Speed Matters: Challenging the Notion of Velocity-Independent Rigidity Using Technological Devices in People with Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
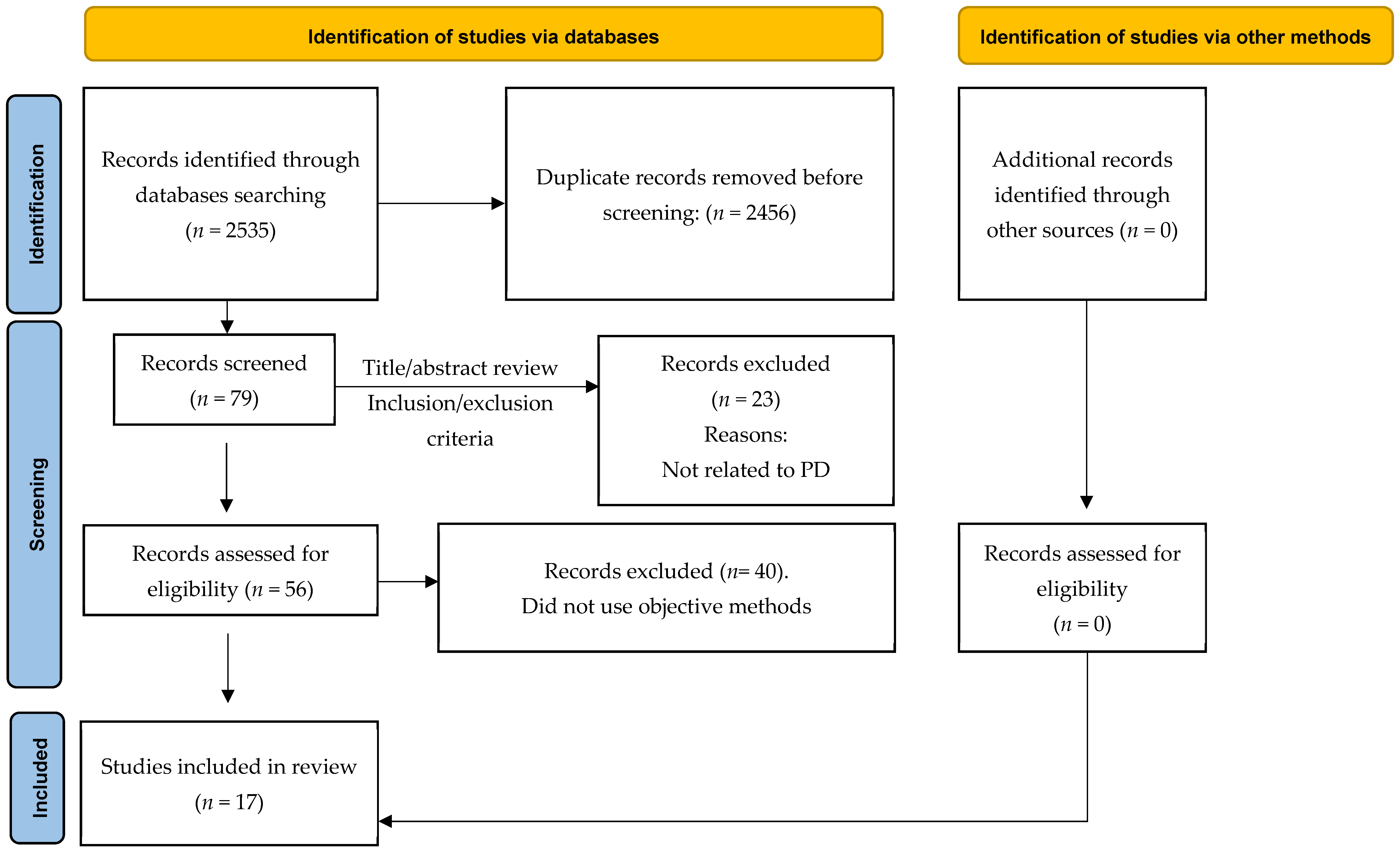
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Analysis
2.4. Assessment of the Methodological Rigor of the Included Studies
3. Results
3.1. Sample Characteristics
3.2. Participant Characteristics by Limb or Anatomical Region Explored
3.3. Objective Methods and Protocols Employed
3.4. Main Findings Regarding Velocity-Dependent Versus Velocity-Independent Responses
3.5. Assessment of Methodological Quality of the Studies
3.6. Strengths, Weaknesses, Opportunities and Threats (SWOT) Analysis of the Included Studies
4. Discussion
4.1. Practical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AXIS | Appraisal Tool for Cross-Sectional Studies |
| EMG | Electromyography |
| H&Y | Hoehn and Yahr |
| LLSR | Long-latency stretch reflex |
| PD | Parkinson’s disease |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| ROM | Range of motion |
| SD | Standard deviation |
| SPASM | Support Programme for Assembly of a Database for Spasticity Measurement |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
References
- Rizzo, M.A.; Hadjimichael, O.C.; Preiningerova, J.; Vollmer, T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult. Scler. 2004, 10, 589–595. [Google Scholar] [CrossRef]
- Sommerfeld, D.K.; Eek, E.U.; Svensson, A.K.; Holmqvist, L.W.; Von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.M.; Karunas, R.S.; Waring, W.P., 3rd. Epidemiology of spasticity following traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1990, 71, 566–569. [Google Scholar] [PubMed]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.H.; Wood, D.E.; Hermens, H.J.; Voerman, G.E.; Johnson, G.R.; Van Wijck, F.; Platz, T.; Gregoric, M.; Hitchcock, R.; Pandyan, A. Theoretical and methodological considerations in the measurement of spasticity. Disabil. Rehabil. 2005, 27, 69–80. [Google Scholar] [CrossRef]
- van den Noort, J.C.; Bar-On, L.; Aertbeliën, E.; Bonikowski, M.; Braendvik, S.M.; Broström, E.W.; Buizer, A.I.; Burridge, J.H.; van Campenhout, A.; Dan, B.; et al. European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch. Eur. J. Neurol. 2017, 24, 981-e38. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Mutch, W.; Strudwick, A.; Roy, S.; Downie, A. Parkinson’s disease: Disability, review, and management. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 675–677. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef]
- Berardelli, A.; Sabra, A.; Hallett, M. Physiological mechanisms of rigidity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1983, 46, 45–53. [Google Scholar] [CrossRef]
- di Biase, L.; Summa, S.; Tosi, J.; Taffoni, F.; Marano, M.; Cascio-Rizzo, A.; Vecchio, F.; Formica, D.; Di Lazzaro, V.; Di Pino, G.; et al. Quantitative Analysis of Bradykinesia and Rigidity in Parkinson’s Disease. Front. Neurol. 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Sánchez, M.D.R.; Moreno-Verdú, M.; Cano-de-la-Cuerda, R. Quantitative Measurement of Rigidity in Parkinson’s Disease: A Systematic Review. Sensors 2020, 20, 880. [Google Scholar] [CrossRef] [PubMed]
- Teräväinen, H.; Tsuim, J.K.C.; Mak, E.; Calne, D.B. Optimal indices for testing parkinsonian rigidity. Can. J. Neurol. Sci. 1989, 16, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Huang, Y.Z.; Chen, J.J.J.; Hwang, I.S. Quantitative analysis of the velocity related pathophysiology of spasticity and rigidity in the elbow flexors. J. Neurol. Neurosurg. Psychiatry 2002, 72, 621–629. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong, E.C.; Hui-Chan, C.W. Quantitative measurement of trunk rigidity in parkinsonian patients. J. Neurol. 2007, 254, 202–209. [Google Scholar] [CrossRef]
- Rothwell, J.C.; Obeso, J.A.; Traub, M.M.; Marsden, C.D. The behaviour of the long-latency stretch reflex in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1983, 46, 35–44. [Google Scholar] [CrossRef]
- Gómez-Soriano, J.; Cano-de-la-Cuerda, R.; Muñoz-Hellin, E.; Ortiz-Gutiérrez, R.; Taylor, J.S. Evaluation and quantification of spasticity: A review of the clinical, biomechanical and neurophysiological methods. Rev. Neurol. 2012, 55, 217–226. [Google Scholar]
- Cano de la Cuerda, R.; Vela, L.; Miangolarra-Page, J.C.; Macías-Macías, Y.; Muñoz-Hellín, E. Quantitative measurement of axial rigidity, functional status and health-related quality of life in patients with Parkinson’s disease. Rev. Neurol. 2010, 51, 193–200. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Fung, V.S.C.; Burne, J.A.; Morris, J.G.L. Objective quantification of resting and activated parkinsonian rigidity: A comparison of angular impulse and work scores. Mov. Disord. 2000, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Asci, F.; Falletti, M.; Zampogna, A.; Patera, M.; Hallett, M.; Rothwell, J.; Suppa, A. Rigidity in Parkinson’s disease: Evidence from biomechanical and neurophysiological measures. Brain 2023, 146, 3705–3718. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Threlkeld, A.J.; Fang, X.; Muthumani, A.; Xia, R. Amplitude- and velocity-dependency of rigidity measured at the wrist in Parkinson’s disease. Clin. Neurophysiol. 2012, 123, 764–773. [Google Scholar] [CrossRef]
- Zetterberg, H.; Frykberg, G.; Gäverth, J.; Lindberg, P. Neural and nonneural contributions to wrist rigidity in Parkinson’s disease: An explorative study using the NeuroFlexor. BioMed Res. Int. 2015, 2015, 276182. [Google Scholar] [CrossRef]
- Xia, R.; Muthumani, A.; Mao, Z.H.; Powell, D.W. Quantification of neural reflex and muscular intrinsic contributions to parkinsonian rigidity. Exp. Brain Res. 2016, 234, 3587–3595. [Google Scholar] [CrossRef]
- Xia, R.; Sun, J.; Threlkeld, A.J. Analysis of interactive effect of stretch reflex and shortening reaction on rigidity in Parkinson’s disease. Clin. Neurophysiol. 2009, 120, 1400–1407. [Google Scholar] [CrossRef]
- Zito, G.A.; Gerber, S.M.; Urwyler, P.; Shamsollahi, M.J.; Pal, N.; Benninger, D.; Nef, T. Development and Pilot Testing of a Novel Electromechanical Device to MeasureWrist Rigidity in Parkinson’s Disease. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4885–4888. [Google Scholar]
- Endo, T.; Yoshikawa, N.; Fujimura, H.; Sakoda, S. Parkinsonian Rigidity Depends on the Velocity of Passive Joint Movement. Park. Dis. 2015, 2015, 961790. [Google Scholar] [CrossRef]
- Nuyens, G.; De Weerdt, W.; Dom, R.; Nieuwboer, A.; Spaepen, A. Torque variations during repeated passive isokinetic movements of the knee in subjects with Parkinson’s disease and healthy control subjects. Park. Relat. Disord. 2000, 6, 87–93. [Google Scholar] [CrossRef]
- Uslu, S.; Gürbüz, M.; Kızılay, F.; Özkaynak, S.; Nüzket, T.; Uysal, H. Amplitude and velocity dependence of patellar pendulum triggered by T reflex in Parkinson’s rigidity. Neurol. Sci. 2021, 42, 3257–3266. [Google Scholar] [CrossRef]
- Huang, H.W.; Ju, M.S.; Lin, C.C. Flexor and extensor muscle tone evaluated using the quantitative pendulum test in stroke and parkinsonian patients. J. Clin. Neurosci. 2016, 27, 48–52. [Google Scholar] [CrossRef]
- Gregoric, M.; Stefanovska, A.; Vodovnik, L.; Rebersek, S.; Gros, N. Rigidity in parkinsonism: Characteristics and influences of passive exercise and electrical nerve stimulation. Funct. Neurol. 1988, 3, 55–68. [Google Scholar]
- Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; Macías-Macías, Y. Isokinetic dynamometry as a technologic assessment tool for trunk rigidity in Parkinson’s disease patients. NeuroRehabilitation 2014, 35, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Quintern, J.; Berger, W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 1981, 104, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.L.; Wiegner, A.W.; Young, R.R. Elastic properties of muscles measured at the elbow in man. II. Patients with parkinsonian rigidity. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1177–1181. [Google Scholar] [CrossRef]
- Anastasopoulos, D.; Maurer, C.; Nasios, G.; Mergner, T. Neck rigidity in Parkinson’s disease patients is related to incomplete suppression of reflexive head stabilization. Exp. Neurol. 2009, 217, 336–346. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Moreno-Verdú, M.; Ferreira-Sánchez, M.D.R.; Macías-Macías, Y.; Miangolarra-Page, J.C. Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study. Sensors 2020, 20, 2482. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; Macías-Macías, Y. Axial rigidity is related to the risk of falls in patients with Parkinson’s disease. NeuroRehabilitation 2017, 40, 569–577. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; Macías-Macías, Y.; Muñoz-Hellín, E. Axial rigidity and quality of life in patients with Parkinson’s disease: A preliminary study. Qual. Life Res. 2011, 20, 817–823. [Google Scholar] [CrossRef]
- Cano-de-la-Cuerda, R.; Pérez-de-Heredia, M.; Miangolarra-Page, J.C.; Muñoz-Hellín, E.; Fernández-de-Las-Peñas, C. Is there muscular weakness in Parkinson’s disease? Am. J. Phys. Med. Rehabil. 2010, 89, 70–76. [Google Scholar] [CrossRef]
- Triolo, G.; Ivaldi, D.; Lombardo, R.; Quartarone, A.; Lo Buono, V. Use of Smartphones and Wrist-Worn Devices for Motor Symptoms in Parkinson’s Disease: A Systematic Review of Commercially Available Technologies. Sensors 2025, 25, 3732. [Google Scholar] [CrossRef]
- Polvorinos-Fernández, C.; Sigcha, L.; Centeno-Cerrato, M.; de Arcas, G.; Grande, M.; Marín, M.; Pareés, I.; Martínez-Castrillo, J.C.; Pavón, I. Evaluation of Free-Living Motor Symptoms in Patients with Parkinson Disease Through Smartwatches: Protocol for Defining Digital Biomarkers. JMIR Res. Protoc. 2025, 14, e72820. [Google Scholar] [CrossRef]
- Ymeri, G.; Salvi, D.; Olsson, C.M.; Wassenburg, M.V.; Tsanas, A.; Svenningsson, P. Quantifying Parkinson’s disease severity using mobile wearable devices and machine learning: The ParkApp pilot study protocol. BMJ Open 2023, 13, e077766. [Google Scholar] [CrossRef]
- Adams, J.L.; Kangarloo, T.; Tracey, B.; O’Donnell, P.; Volfson, D.; Latzman, R.D.; Zach, N.; Alexander, R.; Bergethon, P.; Cosman, J.; et al. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. npj Park. Dis. 2023, 9, 64. [Google Scholar] [CrossRef]
- Liikkanen, S.; Sinkkonen, J.; Suorsa, J.; Kaasinen, V.; Pekkonen, E.; Kärppä, M.; Scheperjans, F.; Huttunen, T.; Sarapohja, T.; Pesonen, U.; et al. Feasibility and patient acceptability of a commercially available wearable and a smart phone application in identification of motor states in parkinson’s disease. PLoS Digit. Health 2023, 2, e0000225. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Pérez-López, C. Commercial devices for monitoring symptoms in Parkinson’s disease: Benefits, limitations and trends. Rev. Neurol. 2024, 79, 229–237. [Google Scholar] [CrossRef] [PubMed]
| Author(s), Year | Population | Clinical Characteristics | Technology Employed | Exploration Protocol | Main Findings |
|---|---|---|---|---|---|
| Teräväinen et al., 1989 [13] | PD patients (n = 20), Controls (n = 12) | H&Y stages were not reported. The patients were treated with a variety of antiparkinsonian drugs including levodopa. Many of them experienced fluctuations of disability. | Electromechanical apparatus | Passive wrist movements angular velocities from 12 to 240 degrees per second and over angular displacements from ±15 to ±30 degrees. | Objective rigidity was more pronounced at faster movement velocities in Parkinson’s patients, whereas normal subjects showed only a modest score increase. Angular velocities of 140–190°/s and displacements of ±25–30° were most sensitive for detecting Parkinsonian rigidity and correlated well with the clinical rigidity score. |
| Fung et al., 2000 [22] | PD patients (n = 20), Controls (n = 10) | H&Y stages (on) ranged from 0 to 4. H&Y stages (off) ranged from 1 to 5. Disease duration ranged from 5 to 30 years. | Electromechanical system + Surface electromyographic (EMG) | Passive wrist movement. The torque motor was operated as a position servo7 to generate sinusoidal perturbations of 60° peak-to-peak amplitude about the 0° position (that is, ±30°) | Angular impulse is a valid objective measure of Parkinsonian rigidity. Activation increases rigidity, but to varying degrees across patients. To improve the sensitivity and reproducibility of clinical rigidity assessments, Parkinson’s rating scales should include separate resting and activated scores. Both elastic and non-elastic forces contribute to the clinical perception of Parkinsonian rigidity. |
| Asci et al., 2023 [23] | PD patients (n = 20), Controls (n = 25) | PD (67.3 ± 6.9 years). 25 age- and sex-matched controls (66.9 ± 7.4 years). Hoehn & Yahr stage 2.1 ± 0.6. UPDRS-III: 21.8 ± 8.2. Upper limb rigidity in UPDRS-III: 2.1 ± 0.8 Disease duration 5.1 ± 2.5 years. | Biomechanical device + EMG | Wrist extensions at seven different angular velocities (5–50–100–150–200–236–280°/s) randomly applied, when ON therapy. Range of motion (ROM) of 50° (i.e., ranging from −20° to +30°). | In patients, objective rigidity progressively increased with rising angular velocities during robot-assisted wrist extensions. Neurophysiological examination revealed increased long-latency reflexes, but not short-latency reflexes or the shortening reaction, in PD compared with control subjects. Long-latency reflexes increased progressively with angular velocity only in people with PD. Specific biomechanical and neurophysiological abnormalities correlated with the clinical rigidity score. |
| Powell et al., 2012 [24] | PD patients (n = 18) | 64.1 ± 9.0 years old. Disease duration ranged from 1 to 14 years. Rigidity (in UPDRS) ranged from 0 to 3 (on) and from 2 to 3 (off). | Electromechanical device + EMG | Passive wrist movement through 60° and 90° ranges of wrist flexion and extension at velocities of 50°/s and 280°/s in both treated and untreated conditions. | Both work scores and angular impulses showed that larger displacement amplitudes and higher velocities were associated with significantly greater rigidity, increased EMG ratio, and higher mean EMG of stretched muscles. Dopaminergic medication was not associated with a reduction in rigidity. Parkinsonian rigidity is modulated by the amplitude and rate of muscle stretch. |
| Zetterberg et al., 2015 [25] | PD patients (n = 25), Controls (n = 14) | Mean age in PD group was 72 ± 5.9 (SD) years, mean time since diagnosis was 7 ± 5.3 (SD) years, and mean age for controls was 73 ± 4.9 (SD) years. | NeuroFlexor device | Passive wrist movements. The range of wrist movement was 50°, starting position at 20° flexion and end position at 30° extension. Two velocities were used: slow 5°/s and fast 236°/s. | The findings suggest that stretch-induced reflex activity, rather than non-neural resistance, is the major contributor to rigidity in wrist muscles in PD. The NeuroFlexor is a potentially valuable clinical and research tool for the quantification of rigidity. |
| Xia et al., 2016 [26] | PD patients (n = 14), Controls (n = 14) | The average ages were 62.6 (±9.1) years for the PD group and 62.9 (±8.5) years for the control group. H&Y ranged from 1 to 4. UPDRS (rigidity) ranged from 0 to 3 (on); and from 2 to 3 (off). Disease duration (years) ranged from 1 to 13. | Electromechanical + EMG | Passive wrist movement at 50º/s. Each subject with PD was tested in Off- and On-medication states. | The results showed that the reflex and intrinsic components were comparable (p > 0.05), and both were greater in PD subjects than in controls (p < 0.05). Medication decreased the reflex component of stiffness (p < 0.01). These findings indicate that both reflex and intrinsic factors contribute to stiffness. |
| Xia et al., 2009 [27] | PD patients (n = 20) | Subjects’ age ranged from 52 to 74 years with an average of 64 ± 7 years. Disease duration (years) ranged from 1 to 11. UPDRS (rigidity) ranged from 0 to 2 (on); and from 2 to 3 (off). | Electromechanical device + EMG | Wrist flexion and extension within ±30º at velocities 50 and 280/s. PD patients were evaluated in Off-medication and On medication states | Both the stretch reflex and the shortening reaction are important determinants of rigidity. |
| Zito et al., 2018 [28] | PD patients (n = 4), Controls (n = 12) | People with PD with a mean age = 50.5 years, SD = 4.2. No more clinical data were showed. | Novel electromechanical wrist device | Passive wrist movement at velocities of 10°/s, 50°/s, and 100°/s. From 7° to −37°. | The comparison of median rigidity between PD patients and HC showed significant differences at all tested velocities, during both flexion and extension. These findings are consistent with the literature, which indicates that the main contributor to rigidity in PD is a stretch-reflex-induced increase in passive movement resistance. |
| Lee et al., 2002 [14] | Stroke patients (n = 12), PD patients (n= 16), Controls (n = 12) | Twelve subjects had spastic hemiparesis, age range 31 to 77 years (mean (SD), 62.2 (6.5) years), with time from onset varying from 8 weeks to 20 years. Sixteen had rigid parkinsonism, age range 27 to 85 years (mean 60.6 (13.5)) and time of onset between 4 weeks and 6 years. The control group consisted of 12 age matched normal persons without a history of neurological disease. | Electromechanical apparatus | Elbow flexors were vertically stretched under four different velocities (40, 80, 120, and 160°/s) through a 75 degrees range of motion. | Velocity-dependence analysis indicates that rigidity and spasticity have approximately equal velocity-dependent properties. To differentiate these two types of hypertonia, position-dependent properties may be used. However, the progressively increasing muscle tone of spasticity differs from the increased (relative to normal) but constant muscle tone observed in parkinsonism. |
| Endo et al., 2015 [29] | PD patients (n = 20) | Mean age: 74.4 ± 6.2 years. The UPDRS rigidity score was 1 in 8 patients, 2 in 9 patients, and 3 in 3 patients. | Muscle tone measurement device. | Passive elbow movement from 10 to 110º at two different velocities, 60°/s and 120°/s. All patients were on medication during the measurements. | The features of rigidity may differ from the conventional definition, which states that rigidity is not dependent on the velocity of joint movement. |
| Huang et al., 2016 [32] | Controls (n = 22), Stoke patients (n = 14), PD patients (n = 21) | Controls: 63.2 (7.5) years. PD: 68.3 (9.9) years. Stroke: 65.1 (8.8) years. | Pendulum test | Elbow joint passive oscillations at spontaneous frequency | No differences were observed between these two patient groups. Hypertonia in Parkinsonian and stroke patients could not be differentiated by the modified pendulum test; elbow extensors exhibited higher muscle tone in both control and patient groups; and hypertonia in both Parkinsonian and stroke patients was velocity dependent. |
| Rothwell et al., 1983 [16] | PD patients (n = 47), Controls (n = 12) | The patients were classified clinically into four groups according to the degree of rigidity at the elbow or tremor. | Electromechanical apparatus + EMG | Upper limb: Long latency stretch reflexes in flexor pollicis longus and triceps brachii was assessed. Six velocities of ramp were used from 15°/s to 600°/s, reaching a maximum displacement of 20º. | Enhanced long-latency stretch reflexes contribute to, but may not be solely responsible for, rigidity in Parkinson’s disease. This technique revealed increased reflex sensitivity of the flexor pollicis longus in both moderately and severely rigid patients, which was not evident using step-torque stretches alone. |
| Nuyens et al., 2000 [30] | PD patients (n = 10), Controls (n = 10) | 65.4 ± 7.41 years had a disease duration of 10 ± 5.23 years. Grades on the modified Hoehn and Yahr staging ranged between 2 and 5. Schwab and England scores varied between 20 and 90%. | Isokinetic dynamometer + EMG | Knee passive flexion-extension at speeds of 60º, 180º and 300º/s in series of ten consecutive movements per velocity. | PD patients demonstrated a greater decrease in resistive torque compared with healthy controls, particularly at higher velocities and during knee flexion. These results contribute to the ongoing debate on whether Parkinsonian rigidity is independent of movement speed and direction. |
| Uslu et al., 2021 [31] | PD patients (n = 40) | Duration of the disease was 5.5 ± 0.67 years. The number of participants in the subgroups was 8 (4 male, 4 female) for the UPDRS 1 group, 16 (11 male, 5 female) for the UPDRS 2 group, 11 (5 male, 6 female) for the UPDRS 3 group and 5 (2 male, 3 female) for the UPDRS 4 group. H&Y ranged from 1 to 4. | Pendulum test + EMG | Knee reflex triggered pendulum at different amplitudes and velocities. | Parkinson’s rigidity has a velocity-dependent component, which correlates negatively with the rigidity scale. |
| Gregoric et al., 1988 [33] | PD patients (n = 7) | -- | Electromechanical apparatus | Rigidity was measured during sinusoidal passive movements of the ankle joint | Velocity-dependent changes were observed, though less pronounced than in spasticity and expressed differently in flexor and extensor muscles: a mild decrease in resistive torque during faster stretching of dorsal flexors and an increase in resistance during stretching of plantar flexors. Dorsal flexors also frequently exhibited shortening reactions. |
| Mak et al., 2007 [15] | PD patients (n = 15), Controls (n = 15) | PD: mean age 64.7 (8.7) years. Hoehn and Yahr staging scores between 2–3 | Isokinetic dynamometry | Trunk movements at 60/s, 75/s, 90/s and 105/s. All tests were performed within 2 h after medication during the ‘‘on’’ phase of the medication cycle. | PD patients exhibited significantly higher muscle tone, indicated by increases in work done and torque at higher movement speeds. Within each subject group, resistive trunk muscle tone increased with passive movement velocity, but the increase was greater in PD patients. Rigidity is therefore not independent of movement speed. |
| Cano-de-la-Cuerda et al., 2014 [34] | PD patients (n = 36) | The mean age of the sample was 62 ± 11 years. Overall, 24 patients were classified as II, 8 as IB (unilateral and axial involvement) and 4 as III in the H&Y staging score. The mean value of disease severity in the UPDRS III was 22 ± 8 points. A total of 26 subjects reached 80%, 7 reached 90% and 3 reached 100% in the Schwab and England scale. The mean disease duration was 55.4 ± 14.3 months | Isokinetic dynamometry | Trunk flexion-extension at 30°/s, 45°/s, 60°/s | Their results suggested that angular velocities of 30°/s, 45°/s, and 60°/s using this objective method provided a valid assessment of trunk rigidity and correlated with disease severity, disease duration, functional status, and quality of life in PD patients. Rigidity appears to increase with assessment speed. |
| AXIS Item/Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teräväinen et al. 1989 [13] | ✓ | ? | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Fung et al. 2000 [22] | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Asci et al. 2023 [23] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Powell et al. 2012 [24] | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Zetterberg et al. 2015 [25] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Xia et al. 2016 [26] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Xia et al. 2009 [27] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Zito et al. 2018 [28] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lee et al. 2002 [14] | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Endo et al. 2015 [29] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Rothwell et al. 1983 [16] | ✓ | ? | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Nuyens et al. 2000 [30] | ✓ | ? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Uslu et al. 2021 [31] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Huang et al. 2016 [32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gregoric et al. 1998 [33] | ✓ | ? | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? | ? | ✗ |
| Mak et al. 2007 [15] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cano-de-la-Cuerda et al. 2014 [34] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| STROBE Item | Teräväinen et al., 1989 [13] | Fung et al., 2000 [22] | Asci et al., 2023 [23] | Powell et al., 2012 [24] | Zetterberg et al., 2015 [25] | Xia et al., 2009 [27] | Xia et al., 2016 [26] | Zito et al., 2018 [28] | Lee et al., 2002 [14] | Endo et al., 2015 [29] | Rothwell et al., 1983 [16] | Nuyens et al., 2000 [30] | Uslu et al., 2021 [31] | Huang et al., 2016 [32] | Gregoric et al., 1998 [33] | Mak et al., 2007 [15] | Cano-de-la-Cuerda et al., 2014 [34] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Design named in title/abstract | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1b | Structured abstract summary | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ⚠ |
| 2 | Background/rationale | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3 | Objectives (with hypotheses) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4 | Design described early | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5 | Setting & dates | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 6a | Eligibility & selection criteria | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ |
| 7 | Defined variables (exposure/outcome/confounders) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 8 | Data sources/measurement methods | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 9 | Efforts to address bias | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ⚠ | ⚠ | ⚠ | ⚠ | ⚠ |
| 10 | Sample size justification | ✗ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ✗ | ⚠ | ✓ | ⚠ | ✗ | ✓ | ✓ |
| 11 | Handling of quantitative variables | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ |
| 12a | Statistical methods, including confounding | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ |
| 12b | Subgroup/interactions methods | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 12c | Methods for missing data | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 12d | Accounting for sampling strategy | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 12e | Sensitivity analyses | ✗ | ✗ | ⚠ | ✗ | ⚠ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 13a | Participant flow numbers | ✗ | ⚠ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 13b | Reasons for non-participation | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ |
| 13c | Flow diagram considered | ✗ | ✗ | ⚠ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 14a | Descriptive data (participant characteristics) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 14b | Missing data per variable | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ |
| 15 | Outcome data (events/summary measures) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 16a | Unadjusted & adjusted estimates | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 16b | Report variable categorization boundaries | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 16c | Translate relative to absolute risk | ✗ | ✗ | ⚠ | ✗ | ⚠ | ⚠ | ⚠ | ✗ | ✗ | ⚠ | ✗ | ✗ | ⚠ | ✗ | ✗ | ⚠ | ⚠ |
| 17 | Other analyses (subgroups/sensitivity) | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| 18 | Key results summary | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 19 | Limitations discussed | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ | ⚠ | ✓ | ✓ |
| 20 | Interpretation in context | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 21 | Generalizability discussed | ⚠ | ⚠ | ✓ | ⚠ | ✓ | ✓ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ⚠ | ⚠ | ✓ | ✓ |
| 22 | Funding and role of funders disclosed | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| Strengths | Weaknesses |
|---|---|
| Use of Advanced Instrumentation: Many studies employed sophisticated biomechanical and neurophysiological tools (e.g., isokinetic dynamometry, NeuroFlexor, EMG-integrated devices), allowing precise quantification of passive resistance and its components. Inclusion of Multiple Joints: The studies covered a broad range of anatomical regions (wrist, elbow, knee, trunk), providing a more comprehensive understanding of rigidity expression across the body. Objective and Quantitative Approaches: Most articles moved beyond subjective clinical scales, using measurable biomechanical indices (e.g., torque, work, angular impulse) to capture rigidity changes with velocity. Recent High-Quality Studies: More recent contributions (e.g., Asci et al., 2023) [23] demonstrated excellent methodological transparency and addressed both reflexive and non-reflexive mechanisms underlying rigidity. | Protocol Heterogeneity: There was substantial variability in passive movement velocities, durations, angles, and devices used, making inter-study comparisons difficult and precluding meta-analysis. Small Sample Sizes: Several studies had limited participant numbers without clear sample size justification, reducing statistical power and generalizability. Incomplete Reporting: Some studies did not fully meet reporting standards (e.g., missing participant flow, recruitment strategy, or blinding), as identified in AXIS and STROBE assessments. Lack of Standardization in Medication State: Medication status (“on” vs. “off” levodopa) was not consistently controlled or reported, even though it significantly influences rigidity. |
| Opportunities | Threats |
| Development of Standardized Protocols: Future studies can build consensus on optimal joint angles, velocities, and outcome measures to assess rigidity and its velocity dependency consistently. Integration of Multimodal Measurements: Combining mechanical, EMG, and neuroimaging data could help disentangle the neural vs. non-neural contributions to rigidity and explore its pathophysiological basis more deeply. Clinical Translation: Rigidity metrics that are velocity-sensitive may aid in differential diagnosis (e.g., spasticity vs. rigidity) or in tracking treatment responses in PD and atypical parkinsonism. Longitudinal Studies: Most current studies are cross-sectional. Longitudinal work could help understand how rigidity and its velocity-dependence evolve with disease progression or treatment. | Technological Accessibility: High-end measurement tools used in some studies may not be widely available in clinical settings, limiting their translational impact. Diagnostic Overlap: In older patients or those with overlapping motor syndromes (e.g., PSP), distinguishing between rigidity and spasticity remains challenging, especially in biomechanical tests alone. Lack of Consensus on Terminology: The inconsistency in how rigidity, tone, and stiffness are defined or operationalized may obscure the interpretation and comparison of findings. Potential Bias in Older Studies: Several early publications lacked methodological rigor or failed to use blinded assessors or validated instruments, which may bias their conclusions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-de-la-Cuerda, R.; Estrada-Barranco, C.; Martín-Casas, P.; Marcos-Antón, S.; Ortiz-Gutiérrez, R.M.; Laguarta-Val, S.; Jiménez-Antona, C. Speed Matters: Challenging the Notion of Velocity-Independent Rigidity Using Technological Devices in People with Parkinson’s Disease: A Systematic Review. Neurol. Int. 2025, 17, 186. https://doi.org/10.3390/neurolint17110186
Cano-de-la-Cuerda R, Estrada-Barranco C, Martín-Casas P, Marcos-Antón S, Ortiz-Gutiérrez RM, Laguarta-Val S, Jiménez-Antona C. Speed Matters: Challenging the Notion of Velocity-Independent Rigidity Using Technological Devices in People with Parkinson’s Disease: A Systematic Review. Neurology International. 2025; 17(11):186. https://doi.org/10.3390/neurolint17110186
Chicago/Turabian StyleCano-de-la-Cuerda, Roberto, Cecilia Estrada-Barranco, Patricia Martín-Casas, Selena Marcos-Antón, Rosa María Ortiz-Gutiérrez, Sofía Laguarta-Val, and Carmen Jiménez-Antona. 2025. "Speed Matters: Challenging the Notion of Velocity-Independent Rigidity Using Technological Devices in People with Parkinson’s Disease: A Systematic Review" Neurology International 17, no. 11: 186. https://doi.org/10.3390/neurolint17110186
APA StyleCano-de-la-Cuerda, R., Estrada-Barranco, C., Martín-Casas, P., Marcos-Antón, S., Ortiz-Gutiérrez, R. M., Laguarta-Val, S., & Jiménez-Antona, C. (2025). Speed Matters: Challenging the Notion of Velocity-Independent Rigidity Using Technological Devices in People with Parkinson’s Disease: A Systematic Review. Neurology International, 17(11), 186. https://doi.org/10.3390/neurolint17110186








