Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease
Abstract
1. Introduction
2. Pathophysiology of PD
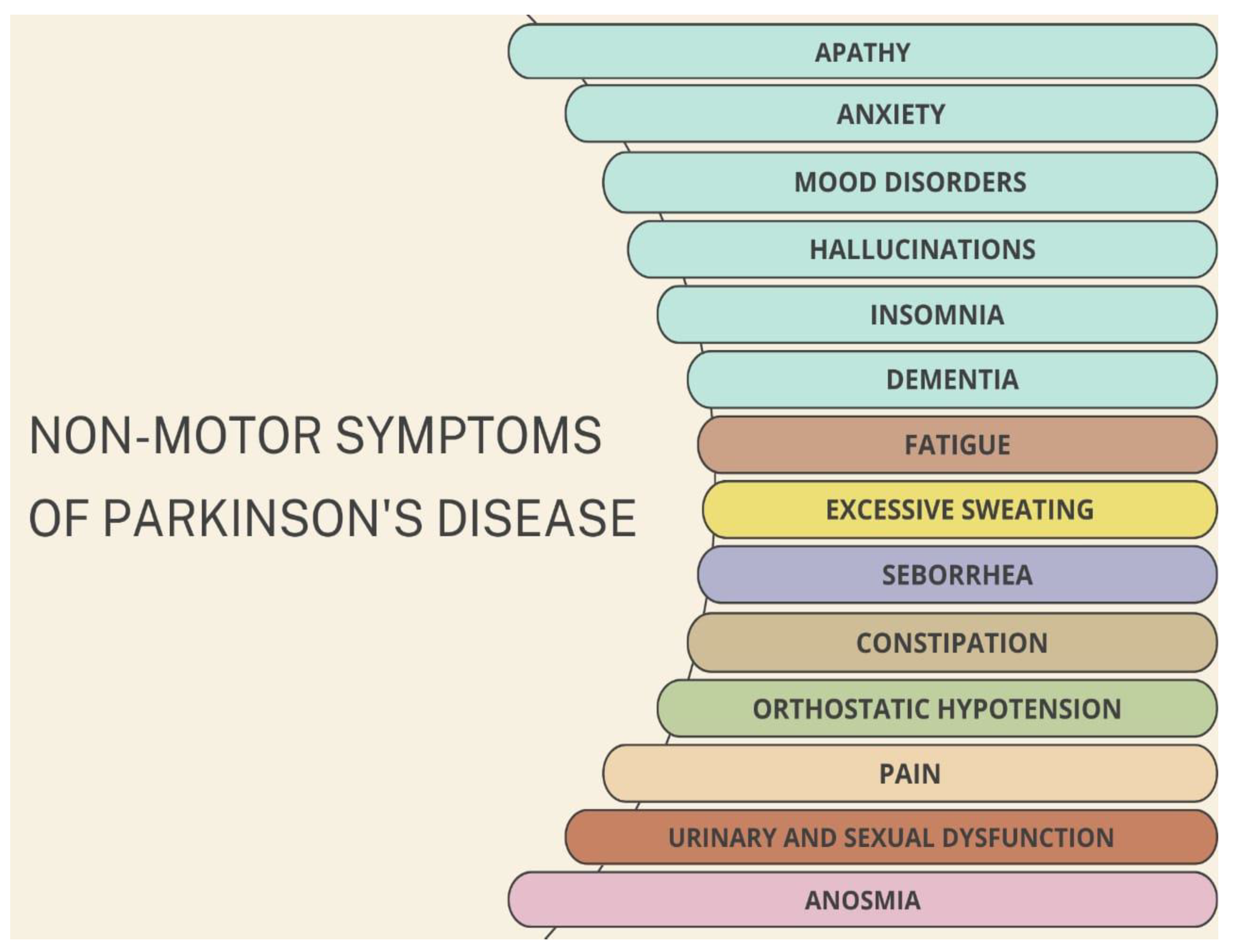
2.1. Clinical Features of PD
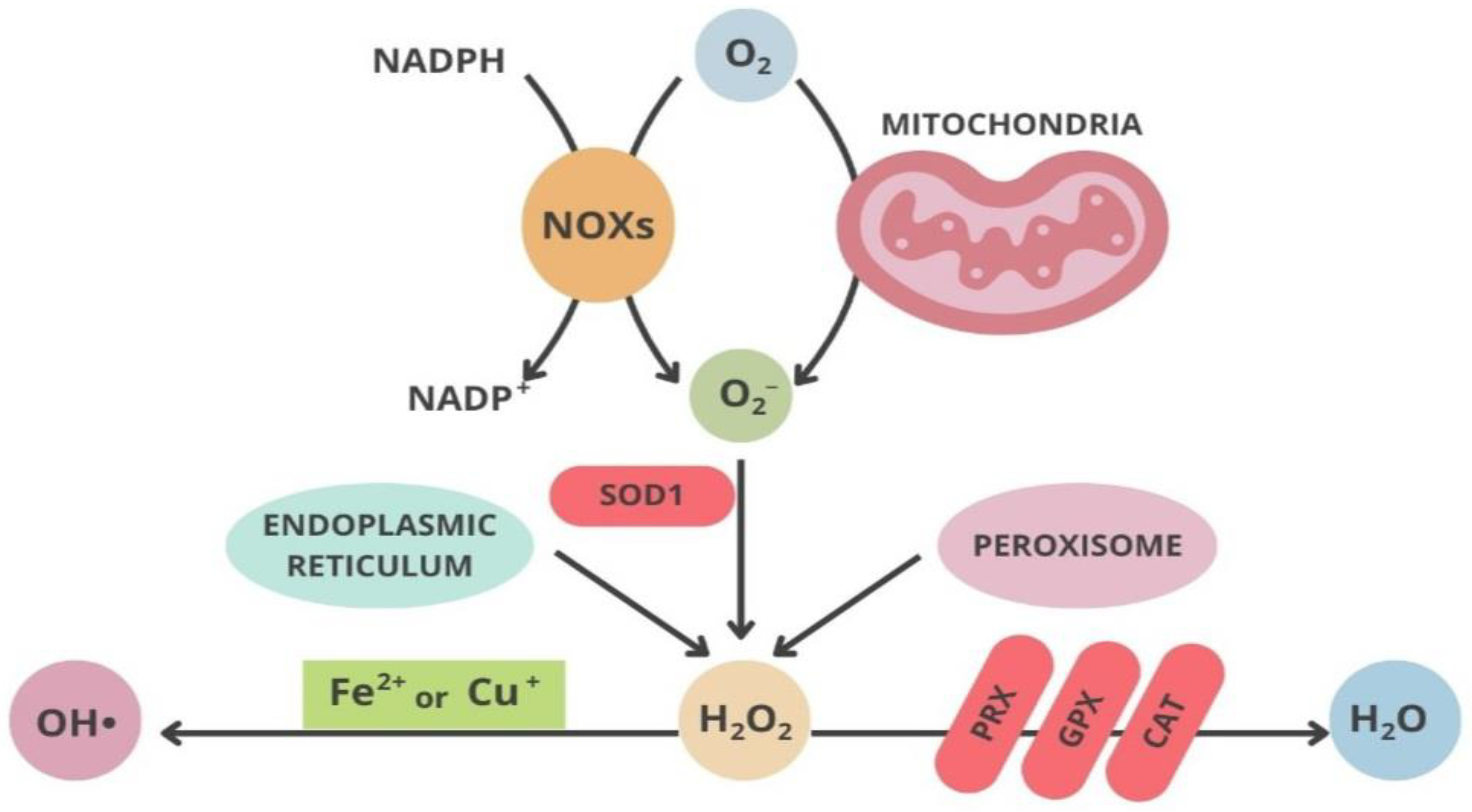
2.2. Mitochondria and Oxidative Stress
2.3. Neuroinflammation in PD
3. Treatments Targeting Oxidative Stress in PD
| Antioxidants | Targeting Oxidative Stress | Reference |
|---|---|---|
(a) Vitamin E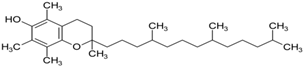 | Interference with iron accumulation reducing neuronal damage and slowing down the progression of PD; protection against iron and MPTP-induced neurodegeneration in mice. | Lan and Jiang, 1997 Jin et al., 2014 |
(b) Creatine | Neuroprotective agents resulting in ameliorated social difficulties; protection against MPTP-induced dopamine reduction in mice. | Jin et al., 2014 Matthews et al., 1999 |
(c) CoQ10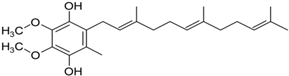 | Inhibition of ROS generation, sustain of mitochondrial membrane potential, slowness of the decline of PD. It slows the decline in subjects with early PD. | Somayajulu et al., 2005 Shults, 2002 |
(d) MitoQ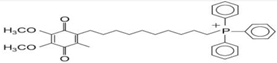 | Reduction in mitochondrial fragmentation, diminishing the activation and translocation of Bax., inhibition of MPTP-induced neurotoxicity in mouse models. | Solesio et al., 2013 Ghosh et al., 2010 |
(e) Quercetin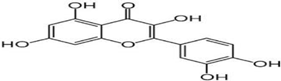 | Reduction in synuclein aggregation, boost of mitochondrial activity, depletion of oxidative stress | Bayazid and Lim, 2022 |
4. Discussion and Conclusions
5. Material and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rapp, T.; Chauvin, P.; Costa, N.; Molinier, L. Health Economic Considerations in Neurodegenerative Disorders. In Imaging in Neurodegenerative Disorders; Saba, L., Ed.; Oxford University Press: Oxford, UK, 2015; pp. 42–53. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Morris, H.R.; Robbins, T.W.; Goedert, M.; Hardy, J.; Ben-Shlomo, Y.; Bolam, P.; Burn, D.; Hindle, J.V.; Brooks, D.; et al. Parkinson’s Disease—The Debate on the Clinical Phenomenology, Aetiology, Pathology and Pathogenesis. J. Park. Dis. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Beal, M.F. Parkinson’s disease. Hum. Mol. Genet. 2007, 16, R183–R194. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- El-Agnaf, O.M.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of Oligomeric Forms of A-synuclein Protein in Human Plasma as a Potential Biomarker for Parkinson’s Disease. FASEB J. 2006, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ferrer, I.; Martinez, A.; Blanco, R.; Dalfo, E.; Carmona, M. Neuropathology of Sporadic Parkinson Disease before the Appearance of Parkinsonism: Preclinical Parkinson Disease. J. Neural Transm. 2011, 118, 821–839. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; D’angelo, M. Neuronal Cells Rearrangement during Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial Clinical Manifestations of Parkinson’s Disease: Features and Pathophysiological Mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef]
- Massano, J.; Bhatia, K.P. Clinical Approach to Parkinson’s Disease: Features, Diagnosis, and Principles of Management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef]
- Bain, P.G. Tremor. Park. Relat. Disord. 2007, 13, S369–S374. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Sethi, K. Levodopa Unresponsive Symptoms in Parkinson Disease. Mov. Disord. 2008, 23, S521–S533. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Dagan, Y.; Hausdorff, J.M. Postural Instability and Fall Risk in Parkinson’s Disease: Impaired Dual Tasking, Pacing, and Bilateral Coordination of Gait during the ‘ON’ Medication State. Exp. Brain Res. 2011, 210, 529–538. [Google Scholar] [CrossRef]
- Spildooren, J.; Vercruysse, S.; Desloovere, K.; Vandenberghe, W.; Kerckhofs, E.; Nieuwboer, A. Freezing of Gait in Parkinson’s Disease: The Impact of Dual-tasking and Turning. Mov. Disord. 2010, 25, 2563–2570. [Google Scholar] [CrossRef]
- Poewe, W. Non-Motor Symptoms in Parkinson’s Disease. Eur. J. Neurol. 2008, 15 (Suppl. S1), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H. Non-Motor Symptoms of Parkinson’s Disease: Dopaminergic Pathophysiology and Treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; Lees, A.J.; Schrag, A. What Are the Most Important Nonmotor Symptoms in Patients with Parkinson’s Disease and Are We Missing Them? Mov. Disord. 2010, 25, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Gaig, C.; Santamaría, J.; Compta, Y. Diagnosis and the Premotor Phase of Parkinson Disease. Neurology 2009, 72, S12–S20. [Google Scholar] [CrossRef]
- Savica, R.; Rocca, W.A.; Ahlskog, J.E. When Does Parkinson Disease Start? Arch. Neurol. 2010, 67, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Hoek, S.; Fon, E.A.; Wade-Martins, R. Mitochondrial Dysfunction and Mitophagy in Parkinson’s: From Familial to Sporadic Disease. Trends Biochem. Sci. 2015, 40, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial Superoxide: Production, Biological Effects, and Activation of Uncoupling Proteins. Free. Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Schapira, A.H. Anthony Hv. Mitochondria in the Aetiology and Pathogenesis of Parkinson’s Disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Hall, C.N.; Klein-Flügge, M.C.; Howarth, C.; Attwell, D. Oxidative Phosphorylation, Not Glycolysis, Powers Presynaptic and Postsynaptic Mechanisms Underlying Brain Information Processing. J. Neurosci. 2012, 32, 8940–8951. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.; Albrecht, J. Changes in the Mitochondrial Antioxidant Systems in Neurodegenerative Diseases and Acute Brain Disorders. Neurochem. Int. 2015, 88, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Collins, Y.; Chouchani, E.T.; James, A.M.; Menger, K.E.; Cochemé, H.M.; Murphy, M.P. Mitochondrial Redox Signalling at a Glance. J. Cell Sci. 2012, 125, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.D., Jr.; Parks, J.K.; Swerdlow, R.H. Complex I Deficiency in Parkinson’s Disease Frontal Cortex. Brain Res. 2008, 1189, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Przedborski, S. Parkinson’s Disease: Animal Models and Dopaminergic Cell Vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Kim, J.-H.; Greenamyre, J. Selective Microglial Activation in the Rat Rotenone Model of Parkinson’s Disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Greenamyre, J.T.; Cannon, J.R.; Drolet, R.; Mastroberardino, P.-G. Lessons from the Rotenone Model of Parkinson’s Disease. Trends Pharmacol. Sci. 2010, 31, 141–142. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.-W. Oxidative Stress and Cellular Pathologies in Parkinson’s Disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Norris, K.L.; Hao, R.; Chen, L.-F.; Lai, C.-H.; Kapur, M.; Shaughnessy, P.J.; Chou, D.; Yan, J.; Taylor, J.P.; Engelender, S.; et al. Convergence of Parkin, PINK1, and α-Synuclein on Stress-Induced Mitochondrial Morphological Remodeling. Biol. Chem. 2015, 290, 13862–13874. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; Dashti, Z.J.S.; Christoffels, A.; Loos, B.; Bardien, S. Evidence for a Common Biological Pathway Linking Three Parkinson’s Disease-causing Genes: Parkin, PINK1 and DJ-1. Eur. J. Neurosci. 2015, 41, 1113–1125. [Google Scholar] [CrossRef]
- Scarffe, L.A.; Stevens, D.A.; Dawson, V.L.; Dawson, T.M. Parkin and PINK1: Much More than Mitophagy. Trends Neurosci. 2014, 37, 315–324. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Müftüoglu, M.; Elibol, B.; Dalmızrak, Ö.; Ercan, A.; Kulaksız, G.; Ögüs, H.; Dalkara, T.; Özer, N. Mitochondrial Complex I and IV Activities in Leukocytes from Patients with Parkin Mutations. Mov. Disord. 2004, 19, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Liu, J.; Hong, X.; Yu, M.; Huang, Y.; Sheng, Z.; Fei, J. Overexpression of Parkin Ameliorates Dopaminergic Neurodegeneration Induced by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine in Mice. PLoS ONE 2012, 7, e39953. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Oelhafen, S.; Hua, H.; Georgiev, O.; Schaffner, W.; Büeler, H. Extended Lifespan of Drosophila Parkin Mutants through Sequestration of Redox-Active Metals and Enhancement of Anti-Oxidative Pathways. Neurobiol. Dis. 2010, 40, 82–92. [Google Scholar] [CrossRef]
- Wang, H.-L.; Chou, A.-H.; Wu, A.-S.; Chen, S.-Y.; Weng, Y.-H.; Kao, Y.-C.; Yeh, T.-H.; Chu, P.-J.; Lu, C.-S. PARK6 PINK1 Mutants Are Defective in Maintaining Mitochondrial Membrane Potential and Inhibiting ROS Formation of Substantia Nigra Dopaminergic Neurons. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 674–684. [Google Scholar] [CrossRef]
- Piccoli, C.; Sardanelli, A.; Scrima, R.; Ripoli, M.; Quarato, G.; D’aprile, A.; Bellomo, F.; Scacco, S.; De Michele, G.; Filla, A.; et al. Mitochondrial Respiratory Dysfunction in Familiar Parkinsonism Associated with PINK1 Mutation. Neurochem. Res. 2008, 33, 2565–2574. [Google Scholar] [CrossRef]
- Gautier, C.A.; Kitada, T.; Shen, J. Loss of PINK1 Causes Mitochondrial Functional Defects and Increased Sensitivity to Oxidative Stress. Proc. Natl. Acad. Sci. USA 2008, 105, 11364–11369. [Google Scholar] [CrossRef]
- Exner, N.; Treske, B.; Paquet, D.; Holmström, K.; Schiesling, C.; Gispert, S.; Carballo-Carbajal, I.; Berg, D.; Hoepken, H.-H.; Gasser, T.; et al. Loss-of-Function of Human PINK1 Results in Mitochondrial Pathology and Can Be Rescued by Parkin. J. Neurosci. 2007, 27, 12413–12418. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Pan, Y.; Price, A.C.; Sterling, W.; Copeland, N.G.; Jenkins, N.A.; Price, D.L.; Lee, M.K. Parkinson’s Disease α-Synuclein Transgenic Mice Develop Neuronal Mitochondrial Degeneration and Cell Death. J. Neurosci. 2006, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Vergnes, L.; Franich, N.R.; Reue, K.; Chesselet, M.-F. Region Specific Mitochondrial Impairment in Mice with Widespread Overexpression of Alpha-Synuclein. Neurobiol. Dis. 2014, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Pukaß, K.; Richter-Landsberg, C. Oxidative Stress Promotes Uptake, Accumulation, and Oligomerization of Extracellular α-Synuclein in Oligodendrocytes. J. Mol. Neurosci. 2014, 52, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Schlachetzki, J.C.; Helling, S.; Bussmann, J.C.; Berlinghof, M.; Schäffer, T.E.; Marcus, K.; Winkler, J.; Klucken, J.; Becker, C.-M. Oxidative Stress-Induced Posttranslational Modifications of Alpha-Synuclein: Specific Modification of Alpha-Synuclein by 4-Hydroxy-2-Nonenal Increases Dopaminergic Toxicity. Mol. Cell. Neurosci. 2013, 54, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ishimori, C.; Takahashi-Niki, K.; Taira, T.; Kim, Y.-C.; Maita, H.; Maita, C.; Ariga, H.; Iguchi-Ariga, S.M.M. DJ-1 Binds to Mitochondrial Complex I and Maintains Its Activity. Biochem. Biophys. Res. Commun. 2009, 390, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shimoji, M.; Thomas, B.; Moore, D.J.; Yu, S.-W.; Marupudi, N.I.; Torp, R.; Torgner, I.A.; Ottersen, O.P.; Dawson, T.M.; et al. Mitochondrial Localization of the Parkinson’s Disease Related Protein DJ-1: Implications for Pathogenesis. Hum. Mol. Genet. 2005, 14, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial Localization of DJ-1 Leads to Enhanced Neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial Dysfunction in Parkinson’s Disease—A Key Disease Hallmark with Therapeutic Potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Li, J.-L.; Lin, T.-Y.; Chen, P.-L.; Guo, T.-N.; Huang, S.-Y.; Chen, C.-H.; Lin, C.-H.; Chan, C.-C. Mitochondrial Function and Parkinson’s Disease: From the Perspective of the Electron Transport Chain. Front. Mol. Neurosci. 2021, 14, 797833. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, J.; Zhang, L.; Peng, Q. Iron Deposition in Parkinson’s Disease: A Mini-Review. Cell. Mol. Neurobiol. 2024, 44, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Y.; Zhang, Y.; Wang, F.; Yu, H.; Zhang, C.; Jiang, Z.; Luo, W. Iron Deposition in Parkinson’s Disease by Quantitative Susceptibility Mapping. BMC Neurosci. 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- You, L.-H.; Li, F.; Wang, L.; Zhao, S.-E.; Wang, S.-M.; Zhang, L.-H.; Duan, X.-L.; Yu, P.; Chang, Y.-Z. Brain Iron Accumulation Exacerbates the Pathogenesis of MPTP-Induced Parkinson’s Disease. Neuroscience 2015, 284, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Alhazmi, H.A.; Hassani, R.; Khuwaja, G.; Maheshkumar, V.; Aldahish, A.; Chidambaram, K. RRole of Ferroptosis Pathways in Neuroinflammation and Neurological Disorders: From Pathogenesis to Treatment. Heliyon 2024, 10, e24786. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Walker, A.J.; Berk, M.; Maes, M.; Puri, B.K. Cell Death Pathways: A Novel Therapeutic Approach for Neuroscientists. Mol. Neurobiol. 2018, 55, 5767–5786. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Vila, M. Mitochondrial Biology and Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, P.; Knight, W.; Guo, Y.; Perlmutter, J.S.; Benzinger, T.L.S.; Morris, J.C.; Xu, J. The Interactions of Dopamine and Oxidative Damage in the Striatum of Patients with Neurodegenerative Diseases. J. Neurochem. 2020, 152, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H. Innate Inflammation in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009373. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Lecca, D.; Mulas, G.; Wardas, J.; Simbula, G.; Spiga, S.; Carta, A.R. Dynamic Changes in Pro- and Anti-Inflammatory Cytokines in Microglia after PPAR-γ Agonist Neuroprotective Treatment in the MPTPp Mouse Model of Progressive Parkinson’s Disease. Neurobiol. Dis. 2014, 71, 280–291. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting Microglia-Mediated Neurotoxicity: The Potential of NOX2 Inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxid. Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Bisaglia, M. Oxidative Stress and Neuroinflammation in Parkinson’s Disease: The Role of Dopamine Oxidation Products. Antioxidants 2023, 12, 955. [Google Scholar] [CrossRef]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and Mitochondrial Dysfunction: A Vicious Circle in Neurodegenerative Disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and Cell Signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef]
- Wolf, A.J.; Reyes, C.N.; Liang, W.; Becker, C.; Shimada, K.; Wheeler, M.L.; Cho, H.C.; Popescu, N.I.; Coggeshall, K.M.; Arditi, M.; et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 2016, 166, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.-J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New Mitochondrial DNA Synthesis Enables NLRP3 Inflammasome Activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Noh, H.; Jeon, J.; Seo, H. Systemic Injection of LPS Induces Region-Specific Neuroinflammation and Mitochondrial Dysfunction in Normal Mouse Brain. Neurochem. Int. 2014, 69, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Smeyne, R.J. MPTP and SNpc DA Neuronal Vulnerability: Role of Dopamine, Superoxide and Nitric Oxide in Neurotoxicity. Minireview. Neurotox. Res. 2005, 7, 193–201. [Google Scholar] [CrossRef]
- Barcia, C.; Bahillo, A.S.; Fernández-Villalba, E.; Bautista, V.; Poza Y Poza, M.; Fernández-Barreiro, A.; Hirsch, E.C.; Herrero, M.-T. Evidence of Active Microglia in Substantia Nigra Pars Compacta of Parkinsonian Monkeys 1 Year after MPTP Exposure. Glia 2004, 46, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The Inflammatory Response in the MPTP Model of Parkinson’s Disease Is Mediated by Brain Angiotensin: Relevance to Progression of the Disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar] [CrossRef]
- Gao, H.-M.; Liu, B.; Hong, J.-S. Critical Role for Microglial NADPH Oxidase in Rotenone-Induced Degeneration of Dopaminergic Neurons. J. Neurosci. 2003, 23, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Monroe, T.O.; Palmieri, M.; Sardiello, M.; Rodney, G.G. Rotenone Induces Neurotoxicity through Rac1-dependent Activation of NADPH Oxidase in SHSY-5Y Cells. FEBS Lett. 2014, 588, 472–481. [Google Scholar] [CrossRef]
- Zhang, W.; Phillips, K.; Wielgus, A.R.; Liu, J.; Albertini, A.; Zucca, F.A.; Faust, R.; Qian, S.Y.; Miller, D.S.; Chignell, C.F.; et al. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson’s Disease. Neurotox. Res. 2011, 19, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Ophof, A.; Broe, M.; Jensen, P.H.; Kettle, E.; Fedorow, H.; Cartwright, M.I.; Griffiths, F.M.; Shepherd, C.E.; Double, K.L. α-Synuclein Redistributes to Neuromelanin Lipid in the Substantia Nigra Early in Parkinson’s Disease. Brain 2005, 128, 2654–2664. [Google Scholar] [CrossRef]
- Li, J.; Scheller, C.; Koutsilieri, E.; Griffiths, F.; Beart, P.M.; Mercer, L.D.; Halliday, G.; Kettle, E.; Rowe, D.; Riederer, P.; et al. Differential Effects of Human Neuromelanin and Synthetic Dopamine Melanin on Neuronal and Glial Cells. J. Neurochem. 2005, 95, 599–608. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Rodriguez-Pallares, J.; Dominguez-Meijide, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain Angiotensin Regulates Iron Homeostasis in Dopaminergic Neurons and Microglial Cells. Exp. Neurol. 2013, 250, 384–396. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Medical Management of Parkinson’s Disease. Pharm. Ther. 2008, 33, 590–606. [Google Scholar]
- De Deurwaerdère, P.; Di Giovanni, G.; Millan, M.J. Expanding the Repertoire of L-DOPA’s Actions: A Comprehensive Review of Its Functional Neurochemistry. Prog. Neurobiol. 2017, 151, 57–100. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Levodopa Strengths and Weaknesses. Neurology 2002, 58 (Suppl. S1), S19–S32. [Google Scholar] [CrossRef]
- Jankovic, J. Motor Fluctuations and Dyskinesias in Parkinson’s Disease: Clinical Manifestations. Mov. Disord. 2005, 20, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Quinn, N. Dyskinesias and Motor Fluctuations in Parkinson’s Disease. Brain 2000, 123, 2297–2305. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current Approaches to the Treatment of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Schott, J.M. Epidemiological, Clinical, and Genetic Characteristics of Early-Onset Parkinsonism. Lancet Neurol. 2006, 5, 355–363. [Google Scholar] [CrossRef]
- Lücking, C.B.; Dürr, A.; Bonifati, V.; Vaughan, J.; De Michele, G.; Gasser, T.; Harhangi, B.S.; Meco, G.; Denèfle, P.; Wood, N.W.; et al. Association between Early-Onset Parkinson’s Disease and Mutations in the Parkin Gene. N. Engl. J. Med. 2000, 342, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Watts, R.L.; Martin, W.; Boroojerdi, B.; SP 512 Rotigotine Transdermal System Clinical Study Group. Transdermal Rotigotine: Double-Blind, Placebo-Controlled Trial in Parkinson Disease. Arch. Neurol. 2007, 64, 676–682. [Google Scholar] [CrossRef]
- Reiter, R.J. Oxidative Processes and Antioxidative Defense Mechanisms in the Aging Brain. FASEB J. 1995, 9, 526–533. [Google Scholar] [CrossRef]
- Odunze, I.N.; Klaidman, L.K.; Adams, J.D. MPTP Toxicity in the Mouse Brain and Vitamin E. Neurosci. Lett. 1990, 108, 346–349. [Google Scholar] [CrossRef]
- Lan, J.; Jiang, D.H. Desferrioxamine and Vitamin E Protect against Iron and MPTP-Induced Neurodegeneration in Mice. J. Neural Transm. 1997, 104, 469–481. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-Targeted Antioxidants for Treatment of Parkinson’s Disease: Preclinical and Clinical Outcomes. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1282–1294. [Google Scholar] [CrossRef]
- Matthews, R.T.; Ferrante, R.J.; Klivenyia, P.; Yanga, L.; Klein, A.M.; Muellera, G.; Kaddurah-Daouk, R.; Beala, M.F. Creatine and Cyclocreatine Attenuate MPTP Neurotoxicity. Exp. Neurol. 1999, 157, 142–149. [Google Scholar] [CrossRef]
- Somayajulu, M.; McCarthy, S.; Hung, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Role of Mitochondria in Neuronal Cell Death Induced by Oxidative Stress; Neuroprotection by Coenzyme Q10. Neurobiol. Dis. 2005, 18, 618–627. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, K.H.; Park, J.; Geum, D.; Kim, K. Mitochondrial Membrane Depolarization and the Selective Death of Dopaminergic Neurons by Rotenone: Protective Effect of Coenzyme Q10. J. Neurochem. 2005, 93, 1199–1208. [Google Scholar] [CrossRef]
- Beal, M.; Matthews, R.T.; Tieleman, A.; Shults, C.W. Coenzyme Q10 Attenuates the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Induced Loss of Striatal Dopamine and Dopaminergic Axons in Aged Mice. Brain Res. 1998, 783, 109–114. [Google Scholar] [CrossRef]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of Coenzyme Q10 in Early Parkinson Disease. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef]
- Idebenone Treatment of Early Parkinson’s Diseasesymptoms (ITEP). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03727295Solesio (accessed on 1 March 2024).
- Solesio, M.E.; Prime, T.A.; Logan, A.; Murphy, M.P.; Arroyo-Jimenez, M.d.M.; Jordán, J.; Galindo, M.F. The Mitochondria-Targeted Anti-Oxidant MitoQ Reduces Aspects of Mitochondrial Fission in the 6-OHDA Cell Model of Parkinson’s Disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 174–182. [Google Scholar] [CrossRef]
- Ghosh, A.; Chandran, K.; Kalivendi, S.V.; Joseph, J.; Antholine, W.E.; Hillard, C.J.; Kanthasamy, A.; Kanthasamy, A.; Kalyanaraman, B. Neuroprotection by a Mitochondria-Targeted Drug in a Parkinson’s Disease Model. Free Radic. Biol. Med. 2010, 49, 1674–1684. [Google Scholar] [CrossRef]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study Group. A Double-blind, Placebo-controlled Study to Assess the Mitochondria-targeted Antioxidant MitoQ as a Disease-modifying Therapy in Parkinson’s Disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Bej, E.; Volpe, A.R.; Cesare, P.; Cimini, A.; D’Angelo, M.; Castelli, V. Therapeutic Potential of Saffron in Brain Disorders: From Bench to Bedside. Phytotherapy Res. 2024, 1–14. [Google Scholar] [CrossRef]
- Castelli, V.; Grassi, D.; Bocale, R.; D’Angelo, M.; Antonosante, A.; Cimini, A.; Ferri, C.; Desideri, G. Diet and Brain Health: Which Role for Polyphenols? Curr. Pharm. Des. 2018, 24, 227–238. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Azam, S.; Cho, D.-Y.; Su-Kim, I.; Choi, D.-K. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, W.; et al. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Lim, B.O. Quercetin Is An Active Agent in Berries against Neurodegenerative Diseases Progression through Modulation of Nrf2/HO1. Nutrients 2022, 14, 5132. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Mirshekar, M.A.; Montazerifar, F.; Saadat, S.; Koushki, A.S.; Maskouni, S.J.; Afsharfar, M.; Arabmoazzen, S. Effects of Quercetin on Spatial Memory, Hippocampal Antioxidant Defense and BDNF Concentration in a Rat Model of Parkinson’s Disease: An Electrophysiological Study. Avicenna J. Phytomed. 2021, 11, 599–609. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-Enhancing Effect of Quercetin in a Rat Model of Parkinson’s Disease Induced by 6-Hydroxydopamine. Evid.-Based Complement. Altern. Med. 2012; 2012, 823206. [Google Scholar] [CrossRef]
- Karuppagounder, S.; Madathil, S.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K. Quercetin Up-Regulates Mitochondrial Complex-I Activity to Protect against Programmed Cell Death in Rotenone Model of Parkinson’s Disease in Rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-Latif, R.N.A.; ElBatsh, M.M.; Emam, M.N. Ameliorative Effect of Quercetin on Neurochemical and Behavioral Deficits in Rotenone Rat Model of Parkinson’s Disease: Modulating Autophagy (Quercetin on Experimental Parkinson’s Disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Rivera-dell Valle, N.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Corvo, M.L.; Jorge, J.C.; Hof, R.V.; Cruz, M.M.; Crommelin, D.J.; Storm, G. Superoxide Dismutase Entrapped in Long-Circulating Liposomes: Formulation Design and Therapeutic Activity in Rat Adjuvant Arthritis. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 227–236. [Google Scholar] [CrossRef]
- Reddy, M.K.; Wu, L.; Kou, W.; Ghorpade, A.; Labhasetwar, V. Superoxide Dismutase-Loaded PLGA Nanoparticles Protect Cultured Human Neurons Under Oxidative Stress. Appl. Biochem. Biotechnol. 2008, 151, 565–577. [Google Scholar] [CrossRef]
- Reddy, M.K.; Labhasetwar, V. Nanoparticle-mediated Delivery of Superoxide Dismutase to the Brain: An Effective Strategy to Reduce Ischemia-reperfusion Injury. FASEB J. 2009, 23, 1384–1395. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Tsai, M.-J.; Wu, P.-C.; Tsai, Y.-H.; Wu, Y.-H.; Fang, J.-Y. Elastic Liposomes as Carriers for Oral Delivery and the Brain Distribution of (+)-Catechin. J. Drug Target. 2011, 19, 709–718. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised Nano-Formulation on the Bioavailability of Hydrophobic Polyphenol, Curcumin, in Freely-Moving Rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J. Enhanced Oral Bioavailability of EGCG Using pH-Sensitive Polymeric Nanoparticles: Characterization and in Vivo Investigation on Nephrotic Syndrome Rats. Drug Des. Dev. Ther. 2018, 12, 2509–2518. [Google Scholar] [CrossRef]
- Lee, W.-H.; Kumar, A.; Rani, A.; Herrera, J.; Xu, J.; Someya, S.; Foster, T.C. Influence of Viral Vector–Mediated Delivery of Superoxide Dismutase and Catalase to the Hippocampus on Spatial Learning and Memory during Aging. Antioxidants Redox Signal. 2012, 16, 339–350. [Google Scholar] [CrossRef]
- Juarez-Moreno, K.; Ayala, M.; Vazquez-Duhalt, R. Antioxidant Capacity of Poly(Ethylene Glycol) (PEG) as Protection Mechanism Against Hydrogen Peroxide Inactivation of Peroxidases. Appl. Biochem. Biotechnol. 2015, 177, 1364–1373. [Google Scholar] [CrossRef]
- Zabiszak, M.; Nowak, M.; Taras-Goslinska, K.; Kaczmarek, M.T.; Hnatejko, Z.; Jastrzab, R. Carboxyl Groups of Citric Acid in the Process of Complex Formation with Bivalent and Trivalent Metal Ions in Biological Systems. J. Inorg. Biochem. 2018, 182, 37–47. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, Y.-P.; Liu, T.-P.; Chien, F.-C.; Chou, C.-M.; Chen, C.-T.; Mou, C.-Y. Approach To Deliver Two Antioxidant Enzymes with Mesoporous Silica Nanoparticles into Cells. ACS Appl. Mater. Interfaces 2016, 8, 17944–17954. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for Oligonucleotide Delivery to the Brain. Bioconjug. Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, X.; Liu, H.; Wang, Y.; Zhang, L.; Sun, Y. Multifunctionality of Self-Assembled Nanogels of Curcumin-Hyaluronic Acid Conjugates on Inhibiting Amyloid β-Protein Fibrillation and Cytotoxicity. React. Funct. Polym. 2016, 104, 22–29. [Google Scholar] [CrossRef]
- Feng, T.; Du, Y.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of Antioxidant Activity of Chitosan by Irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front. Neurosci. 2017, 11, 82. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Kesidou, D.; Moura, F.; Felli, E.; Song, W. Redox-Responsive Nanobiomaterials-Based Therapeutics for Neurodegenerative Diseases. Small 2020, 16, e1907308. [Google Scholar] [CrossRef]
- Büning, H.; Schmidt, M. Adeno-Associated Vector Toxicity—To Be or Not to Be? Mol. Ther. 2015, 23, 1673–1675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bej, E.; Cesare, P.; Volpe, A.R.; d’Angelo, M.; Castelli, V. Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease. Neurol. Int. 2024, 16, 502-517. https://doi.org/10.3390/neurolint16030037
Bej E, Cesare P, Volpe AR, d’Angelo M, Castelli V. Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease. Neurology International. 2024; 16(3):502-517. https://doi.org/10.3390/neurolint16030037
Chicago/Turabian StyleBej, Erjola, Patrizia Cesare, Anna Rita Volpe, Michele d’Angelo, and Vanessa Castelli. 2024. "Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease" Neurology International 16, no. 3: 502-517. https://doi.org/10.3390/neurolint16030037
APA StyleBej, E., Cesare, P., Volpe, A. R., d’Angelo, M., & Castelli, V. (2024). Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease. Neurology International, 16(3), 502-517. https://doi.org/10.3390/neurolint16030037








