Successful Consolidation/Maintenance Therapy with Single Agent Ibrutinib for Primary CNS Lymphoma after Initial Induction Therapy
Abstract
:1. Introduction
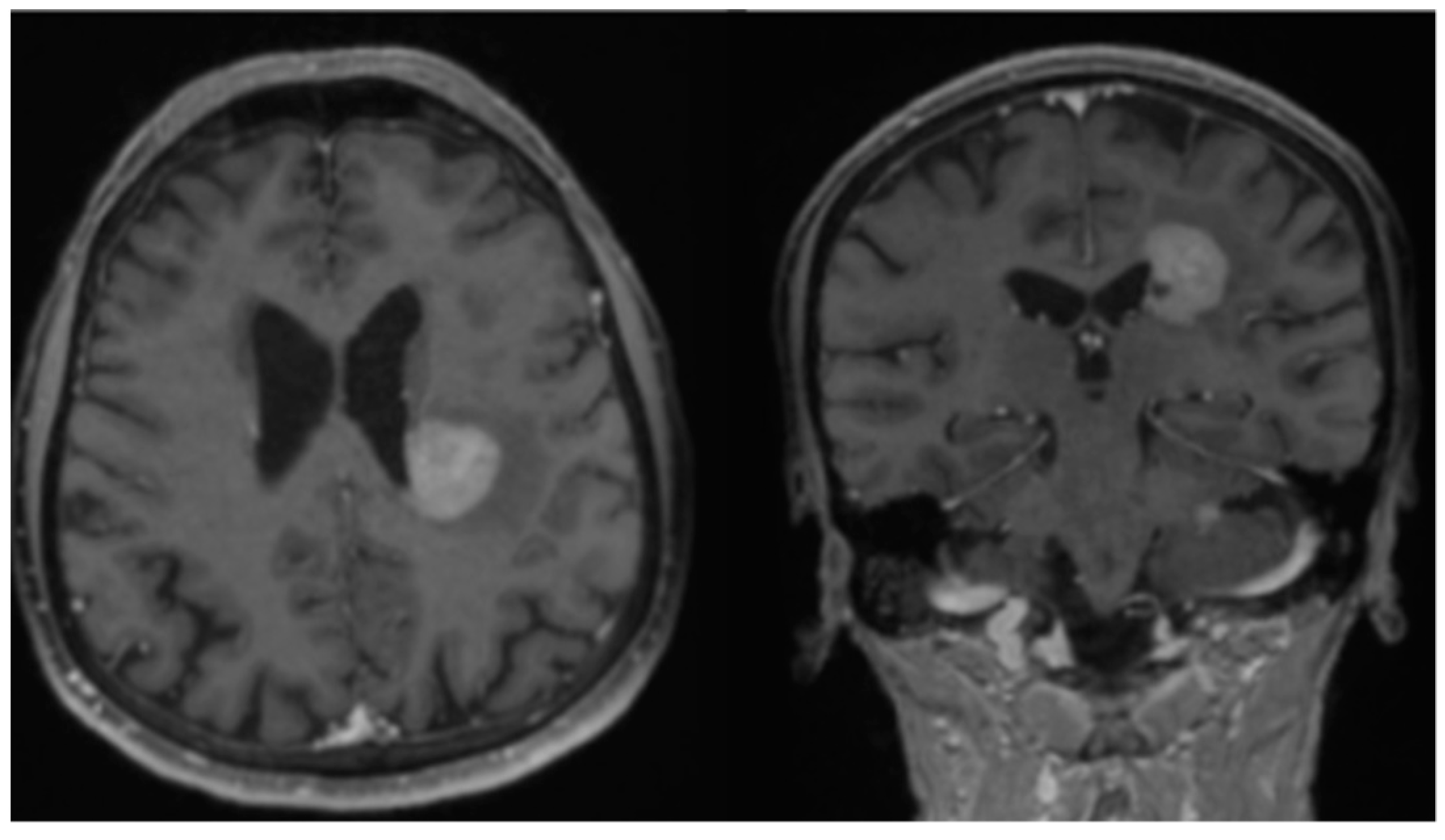
2. Case Report
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiels, M.S.; Pfeiffer, R.M.; Besson, C.; Clarke, C.A.; Morton, L.M.; Nogueira, L.; Pawlish, K.; Yanik, E.; Suneja, G.; Engels, E.A. Trends in primary central nervous system lymphoma incidence and survival in the U. S. Br. J. Haematol. 2016, 174, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Baumgarten, L.; Illerhaus, G.; Korfel, A.; Schlegel, U.; Deckert, M.; Dreyling, M. The Diagnosis and Treatment of Primary CNS Lymphoma. Dtsch. Ärzteblatt Int. 2018, 115, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bataille, B.; Delwail, V.; Menet, E.; Vandermarcq, P.; Ingrand, P.; Wager, M.; Guy, G.; Lapierre, F. Primary intracerebral malignant lymphoma: Report of 248 cases. J. Neurosurg. 2000, 92, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Küker, W.; Nägele, T.; Korfel, A.; Heckl, S.; Thiel, E.; Bamberg, M.; Weller, M.; Herrlinger, U. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J. Neurooncol. 2005, 72, 169–177. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef]
- Mendez, J.S.; Ostrom, Q.; Gittleman, H.; Kruchko, C.; DeAngelis, L.; Barnholtz-Sloan, J.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 2.2019). Available online: http://medi-guide.meditool.cn/ymtpdf/539E4DAA-3FE5-BA65-06CC-E497DB9B1857.pdf (accessed on 15 August 2021).
- Thiel, E.; Korfel, A.; Martus, P.; Kanz, L.; Griesinger, F.; Rauch, M.; Röth, A.; Hertenstein, B.; von Toll, T.; Hundsberger, T.; et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010, 11, 1036–1047. [Google Scholar] [CrossRef] [Green Version]
- Illerhaus, G.; Kasenda, B.; Ihorst, G.; Egerer, G.; Lamprecht, M.; Keller, U.; Wolf, H.-H.; Hirt, C.; Stilgenbauer, S.; Binder, M.; et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: A prospective, single-arm, phase 2 trial. Lancet Haematol. 2016, 3, e388–e397. [Google Scholar] [CrossRef]
- Omuro, A.; Correa, D.D.; DeAngelis, L.M.; Moskowitz, C.H.; Matasar, M.J.; Kaley, T.J.; Gavrilovic, I.T.; Nolan, C.; Pentsova, E.; Grommes, C.C.; et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015, 125, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J. Imbruvica FDA Approval History. 2021. Available online: https://www.drugs.com/history/imbruvica.html (accessed on 25 June 2022).
- Chen, F.; Pang, D.; Guo, H.; Ou, Q.; Wu, X.; Jiang, X.; Wei, X.; Liu, S.; Huang, L.; Liang, Z.; et al. Clinical outcomes of newly diagnosed primary CNS lymphoma treated with ibrutinib-based combination therapy: A real-world experience of off-label ibrutinib use. Cancer Med. 2020, 9, 8676–8684. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soussain, C.; Choquet, S.; Blonski, M.; Leclercq, D.; Houillier, C.; Rezai, K.; Bijou, F.; Houot, R.; Boyle, E.; Gressin, R.; et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur. J. Cancer 2019, 117, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Wolfe, J.; Gavrilovic, I.; Kaley, T.; Stone, J.; Daras, M.; Nolan, C.; Pentsova, E.; Hatzoglou, V.; Mellinghoff, I.; et al. Phase II of single-agent Ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Blood 2018, 132 (Suppl. S1), 2965. [Google Scholar] [CrossRef]
- Batchelor, T.; Carson, K.; O’Neill, A.; Grossman, S.A.; Alavi, J.; New, P.; Hochberg, F.; Priet, R. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J. Clin. Oncol. 2003, 21, 1044–1049. [Google Scholar] [CrossRef]
- Enting, R.H.; Demopoulos, A.; DeAngelis, L.M.; Abrey, L.E. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004, 63, 901–903. [Google Scholar] [CrossRef] [Green Version]
- Grommes, C.; Tang, S.S.; Wolfe, J.; Kaley, T.J.; Daras, M.; Pentsova, E.I.; Piotrowski, A.F.; Stone, J.; Lin, A.; Nolan, C.P.; et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019, 133, 436–445. [Google Scholar] [CrossRef]
- Narita, Y.; Nagane, M.; Mishima, K.; Terui, Y.; Arakawa, Y.; Yonezawa, H.; Asai, K.; Fukuhara, N.; Sugiyama, K.; Shinojima, N.; et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021, 23, 122–133. [Google Scholar] [CrossRef]
- Bruno, A.; Boisselier, B.; Labreche, K.; Marie, Y.; Polivka, M.; Jouvet, A.; Adam, C.; Figarella-Branger, D.; Miquel, C.; Eimer, S.; et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget 2014, 5, 5065–5075. [Google Scholar] [CrossRef] [Green Version]
- Nepal, G.; Khurana, M.; Bucheli, D.H.; Bhandari, S.; Joshi, U.; Bhagat, R.; Rehrig, J.H.; Pudasainee, P.; Shing, Y.K.; Ortiz, J.F.; et al. Ibrutinib in Refractory or Relapsing Primary Central Nervous System Lymphoma: A Systematic Review. Neurol. Int. 2022, 14, 99–108. [Google Scholar] [CrossRef]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Mahadevan, A.; Rao, C.R.; Shanmugham, M.; Shankar, S.K. Primary central nervous system diffuse large B-cell lymphoma in the immunocompetent: Immunophenotypic subtypes and Epstein-Barr virus association. J. Neurosci. Rural Pract. 2015, 6, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Bota, D.; Kong, X.-T. Successful Consolidation/Maintenance Therapy with Single Agent Ibrutinib for Primary CNS Lymphoma after Initial Induction Therapy. Neurol. Int. 2022, 14, 574-580. https://doi.org/10.3390/neurolint14030046
Du S, Bota D, Kong X-T. Successful Consolidation/Maintenance Therapy with Single Agent Ibrutinib for Primary CNS Lymphoma after Initial Induction Therapy. Neurology International. 2022; 14(3):574-580. https://doi.org/10.3390/neurolint14030046
Chicago/Turabian StyleDu, Steven, Daniela Bota, and Xiao-Tang Kong. 2022. "Successful Consolidation/Maintenance Therapy with Single Agent Ibrutinib for Primary CNS Lymphoma after Initial Induction Therapy" Neurology International 14, no. 3: 574-580. https://doi.org/10.3390/neurolint14030046
APA StyleDu, S., Bota, D., & Kong, X.-T. (2022). Successful Consolidation/Maintenance Therapy with Single Agent Ibrutinib for Primary CNS Lymphoma after Initial Induction Therapy. Neurology International, 14(3), 574-580. https://doi.org/10.3390/neurolint14030046





