Robot Assisted Gait Training in a Patient with Ataxia
Abstract
:1. Introduction
- reduced cadence and speed
- reduced step and stride length
- increased stance time
- increased step and stride time
- increased double limb support time
- increased step/stride length variability and increased stride time variability, which represent a compensatory strategy for trunk instability [8].
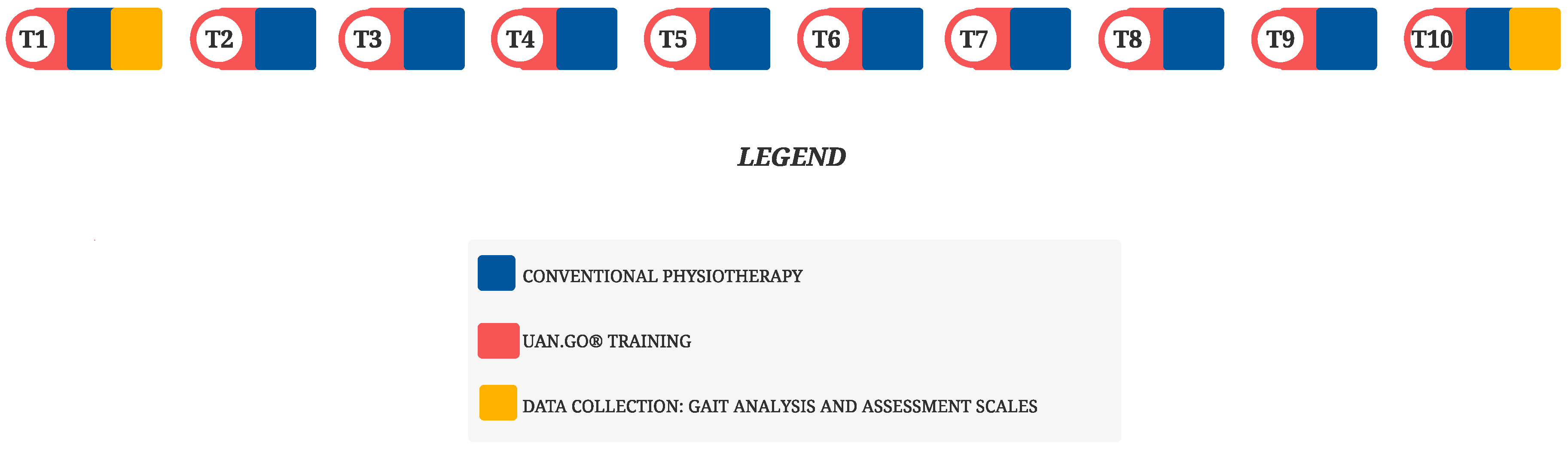
2. Materials and Methods
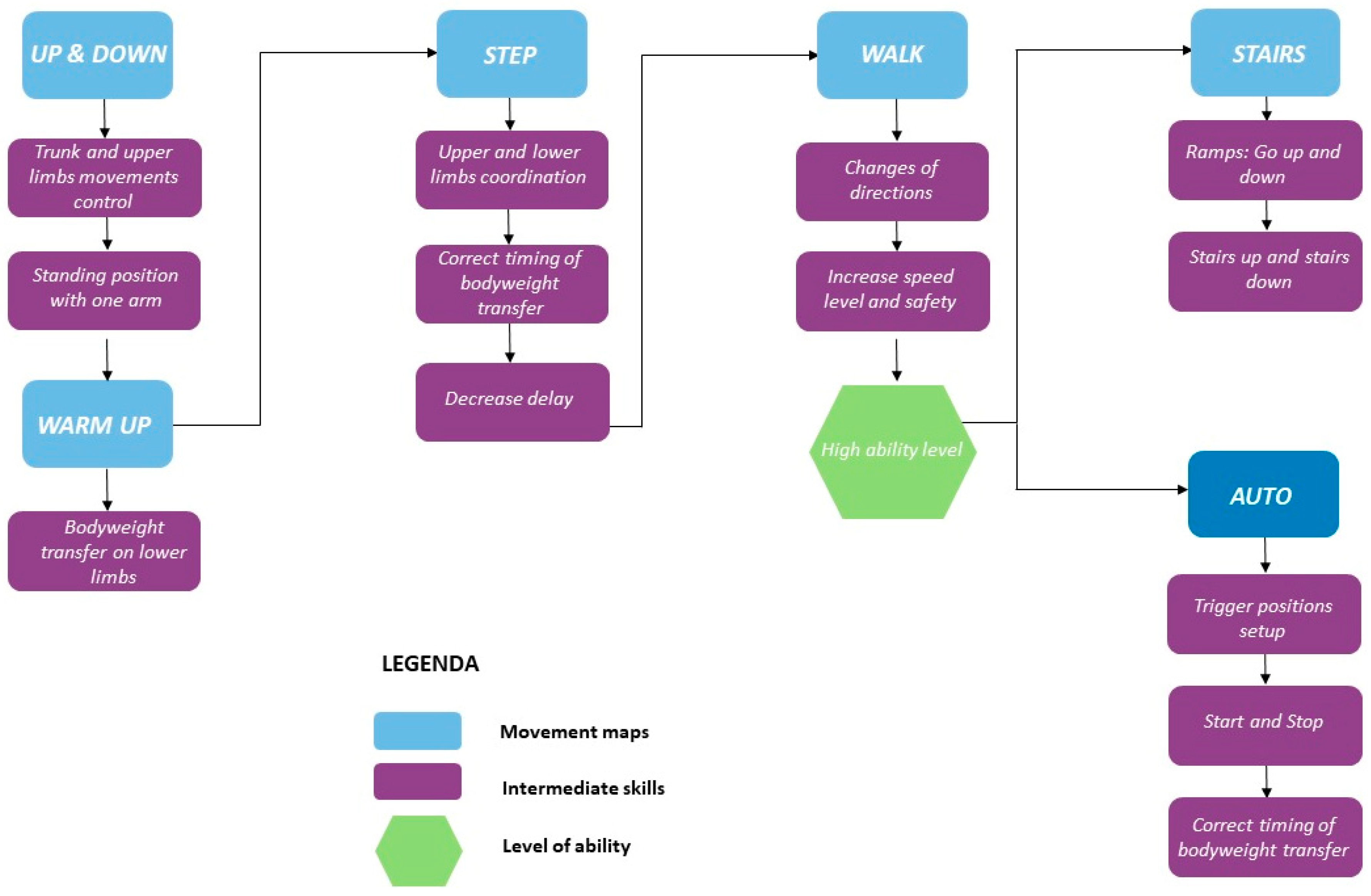
Therapeutic Intervention
3. Results
- Sitting-standing position transfer: in T10, the subject was able to get up without the use of hands, and to stabilize himself in the new taken posture without any help.
- Standing-sitting position transfer: in T10, the subject could sit without danger of falling and with minimal use of upper limbs, without the need to control the drop with hands. In T1, the subject managed to sit with the help of upper limbs.
- Standing position with closed eyes: in T10, the subject could maintain a standing position with eyes closed, fixed for 10 s in safety. In T1, he could not perform the task and needed other person’s supervision.
- Picking up an object placed in front of the feet: in T10, the subject was able to pick it up in a safe and easy way. In T1, he could not pick it up alone.
- Rotating 360° in standing position: in T10, the subject could rotate completely and safely in a single direction in 4 s or less (pivot on left leg and left rotation). In T1, he could perform this movement very slowly.
- Put feet alternatively on a step during standing position: in T10, the subject could carry out more than two movements and needed minimal assistance. In T1, assistance was essential to prevent falls.
- Standing tandem position: in T10, the subject could put one foot in front of the other (not in tandem position) and maintain this position for 30 s. In T1, he could complete a small step.
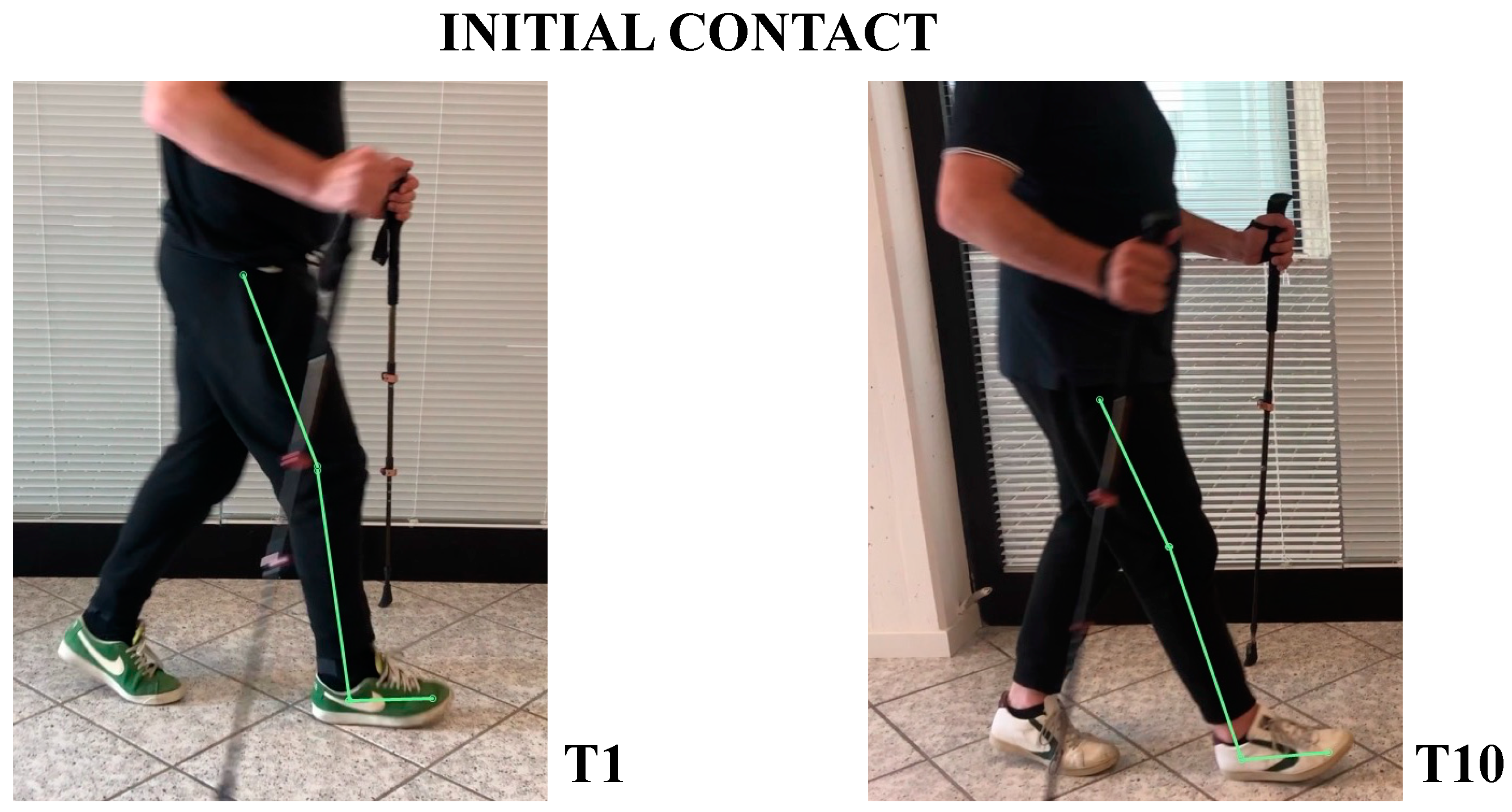
- In initial contact phase, trunk was flexed, hip and knee were flexed; forefoot, midfoot, and backfoot touched the ground at the same time (Figure 5).
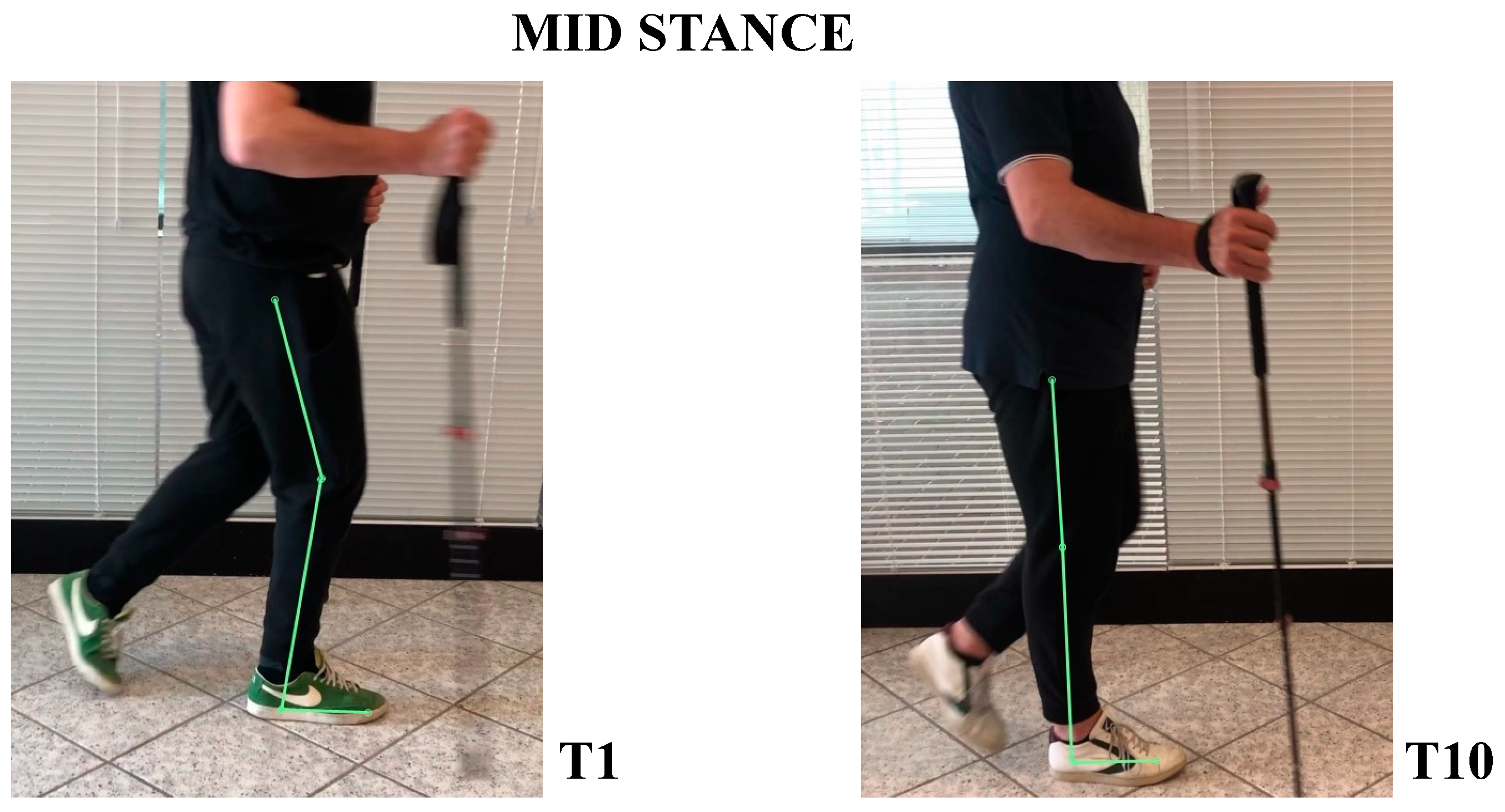
- In mid-stance phase, trunk, hip, and knee flexion persisted, while ankle was dorsiflexed (Figure 6).
- In the pre-swing phase, hip was in a neutral position on the sagittal plane, knee was flexed, and foot was equinus (Figure 7).
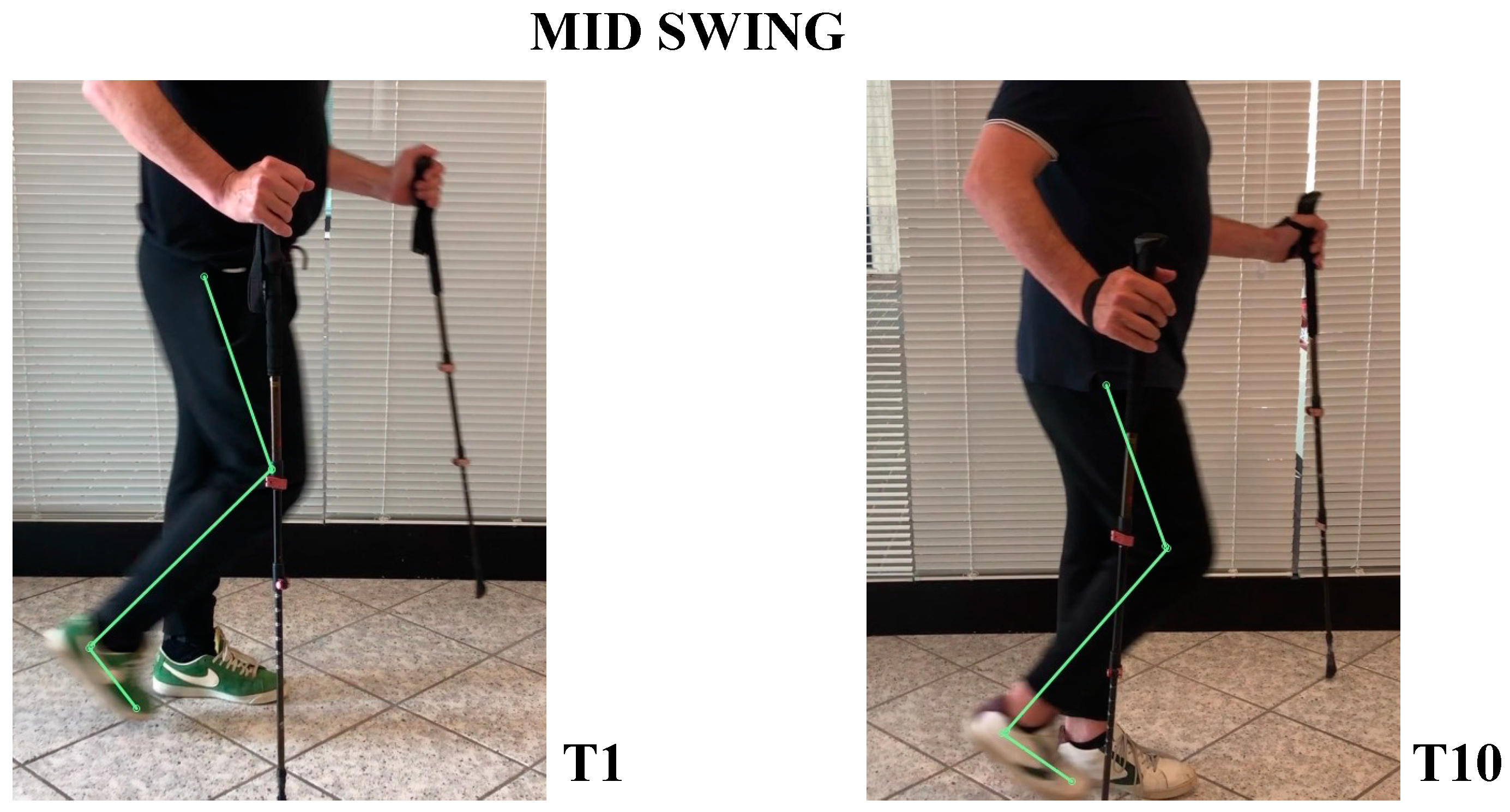
- In the mid-swing phase, hip was slightly flexed and knee had a marked flexion to compensate for the forefoot fall in equinus (Figure 8).
- At initial contact, hip was flexed, knee was extended, and foot performed “heel strike” on the ground with slight ankle dorsiflexion (Figure 5).
- In the mid-stance phase, hip and foot were in neutral position, while knee was extended (Figure 6)
- In the pre-swing phase, hip went to extension, knee reached very few degrees of flexion, while foot was in dorsal flexion (Figure 7)
- In the mid-swing phase, hip and knee were flexed, foot maintained neutral position with a reduced fall of the forefoot compared to the initial evaluation (Figure 8)
4. Discussion
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariotti, C.; Fancellu, R.; Di Donato, S. An overview of the patient with ataxia. J. Neurol. 2005, 252, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Xia, G. Ataxia. Continuum 2016, 22, 1208–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruano, L.; Melo, C.; Silva, M.C.; Coutinho, P. The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology 2014, 42, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Cruccu, G. Sapienza Neurology, 3rd ed.; Società Editrice Esculapio: Bologna, Italy, 2019; pp. 344–347. [Google Scholar]
- Akbar, U.; Ashizawa, T. Ataxia. Neurol. Clin. 2015, 33, 225–248. [Google Scholar] [CrossRef] [Green Version]
- Hafiz, S.; De Jesus, O. Ataxia. In Stas Pearl [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Byung-Euk, J.; Lee, C.N.; Park, K.W. Prevalence rate and functional status of cerebellar ataxia in Korea. Cerebellum 2012, 11, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Marquer, A.; Barbieri, G.; Pérennou, D. The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Ann. Phys. Rehabil. Med. 2014, 57, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, H.I.; Volpe, B.T. Rehabilitation robotics. Handb. Clin. Neurol. 2013, 110, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Goffredo, M.; Guanziroli, E.; Pournajaf, S.; Gaffuri, M.; Gasperini, G.; Filoni, S.; Baratta, S.; Damiani, C.; Franceschini, M.; Molteni, F.; et al. Overground wearable powered exoskeleton for gait training in subacute stroke subjects: Clinical and gait assessments. Eur. J. Phys. Rehabil. Med. 2019, 55, 710–721. [Google Scholar] [CrossRef]
- Swinnen, E.; Beckwée, D.; Meeusen, R.; Baeyens, J.-P.; Kerckhofs, E. Does robot-assisted gait rehabilitation improve balance in stroke patients? A systematic review. Top Stroke Rehabil. 2014, 21, 87–100. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Enrich the environment to empower the brain. Trends Neurosci. 2009, 32, 233–239. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Russo, E.F.; Russo, M.; Masiero, S.; Piccione, F.; Calabrò, R.S.; Filoni, S. Effects on mobility training and de-adaptations in subjects with Spinal Cord Injury due to a Wearable Robot: A preliminary report. BMC Neurol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AVEN. Guideline for the Identification of Experimental Thesis to Be Submitted to the Ethics Committee Approval. Available online: https://www.aou.mo.it/ComitatoEticoAVEN (accessed on 11 September 2021).
- Khan, A.S.; Livingstone, D.C.; Hurd, C.L.; Duchcherer, J.; Misiaszek, J.E.; Gorassini, M.A.; Manns, P.J.; Yang, J.F. Retraining walking over ground in a powered exoskeleton after spinal cord injury: A prospective cohort study to examine functional gains and neuroplasticity. J. Neuroeng. Rehabil. 2019, 16, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, A.E.; Maher, J.L.; Baunsgaard, C.B.; Nash, M.S. Clinician-Focused Overview of Bionic Exoskeleton Use After Spinal Cord Injury. Top Spinal Cord Inj. Rehabil. 2017, 23, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Pais-Vieira, C.; Allahdad, M.; Neves-Amado, J.; Perrotta, A.; Morya, E.; Moioli, R.; Shapkova, E.; Pais-Vieira, M. Method for positioning and rehabilitation training with the ExoAtlet® powered exoskeleton. MethodsX 2020, 7, 100849. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.-S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Bürk, K.; Schulz, S.R.; Schulz, J.B. Monitoring progression in Friedreich ataxia (FRDA): The use of clinical scales. J. Neurochem. 2013, 126 (Suppl. 1), 118–124. [Google Scholar] [CrossRef]
- Ottonello, M.F.G.; Benevolo, E.; Sessarego, P.D.D. Psychometric evaluation of the Italian version of the Berg Balance Scale in rehabilitation inpatients. Eur. Medicophys. 2003, 39, 181–189. [Google Scholar]
- Gervasoni, E.; Jonsdottir, J.; Montesano, A.; Cattaneo, D. Minimal Clinically Important Difference of Berg Balance Scale in People With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 337–340.e2. [Google Scholar] [CrossRef]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: A multicenter, retrospective, observational study. Top Stroke Rehabil. 2022, 29, 1–7. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, S.; Bird, T.D. Hereditary ataxias: Overview. Genet Med. 2013, 15, 673–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Han, J.-Y.; Song, M.-K.; Choi, I.-S.; Park, H.-K. Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report. Sensors 2021, 21, 4874. [Google Scholar] [CrossRef] [PubMed]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PM&R 2018, 10 (Suppl. 2), S174–S188. [Google Scholar] [CrossRef] [Green Version]
- Langan, J.; Subryan, H.; Nwogu, I.; Cavuoto, L. Reported use of technology in stroke rehabilitation by physical and occupational therapists. Disabil. Rehabil. Assist. Technol. 2018, 13, 641–647. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, J.-H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar] [CrossRef]
- Belas Dos Santos, M.; Barros de Oliveira, C.; Dos Santos, A.; Garabello Pires, C.; Dylewski, V.; Arida, R.M. A Comparative Study of Conventional Physiotherapy versus Robot-Assisted Gait Training Associated to Physiotherapy in Individuals with Ataxia after Stroke. Behav. Neurol. 2018, 2018, 2892065. [Google Scholar] [CrossRef]
- Portaro, S.; Russo, M.; Bramanti, A.; Leo, A.; Billeri, L.; Manuli, A.; La Rosa, G.; Naro, A.; Calabrò, R.S. The role of robotic gait training and tDCS in Friedrich ataxia rehabilitation: A case report. Medicine 2019, 98, e14447. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Upper limbs able to handle a walker | History of severe neurological diseases associated with severe systemic diseases (e.g., infections, circulatory or heart problems, lung problems) |
| Absence of unconsolidated fractures | Presence of pressure sores |
| Good general health | Severe spasticity |
| Height between 155 and 195 cm | Heterotopic ossifications that reduce ROM |
| Weight not exceeding 100 kg | Spinal instability or pelvic or AAII fractures not healed |
| Good bone mineral density | Important retractions |
| Psychiatric or cognitive problems that can interfere with the correct use of the device |
| T1 | T10 | |
|---|---|---|
| SARA | 11 | 9 |
| Gait | 4 | 3 |
| Stance | 2 | 1 |
| Sitting | 0 | 0 |
| Speech disturbance | 1 | 1 |
| Finger chase | 1 | 1 |
| Nose-finger test | 1 | 1 |
| Fast alternating hand movements | 1 | 1 |
| Heel-shin slide | 1 | 1 |
| BBS | 41 | 47 |
| WALKED DISTANCE IN 6MWT | 144. 76 m | 160. 27 m |
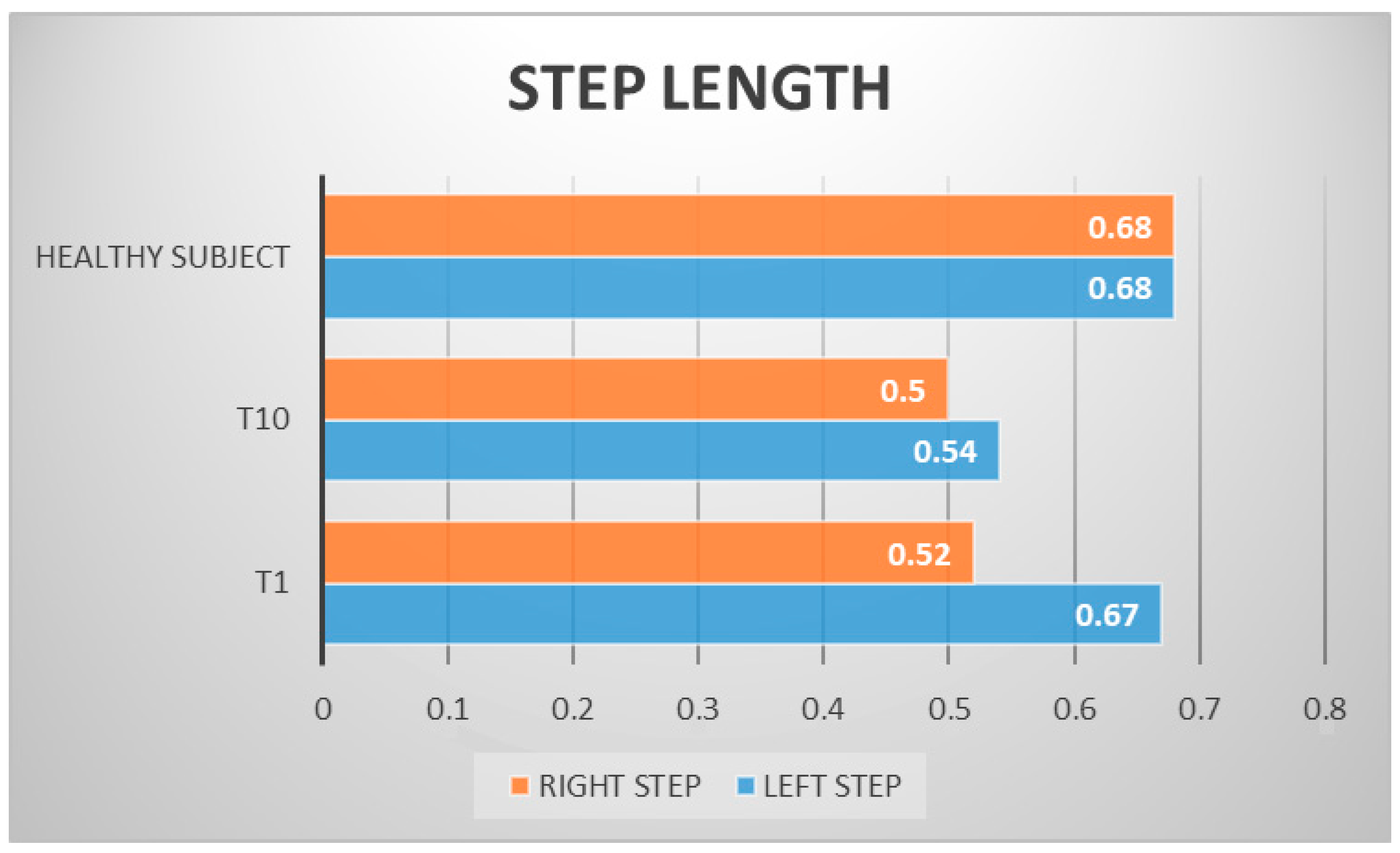
| LEFT STEP LENGTH | 0.67 m | 0.54 m |
| RIGHT STEP LENGTH | 0.52 m | 0.50 m |
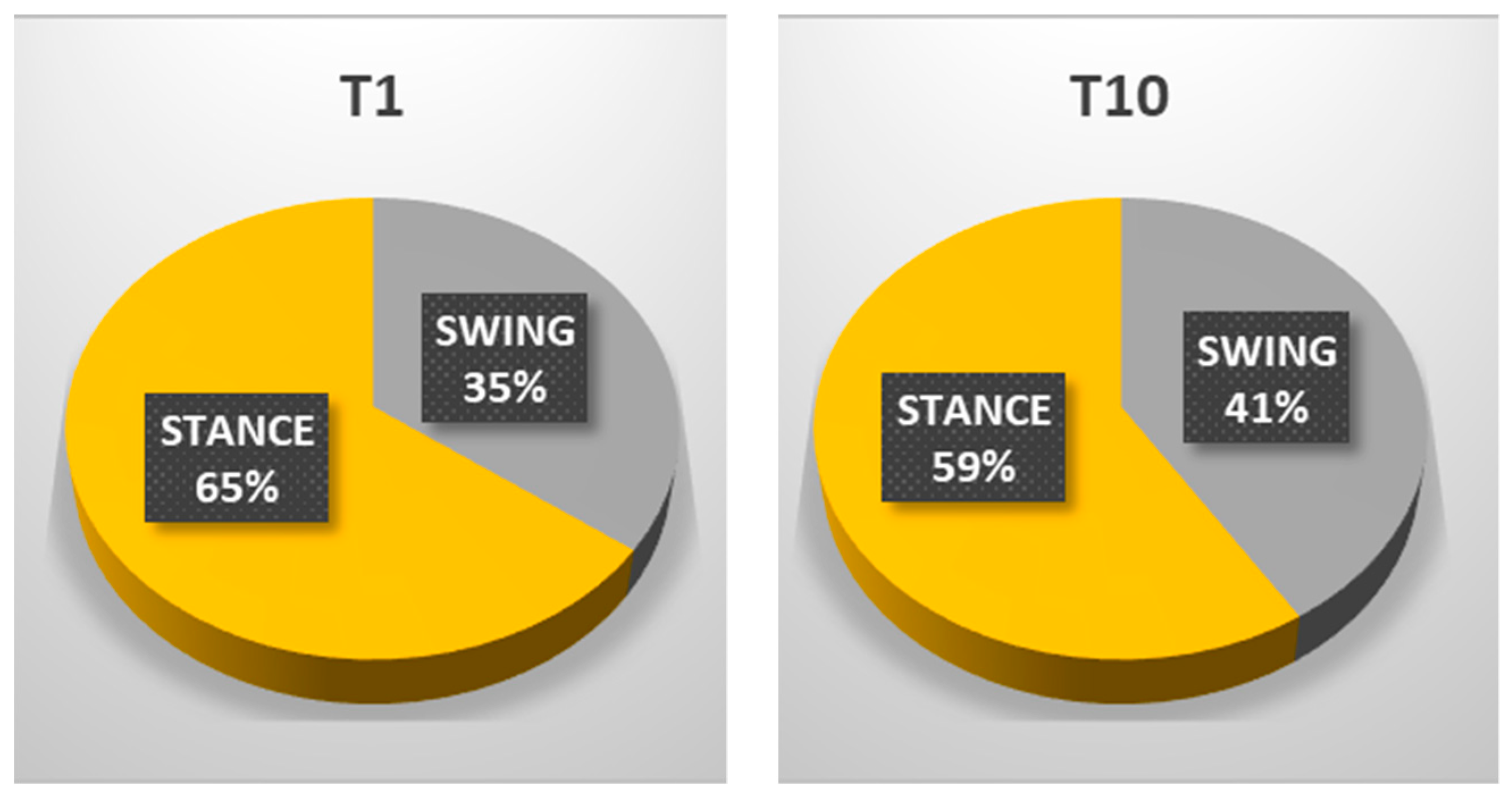
| STANCE % | 65.54% | 59.01% |
| SWING % | 35.46% | 40.99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, G.; Sesenna, G.; Marina, M.; Ricci, E.; Ciardi, G. Robot Assisted Gait Training in a Patient with Ataxia. Neurol. Int. 2022, 14, 561-573. https://doi.org/10.3390/neurolint14030045
Lamberti G, Sesenna G, Marina M, Ricci E, Ciardi G. Robot Assisted Gait Training in a Patient with Ataxia. Neurology International. 2022; 14(3):561-573. https://doi.org/10.3390/neurolint14030045
Chicago/Turabian StyleLamberti, Gianfranco, Gianluca Sesenna, Martina Marina, Emanuela Ricci, and Gianluca Ciardi. 2022. "Robot Assisted Gait Training in a Patient with Ataxia" Neurology International 14, no. 3: 561-573. https://doi.org/10.3390/neurolint14030045
APA StyleLamberti, G., Sesenna, G., Marina, M., Ricci, E., & Ciardi, G. (2022). Robot Assisted Gait Training in a Patient with Ataxia. Neurology International, 14(3), 561-573. https://doi.org/10.3390/neurolint14030045






