Abstract
Background: In this study, we aimed to investigate the incidence of fecal incontinence (FI) after severe acquired brain injuries (sABIs) and to determine whether this symptom can lead to an inability to return home after rehabilitation. Methods: This was a retrospective observational cohort study. In total, 521 acute sABI inpatients were enrolled from the Department of Neurorehabilitation at an academic tertiary care hospital. Patients were divided into two groups, with and without FI, at the end of the rehabilitation phase. The primary and secondary endpoints were the incidence of persistent FI and any difference in the discharge destination. Results: Upon admission, new-onset FI was found in 443 (85%) patients, of which 38% had traumatic sABI. Moreover, 62.7% of all patients had FI upon admission. At discharge, 53.3% (264/495) of patients still had FI. Of these, 75.4% (199/264) had a Rancho Level of Cognitive Functioning Scale (LCFS) ≥3. A statistically significant correlation between FI at discharge and the presence of frontal lesions, autonomic crises, and increased LCFS scores was noted. Among the patients discharged to their homes, the proportion with persistent FI was lower (34% vs. 53.3). Conclusions: FI was significantly persistent after sABI, even after recovery from unconsciousness, and must be considered as a consequence of, rather than an independent risk factor for, unfavorable outcomes.
1. Introduction
A severe acquired brain injury (sABI) is usually defined as a traumatic or non-traumatic pathologic condition of the central nervous system (CNS) [1]. Following sABI, some patients can suffer from a state of coma (Glasgow Coma Scale ≤8) of variable duration, with a broad spectrum of impairments affecting physical and neurocognitive functioning, representing a significant cause of lifelong disability [2]. One of the most disabling and self-limiting complications is represented by altered nervous control of the bowel. This is known as neurogenic bowel dysfunction (NBD), which refers to the impairment of voluntary control over bowel function caused by CNS disorders. This condition manifests as a range of bowel symptoms, primarily characterized by fecal incontinence (FI) and/or constipation [3,4].
1.1. Neurologic Bowel System Control
The CNS plays a pivotal role in regulating various aspects of gastrointestinal regulation, including muscular processes, sensory perception, storage, and excretion [5]. The CNS and the enteric nervous system (ENS) engage in an intricate and ongoing connection. This interaction mostly occurs through the sympathetic prevertebral ganglia, pelvic, and vagus nerve pathways, which are located within the gastroenteric wall. The CNS plays a crucial role in regulating contractile and secretive functions in the upper gastrointestinal tract. Additionally, it is engaged in controlling motility in the lower tract, blood flow, and electrolyte transfer through reflex circuits mediated by ENS neurons. The ENS is the primary determinant in regulating intestinal function. It is comprised of around 500,000 neurons that are distributed throughout Meissner’s plexus, which governs intestinal secretions, and Auerbach’s plexus, which controls the motor activity of the entire intestine [6].
The intricate neural system possesses the ability to integrate various reflex activities throughout the digestive tract, exhibiting both excitatory and inhibitory functions. This characteristic highlights its independence from both the central nervous system and the peripheral nervous system. This particular circumstance enables us to accurately designate it as the “enteric nervous system” [7].
As can be inferred from the control mechanisms of gastrointestinal function, conditions related to CNS (central nervous system) alterations are often accompanied by intestinal neuronal dysfunctions.
The CNS governs defecation by exerting control through lumbosacral centers in the spinal cord [7]. The regulation of the anorectal system’s physiological function is facilitated by brain control in conjunction with anatomical structures and the innervation of both somatic and visceral peripheral components. In contrast to the extensive literature on spinal and peripheral innervation, our understanding of brain mechanisms governing anorectal continence remains limited [8]. The rectum functions as a storage compartment for both solid and liquid fecal matter, as well as the gases generated by the small and large intestines. Its primary role is to facilitate the effective evacuation of these contents. The presence of both smooth and striated muscle sphincters contributes to fecal continence maintenance. The cerebral structures responsible for maintaining urine continence also play a role in regulating fecal continence and evacuation mechanisms [9].
The physiological process of filling and emptying, which is subject to voluntary control, is contingent upon the transmission of information from the periphery to the brain. Any circumstance that interferes with the cognition, transmission, or processing of these data at the brain level has the potential to result in malfunction of the lower gastrointestinal tract [10,11,12].
The progress made in imaging techniques has facilitated the acquisition of knowledge pertaining to the cerebral regions that govern anorectal continence. The condition of rectal distension, which is similar to the introduction of a fecal bolus due to a high-amplitude propagated contraction (HAPC), elicits bilateral activity in the insula, anterior cingulate gyrus, secondary somatosensory cortex, and thalamus [13,14]. Motor areas such as M1, the supplementary motor area, and the cerebellum are specifically activated alone in response to anal stimulation. This activation is most likely a reflexive reaction to the expansion of the rectum, and it typically happens with a delay of around six seconds. Passive fecal continence is established through reflex motor activity, which serves to confine the fecal bolus within the rectal ampulla. The activation of the motor cortex in the supplementary motor region, as well as the primary somatosensory cortex and insula, occurs when the external anal sphincter undergoes voluntary contraction, particularly when this contraction is repeated [15].
Recent research has additionally demonstrated the simultaneous activation of brain regions responsible for regulating the external anal sphincter and the regulatory regions associated with the long flexor of the hallux [7]. The intricate coordination of cerebral functions, including continence, lower limb movement, and respiration, highlights the intricate nature of the control systems responsible for maintaining continence at the brain level. This integration appears to be linked to the physiological imperative of sustaining continence [16].
The confirmation of the overlap control between intestinal and bladder functions is supported by the control pathways present in the brainstem and spinal cord, as well as the peripheral innervation facilitated by the pudendal nerve, which is shared by both functions [17].
There is evidence that suggests the existence of a pontine defecation center, similar to the pontine micturition center (PMC), which regulates the distal colon, rectum, and internal anal sphincter. Additionally, the pontine continence center (PCC) seems to be responsible for controlling the external anal sphincter to maintain fecal continence [18].
1.2. Rationale and Aim of This Work
To date, limited information is available about FI persistence and risk factors in patients after sABI. Authors have also described that, during the acute phase, FI occurs in 23–70% of the sABI population [3,4,5,6] and persists in the chronic phase in 5–15% of cases [7,8,9,10,11,12,13,14,15,16]. Constipation has been reported in the chronic phase in 23% of patients [17]. Impaired consciousness can make bowel continence assessment difficult, and in critically ill patients, enteral nutrition (EN) [18] is associated with diarrhea, which is one of the most frequent causes of FI [19,20], often as a side effect of other treatments (e.g., antibiotics, osmolar compounds, cardiac drugs) in intensive care units [21]. Incontinence can prolong a patient’s stay in a rehabilitation setting and substantially hinder recovery from acquired brain injury [22,23,24,25]. Furthermore, FI can also lead to adverse effects such as skin disintegration, decubitus ulcers, and skin infections [23].
Thus, in the present study, we aimed to better understand FI incidence and its effects on rehabilitation outcomes after acute brain injury rehabilitation, to identify clinical variables associated with it, and to determine whether FI is an independent risk factor for poor functional recovery and for the discharge setting.
2. Materials and Methods
This was a retrospective and observational cohort study conducted in accordance with the STROBE guidelines statement [26]; it was conducted in a tertiary care rehabilitation hospital. According to the Aven guidelines, no ethics committee permission was required since the collection and subsequent processing of data was carried out anonymously, agglomerated, and not traceable to individual patients [27]. Despite the retrospective nature of this study, informed consent was collected from each patient/legal guardian at the beginning of the research process; this procedure ensured patients’ agreement to use their data in a clinical scope (inpatient rehabilitation procedures) as well as a research scope [27].
Data were collected from a medical chart review of all consecutive acute traumatic and non-traumatic sABIs from January 2005 to December 2018, regardless of the severity of the cognitive impairment. Patients with a history of other neurological diseases before or concomitant with the onset of a sABI (e.g., polytrauma with spinal cord lesion), a history of pre-existing FI or bowel inflammatory disease, lower bowel tract surgery, or pelvic cancer were excluded. The neurorehabilitation program included a scheduled and individualized diet, control of systemic and localized infections, and management of bladder disorders, bowel disorders, autonomic disorders, postural control, and mobility impairment. Patients underwent 3 h of rehabilitative treatment daily. In addition, speech/swallowing therapy was performed daily. Patients were treated daily with passive joint mobilization and placed upright on a tilt table. Moreover, sABIs were classified upon admission using the Italian version of the Rancho Level of Cognitive Functioning Scale (LCFS) [28,29].
The LCFS is one of the earliest developed scales for assessing cognitive functions after sABI. This scale classifies outcomes into eight levels, from no response (level I) to a purposeful and appropriate response (level VIII). The LCFS was administered on first admission and again at discharge.
The type of sABI was classified as traumatic/vascular/neoplastic/infection-related upon admission via a CT scan. Demographic variables and comorbidities such as frontal lobe lesions, pelvic fractures, paroxysmal sympathetic hyperactivity, Clostridioides difficile infections, healthcare-associated infections (HAIs), the presence and duration of tube feeding (nasogastric tube (NGT), percutaneous endoscopy gastrostomy (PEG)) upon admission and during recovery, length of hospitalization, and type of discharge were recorded. Fecal impaction was ascertained through a standard digital rectal examination consisting of three specific phases: (1) inspection of the anus and the neighboring tissues; (2) evaluation of the anocutaneous reflex; and (3) digital palpation. With the patient oriented in the left lateral position and the hips flexed at a 90° angle, an in-depth inspection of the anus and its surrounding tissues was undertaken. This inspection was designed to determine the presence of skin excoriation, skin tags, anal fissures, scars, or hemorrhoids and was conducted with appropriate lighting. Furthermore, an assessment of the sacral region was conducted: sacral reflexes required gently stroking the skin proximate to the anal area, progressing inward from all four quadrants, utilizing a stick tipped with a cotton swab. A standard reaction resulted in the swift contraction of the perianal skin, anoderm, and the external anal sphincter. This described reflex response is typically known as the anocutaneous reflex. If the cotton swab did not induce a reaction, the stick’s wooden end was used to invoke a more intense and distinct sensation. Following this, digital palpation was performed by methodically inserting a lubricated, gloved index finger into the rectal chamber. Assessing the resting sphincter tone consisted of categorizing its condition as normal, diminished, or augmented.
A three-week bowel diary assessed bowel behavior and was recorded upon admission and a week before discharge [30,31] (Figure 1).

Figure 1.
Bowel diary.
This study aimed to determine whether new-onset FI episodes during the neurorehabilitation program undertaken after an acute sABI are a predictor for discharge settings and whether this condition and its persistence at discharge are dependent on clinical conditions during the rehabilitation phase.
Patient data were coded to identify certain factors—including feeding mode, sABI etiology, LCFS upon rehabilitation admission, FI upon rehabilitation admission, frontal lobe lesions, pelvic lesions, paroxysmal sympathetic hyperactivity, C. difficile infections, and healthcare-associated infections as independent variables—that could potentially influence the persistence of FI after an sABI at discharge from rehabilitation (the dependent variable), and whether FI is a predictor for the type of discharge setting.
Data were processed using JMP10 (SAS Institute Inc., Cary, NC, USA). Multivariable logistic regression was used for continuous variables, and chi-squared tests were used for nominal or categorical variables. Statistical analysis was completed in consultation with a biostatistician.
3. Results
A total of 531 patients (156 women and 365 men; median age, 48 years) consecutively admitted to our neurorehabilitation unit were included in this study. Ten patients were excluded because of pre-existing FI (n = 3), concomitant spinal cord injury (n = 3), previous colorectal surgery (n = 2), or irritable bowel syndrome (n = 2). The mean time from the severe brain injury to admission was 27.6 ± 11.8 days, with a mean length of stay in the neurorehabilitation unit of 70.5 ± 35.9 days. Table 1 shows the baseline demographic and clinical characteristics of the patients.

Table 1.
Demographic and clinical data of study participants (n = 521).
Upon admission, 443 (85%) patients showed FI, of whom 38% had a traumatic sABI. The number of FI episodes upon admission per week was 8 ± 6.3 (mean ± SD). The percentage of patients affected by FI who showed a partial recovery of consciousness level (LCFS ≥ 3) at admission to rehabilitation was 62.7%.
The patients were mainly affected by frontal lobe lesions (40.1%). Concomitant pelvic lesions in polytrauma were observed in 10.9% of the cases, and episodes of paroxysmal sympathetic hyperactivity were observed in 18.8% of the cases. Hospital-care-related infections were documented in 30.3% of patients with sABI, of whom 7.8% experienced C. difficile infections. During hospitalization, 26 deaths occurred, mainly from sepsis and neurological relapses.
Many patients (72.8%) were fed using PEG during recovery, whereas 18.2% were fed orally or with a mixed regimen. At discharge, 53.3% (264/495) of patients still had FI. Of those, 75.4% (199/264) had LCFS scores ≥ 3. The number of FI episodes at discharge in the last week was 4 ± 4.3 (mean ± SD).
When using dichotomous variables of any predictors that could modify the probability that the patient had FI at discharge (whether it was present at the time of admission or not), the logistic analysis only highlighted the presence of frontal lesions, the presence of autonomic crises in the acute phase, and LCFS scores at discharge as variables that were hierarchically more important, thus classifying others as less important (Table 2).

Table 2.
Logistic model variables for fecal incontinence at discharge from rehabilitation. OR—odds ratio; CI—confidence interval; χ2—chi-squared value.
On the other hand, the following variables were not deemed significant: cause of sABI; LCFS; presence of FI and pelvic lesions (data collected at admission); duration of feeding via PEG or NGT in the acute phase; persistence of paroxysmal sympathetic hyperactivity; and presence of related infections at the time of treatment (data collected during the rehabilitation phase).
Patients with frontal lesions had a significantly higher risk of FI at discharge than those without lesions (p < 0.0001). The presence of autonomic crises in the acute phase further increased a patient’s chances of FI at discharge, with a doubled probability compared to patients who did not show paroxysmal sympathetic hyperactivity in the acute phase (p < 0.0001).
Regardless of the other predictors’ configurations, an increase in the LCFS level (increased level of cognitive function) from baseline was correlated with a reduction in the probability of FI at discharge. Research on potential correlations with various clinical factors using multiple regression to highlight predictors that can determine a change in the LCFS score at discharge, compared with those obtained at the time of admission, isolated the etiology, LCFS at admission, duration in days of feeding via NGT or PEG, and persistence of autonomic crises as hierarchically more relevant variables (Table 3).

Table 3.
Regression model variables for LCFS at discharge.
Higher LCFS scores at admission were correlated with higher scores at discharge. Regarding etiology, the most significant increase correlated with traumatic pathogenesis, while anoxic pathogenesis correlated with lower LCFS scores at discharge compared to admission. An increase in the duration of feeding via NGT or PEG had a consistent and decreasing effect on the LCFS score at discharge. In particular, FI at admission did not appear to correlate with a low LCFS score at discharge; these results are consistent with what is reported in the literature [13].
Finally, we evaluated whether the presence of FI at discharge influenced the post-rehabilitation destination of patients. The data in Table 3 indicate a difference between the proportion of patients with or without FI at discharge compared with the discharge setting, as shown in Figure 2. Among patients discharged to their homes at the end of the rehabilitation period, the proportion with persistent FI was lower than the general mean (34% vs. 53.3%); for those sent to long-term care, the proportion affected by persistent FI was higher than the general mean (87% vs. 53.3%). A similar trend was also found in transfers to NSV (vegetative state care), NAC (highly complex neurological care) (94% vs. 53.3%), and other acute care facilities (77% vs. 53.3%). The numbers of patients transferred to other rehabilitation facilities were similar to the general mean (40% vs. 53.3%). All of the findings above were statistically significant (p < 0.0001). Therefore, it is clear that there was a correlation between persistent FI at discharge and the setting to which patients were transferred (Figure 2).
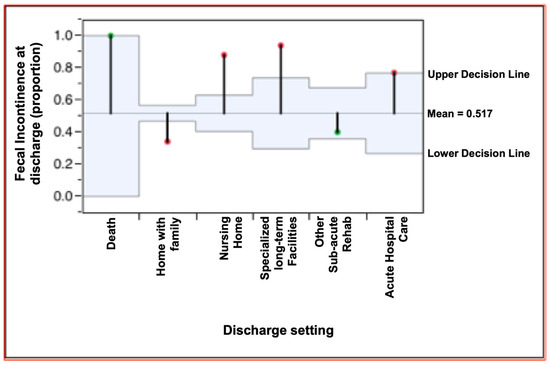
Figure 2.
Fecal incontinence proportion and discharge setting: chi-squared analysis.
4. Discussion
The present study was conducted in accordance with the STROBE statement [26]. The first interesting result was the high percentage of sABI patients with FI at admission to the neurorehabilitation unit, with an overall percentage of approximately 85.5% and approximately 62.7% for patients who recovered with an LCFS ≥3 (“localized response”). This partially diverges from the literature, where FI prevalence is estimated between 2.2% and 20.7% and reaches 50% in institutionalized individuals [31,32,33]; during the acute phase, FI has been reported to be between 23% and 70% [3,4,5] and persist during the chronic phase for 5% to 15% of patients [6,7,8,9,10,11,12,13,14,15]. A possible explanation is that our cohort also included participants with consciousness disorders, which may have negatively influenced our clinical evaluation.
Concerning the correlation we observed between FI persistence at discharge and EN duration, it is known that sABIs involve increased energy expenditure, increased weight, and muscle mass loss, along with the risk of impaired immune defenses. Several factors contribute to the onset of NBD in response to severe comorbidities during the acute phase: hypoperfusion (the need to infuse large quantities of fluid to support cardiovascular function, particularly because of intestinal edema, with reduced absorption, mobility, and lymphatic stagnation); sedation; and the use of vasoactive agents, which contribute to a slower transit speed with a reduction in absorption and constipation in 70% of cases [34]. Early EN is a crucial therapeutic strategy for maintaining intestinal mucosa and microbiome integrity [35], together with the immune responses that depend on it [36]. However, these benefits are not considered cost-effective, given possible risks and adverse events (e.g., diarrhea, delayed gastric emptying, aspiration pneumonia) [37,38]. Even after the acute phase, with the continuation of EN, alterations in intestinal transit, gastric secretion, and impaired intestinal absorption may be observed, especially when the characteristics of the ingredients are not modified [39]. Simultaneous modifications of the brain–gut axis [40], with a significant reduction in the diversity of microbiome components and the appearance of dysautonomia following the release of noradrenaline after sABI [41], results in need to reevaluate etiological aspects, which are often connected exclusively to the lesion sites of brain injuries and the therapies in place rather than the actual intestinal changes.
Concerning the persistence of FI at discharge, in our study, the data correlated exclusively with frontal damage, autonomic crises in the acute phase, and the LCFS score at discharge. The lack of correlation with other clinical indicators, both in the acute phase and during rehabilitation, including healthcare-related infections, such as Clostridioides, appears significant. In our study, the presence of healthcare-related infections did not seem to correlate with FI persistence at discharge from the neurorehabilitation program. The published literature shows correlations between pulmonary infections and acute gastrointestinal failure (AGF) [42], probably due to a compromised mucosal immune system being common to both [43]. Another interesting aspect of AGF seems to be its correlation with frontal damage, which correlates with the persistence of FI at discharge from neurorehabilitation, according to our data [44]. A similar observation concerning gastrointestinal tract disorders has been reported in a study of right frontal lobe lesions of the limbic system and ventromedial hypothalamus, which are characterized by increased serotonin and noradrenaline values in the same areas [45]. Our data revealed a correlation between the persistence of FI at discharge and the presence of paroxysmal sympathetic hyperactivity (PSH) in the acute phase. The hyperadrenergic syndrome is secondary to alterations of the central autonomic pathways rather than focal damage and is caused by a lack of hypothalamic inhibition of sympathetic and even non-nociceptive output, with a disconnection of the insular cortex [45]. PSH commonly presents with symptoms of tachycardia, excessive sweating, tachypnea, and increased temperature and is often associated with postural changes in decortication or decerebration [46]. After sABI, 60–90% of patients have at least one episode of dysautonomia in the first weeks after the acute event [47], although it also occurs frequently during rehabilitation [48,49]. The persistence of dysautonomia does not appear to correlate with the presence of other severe symptoms [48]; although it is frequently associated with disturbances of consciousness and extended hospital stays, its influence on prognosis is uncertain [48,50,51]. This is also consistent with the correlation we observed between the persistence of FI and the LCFS score at discharge. LCFS has been proposed and validated for monitoring functional and cognitive recovery after sABI [52]. Therefore, we believe that our results should not be interpreted as a direct correlation between PSH, a low LCFS score, and persistent FI at discharge but rather as the coexistence of symptoms in patients with a more compromised clinical condition.
These data have already been observed in the literature: in a 5-years retrospective study, Safaz [15] reported that the mean Mini-Mental State Examination (MMSE) scores and the median LCFS scores of patients with fecal or urinary incontinence were the same as those without incontinence.
Regarding our observed correlation between discharge planning and persistent FI at discharge from neurorehabilitation, it has been reported that FI may be responsible for reduced social reintegration [12] and poor clinical recovery [22], increasing the need for institutionalization [53,54].
In patients with sABI, fecal incontinence presents significant challenges. It imposes a heightened care burden due to the need for regular hygiene management and monitoring, and associated secondary risks further complicate home care. Additionally, the psychological impact on caregivers and family members is profound. Given these complexities, FI often necessitates institutionalized care at discharge, as opposed to home settings.
A final consideration about FI management is also due: transanal irrigation (TAI), administered either by healthcare professionals or by appropriately trained caregivers, represents a useful tool to manage such a disabling impairment. This intervention provides a means to regularly irrigate the bowel, thereby scheduling evacuations by thoroughly emptying the distal colonic segment. As a result, it aids in preventing episodes of fecal incontinence between irrigation sessions, offering a structured approach to bowel care and enhancing patient comfort and dignity [55].
There are devices designed for TAI intended for bedridden patients. These devices feature a closed irrigation and fecal collection system, ensuring that bed linens and pads remain uncontaminated. This allows for a simplified and hygienic procedure. Such streamlining could also facilitate caregivers in the home-based management of hygiene [7,55,56]. In recent years, strategies for managing neurogenic lower intestinal dysfunctions have been progressively enriched with useful tools, such as TAI systems performed at the patient’s bedside. Nevertheless, there are no specific studies about TAI in patients with sABI. Therefore, the authors advocate the development of protocols for the management of FI in the ABI population that can include TAI as a specific tool for reducing the time and cost of hygiene management.
One limitation of our study was the absence of follow-ups, which may have resulted in inaccurately recording persistent FI after discharge. It would be interesting to evaluate how intestinal function changes after a change in setting at discharge, both in patients who return home and those who move to specialized facilities. The second limitation was the inability to analyze the intestinal microbiome in order to evaluate the impact of dysbiosis on the brain–gut axis and fecal continence.
Moreover, we used LCFS to assess cognitive functioning because this study was designed before 2005, and the CRS-R (Coma Recovery Scale-Revised, at the moment, the best standardized neurobehavioral assessment measure designed for patients with consciousness disorders) was validated in the Italian language in 2007. Currently, we are exploring the relationship between fecal incontinence and DOC using the CRS-R.
Another potential limitation is that the data were sourced solely from a single academic tertiary care hospital, possibly not encompassing the entire spectrum of sABI cases; for this reason, future studies should also involve additional sABI reference centers in order to conduct multi-center investigations.
Future research should examine the pathophysiological mechanisms of NBD in patients with sABI, with the aim of better understanding the issue and identifying optimal care and management strategies. For this reason, if subsequent prospective studies demonstrate that return of bowel and/or bladder continence and cognition are useful in predicting long-term sABI outcome, potential applications may include early determination of rehabilitation services targeted at specific functional deficits, vocational rehabilitation planning, intervention development, resource allocation, cost projection, and family counseling.
5. Conclusions
In conclusion, we found significant persistent FI after sABI, even in patients recovering from unconsciousness. Predictive factors for the persistence of FI upon discharge from neurorehabilitation were the frontal lesion’s location, the presence of autonomic crises in the acute phase, and the LCFS score at discharge. FI occurring after sABI and persisting after rehabilitation treatment was more likely in patients who were referred to long-term care facilities and residential units. These data seem to agree with the findings of previous studies [3,8], but the correlation found in this study seems to be more predictive of disability following sABI; thus, it mainly represents a consequence of reduced functional recovery rather than an independent risk factor for unfavorable outcomes after sABI [10].
According to our data, the role of FI in the setting at discharge seems to be a significant and independent risk factor, and in our opinion, this depends on the caregiving needs that fecal incontinence management requires.
A structured simultaneous evaluation plan, both for an early rehabilitative approach and for a positive prediction of recovery, is needed for both NBD and neurogenic urinary dysfunction [54,56].
Author Contributions
Conceptualization, E.A. and G.L.; methodology, D.G. and G.L.; software, L.P. and G.C.; validation, L.P.; formal analysis, G.L.; investigation, E.A. and D.G.; resources, G.L.; data curation, G.L.; writing—original draft preparation, D.G., G.C. and G.L.; writing—review and editing, L.P., E.A. and G.L.; visualization, L.P.; supervision, D.G. and G.C.; project administration, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. According to the Aven guidelines, no ethics committee permission was required since the collection and subsequent processing of data was carried out anonymously, agglomerated, and not traceable to individual patients.
Informed Consent Statement
Due to the retrospective nature of this study, informed consent was collected from each patient/legal guardian at the beginning of the research activity, thus ensuring that the data were transferred from clinical to research purposes.
Data Availability Statement
All supporting data are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Avesani, R.; Roncari, L.; Khansefid, M.; Formisano, R.; Boldrini, P.; Zampolini, M.; Ferro, S.; De Tanti, A.; Dambruoso, F. The Italian National Registry of severe acquired brain injury: Epidemiological, clinical and functional data of 1469 patients. Eur. J. Phys. Rehabil Med. 2013, 49, 611–618. [Google Scholar] [PubMed]
- Quander, C.R.; Morris, M.C.; de Leon, C.F.M.; Bienias, J.L.; Evans, D.A. Association of fecal incontinence with physical disability and impaired cognitive function. Am. J. Gastroenterol. 2006, 101, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, J.C.; Andrews, K.; Richards, B.; Laycock, P.J. Incidence and correlates of incontinence in stroke patients. J. Am. Geriatr. Soc. 1985, 33, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Nakase-Richardson, R.; Hammond, F.M.; McNamee, S.; Giacino, J.T.; Kalmar, K.; Greenwald, B.D.; Yablon, S.A.; Horn, L.J. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the national institute on disability and rehabilitation research traumatic brain injury model systems. Arch. Phys. Med. Rehabil. 2013, 94, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, D.; Gozzerino, F.; Antoniono, E.; Lamberti, G. Anal incontinence and severe acquired brain injury: A retrospective study of 347 rehabilitation inpatients. Pelviperineology 2017, 36, 13–16. [Google Scholar]
- Aadal, L.; Mortensen, J.; Kellenberger, S.; Nielsen, J.F. Lower Bowel Dysfunction Following Acquired Brain Injury: A Challenge During Rehabilitation. Gastroenterol. Nurs. 2019, 42, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A. Rehabilitation in practice: Managing neurogenic bowel dysfunction. Clin. Rehabil. 2010, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Hewer, R.L. Functional abilities after stroke: Measurement, natural history and prognosis. J. Neurol. Neurosurg. Psychiatry 1987, 50, 177–182. [Google Scholar] [CrossRef]
- Nakayama, H.; Jørgensen, H.; Pedersen, P.; Raaschou, H.; Olsen, T. Prevalence and risk factors of incontinence after stroke. Stroke 1997, 28, 58–62. [Google Scholar] [CrossRef]
- Bliss, D.Z.; Johnson, S.; Savik, K.; Clabots, C.R.; Gerding, D.N. Fecal incontinence in hospitalized patients who are acutely Ill. Nurs. Res. 2000, 49, 101–108. [Google Scholar] [CrossRef]
- Baztán, J.J.; Domenech, J.R.; González, M. New-onset fecal incontinence after stroke: Risk factor or consequence of poor outcomes after rehabilitation? Stroke 2003, 34, e101–e102. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.; Chuo, A.; Kong, K.H. Urinary incontinence after traumatic brain injury: Incidence, outcomes and correlates. Brain Inj. 2003, 17, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Foxx-Orenstein, A.; Kolakowsky-Hayner, S.; Marwitz, J.H.; Cifu, D.X.; Dunbar, A.; Englander, J.; Francisco, G. Incidence, risk factors, and outcomes of fecal incontinence after acute brain injury: Findings from the traumatic brain injury model systems national database. Arch. Phys. Med. Rehabil. 2003, 84, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Harari, D.; Coshall, C.; Rudd, A.G.; Wolfe, C.D. New-onset fecal incontinence after stroke: Prevalence, natural history, risk factors, and impact. Stroke 2003, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Safaz, I.; Alaca, R.; Yasar, E.; Tok, F.; Yilmaz, B. Medical complications, physical function and communication skills in patients with traumatic brain injury: A single centre 5-year experience. Brain Inj. 2008, 22, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.M.; Liu, C.; Cheesman, A.L.; Ritter, A.; Thompson, S.; Greenwood, R. Incontinence after brain injury: Prevalence, outcome and multidisciplinary management on a neurological rehabilitation unit. Clin. Rehabil. 2006, 20, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Doshi, V.S.; Say, J.H.; Young, S.H.-Y.; Doraisamy, P. Complications in stroke patients: A study carried out at the Rehabilitation Medicine Service, Changi General Hospital. Singapore Med. J. 2003, 44, 643–652. [Google Scholar] [PubMed]
- García, C.B.; Binks, R.; De Luca, E.; Dierkes, C.; Franci, A.; Gallart, E.; Niederalt, G.; Wyncoll, D. Prevalence, management and clinical challenges associated with acute faecal incontinence in the ICU and critical care settings: The FIRST™ cross-sectional descriptive survey. Intensiv. Crit. Care Nurs. 2012, 28, 242–250. [Google Scholar] [CrossRef]
- Whelan, K.; Schneider, S.M. Mechanisms, prevention, and management of diarrhea in enteral nutrition. Curr. Opin. Gastroenterol. 2011, 27, 152–159. [Google Scholar] [CrossRef]
- Wishin, J.; Gallagher, T.J.; McCann, E. Emerging options for the management of fecal incontinence in hospitalized patients. J. Wound Ostomy Cont. Nurs. 2008, 35, 104–110. [Google Scholar] [CrossRef]
- Thaha, M.A.; Abukar, A.A.; Thin, N.N.; Ramsanahie, A.; Knowles, C.H. Sacral nerve stimulation for faecal incontinence and consti-pation in adults. Cochrane Database Syst. Rev. 2015, 8, CD004464. [Google Scholar]
- Dorman, B.P.; Hill, C.; McGrath, M.; Mansour, A.; Dobson, D.; Pearse, T.; Singleton, J.; Al-Omoush, A.; Barry, M.; Colongon, A.R.; et al. Bowel management in the intensive care unit. Intensiv. Crit. Care Nurs. 2004, 20, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Costilla, V.C.; Foxx-Orenstein, A.E.; Mayer, A.P.; Crowell, M.D. Office-based management of fecal incontinence. Gastroenterol. Hepatol. 2013, 9, 423–433. [Google Scholar]
- Beitz, J.M. Fecal incontinence in acutely and critically ill patients: Options in management. Ostomy Wound Manag. 2006, 52, 56–58. [Google Scholar]
- Ritzema, J. Bowel management systems in critical care: A service evaluation. Nurs. Stand. 2017, 31, 42–49. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- AVEN. Guideline for the Identification of Experimental Thesis to Be Submitted to the Ethics Committee Approval. Available online: https://www.aou.mo.it/ComitatoEticoAVEN (accessed on 1 September 2023).
- Hagen, C.; Malkmus, D.; Durham, P. Levels of Cognitive Functioning; Rancho Los Amigos Hospital: Downey, CA, USA, 1972. [Google Scholar]
- Lavezzi, S.; Bargellesi, S.; Cassio, A.; De Tanti, A.; Gatta, G.; Hakiti, B.; Lombardi, F.; Montis, A.; Posteraro, F.; Scarponi, F.; et al. Redefining a minimal rehabilitation assessment protocol for severe acquired brain injuries. Eur. J. Rehabil. Med. 2022, 58, 584–591. [Google Scholar]
- International Continence Society. Annual Meeting 2018, W6: Hands-on Workshop on Rectal Balloon Training and Transanal Irrigation in the Management of Lower Bowel Dysfunction. Available online: https://www.ics.org/2018/session/3849 (accessed on 28 August 2018).
- Juul, T.; Bazzocchi, G.; Coggrave, M.; Johannesen, I.L.; Hansen, R.B.M.; Thiyagarajan, C.; Poletti, E.; Krogh, K.; Christensen, P. Reliability of the international spinal cord injury bowel function basic and extended data sets. Spinal Cord 2011, 49, 886–891. [Google Scholar] [CrossRef]
- Menees, S.B.; Almario, C.V.; Spiegel, B.M.; Chey, W.D. Prevalence of and Factors Associated with Fecal Incontinence: Results from a Population-Based Survey. Gastroenterology 2018, 154, 1672–1681.e3. [Google Scholar] [CrossRef]
- Meinds, R.J.; van Meegdenburg, M.M.; Trzpis, M.; Broens, P.M. On the prevalence of constipation and fecal incontinence, and their co-occurrence, in the Netherlands. Int. J. Color. Dis. 2016, 32, 475–483. [Google Scholar] [CrossRef]
- Taylor, S.J.; Fettes, S.B.; Jewkes, C.; Nelson, R.J. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit. Care Med. 1999, 27, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Early Enteral Nutrition in Patients Undergoing Sustained Neuro-muscular Blockade: A Propensity-Matched Analysis Using a Nationwide Inpatient Database. Crit. Care Med. 2019, 47, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Savino, P. Knowledge of Constituent Ingredients in Enteral Nutrition Formulas Can Make a Difference in Patient Response to Enteral Feeding. Nutr. Clin. Pract. 2017, 33, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Berger, M.M. Early or Late Feeding after ICU Admission? Nutrients 2017, 9, 1278. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Pitta, M.R.; Campos, F.M.; Monteiro, A.G.; Cunha, A.G.F.; Porto, J.D.; Gomes, R.R. Tutorial on Diarrhea and Enteral Nutrition: A Comprehensive Step-By-Step Approach. JPEN J. Parenter Enteral Nutr. 2019, 43, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Kharrazian, D. Traumatic Brain Injury and the Effect on the Brain-Gut Axis. Altern. Ther. Health Med. 2015, 21, 538–542. [Google Scholar]
- Lv, L.-Q.; Hou, L.-J.; Yu, M.-K.; Qi, X.-Q.; Chen, H.-R.; Chen, J.-X.; Hu, G.-H.; Luo, C.; Lu, Y.-C. Risk factors related to dysautonomia after severe traumatic brain injury. J. Trauma: Inj. Infect. Crit. Care 2011, 71, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Shi, N.; Wan, Y.; Mei, F.; Qiu, B.; Bao, Y.; Zhang, Y.; Hao, J.; He, J.; Peng, X. Risk Factors of Acute Gastrointestinal Failure in Critically Ill Patients with Traumatic Brain Injury. J. Craniofacial Surg. 2020, 31, e176–e179. [Google Scholar] [CrossRef]
- McDermott, M.R.; Bienenstock, J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 1979, 122, 1892–1898. [Google Scholar] [CrossRef]
- Trummer, M.; Eustacchio, S.; Unger, F.; Tillich, M.; Flaschka, G. Right hemispheric frontal lesions as a cause for anorexia nervosa report of three cases. Acta Neurochir. 2002, 144, 797–801. [Google Scholar] [CrossRef]
- Hinson, H.E.; Puybasset, L.; Weiss, N.; Perlbarg, V.; Benali, H.; Galanaud, D.; Lasarev, M.; Stevens, R.D.; NICER Consortium. Neuro-anatomical basis of paroxysmal sympathetic hyperactivity: A diffusion tensor imaging analysis. Brain Inj. 2015, 29, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Baguley, I.J.; Perkes, I.E.; Fernandez-Ortega, J.F.; Rabinstein, A.A.; Dolce, G.; Hendricks, H.T. Consensus Working Group. Paroxysmal sympathetic hyperactivity after acquired brain injury: Consensus on conceptual definition, nomenclature, and diagnostic criteria. J. Neurotrauma 2014, 31, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Baguley, I.J.; Nicholls, J.L.; Felmingham, K.L.; Crooks, J.; Gurka, J.A.; Wade, L.D. Dysautonomia after traumatic brain injury: A forgotten syndrome? J. Neurol. Neurosurg. Psychiatry 1999, 67, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Lucca, L.F.; Pignolo, L.; Leto, E.; Ursino, M.; Rogano, S.; Cerasa, A. Paroxysmal Sympathetic Hyperactivity Rate in Vegetative or Minimally Conscious State after Acquired Brain Injury Evaluated by Paroxysmal Sympathetic Hyperactivity Assessment Measure. J. Neurotrauma 2019, 36, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Hinson, H.E.; Baguley, I.J. Autonomic dysfunction syndromes after acute brain injury. Handb. Clin. Neurol. 2015, 128, 539–551. [Google Scholar] [PubMed]
- Laxe, S.; Terré, R.; León, D.; Bernabeu, M. How does dysautonomia influence the outcome of traumatic brain injured patients admitted in a neurorehabilitation unit? Brain Inj. 2013, 27, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-Q.; Hou, L.-J.; Yu, M.-K.; Qi, X.-Q.; Chen, H.-R.; Chen, J.-X.; Hu, G.-H.; Luo, C.; Lu, Y.-C. Prognostic influence and magnetic resonance imaging findings in paroxysmal sympathetic hyperactivity after severe traumatic brain injury. J. Neurotrauma 2010, 27, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Gouvier, W.D.; Blanton, P.D.; Laporte, K.K.; Nepomuceno, C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef]
- Lucente, G.; Corral, J.; Rodríguez-Esparragoza, L.; Castañer, S.; Ortiz, H.; Piqueras, A.; Broto, J.; Hernández-Pérez, M.; Domenech, S.; Martinez-Piñeiro, A.; et al. Current Incidence and Risk Factors of Fecal Incontinence After Acute Stroke Affecting Functionally Independent People. Front. Neurol. 2021, 12, 755432. [Google Scholar] [CrossRef]
- Van Almenkerk, S.; Smalbrugge, M.; Depla, M.F.; Eefsting, J.A.; Hertogh, C.M. What predicts a poor outcome in older stroke sur-vivors? A systematic review of the literature. Disabil. Rehabil. 2013, 35, 1774–1782. [Google Scholar] [CrossRef]
- Boman, E.; Nylander, M.; Oia, J.; Olofsson, B. Transanal irrigation for people with neurogenic bowel dysfunction: An integrative literature review. Gastroenterol. Nurs. 2022, 45, 211–230. [Google Scholar] [CrossRef]
- Zandalasini, M.; Pelizzari, L.; Ciardi, G.; Giraudo, D.; Guasconi, M.; Paravati, S.; Lamberti, G.; Frizziero, A. Bowel Dysfunctions after Acquired Brain Injury: A Scoping Review. Front. Hum. Neurosci. 2023, 17, 1146054. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).