A Case Series of Four Patients with Artery of Percheron Occlusion over a Three-Month Period
Abstract
:1. Introduction
2. Case Presentation
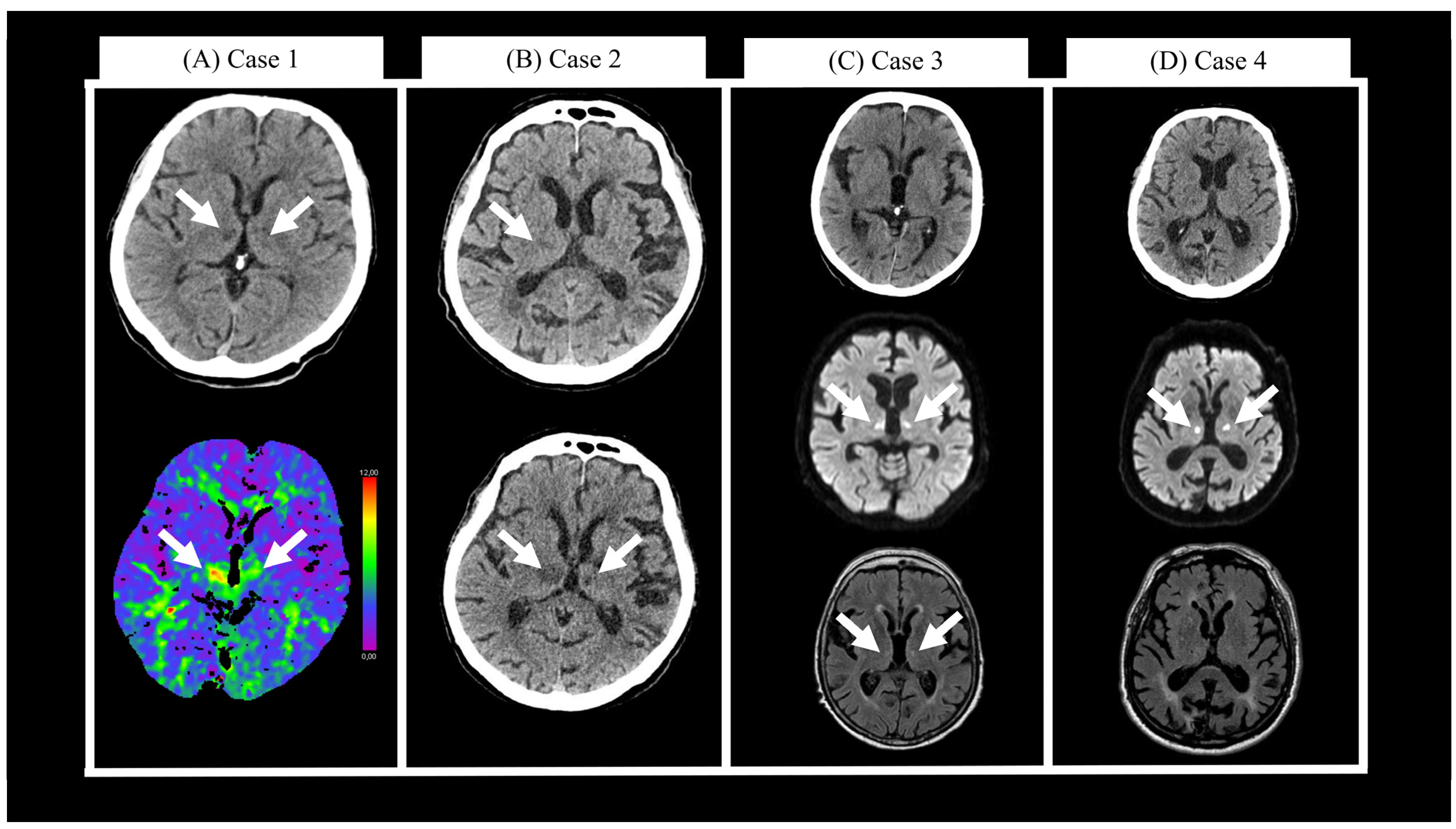
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
| Patient | Sex | Age | Time from Symptom Onset to Arrival [h] | Time from Arrival to Diagnosis [h] | Presenting Symptoms | Infarction Topography | Acute Treatment | mRS after 3 Months |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | 1 | 25 | Somnolence; vertical gaze palsy; dysarthria; gait instability | Paramedian bilateral thalami; anterior mesencephalon | LMWH, Aspirin | 1 |
| 2 | M | 74 | 1 | 21 | Impaired consciousness | Bilateral medial thalami | LMWH, Aspirin | 6 |
| 3 | F | 73 | 1.5 | 7 | Somnolence; right pupil widening; vertical gaze palsy; diplopia; orofacial dyskinesia; mild ataxia in all four limbs | Bilateral medial thalami; anteromedial mesencephalon | Apixaban | 4 |
| 4 | M | 81 | 13.5 (TIA), wake-up stroke | 0.5 | Diplopia; gait instability; somnolence; vertical gaze palsy | Bilateral medial and ventral thalami; superior mesencephalon | IVT, aspirin, clopidogrel | 4 |
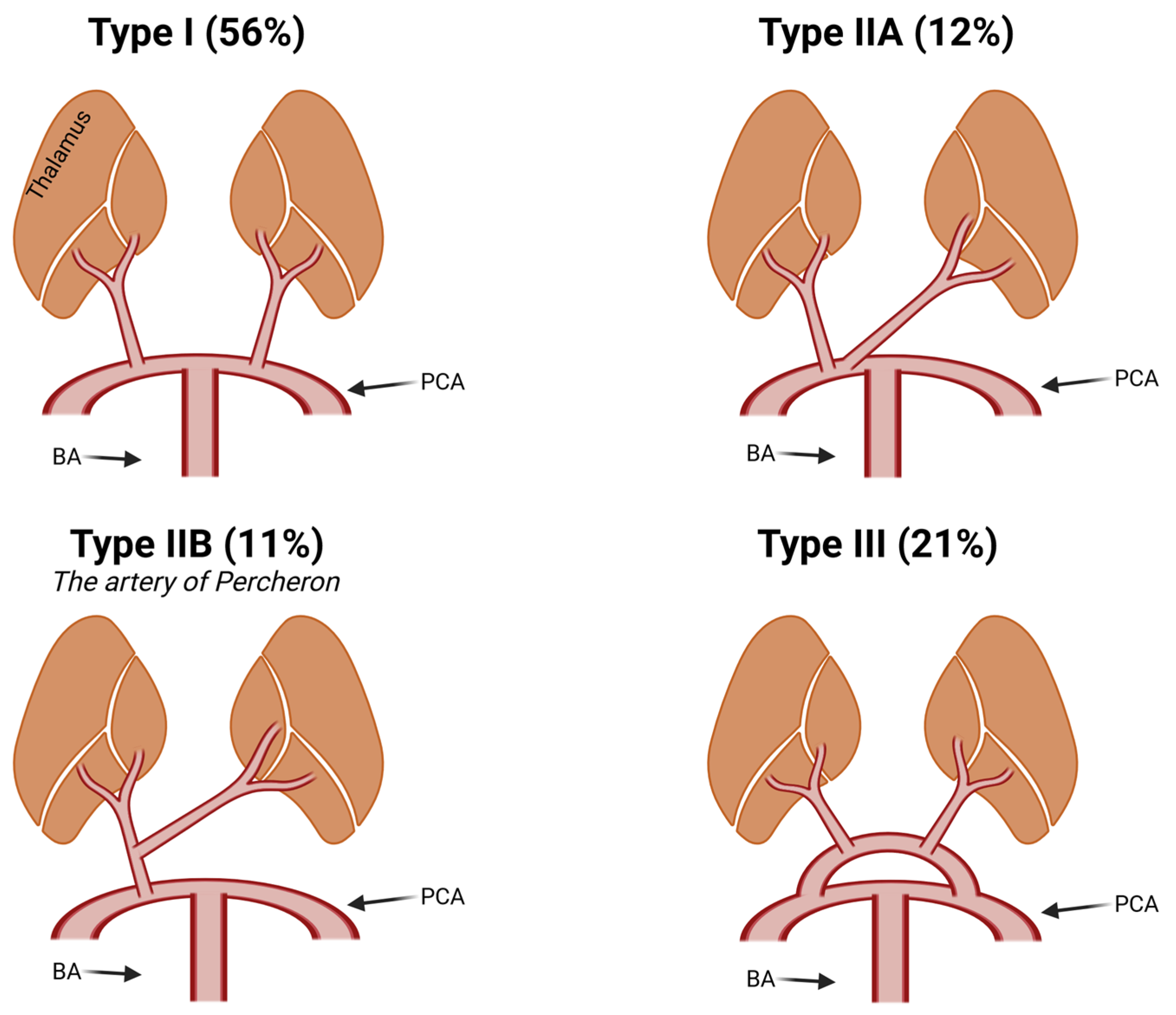
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nouh, A.; Remke, J.; Ruland, S. Ischemic Posterior Circulation Stroke: A Review of Anatomy, Clinical Presentations, Diagnosis, and Current Management. Front. Neurol. 2014, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Arauz, A.; Patiño-Rodríguez, H.M.; Vargas-González, J.C.; Arguelles-Morales, N.; Silos, H.; Ruiz-Franco, A.; Ochoa, M.A. Clinical Spectrum of Artery of Percheron Infarct: Clinical–Radiological Correlations. J. Stroke Cerebrovasc. Dis. 2014, 23, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Kocaeli, H.; Yılmazlar, S.; Kuytu, T.; Korfalı, E. The artery of Percheron revisited: A cadaveric anatomical study. Acta Neurochir. 2013, 155, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ciacciarelli, A.; Francalanza, I.; Giammello, F.; Galletta, K.; Toscano, A.; Musolino, R.F.; Granata, F.; La Spina, P. Prevalence, clinical features, and radiological pattern of artery of Percheron infarction: A challenging diagnosis. Neurol. Sci. 2023, 44, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Michel, P.; Bogousslavsky, J. Anteromedian, Central, and Posterolateral Infarcts of the Thalamus. Stroke 2004, 35, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Caballero, P.E. Bilateral Paramedian Thalamic Artery Infarcts: Report of 10 Cases. J. Stroke Cerebrovasc. Dis. 2010, 19, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, L.; Duan, Y.; Zhang, J.; Zhang, M.; Cai, X. Assessment of Percheron infarction in images and clinical findings. J. Neurol. Sci. 2017, 383, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M. The thalamus is more than just a relay. Curr. Opin. Neurobiol. 2007, 17, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Lewis, L.D.; Garrett, D.D.; Hwang, K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 2023, 24, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. Vascular Syndromes of the Thalamus. Stroke 2003, 34, 2264–2278. [Google Scholar] [CrossRef] [PubMed]
- Percheron, G. The Anatomy of the Arterial Supply of the Human Thalamus and Its Use for the Interpretation of the Thalamic Vascular Pathology. Z. Neurol 1973, 205, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hung, J.; Lin, S. Percheron Artery-Plus Syndrome: A Syndrome Beyond Stroke Chameleon. J. Nippon Med. Sch. 2021, 88, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, N.A.; Wright, B.; Castillo, M.; Fischbein, N.J.; Glastonbury, C.M.; Hildenbrand, P.G.; Wiggins, R.H.; Quigley, E.P.; Osborn, A.G. Artery of Percheron Infarction: Imaging Patterns and Clinical Spectrum. Am. J. Neuroradiol. 2010, 31, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Flowers, J.; Gandhi, S.; Guduguntla, L.; Yang, A.; Moudgil, S. Artery of Percheron Strokes: Three Cases in Three Months. Cureus 2022, 14, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Lamot, U.; Ribaric, I.; Popovic, K.S. Artery of Percheron infarction: Review of literature with a case report. Radiol. Oncol. 2015, 49, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Katyal, A.; Calic, Z.; Killingsworth, M.; Bhaskar, S.M.M. Diagnostic and prognostic utility of computed tomography perfusion imaging in posterior circulation acute ischemic stroke: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Bivard, A.; Krishnamurthy, V.; Levi, C.R.; Parsons, M.W. Whole-Brain CT Perfusion to Quantify Acute Ischemic Penumbra and Core. Radiology 2016, 279, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Cheng, B.; Ebinger, M.; Hao, Q.; Tourdias, T.; Wu, O.; Kim, J.S.; Breuer, L.; Singer, O.C.; Warach, S.; et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): A multicentre observational study. Lancet Neurol. 2011, 10, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, X.; Gang, C.; Wang, J. Acute percheron infarction: A precision learning. BMC Neurol. 2022, 22, 207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perovnik, M.; Pretnar Oblak, J.; Frol, S. A Case Series of Four Patients with Artery of Percheron Occlusion over a Three-Month Period. Neurol. Int. 2023, 15, 1352-1358. https://doi.org/10.3390/neurolint15040085
Perovnik M, Pretnar Oblak J, Frol S. A Case Series of Four Patients with Artery of Percheron Occlusion over a Three-Month Period. Neurology International. 2023; 15(4):1352-1358. https://doi.org/10.3390/neurolint15040085
Chicago/Turabian StylePerovnik, Matej, Janja Pretnar Oblak, and Senta Frol. 2023. "A Case Series of Four Patients with Artery of Percheron Occlusion over a Three-Month Period" Neurology International 15, no. 4: 1352-1358. https://doi.org/10.3390/neurolint15040085
APA StylePerovnik, M., Pretnar Oblak, J., & Frol, S. (2023). A Case Series of Four Patients with Artery of Percheron Occlusion over a Three-Month Period. Neurology International, 15(4), 1352-1358. https://doi.org/10.3390/neurolint15040085





