Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
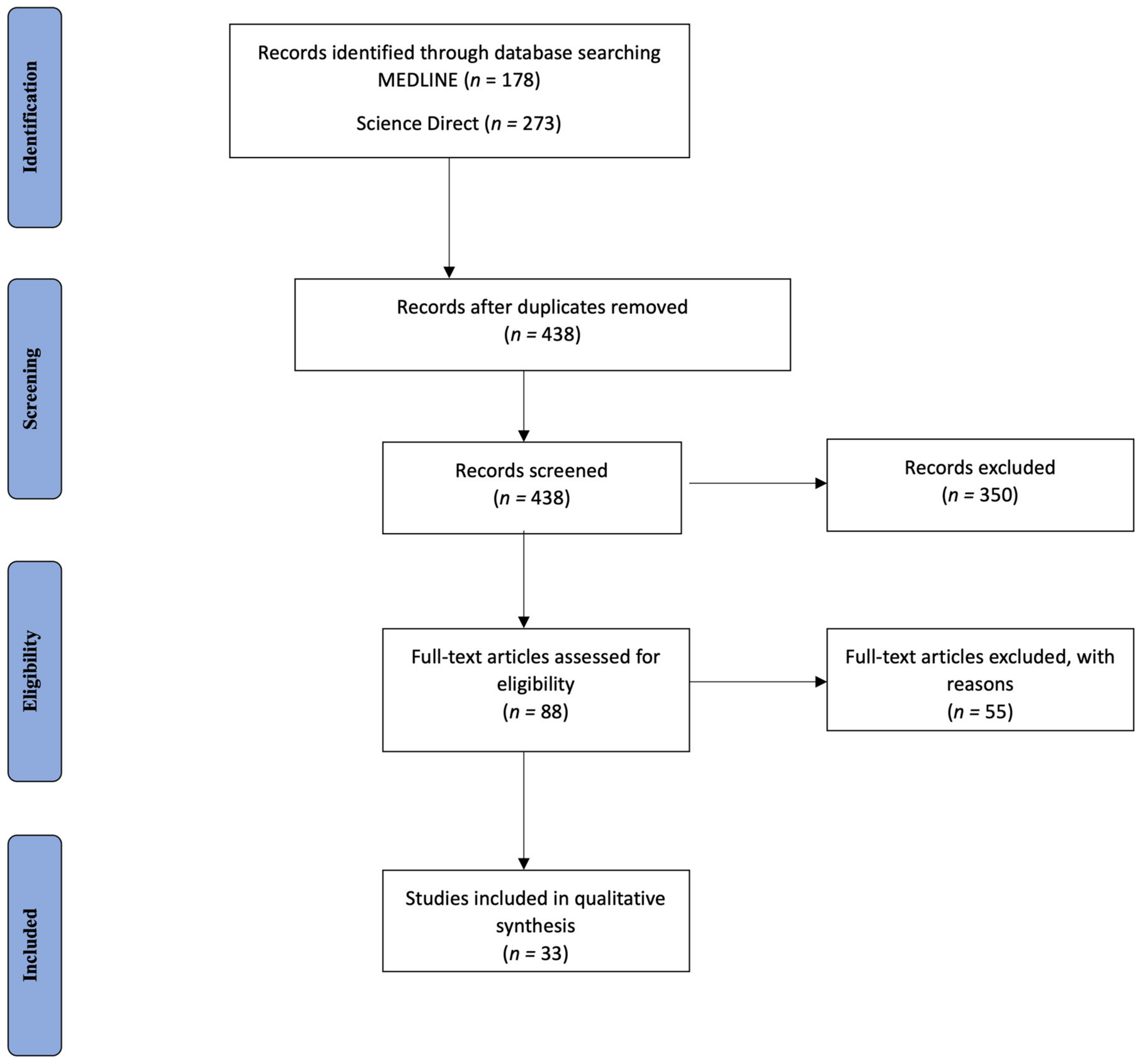
3.1. Database Search
3.2. Study Characteristics
3.3. Study Design
3.4. Stroke Patient Groups
3.5. Demographic and Clinical Profiles
3.6. Time of Sampling
3.7. Scales of Stroke Severity and Prognosis/Clinical Outcome
3.8. Presentation of Main Findings
3.8.1. Abundant NET Formation in AIS Patients’ Thrombi and Peripheral Blood
3.8.2. Correlation between Elevated Neutrophil Total Count, NET Marker Levels in Plasma, and Ischemic Stroke Severity Functional Outcome
3.8.3. Link between NETs Presence, the Age of Thrombi, and Success of Recanalization Therapies
3.8.4. NETs’ Correlation with AIS of Cardioembolic or Cryptogenic Origin
3.8.5. NETs Correlate with Worse Functional Outcome in SAH and ICH
4. Discussion
4.1. NETs Presence in Stroke Patients’ Thrombi, Peripheral Blood, and Brain Tissue: Timing and Pathophysiological Implications
4.2. Neutrophil Count, NLR, and NETs as Biomarkers of Stroke Severity, Unfavorable Outcomes, and All-Cause Mortality
4.3. NETs as a Prognostic Tool for Predicting Response to Recanalization Therapies
4.4. NETs as Predictor of Stroke Risk
4.5. NET Levels as an Indirect Marker of Stroke Etiology
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Vallés, J.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Lago, A.; Moscardó, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.; Skendros, P.; Konstantinides, S.; Ritis, K. Traps N’ Clots: NET-Mediated Thrombosis and Related Diseases. Thromb. Haemost. 2020, 120, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Kambas, K.; Chrysanthopoulou, A.; Vassilopoulos, D.; Apostolidou, E.; Skendros, P.; Girod, A.; Arelaki, S.; Froudarakis, M.; Nakopoulou, L.; Giatromanolaki, A.; et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann. Rheum. Dis. 2014, 73, 1854–1863. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Orbán-Kálmándi, R.; Árokszállási, T.; Fekete, I.; Fekete, K.; Héja, M.; Tóth, J.; Sarkady, F.; Csiba, L.; Bagoly, Z. A Modified in vitro Clot Lysis Assay Predicts Outcomes in Non-traumatic Intracerebral Hemorrhage Stroke Patients—The IRONHEART Study. Front. Neurol. 2021, 12, 613441. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.-W.; Cho, Y.H.; Oh, M.J.; Seo, W.-K.; Kim, G.-M.; Ahn, M.-J. Circulating DNAs, a Marker of Neutrophil Extracellular Traposis and Cancer-Related Stroke: The OASIS-Cancer Study. Stroke 2019, 50, 2944–2947. [Google Scholar] [CrossRef]
- Genchi, A.; Semerano, A.; Schwarz, G.; Dell’acqua, B.; Gullotta, G.S.; Sampaolo, M.; Boeri, E.; Quattrini, A.; Sanvito, F.; Diamanti, S.; et al. Neutrophils predominate the immune signature of cerebral thrombi in COVID-19 stroke patients. Acta Neuropathol. Commun. 2022, 10, 14. [Google Scholar] [CrossRef]
- Chen, S.H.; Scott, X.O.; Marcelo, Y.F.; Almeida, V.W.; Blackwelder, P.L.; Yavagal, D.R.; Peterson, E.C.; Starke, R.M.; Dietrich, W.D.; Keane, R.W.; et al. Netosis and Inflammasomes in Large Vessel Occlusion Thrombi. Front. Pharmacol. 2021, 11, 607287. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Jeong, I.H.; An, G.D.; Woo, K.S.; Kim, K.H.; Kim, J.M.; Yun, S.H.; Park, J.I.; Cha, J.K.; Kim, M.H.; et al. Evaluation of neutrophil extracellular traps as the circulating marker for patients with acute coronary syndrome and acute ischemic stroke. J. Clin. Lab. Anal. 2020, 34, e23190. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, R.; Liu, C.; Zhou, P.; Li, J.; Wang, Y.; Zhao, X.; Zhao, H.; Song, L.; Yan, H. Associations of NETs with inflammatory risk and atherosclerotic severity in ST-segment elevation myocardial infarction. Thromb. Res. 2021, 203, 5–11. [Google Scholar] [CrossRef] [PubMed]
- De Buhr, N.; Baumann, T.; Werlein, C.; Fingerhut, L.; Imker, R.; Meurer, M.; Götz, F.; Bronzlik, P.; Kühnel, M.P.; Jonigk, D.D.; et al. Insights into Immunothrombotic Mechanisms in Acute Stroke due to Vaccine-Induced Immune Thrombotic Thrombocytopenia. Front. Immunol. 2022, 13, 879157. [Google Scholar] [CrossRef]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Demers, M.; Blomgren, B.; Wong, S.L.; von Arbin, M.; von Heijne, A.; Laska, A.C.; Wallén, H.; Wagner, D.D.; Aspberg, S. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 2016, 139, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Z.; Farkas, V.J.; Gubucz, I.; Szabó, L.; Bálint, K.; Tenekedjiev, K.; Nagy, A.I.; Sótonyi, P.; Hidi, L.; Nagy, Z.; et al. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb. Res. 2019, 175, 46–52. [Google Scholar] [CrossRef]
- Desilles, J.; Nomenjanahary, M.S.; Consoli, A.; Ollivier, V.; Faille, D.; Bourrienne, M.; Dupont, S.; Di Meglio, L.; Escalard, S.; Maier, B.; et al. Impact of COVID-19 on thrombus composition and response to thrombolysis: Insights from a monocentric cohort population of COVID-19 patients with acute ischemic stroke. J. Thromb. Haemost. 2022, 20, 919–928. [Google Scholar] [CrossRef]
- Novotny, J.; Oberdieck, P.; Titova, A.; Pelisek, J.; Chandraratne, S.; Nicol, P.; Hapfelmeier, A.; Joner, M.; Maegdefessel, L.; Poppert, H.; et al. Thrombus NETcontent isassociated with clinical outcome in stroke and myocardial infarction. Neurology 2020, 94, e2346–e2360. [Google Scholar] [CrossRef]
- Zeng, H.; Fu, X.; Cai, J.; Sun, C.; Yu, M.; Peng, Y.; Zhuang, J.; Chen, J.; Chen, H.; Yu, Q.; et al. Neutrophil Extracellular Traps may be a Potential Target for Treating Early Brain Injury in Subarachnoid Hemorrhage. Transl. Stroke Res. 2022, 13, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Datsi, A.; Piotrowski, L.; Markou, M.; Köster, T.; Kohtz, I.; Lang, K.; Plöttner, S.; Käfferlein, H.U.; Pleger, B.; Martinez, R.; et al. Stroke-derived neutrophils demonstrate higher formation potential and impaired resolution of CD66b + driven neutrophil extracellular traps. BMC Neurol. 2022, 22, 186. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Larco, J.A.; Mereuta, M.O.; Liu, Y.; Fitzgerald, S.; Dai, D.; Kadirvel, R.; Savastano, L.; Kallmes, D.F.; Brinjikji, W. Diverse thrombus composition in thrombectomy stroke patients with longer time to recanalization. Thromb. Res. 2022, 209, 99–104. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, M.; Liu, Q.; Liu, J.; Cui, Y. Neutrophil extracellular traps induce thrombogenicity in severe carotid stenosis. Immun. Inflamm. Dis. 2021, 9, 1025–1036. [Google Scholar] [CrossRef]
- Puy, L.; Corseaux, D.; Perbet, R.; Deramecourt, V.; Cordonnier, C.; Bérézowski, V. Neutrophil extracellular traps (NETs) infiltrate haematoma and surrounding brain tissue after intracerebral haemorrhage: A post-mortem study. Neuropathol. Appl. Neurobiol. 2021, 47, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, F.; Zhang, Y.; Zhou, Y.; Xu, X.; Zhang, X.; Zhao, Y. Neutrophil extracellular traps increased by hyperglycemia exacerbate ischemic brain damage. Neurosci. Lett. 2020, 738, 135383. [Google Scholar] [CrossRef]
- Pir, G.J.; Parray, A.; Ayadathil, R.; Pananchikkal, S.V.; Mir, F.A.; Muhammad, I.; Abubakar, A.; Amir, N.; Hussain, S.; Haroon, K.H.; et al. Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi. Int. J. Mol. Sci. 2022, 23, 14477. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Ondracek, A.S.; Lang, I.M. Neutrophil Extracellular Traps in Atherosclerosis and Thrombosis. In Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; Von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK584303/ (accessed on 19 January 2023). [CrossRef]
- Genchi, A.; Semerano, A.; Gullotta, G.S.; Strambo, D.; Schwarz, G.; Bergamaschi, A.; Panni, P.; Simionato, F.; Scomazzoni, F.; Michelozzi, C.; et al. Cerebral thrombi of cardioembolic etiology have an increased content of neutrophil extracellular traps. J. Neurol. Sci. 2021, 423, 117355. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, C.; Xu, W.; Li, W.; Feng, Z.; Zhang, X.; Zhao, K. Edaravone Dexborneol Downregulates Neutrophil Extracellular Trap Expression and Ameliorates Blood-Brain Barrier Permeability in Acute Ischemic Stroke. Mediat. Inflamm. 2022, 2022, 3855698. [Google Scholar] [CrossRef]
- De Vries, J.J.; Autar, A.S.A.; van Dam-Nolen, D.H.K.; Donkel, S.J.; Kassem, M.; van der Kolk, A.G.; van Velzen, T.J.; Kooi, M.E.; Hendrikse, J.; Nederkoorn, P.J.; et al. Association between plaque vulnerability and neutrophil extracellular traps (NETs) levels: The Plaque at RISK study. PLoS ONE 2022, 17, e0269805. [Google Scholar] [CrossRef]
- Essig, F.; Kollikowski, A.M.; Pham, M.; Solymosi, L.; Stoll, G.; Haeusler, K.G.; Kraft, P.; Schuhmann, M.K. Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7387. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.-J.; Ha, J.; Lee, H.; Kwon, I.; Kim, S.; Kim, Y.D.; Nam, H.S.; Lee, H.S.; Song, T.-J.; Choi, H.-J.; et al. Neutrophil Recruitment in Arterial Thrombus and Characteristics of Stroke Patients with Neutrophil-Rich Thrombus. Yonsei Med. J. 2022, 63, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, S.; Hu, M.; Huang, F.; Zhu, Q.; Qiu, W.; Hu, X.; Colello, J.; Zheng, S.G.; Lu, Z. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl. Stroke Res. 2020, 11, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, T.; Jin, J.; Liu, Y.; Li, B.; Sun, Q.; Tian, J.; Zhao, H.; Liu, Z.; Ma, S.; et al. Interactions between neutrophil extracellular traps and activates enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicone 2020, 53, 102671. [Google Scholar] [CrossRef]
- Lin, C.; Memon, R.; Sui, J.; Zheng, X.L. Identification of Biomarkers in Patients with Thrombotic Thrombocytopenic Purpura Presenting with Large and Small Ischemic Stroke. Cerebrovasc. Dis. Extra 2021, 11, 29–36. [Google Scholar] [CrossRef]
- Arroyo, A.B.; Reyes-García, A.M.d.L.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients with Atrial Fibrillation. Arter. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Y.; Du, J.; Liu, H.; Chen, X.; Li, M.; Xiang, M.; Wang, C.; Wu, X.; Liu, L.; et al. Neutrophil extracellular traps contribute to tissue plasminogen activator resistance in acute ischemic stroke. FASEB J. 2021, 35, e21835. [Google Scholar] [CrossRef]
- Kitano, T.; Hori, Y.; Okazaki, S.; Shimada, Y.; Iwamoto, T.; Kanki, H.; Sugiyama, S.; Sasaki, T.; Nakamura, H.; Oyama, N.; et al. An Older Thrombus Delays Reperfusion after Mechanical Thrombectomy for Ischemic Stroke. Thromb. Haemost. 2022, 122, 415–426. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil ex-tracellular traps in ischemic stroke thrombi. Ann Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef]
- Mołek, P.; Ząbczyk, M.; Malinowski, K.P.; Natorska, J.; Undas, A. Markers of NET formation and stroke risk in patients with atrial fibrillation: Association with a prothrombotic state. Thromb. Res. 2022, 213, 1–7. [Google Scholar] [CrossRef]
- Witsch, J.; Spalart, V.; Martinod, K.; Schneider, H.M.; Oertel, J.; Geisel, J.; Hendrix, P.; Hemmer, S. Neutrophil Extracellular Traps and Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. Crit. Care Explor. 2022, 4, e0692. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, e154225. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.; Beinrohr, L.; Gubucz, I.; Szabó, L.; Tenekedjiev, K.; Nikolova, N.; Nagy, A.I.; Hidi, L.; Sótonyi, P.; Szikora, I.; et al. Fibrin to von Willebrand factor ratio in arterial thrombi is associated with plasma levels of inflammatory biomarkers and local abundance of extracellular DNA. Thromb. Res. 2022, 209, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Gkaliagkousi, E.; Lazaridis, A.; Arelaki, S.; Pateinakis, P.; Ntinopoulou, M.; Mitsios, A.; Antoniadou, C.; Argyriou, C.; Georgiadis, G.S.; et al. Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. J. Clin. Investig. 2021, 6, e148668. [Google Scholar] [CrossRef]
- Shimonaga, K.; Matsushige, T.; Takahashi, H.; Hashimoto, Y.; Yoshiyama, M.; Ono, C.; Sakamoto, S. Peptidylarginine Deiminase 4 as a Possible Biomarker of Plaque Instability in Carotid Artery Stenosis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105816. [Google Scholar] [CrossRef]
- Drieu, A.; Levard, D.; Vivien, D.; Rubio, M. Anti-inflammatory treatments for stroke: From bench to bedside. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789854. [Google Scholar] [CrossRef]
- Krams, M.; Lees, K.R.; Hacke, W.; Grieve, A.P.; Orgogozo, J.M.; Ford, G.A. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): An adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 2003, 34, 2543–2548. [Google Scholar] [CrossRef]
- Coveney, S.; McCabe, J.J.; Murphy, S.; O’Donnell, M.; Kelly, P.J. Anti-inflammatory therapy for preventing stroke and other vascular events after ischaemic stroke or transient ischaemic attack. Cochrane Database Syst. Rev. 2020, 5, CD012825. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; González-Gay, M.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef]
- Mitsios, A.; Chrysanthopoulou, A.; Arampatzioglou, A.; Angelidou, I.; Vidali, V.; Ritis, K.; Skendros, P.; Stakos, D. Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps. Int. J. Mol. Sci. 2020, 21, 3625. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Germanidis, G.; Mastellos, D.C.; Antoniadou, C.; Gavriilidis, E.; Kalopitas, G.; Samakidou, A.; Liontos, A.; Chrysanthopoulou, A.; Ntinopoulou, M.; et al. Complement C3 inhibition in severe COVID-19 using compstatin AMY-101. Sci. Adv. 2022, 8, eabo2341. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaptsi, E.; Merkouris, E.; Polatidou, E.; Tsiptsios, D.; Gkantzios, A.; Kokkotis, C.; Petridis, F.; Christidi, F.; Karatzetzou, S.; Karaoglanis, C.; et al. Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path. Neurol. Int. 2023, 15, 1212-1226. https://doi.org/10.3390/neurolint15040076
Liaptsi E, Merkouris E, Polatidou E, Tsiptsios D, Gkantzios A, Kokkotis C, Petridis F, Christidi F, Karatzetzou S, Karaoglanis C, et al. Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path. Neurology International. 2023; 15(4):1212-1226. https://doi.org/10.3390/neurolint15040076
Chicago/Turabian StyleLiaptsi, Eirini, Ermis Merkouris, Efthymia Polatidou, Dimitrios Tsiptsios, Aimilios Gkantzios, Christos Kokkotis, Foivos Petridis, Foteini Christidi, Stella Karatzetzou, Christos Karaoglanis, and et al. 2023. "Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path" Neurology International 15, no. 4: 1212-1226. https://doi.org/10.3390/neurolint15040076
APA StyleLiaptsi, E., Merkouris, E., Polatidou, E., Tsiptsios, D., Gkantzios, A., Kokkotis, C., Petridis, F., Christidi, F., Karatzetzou, S., Karaoglanis, C., Tsagkalidi, A.-M., Chouliaras, N., Tsamakis, K., Protopapa, M., Pantazis-Pergaminelis, D., Skendros, P., Aggelousis, N., & Vadikolias, K. (2023). Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path. Neurology International, 15(4), 1212-1226. https://doi.org/10.3390/neurolint15040076








