
| 0–4 | −4.71 * | 4.50 * | −7.52 * | 5.59 * | 1.29 |
| 5–10 | 4.05 * | −2.87 | 4.27 * | −4.61 * | −0.45 |
| ≥11 | 1.95 | −3.04 * | 5.69 * | −2.55 | −1.31 |
| mRS at 90 Days | Coef. | Std. Err. | t-Value | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age | |||||
| 45–60 | −0.021 | 0.059 | −0.35 | (−0.14, 0.096) | 0.72 |
| >60 | 0.037 | 0.061 | 0.61 | (−0.08, 0.157) | 0.55 |
| Gender | |||||
| Male | −0.098 | 0.046 | −2.11 | (−0.19, −0.007) | 0.04 |
| Ethnicity | |||||
| India | −0.016 | 0.034 | -0.48 | (−0.08, 0.051) | 0.64 |
| Nepal | 0.077 | 0.066 | 1.17 | (−0.05, 0.206) | 0.24 |
| Pakistan | 0.034 | 0.046 | 0.75 | (−0.06, 0.124) | 0.46 |
| Sri Lanka | −0.081 | 0.061 | −1.33 | (−0.20, 0.038) | 0.18 |
| Smoking | |||||
| Yes | −0.015 | 0.033 | −0.45 | (−0.08, 0.050) | 0.65 |
| Diabetes | |||||
| Known | 0.045 | 0.082 | 0.55 | (−0.12, 0.205) | 0.58 |
| Undiagnosed | −0.019 | 0.103 | −0.19 | (−0.22, 0.183) | 0.85 |
| Hypertension | |||||
| Known | 0.025 | 0.042 | 0.60 | (−0.06, 0.108) | 0.55 |
| Undiagnosed | 0.53 | ||||
| Dyslipidemia | |||||
| Known | 0.056 | 0.037 | 1.50 | (−0.02, 0.129) | 0.14 |
| Undiagnosed | 0.103 | 0.037 | 2.80 | (0.03, 0.175) | 0.005 |
| NIHSS Admission | |||||
| 5–10 | 0.293 | 0.033 | 8.87 | (0.23, 0.357) | <0.001 |
| 11–40 | 0.603 | 0.045 | 13.5 | (0.52, 0.691) | <0.001 * |
| Diabetic Therapy | |||||
| Yes | −0.143 | 0.058 | −2.47 | (−0.26, −0.029) | 0.014 |
| Statin | |||||
| Yes | −0.016 | 0.037 | −0.44 | (−0.09, 0.057) | 0.66 |
| Antihypertensive | |||||
| Yes | 0.059 | 0.037 | 1.61 | (−0.01, 0.131) | 0.11 |
| Mortality at 90 Days | Coef. | Std. Err. | t-Value | 95% CI | p-Value |
| Age | |||||
| 45–60 | −0.006 | 0.026 | −0.23 | (−0.06, 0.045) | 0.82 |
| >60 | 0.002 | 0.027 | 0.08 | (−0.05, 0.054) | 0.94 |
| Gender | |||||
| Male | −0.025 | 0.020 | −1.23 | (−0.06, 0.015) | 0.22 |
| Ethnicity | |||||
| India | 0.005 | 0.015 | 0.34 | (−0.02, 0.034) | 0.73 |
| Nepal | 0.041 | 0.029 | 1.42 | (−0.02, 0.097) | 0.16 |
| Pakistan | 0.027 | 0.020 | 1.38 | (−0.01, 0.066) | 0.17 |
| Sri Lanka | 0.005 | 0.026 | 0.19 | (−0.05, 0.057) | 0.85 |
| Smoking | |||||
| Yes | 0.012 | 0.015 | 0.85 | (−0.02, 0.041) | 0.40 |
| Diabetes | |||||
| Known | −0.007 | 0.035 | −0.19 | (−0.08, 0.063) | 0.85 |
| Undiagnosed | 0.009 | 0.045 | 0.19 | (−0.08, 0.097) | 0.85 |
| Hypertension | |||||
| Known | −0.016 | 0.018 | −0.87 | (−0.05, 0.020) | 0.38 |
| Undiagnosed | −0.021 | 0.024 | −0.88 | (−0.07, 0.026) | 0.38 |
| Dyslipidemia | |||||
| Known | −0.019 | 0.016 | −1.15 | (−0.05, 0.013) | 0.25 |
| Undiagnosed | −0.013 | 0.016 | −0.82 | (−0.04, 0.018) | 0.41 |
| NIHSS Admission | |||||
| 5–10 | 0.001 | 0.014 | 0.10 | (−0.03, 0.030) | 0.92 |
| 11–40 | 0.125 | 0.019 | 6.44 | (0.09, 0.164) | <0.001 * |
| Diabetic Therapy | |||||
| Yes | 0.019 | 0.025 | 0.77 | (−0.03, 0.069) | 0.44 |
| Statin | |||||
| Yes | 0.016 | 0.016 | 0.96 | (−0.02, 0.048) | 0.34 |
| Antihypertensive | |||||
| Yes | 0.001 | 0.016 | 0.08 | (−0.03, 0.033) | 0.94 |
| mRS at 90 days | |||||
| Obs | R-sq | ||||
| 879 | 0.272 | ||||
| Parms | F | ||||
| 24 | 13.92 | ||||
| Root-mean-square deviation | Prob > F | ||||
| 0.396 | <0.001 | ||||
| Mortality at 90 days | |||||
| Obs | R-sq | ||||
| 879 | 0.091 | ||||
| Parms | F | ||||
| 24 | 3.70 | ||||
| Root-mean-square deviation | Prob > F | ||||
| 0.173 | <0.001 | ||||
| Variable | aOR | p-Value | 95% CI |
|---|---|---|---|
| (a) | |||
| Age | |||
| >60 | 0.73 | 0.34 | 0.38, 1.40 |
| 45–60 | 0.75 | 0.35 | 0.42, 1.36 |
| <45 | - | - | - |
| Gender | |||
| Male | 1.23 | 0.76 | 0.34, 4.40 |
| Female | - | - | - |
| Smoking | |||
| Yes | 1.21 | 0.36 | 0.81, 1.82 |
| NIHSS Admission | |||
| 5–10 | 5.35 | <0.001 * | 3.49, 8.22 |
| 11–40 | 30.67 | <0.001 * | 17.60, 53.42 |
| Diabetes | |||
| Known | 1.38 | 0.15 | 0.89, 2.15 |
| Undiagnosed | 0.86 | 0.61 | 0.48, 1.55 |
| Hypertension | |||
| Known | 1.37 | 0.18 | 0.86, 2.17 |
| Undiagnosed | 0.98 | 0.94 | 0.53, 1.82 |
| Dyslipidemia | |||
| Known | 1.21 | 0.57 | 0.62, 2.38 |
| Undiagnosed | 1.24 | 0.31 | 0.82, 1.88 |
| TOAST | |||
| Small Vessel Disease | 0.36 | 0.02 * | 0.15, 0.84 |
| Large Vessel Disease | 0.67 | 0.37 | 0.28, 1.61 |
| Cardioembolic Stroke | 0.66 | 0.37 | 0.27, 1.63 |
| Stroke of Determined Etiology | 0.37 | 0.08 | 0.12, 1.14 |
| (b) | |||
| Age | |||
| >60 | 1.22 | 0.40 | 0.77, 1.95 |
| 45–60 | 1.26 | 0.30 | 0.81, 1.95 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.88 | 0.57 | 0.57, 1.36 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.70 | 0.03 * | 0.52, 0.96 |
| NIHSS Admission | |||
| 5–10 | 5.31 | <0.001 * | 4.00, 7.05 |
| 11–40 | 16.57 | <0.001 * | 11.48, 23.93 |
| Diabetes | |||
| Known | 1.62 | <0.001 * | 1.20, 2.18 |
| Undiagnosed | 1.49 | 0.047 * | 1.01, 2.20 |
| Hypertension | |||
| Known | 1.34 | 0.07 | 0.98, 1.84 |
| Undiagnosed | 1.23 | 0.31 | 0.82, 1.85 |
| Dyslipidemia | |||
| Known | 0.85 | 0.42 | 0.56, 1.28 |
| Undiagnosed | 0.87 | 0.36 | 0.65, 1.17 |
| TOAST | |||
| Small Vessel Disease | 0.50 | 0.03 * | 0.27, 0.92 |
| Large Vessel Disease | 0.95 | 0.88 | 0.51, 1.78 |
| Cardioembolic Stroke | 0.61 | 0.13 | 0.32, 1.16 |
| Stroke of Determined Etiology | 0.50 | 0.06 | 0.24, 1.02 |
| (c) | |||
| Age | |||
| >60 | 0.68 | 0.53 | 0.21, 2.24 |
| 45–60 | 1.19 | 0.56 | 0.66, 2.15 |
| <45 | - | - | - |
| Gender | |||
| Male | 2.52 | 0.50 | 0.18, 35.86 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.53 | 0.06 | 0.28, 1.02 |
| NIHSS Admission | |||
| 5–10 | 7.35 | <0.001 * | 4.10, 13.18 |
| 11–40 | 23.06 | <0.001 * | 10.87, 48.91 |
| Diabetes | |||
| Known | 1.61 | 0.14 | 0.85, 3.03 |
| Undiagnosed | 1.11 | 0.78 | 0.56, 2.20 |
| Hypertension | |||
| Known | 1.62 | 0.11 | 0.90, 2.94 |
| Undiagnosed | 1.06 | 0.90 | 0.49, 2.25 |
| Dyslipidemia | |||
| Known | 0.90 | 0.85 | 0.31, 2.60 |
| Undiagnosed | 0.97 | 0.93 | 0.54, 1.74 |
| TOAST | |||
| Small Vessel Disease | 0.61 | 0.35 | 0.21, 1.73 |
| Large Vessel Disease | 0.92 | 0.88 | 0.32, 2.71 |
| Cardioembolic Stroke | 0.89 | 0.84 | 0.29, 2.71 |
| Stroke of Determined Etiology | 0.32 | 0.10 | 0.08, 1.26 |
| (d) | |||
| Age | |||
| >60 | 1.71 | 0.25 | 0.69, 4.24 |
| 45–60 | 1.01 | 0.98 | 0.39, 2.65 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.97 | 0.91 | 0.52, 1.80 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.69 | 0.22 | 0.38, 1.25 |
| NIHSS Admission | |||
| 5–10 | 3.16 | <0.001 * | 1.91, 5.24 |
| 11–40 | 17.48 | <0.001 * | 8.54, 35.78 |
| Diabetes | |||
| Known | 1.08 | 0.77 | 0.65, 1.79 |
| Undiagnosed | 1.58 | 0.31 | 0.65, 3.87 |
| Hypertension | |||
| Known | 1.07 | 0.82 | 0.60, 1.90 |
| Undiagnosed | 0.49 | 0.13 | 0.20, 1.22 |
| Dyslipidemia | |||
| Known | 1.13 | 0.66 | 0.65, 1.98 |
| Undiagnosed | 1.01 | 0.99 | 0.55, 1.83 |
| TOAST | |||
| Small Vessel Disease | 0.64 | 0.38 | 0.23, 1.74 |
| Large Vessel Disease | 0.73 | 0.56 | 0.26, 2.09 |
| Cardioembolic Stroke | 1.00 | 0.99 | 0.35, 2.81 |
| Stroke of Determined Etiology | 0.27 | 0.044 * | 0.07, 0.97 |
| (e) | |||
| Age | |||
| >60 | 3.21 | 0.12 | 0.75, 13. 84 |
| 45–60 | 2.71 | 0.16 | 0.67, 10.99 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.55 | 0.35 | 0.16, 1.90 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.77 | 0.54 | 0.33, 1.79 |
| NIHSS Admission | |||
| 5–10 | 3.10 | 0.006 * | 1.38, 6.92 |
| 11–40 | 29.90 | <0.001 * | 10.27, 87.04 |
| Diabetes | |||
| Known | 0.72 | 0.44 | 0.31, 1.67 |
| Undiagnosed | 2.18 | 0.15 | 0.76, 6.23 |
| Hypertension | |||
| Known | 3.28 | 0.01 * | 1.32, 8.14 |
| Undiagnosed | 3.57 | 0.03 * | 1.12, 11.41 |
| Dyslipidemia | |||
| Known | 0.24 | 0.07 | 0.05, 1.12 |
| Undiagnosed | 0.78 | 0.55 | 0.36, 1.73 |
| TOAST | |||
| Small Vessel Disease | 2.52 | 0.49 | 0.18, 35.30 |
| Large Vessel Disease | 3.39 | 0.36 | 0.24, 47.27 |
| Cardioembolic Stroke | 2.06 | 0.61 | 0.13, 32.12 |
| Stroke of Determined Etiology | 1.55 | 0.77 | 0.09, 27.37 |
| Variable | aOR | p-Value | 95% CI |
|---|---|---|---|
| (a) | |||
| Age | |||
| >60 | 3.36 | 0.13 | 0.69, 16.30 |
| 45–60 | 3.31 | 0.09 | 0.83, 13.25 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.37 | 0.32 | 0.05, 2.65 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.38 | 0.10 | 0.12, 1.20 |
| NIHSS Admission | |||
| 5–10 | 2.30 | 0.32 | 0.44, 12.04 |
| 11–40 | 19.75 | <0.001 * | 5.58, 69.87 |
| Diabetes | |||
| Known | 1.02 | 0.96 | 0.40, 2.61 |
| Undiagnosed | 0.78 | 0.71 | 0.20, 2.95 |
| Hypertension | |||
| Known | 0.60 | 0.30 | 0.23, 1.57 |
| Undiagnosed | 0.33 | 0.14 | 0.08, 1.43 |
| Dyslipidemia | |||
| Known | 0.32 | 0.30 | 0.04, 2.76 |
| Undiagnosed | 0.37 | 0.07 | 0.13, 1.08 |
| TOAST | |||
| Small Vessel Disease | - | - | - |
| Large Vessel Disease | 0.45 | 0.32 | 0.09, 2.17 |
| Cardioembolic Stroke | 1.29 | 0.73 | 0.29, 5.72 |
| Stroke of Determined Etiology | 0.94 | 0.95 | 0.15, 5.85 |
| (b) | |||
| Age | |||
| >60 | 2.30 | 0.31 | 0.47, 11.30 |
| 45–60 | 2.65 | 0.21 | 0.58, 12.18 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.56 | 0.22 | 0.23, 1.39 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.18 | 0.02 * | 0.04, 0.78 |
| NIHSS Admission | |||
| 5–10 | 1.60 | 0.31 | 0.64, 3.97 |
| 11–40 | 5.85 | <0.001 * | 2.70, 12.69 |
| Diabetes | |||
| Known | 1.34 | 0.44 | 0.64, 2.79 |
| Undiagnosed | 1.06 | 0.92 | 0.36, 3.11 |
| Hypertension | |||
| Known | 1.90 | 0.11 | 0.86, 4.19 |
| Undiagnosed | 0.46 | 0.33 | 0.10, 2.20 |
| Dyslipidemia | |||
| Known | 0.53 | 0.21 | 0.20, 1.41 |
| Undiagnosed | 0.54 | 0.17 | 0.23, 1.29 |
| TOAST | |||
| Small Vessel Disease | 0.15 | 0.02 * | 0.03, 0.76 |
| Large Vessel Disease | 1.03 | 0.97 | 0.27, 3.95 |
| Cardioembolic Stroke | 0.93 | 0.91 | 0.24, 3.60 |
| Stroke of Determined Etiology | 0.40 | 0.30 | 0.07, 2.26 |
| (c) | |||
| Age | |||
| >60 | 0.12 | 0.29 | 0.002, 6.22 |
| 45–60 | 0.22 | 0.21 | 0.02, 2.32 |
| <45 | - | - | - |
| Gender | |||
| Male | - | - | - |
| Female | - | - | - |
| Smoking | |||
| Yes | - | - | - |
| NIHSS Admission | |||
| 5–10 | - | - | - |
| 11–40 | 26.19 | 0.044 * | 1.09, 628.9 |
| Diabetes | |||
| Known | 53.5 | 0.006 * | 3.16, 904.34 |
| Undiagnosed | - | - | - |
| Hypertension | |||
| Known | 3.94 | 0.26 | 0.35, 43.79 |
| Undiagnosed | - | - | - |
| Dyslipidemia | |||
| Known | 3.08 | 0.51 | 0.11, 86.82 |
| Undiagnosed | - | - | - |
| TOAST | |||
| Small Vessel Disease | 0.15 | 0.39 | 0.002, 11.00 |
| Large Vessel Disease | 0.26 | 0.50 | 0.10, 13.00 |
| Cardioembolic Stroke | 0.39 | 0.62 | 0.10, 15.94 |
| Stroke of Determined Etiology | - | - | - |
| (d) | |||
| Age | |||
| >60 | 0.76 | 0.71 | 0.17, 3.30 |
| 45–60 | 0.26 | 0.13 | 0.04, 1.51 |
| <45 | - | - | - |
| Gender | |||
| Male | 1.05 | 0.95 | 0.29, 3.79 |
| Female | - | - | - |
| Smoking | |||
| Yes | 2.32 | 0.16 | 0.71, 7.54 |
| NIHSS Admission | |||
| 5–10 | 1.52 | 0.57 | 0.36, 6.45 |
| 11–40 | 15.59 | <0.001 * | 5.19, 46.85 |
| Diabetes | |||
| Known | 1.58 | 0.38 | 0.57, 4.41 |
| Undiagnosed | 0.45 | 0.51 | 0.04, 4.97 |
| Hypertension | |||
| Known | 0.69 | 0.54 | 0.21, 2.25 |
| Undiagnosed | 0.20 | 0.17 | 0.02, 2.04 |
| Dyslipidemia | |||
| Known | 0.69 | 0.55 | 0.21, 2.28 |
| Undiagnosed | 1.18 | 0.80 | 0.32, 4.30 |
| TOAST | |||
| Small Vessel Disease | - | - | - |
| Large Vessel Disease | 2.16 | 0.31 | 0.49, 9.47 |
| Cardioembolic Stroke | 4.86 | 0.03 * | 1.21, 19.46 |
| Stroke of Determined Etiology | - | - | - |
| (e) | |||
| Age | |||
| >60 | 2.28 | 0.55 | 0.15, 33.59 |
| 45–60 | 0.82 | 0.88 | 0.07, 10.17 |
| <45 | - | - | - |
| Gender | |||
| Male | 0.53 | 0.62 | 0.04, 6.62 |
| Female | - | - | - |
| Smoking | |||
| Yes | 0.19 | 0.19 | 0.02, 2.26 |
| NIHSS Admission | |||
| 5–10 | 1.02 | 0.99 | 0.08, 13.44 |
| 11–40 | 14.00 | 0.008 * | 1.98, 99.09 |
| Diabetes | |||
| Known | 1.93 | 0.58 | 0.19, 19.58 |
| Undiagnosed | 1.39 | 0.79 | 0.13, 14.64 |
| Hypertension | |||
| Known | 0.66 | 0.71 | 0.08, 5.72 |
| Undiagnosed | - | - | - |
| Dyslipidemia | |||
| Known | 1.20 | 0.90 | 0.08, 18.65 |
| Undiagnosed | 0.34 | 0.32 | 0.04, 2.86 |
| TOAST | |||
| Small Vessel Disease | - | - | - |
| Large Vessel Disease | 4.97 | 0.23 | 0.36, 68.85 |
| Cardioembolic Stroke | 6.31 | 0.16 | 0.47, 84.61 |
| Stroke of Determined Etiology | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, K.H.; Akhtar, N.; Imam, Y.; Uddin, M.G.; Joseph, S.; Morgan, D.; Babu, B.; Uy, R.T.; Shuaib, A. Epidemiological and Clinical Characteristics of Acute Stroke in a Multi-Ethnic South Asian Population. Neurol. Int. 2025, 17, 140. https://doi.org/10.3390/neurolint17090140
Tran KH, Akhtar N, Imam Y, Uddin MG, Joseph S, Morgan D, Babu B, Uy RT, Shuaib A. Epidemiological and Clinical Characteristics of Acute Stroke in a Multi-Ethnic South Asian Population. Neurology International. 2025; 17(9):140. https://doi.org/10.3390/neurolint17090140
Chicago/Turabian StyleTran, Kim H., Naveed Akhtar, Yahia Imam, Md Giass Uddin, Sujatha Joseph, Deborah Morgan, Blessy Babu, Ryan Ty Uy, and Ashfaq Shuaib. 2025. "Epidemiological and Clinical Characteristics of Acute Stroke in a Multi-Ethnic South Asian Population" Neurology International 17, no. 9: 140. https://doi.org/10.3390/neurolint17090140
APA StyleTran, K. H., Akhtar, N., Imam, Y., Uddin, M. G., Joseph, S., Morgan, D., Babu, B., Uy, R. T., & Shuaib, A. (2025). Epidemiological and Clinical Characteristics of Acute Stroke in a Multi-Ethnic South Asian Population. Neurology International, 17(9), 140. https://doi.org/10.3390/neurolint17090140






