Abstract
Left ventricular non-compaction (LVNC) is an extremely heterogeneous disorder with a highly variable clinical presentation, morphologic appearance at imaging testing, and prognosis. It is still unclear whether LVNC should be classified as a separate cardiomyopathy or if it is a mere morphological trait shared by many phenotypically distinct cardiomyopathies. Moreover, the hypertrabeculated phenotype may be reversible in some cases, possibly reflecting the left ventricular physiological response of the cardiac muscle to chronic overload. The current diagnostic criteria have several limitations, leaving many patients in a grey area. Here, we review the available literature on LVNC in order to provide an overview of the current knowledge on this complex disorder.
1. Introduction
Left ventricular non-compaction (LVNC) is a structural anomaly of the left ventricle, characterized by prominent trabeculations and deep intertrabecular recesses in continuity with the ventricular cavity. LVNC morphology may be found in isolation in functionally normal hearts, or in the context of ventricular dilation and dysfunction. LVNC may also overlap with other cardiomyopathies phenotypes. Over the last 20 years, technological advances and improved imaging definition have allowed the recognition of trabeculations in an increasing number of patients, and several echocardiographic and cardiac magnetic resonance (CMR)-based diagnostic criteria have been proposed. However, whether LVNC should be considered a distinct cardiomyopathy or simply a morphological trait shared by other cardiomyopathies is still controversial.
Search Strategy and Selection Criteria
The PubMed database was systematically searched for published articles in English until November 2021, entering specific keywords in relation to the issues of interest for the purpose of this review. We excluded outdated textbook chapters. Recommendations from ESC guidelines and consensus documents related to the topic were reviewed for inclusion in the present paper.
2. Morphological Features and Pathogenesis
Trabeculations are generally located mainly at the apex, which may have a spongy aspect in extreme cases. The anatomical features of explanted hearts from patients with LVNC are a luminal meshwork of thick endocardial bands, filaments and trabeculations with deep recesses into the LV cavity, forming a discrete trabecular inner layer with a thin, external, compacted layer [1]. The histological findings of LVNC are variably reported as being normal or as showing subendocardial or myocardial fibrosis, myocardial disorganization, hypertrophy, myocardial degeneration, intramural thrombi, myocardium scars, and signs of inflammation [2,3]. In addition, nuclear imaging techniques have shown reduced cardiac perfusion in patients with LVNC in both adults [4] and children [3], suggesting that microcirculatory dysfunction may be part of the disorder. Despite a recent widespread interest in LVNC, the physiopathological mechanisms behind this complex disease are still largely unknown. The most accredited theory of the congenital origin of LVNC derives from the observation of marked trabeculations during the early embryological evolution of the heart. At this stage, in the absence of a discrete coronary circulation, trabeculations guarantee myocardial perfusion [5]. Between 5 and 8 weeks of age, after the development of coronary arteries, a progressive process of ventricular compaction occurs, starting from the base and moving towards the apex. Therefore, LVNC could be the result of an arrest of the normal ventricular development during foetal life. The constant involvement of the apical region [6] and the frequent association with congenital heart disease seem to confirm this hypothesis. However, recent findings of reversible hypertrabeculation in healthy individuals [7,8] support the hypothesis that LVNC may be an acquired trait in response to increased ventricular loading conditions.
3. Etiology: A Genetic Disease of the Cardiac Muscle
LVNC exists as both a sporadic and familial disease. The familial form has been reported in 18–29% [2,9] of adults with LVNC, and in up to 44% [3] of paediatric cases. The familial form is a genetically heterogeneous disorder with several causative mutations in the genes encoding for sarcomeric (ACTC1, MYH7, MYBPC3, TNNT2, and TPM1), mitochondrial, desmosomal (DSP and PKP2), cytoskeletal, z-disk (LDB3), nuclear envelope (LMNA) [10], and ion channel proteins [11]. Mutations in the NOTCH signalling pathway, which is also involved in congenital heart diseases, have also been advocated. A significant number of mutations are in common with dilated and hypertrophic cardiomyopathy [12]. The inheritance pattern is mainly autosomic dominant or X-linked, but autosomic recessive and matrilineal transmission have also been described. Mutation in the mitochondrial protein tafazzin, which is involved in ATP production and utilization, is causative of the X-linked Barth Syndrome, characterized by a form of skeletal myopathy which is frequently associated with cardiomyopathy, typically LVNC, presenting in the first months of life with poor sucking, growth failure, tachypnea, and marked ventricular dilatation [13]. LVNC has also been described in genetic neuromuscular disorders, including Duchenne muscular dystrophy, and metabolic disorders, such as Danon Syndrome and Anderson–Fabry disease [14], as well as in chromosome anomalies such as trisomies 18 and 13, NSD1 mutation (Sotos syndrome), and PMP22 duplication (Charcot-Marie-Tooth disease type 1A) [15]. In the present state, no specific guidelines for genetic tests in isolated LVNC are available. However, according to the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Consensus Statement on genetic testing for the cardiomyopathies, mutation-specific genetic testing is recommended for family members following the identification of the LVNC mutation in an index case [16].
4. LVNC as a Spectrum: The Issue of the Reversible Hypertrabeculated Phenotype
In the last 10 years, increasing cases of marked trabeculated LV have been reported in conditions of increased afterload. Gati et al. demonstrated the higher prevalence of LV trabeculation among athletes, with 8.1% of athletes fulfilling the conventional criteria for LVNC [17]. More recently, increased trabeculations, which are most pronounced at the peak-training period compared to detraining period, were described in professional basketball players [8], suggesting that trabeculations may be part of the physiological changes of the athlete’s heart. Increased trabeculations were also reported in pregnancy. Gati et al. followed 102 women during pregnancy [7]: 26 women (25.4%) showed increased trabeculations, and eight women developed sufficient trabeculations to fulfill LVNC echo criteria; in 73% of the women, the complete regression of trabeculations occurred during the post-partum period [7]. Acquired LV hypertrabeculation may also be found during follow-up in patients with congenital heart disease in response to increased ventricular loading, as described below. The observation of the acquired and, in some cases, partially reversible non-compaction phenotype in athletes, pregnant women, and sickle cell anemia [18] and chronic renal failure [19] supported the hypothesis that trabeculae may be a physiological response to ventricular overload, giving rise to an animated debate on whether the presence of this morphological trait with no features of systolic and/or diastolic dysfunction should be regarded as an early form of cardiomyopathy. In this context, a large prospective study by Zemrak et al. investigated 2742 subjects without known cardiovascular disease with CMR, reporting no relevant changes in LV function after 10 years follow-up in those fulfilling the CMR criteria for LVNC at the baseline [20], further corroborated the theory of that hypertrabeculated LV should not be considered, in itself, a muscle disease. Moreover, the presence of a LVNC morphology in the healthy population, with the potential for regression, supports the hypothesis that LVNC should actually be deemed a wide morpho-functional spectrum from a purely morphological characteristic with a benign course to a structural muscular disease with a poor prognosis. Therefore, the main issue remains where the cut-off between normality and disease should be placed (Figure 1).
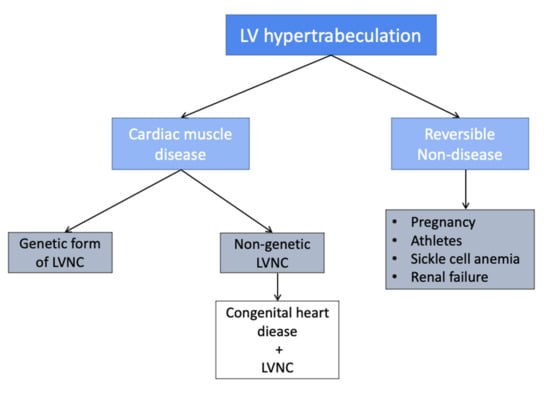
Figure 1.
LV hypertrabeculation classification.
5. LVNC Classification
A cardiomyopathy is defined as a primary or idiopathic heart muscle disease. A position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases listed LVNC among unclassified cardiomyopathies, arguing that it is still unclear whether LVNC exists as a separate cardiomyopathy, or if it is a mere morphological trait shared by many phenotypically distinct cardiomyopathies [21]. Conversely, the American Heart Association recognized LVNC as a primary, genetic cardiomyopathy [22]. An alternative nosology proposed by Arbustini et al. [23] aimed to classify any cardiomyopathy phenotypes by addressing five main features, including morphofunctional characteristics (M), organ involvement (O), a genetic pattern (G), etiological annotation (E), and functional status (S) (AHA/ACA stage or NYHA class). The MOGE(S) classification allows us to distinguish LVNC with LV dilation and dysfunction (MLVNC + D) or with LV hypertrophy (MLVNC + H) from a pure LVNC trait with normal ventricular function (MLVNC).
6. Epidemiology
LVNC has been considered a rare condition in the past, but its diffusion was probably underestimated. In the last two decades, LVNC diagnosis has shown an increasing trend, likely due to more awareness of the disorder along with improved imaging technologies. However, there is still a lack of a universally accepted cut-off for pathological trabeculations for the diagnosis of LVNC. Therefore, the real prevalence of LVNC remains difficult to define. The finding of isolated LVNC in an adult echo lab has been reported to be between 0.14% [24] and 0.24% [25]. The prevalence rises to 2.7% among adult patients with heart failure [26]. LVNC is diagnosed in 1.27% of children referred for echocardiography [27], with LVNC accounting for 9.2–9.5% of cardiomyopathies in the paediatric population [28,29].
7. LVNC and Congenital Heart Disease
Congenital heart diseases (CHD) are frequently associated with LVNC, in keeping with the congenital theory of non-compaction (Figure 2 and Figure 3). This association has been reported in 12% of cases among adults. Trabeculations need to be carefully distinguished from sinusoids, which are also frequent in congenital heart disease, and result from the continuity between a ventricular cavity and coronary capillaries. In the CHD spectrum, a ventricular septal defect is the most common association (up to 50%) [30], followed by Ebstein’s anomaly (15%), aortic coarctation (3%) [31] and Tetralogy of Fallot (2%) [32], and, more rarely, a double outlet right ventricle [33]. Moreover, LVNC has been described in around a third of patients with a left-ventricle type univentricular heart [34,35], and may likely influence the outcome being associated with impaired LV function [33]. LVNC in adult CHD patients may be present from childhood, or may become more evident during follow-up, as a result of LV overload. In children, the occurrence of LVNC with congenital defects has been reported in between 27% [36] and 62% [28] of cases. Ventricular septal defects and left and right outflow obstructive lesions are the most frequent defects found in childhood in association with LVNC [18]. In a recent CMR-based study, LVNC was demonstrated in 37% of patients with univentricular heart disease and left ventricular morphology, and was associated with a significantly reduced ejection fraction compared to those with normal ventricular morphology [34]. LVNC seems to aggravate the prognosis of congenital heart defects. Ramachandran et al. studied the impact of concomitant LVNC on perioperative outcomes in children undergoing cardiac surgery for congenital heart disease in terms of death, cardiac arrest, or the need for ECMO or transplantation, and demonstrated a significantly longer length of hospitalization and a higher incidence of complications in the LVNC-CHD group compared to patients with no myocardial abnormalities [37].
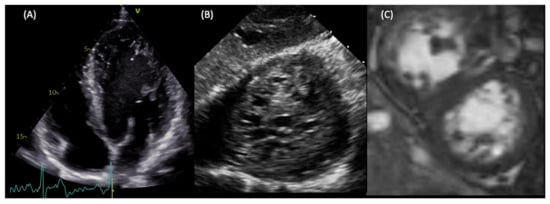
Figure 2.
LVNC in a young patient with no cardiological symptoms. The patients presented at the ER with paroxysmal atrial fibrillation. Transthoracic echocardiography showed hypertrabeculated LV (A). During a transoesophageal echocardiogram to rule out intra-atrial clots, the deep transgastric view at 0 degrees demonstrated the spongy aspect of the apex (B). CMR confirmed the diagnosis of LVNC using the Peterson criteria (C), and showed a mildly reduced ejection fraction.
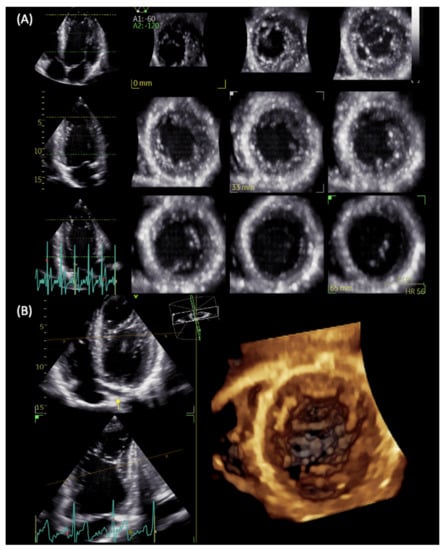
Figure 3.
LVNC in a patient with repaired aortic coarctation. Three-dimensional echocardiography with full-volume acquisition allows the better visualization of multiple apical intertrabecular recesses with multislice (A) and 3D reconstructions (B).
8. Clinical Presentation and Instrumental Findings at Diagnosis
LVNC is characterized by an extremely variable clinical presentation and a wide range of possible morphological expressions. The challenging diagnostic pathway accounts for a mean diagnostic delay of 6.43 ± 3 years, as reported by the French LVNC registry, with 32% of cases being diagnosed after 5 years or more [38]. The reported mean age at diagnosis varies from 37 ± 17 [39] to 45 ± 17 [40], and only in a quarter of cases does the first presentation occur in patients aged >60 years [35]. From the published cases, most patients were symptomatic at the time of diagnosis; however, many studies were likely biased from the inclusion mainly of those with advanced disease. Heart failure was the first presentation in up to 62% [16] of cases, and in 98% of the black population [41]. The initial NYHA class III-IV was reported in up to 50% of patients, and asymptomatic patients are more likely younger and with better systolic function [42]. Other reported findings which may raise the suspicion of LVNC before—and triggering—additional investigations are rhythm disorder, the screening of familiars, and embolic events [40]. ECG may reveal non-specific anomalies in 91% of patients, including left bundle branch block, poor R wave progression, ST-segment change, pathological Q waves, and T wave inversion. Moreover, a non-negligible proportion of patients may present with atrial fibrillation [40]. LVNC is also associated with Wolff-Parkinson-White [3,43].
On the basis of echocardiographic findings, different LVNC phenotypes have been described: isolated hypertrabeculations, dilated LVNC, hypertrophic LVNC, restrictive LVNC, and LVNC associated with congenital heart disease. Isolated right ventricular non-compaction [44,45,46], and biventricular non-compaction [47] have rarely been reported and diagnosed using the criteria [44]. Biventricular involvement may be associated with CHD [48], and has been described to lead to worse outcomes [49]. A genetic variant of MYH7 has been reported in patients with biventricular disease presenting with restrictive physiology [50] or a dilated phenotype and severe systolic dysfunction [51], requiring early transplantation. Dilated LV and impaired systolic function were reported in most patients at the time of LVNC diagnosis [41,52]. The mean initial ejection fraction(EF) found by different authors is between 41% [42,52] and 46% [41]. Improved diagnostic pathways and a greater awareness of the disorder are likely responsible for the improved mean EF at diagnosis in the latest series compared with initial reports by Oechslin et al. [2]. An advanced diastolic dysfunction demonstrated by an LV-restrictive filling pattern on echo has been reported in up to 50% [41] of cases at diagnosis. The paediatric form of LVNC is diagnosed at a median of 5 years of age [12]. Among children, there is high prevalence of neuromuscular disorders, including Becker’s muscular dystrophy and Friedreich’s ataxia [53]; therefore, a complete neurological assessment is reasonable at the time of diagnosis. Pignatelli et al. reported a higher proportion of symptomatic patients among children at the first presentation of LVNC compared to the adult population [28]. In various publications, an initial presentation with heart failure was reported between 20% [12] and 83% [29]. Other reasons for an initial consultation were arrhythmias in 17%, the evaluation of murmurs in 19%, an abnormal ECG in 10%, complaints of chest pain in 9%, family screening in 14%, and cardiomegaly noted on chest radiography in 7% of patients [54]. Echocardiography showed dilated LV in 93% [29] and systolic dysfunction in up to 88% [28] of children at diagnosis, while impaired diastolic function was present in only 10% of paediatric cases [12] (Figure 4).
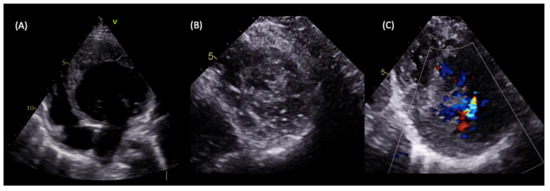
Figure 4.
LVNC in a 5-year-old patient with a family history of dilated cardiomyopathy presenting with heart failure symptoms. Transthoracic echocardiography showed dilated LV with severely impaired systolic function and an estimated ejection fraction of 30% (A), the spongy aspect of the apex on echocardiographic short axis view (B), and perfused intertrabecular recesses seen on a Colour Doppler (C), which are compatible with LVNC diagnosis according to both the Jenni and Stöllberger criteria.
9. Diagnosis
9.1. LVNC Diagnostic Criteria
Numerous sets of LVNC diagnostic criteria have been proposed over the years. Echocardiography, despite its possible pitfalls, is largely diffused with an excellent price–benefit relation, and remains the first-level imaging modality for the suspected or confirmed diagnosis of LVNC, and is also useful during follow-up. CMR is the second-line imaging modality for LVNC diagnosis, offering the high-resolution visualization of the trabecular portion of the LV and a better view of the apical region with a cine short-axis steady-state free precession (SSFP) sequence. Therefore, CMR should be performed in all patients with suspected or confirmed LVNC diagnosis by echocardiography in order to assess the extension of the non-compaction, and to evaluate the ventricular volume and function during follow-up. A recent meta-analysis of 59 studies showed that, in CMR-based studies, the pooled LVNC prevalence was 12-fold higher compared to echo-based studies, reflecting higher sensitivity [55]. Furthermore, CMR provides data on the presence and the extension of fibrosis with late gadolinium enhancement (LGE) imaging. LGE uptake has been associated with systolic function and symptom severity [56], and is the best predictor of adverse events in patients with LVNC [57]. The main echo and CMR-based diagnostic criteria are summarized in Table 1. Novel echocardiographic techniques are currently emerging as important tools in addition to basal standard echocardiographic protocol in order to improve diagnostic sensibility, or to provide further functional data. In particular, contrast administration is helpful in the case of a difficult echo window, in order to enhance the endocardial border and trabeculations’ definition, to demonstrate perfused intertrabecular recesses, and to exclude thrombotic formations [58]. Three-dimensional echocardiography is a useful tool that offers high spatial resolution and allows accurate measurements of LV volumes before and after trabeculation exclusion to quantify the extension of non-compacted myocardium (Figure 4). Speckle tracking echocardiography allows the early recognition of subclinical LV impairment in cases with normal systolic function in both children [59] and adults [60] with LVNC. Furthermore, in patients with LVNC, reduced global radial, circumferential, and longitudinal strain values were related to a worse prognosis compared with patients with normal strain values [61]. Moreover, a peculiar regional deformation pattern with apical sparing in patients in LVNC may be potentially helpful in differential diagnosis with dilated cardiomyopathy [62,63]. Conversely, the apical–basal strain gradient seems to be more pronounced in patients with hypertrophic cardiomyopathy compared with LVNC patients [64]. In addition, advanced speckle tracking analysis enables the evaluation of LV rotation movement. Basal and apical twist in the same direction leading to LV “solid body rotation” with a near-absent LV twist occurs in 53.3% [65] to 88% [66] of patients with LVNC, and may be an additional functional parameter to differentiate LVNC from dilated cardiomyopathy with good sensitivity and specificity (88% and 78%, respectively [65,66]). Abnormal LV rotation may contribute to LV dysfunction in LVNC patients, and supports the theory of congenital muscular disorder in non-compaction myocardium. A stepwise diagnostic pathway with the use of multimodality imaging is proposed in Figure 5.

Table 1.
LVNC diagnostic criteria.
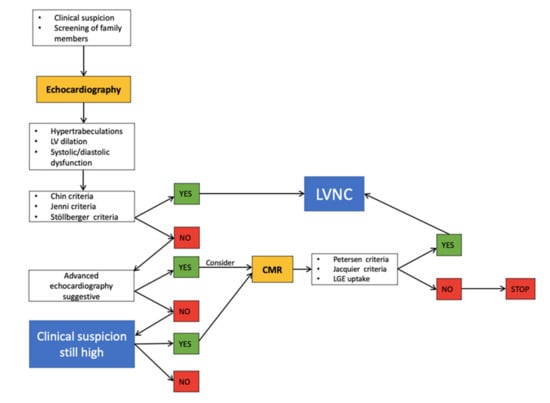
Figure 5.
Proposed stepwise diagnostic pathway.
9.2. Limitations of the Current Diagnostic Criteria
The current diagnostic criteria are derived from empiric observations in small groups of patients: the original studies included eight patients for the Chin criteria, seven patients for the Jenni and Peterson criteria, and 16 patients for the Jacquier criteria; none of them were systematically validated in a large cohort against a control group. Kohli et al. demonstrated the poor correlation among different sets of echocardiographic criteria, with only 30% of patients fulfilling all of them. Moreover, 8% of the healthy controls and 23% of the patients with heart failure also met at least one of the three sets of criteria to diagnose LVNC [5]. CMR criteria do not appear superior: a highly variable disease prevalence was found using different diagnostic criteria ina cohort of 700 patients, and the diagnosis of LVNC—by any CMR criteria set—was not associated with adverse clinical events after nearly 7 years of observation [76]. Inter-observer reproducibility is another major concern: the interobserver disagreement ranges between 43 and 35% of cases [77]. The current diagnostic criteria appear too sensitive, especially for the Afro-American population [5,6,20]. However, the main limitation is that both CMR and echo criteria are based solely on morphological features. Zemrak et al., demonstrated an unexpectedly high percentage of subjects fulfilling CMR criteria in a healthy population, highlighting the importance of the consideration of the pre-test probability of LVNC along with the diagnostic criteria [20]. Multiparametric evaluation including ECG, a complete echocardiography protocol with the use of new echo techniques, a cardiopulmonary test, 24-h Holter monitoring, and CMR with a study of late gadolinium enhancement may improve the diagnostic specificity for LVNC.
10. Outcome
The current knowledge about the disease course is extremely limited, leading to severe difficulties in identifying patients who are at a higher risk of adverse events. Older studies reported a higher incidence of negative outcomes, reflecting the improved efficacy of medical therapy in the latest years. The typical complications in patients with LVNC are summarized by the classical triad of heart failure, ventricular arrhythmias and systemic embolic events. Admissions for heart failure episodes occurred in up to 53% of the patients during follow-up. Although it is assumed that non-compacted myocardium is more prone to thrombus formation, this appears to be a rare event in LVNC [78]. The reported incidence of systemic thromboembolic events in LVNC—including stroke, transient ischemic attack, and mesenteric ischemia—varies between 21% [3] and 5% [39,41] in more recent papers. During follow-up, atrial fibrillation occurred in 7% of patients [72], while sustained and non-sustained ventricular tachycardia episodes were reported in up to 33% [24] and 6% of cases [41], respectively. In 66 affected children, the occurrence of congestive heart failure at 1 year of follow-up was 68%, irrespectively of the coexistence of a congenital heart disease [27]. The cardiovascular mortality rate was initially reported at 35% after a median follow-up of 3.7 years [2]. However, two more recent studies based on a larger cohort estimated mortality at 11–22% [41,78] after a similar follow-up length. In analogy, sudden death, initially complicating 18% [2] of cases, has been progressively reported less frequently, lately being found at 1–4% [41,78]. Independent predictors of cardiovascular death or heart transplantation include NYHA class III–IV, sustained ventricular arrhythmias, left atrial size [41], an impaired left ventricular ejection fraction, a dilated left ventricular end-diastolic diameter, reduced systolic blood pressure, and pulmonary hypertension [64]. CMR evidence of LGE has also been related to a worse prognosis [58]. However, from a recent meta-analysis of 28 studies including 2501 LVNC patients, EF appears to be the strongest predictor of death [79]. Right ventricular function needs also attention: right ventricular dysfunction has recently been associated with a higher risk of events in patients with biventricular involvement [80]. Pregnancy in women with LVNC is often complicated by heart failure and arrhythmias. Therefore, preconception genetic counseling with the consideration of genetic testing should be provided, as most of the genes associated with LVNC cardiomyopathy have overlap with other cardiomyopathies, and are associated with an autosomal dominant pattern of inheritance [81]. In children with LVNC, adverse outcomes including death or transplant may occur in a variable percentage of patients. The Australian registry for cardiomyopathy in childhood reported a worse prognosis in the long term of LVNC compared to dilated cardiomyopathy in the paediatric population [9]. The mortality rate was specifically higher in children presenting in the first year of life in all of the studies (the highest reported mortality was 30.4% [52]), suggesting that the neonatal form of LVNC represents a more malignant phenotype. A lower proportion of negative outcomes was reported in Japanese and Chinese children with LVNC [3,29]. In all of the studies involving a paediatric population, initial presentation with reduced ventricular function was related to increased mortality risk [3,30,64,78]. Other factors predicting poor outcomes were younger age at diagnosis [62,78] dilated LV [3,9,62], reduced strain values [62], ventricular arrhythmias [28,55], and raised end-diastolic pressure and mean pulmonary arterial pressure with invasive measurements [2].
11. Management
The medical treatment of patients with LVNC and decompensated systolic/diastolic dysfunction should follow international guidelines for the management of heart failure [82]. Medical therapy should also be administrated in asymptomatic patients with echocardiographic evidence of systolic dysfunction in order to prevent or delay the development of heart failure. In patients who remain symptomatic despite optimal medical therapy, CRT should be considered, when appropriate. ICD implantation for primary prevention of sudden cardiac death in patients in all LVNC has been proposed but is still controversial. The current guidelines recommend ICD implantation when EF is ≤35% despite optimal medical therapy, and in patients who recovered from symptomatic VT [82]. Anticoagulation treatment is recommended in those with a history of atrial fibrillation, previous embolic events, known ventricular thrombi, or severe systolic dysfunction with hypo/akinetic apex, irrespective of thrombotic risk scores [78]. However, despite the absence of evidence of a clear benefit, the routine administration of anticoagulation has recently been proposed [83] and may be considered in LVNC patients, according to the latest guidelines [82].
12. Conclusions
LVNC is an extremely heterogeneous disorder with a highly variable clinical presentation, morphologic appearance in imaging tests, and prognosis. Trabeculations may be found in the context of ventricular overload, with these being reversible in some cases. For this reason, whether hypertrabeculated LV fulfilling the diagnostic criteria in the absence of symptoms, familiar history or signs of ventricular dysfunction should be considered an early form of cardiomyopathy is still matter of debate. It can be speculated that LVNC comprises a wide spectrum from a purely morphological trait with no heamodynamic consequences to a real muscular disease, potentially associated with adverse outcomes. Where to place a cut-off for disease diagnosis remains to be determined. The current evidence suggests that LVNC in children may be a more severe disease with higher mortality, especially for those presenting in the first year of life with ventricular dysfunction. Further studies are needed to define the disease features in order to identify patients at higher risk of death or complications. Novel imaging techniques may be helpful in differential diagnosis, providing essential functional data related to prognosis. International collaborations are warranted in order to ensure the inclusion of a greater number of patients, aiming to elaborate new diagnostic criteria and validate a clear diagnostic path for LVNC, including patients’ personal and familiar history, and a multiparametric evaluation with morphologic and functional data.
Author Contributions
Conceptualization, F.F., N.B. and R.B.; methodology, N.B. and G.D.C.; resources, F.V. and R.B.; writing—original draft preparation, F.F. and G.S.; writing—review and editing, B.S. and G.D.C.; visualization, F.V.; supervision, B.S. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Freedom, R.M.; Yoo, S.-J.; Perrin, D.; Taylor, G.; Petersen, S.; Anderson, R.H. The morphological spectrum of ventricular noncompaction. Cardiol. Young 2005, 15, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef]
- Ichida, F.; Hamamichi, Y.; Miyawaki, T.; Ono, Y.; Kamiya, T.; Akagi, T.; Hamada, H.; Hirose, O.; Isobe, T.; Yamada, K.; et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J. Am. Coll. Cardiol. 1999, 34, 233–240. [Google Scholar] [CrossRef]
- Jenni, R.; Wyss, C.A.; Oechslin, E.N.; Kaufmann, P.A. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J. Am. Coll. Cardiol. 2002, 39, 450–454. [Google Scholar] [CrossRef]
- Dusek, J.; Ostádal, B.; Duskova, M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch. Pathol. 1975, 99, 312–317. [Google Scholar]
- Kohli, S.K.; Pantazis, A.A.; Shah, J.S.; Adeyemi, B.; Jackson, G.; McKenna, W.J.; Sharma, S.; Elliott, P.M. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur. Hear. J. 2007, 29, 89–95. [Google Scholar] [CrossRef]
- Gati, S.; Papadakis, M.; Papamichael, N.D.; Zaidi, A.; Sheikh, N.; Reed, M.; Sharma, R.; Thilaganathan, B.; Sharma, S. Reversible De Novo Left Ventricular Trabeculations in Pregnant Women. Circulation 2014, 130, 475–483. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Bonifazi, M.; Mondillo, S. Exercise-induced left-ventricular hypertrabeculation in athlete’s heart. Int. J. Cardiol. 2015, 181, 320–322. [Google Scholar] [CrossRef]
- Probst, S.; Oechslin, E.; Schuler, P.; Greutmann, M.; Boyé, P.; Knirsch, W.; Berger, F.; Thierfelder, L.; Jenni, R.; Klaassen, S. Sarcomere Gene Mutations in Isolated Left Ventricular Noncompaction Cardiomyopathy Do Not Predict Clinical Phenotype. Circ. Cardiovasc. Genet. 2011, 4, 367–374. [Google Scholar] [CrossRef]
- Protonotarios, A.; Elliott, P.M. Left ventricular non-compaction: Have we reached the limits of conventional imaging? Eur. Hear. J. 2019, 41, 1437–1438. [Google Scholar] [CrossRef]
- Shan, L.; Makita, N.; Xing, Y.; Watanabe, S.; Futatani, T.; Ye, F.; Saito, K.; Ibuki, K.; Watanabe, K.; Hirono, K. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol. Genet. Metab. 2008, 93, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Hear. J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Hirono, K.; Hata, Y.; Nakazawa, M.; Momoi, N.; Tsuji, T.; Matsuoka, T.; Ayusawa, M.; Abe, Y.; Hayashi, T.; Tsujii, N.; et al. Clinical and Echocardiographic Impact of Tafazzin Variants on Dilated Cardiomyopathy Phenotype in Left Ventricular Non-Compaction Patients in Early Infancy. Circ. J. 2018, 82, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Slany, J. Is left ventricular hypertrabeculation/ noncompaction a cardiac manifestation of Fabry?s disease? Z. Kardiol. 2003, 92, 966–969. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.H.; Hamilton, R.M.; et al. HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011, 13, 1077–1109. [Google Scholar] [CrossRef]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- Piga, A.; Longo, F.; Musallam, K.M.; Veltri, A.; Ferroni, F.; Chiribiri, A.; Bonamini, R. Left ventricular noncompaction in patients with β-thalassemia: Uncovering a previously unrecognized abnormality. Am. J. Hematol. 2012, 87, 1079–1083. [Google Scholar] [CrossRef]
- Markovic, N.; Dimkovic, N.; Damjanovic, T.; Loncar, G. Isolated ventricular noncompaction in patients with chronic renal failure. Clin. Nephrol. 2008, 70, 72–76. [Google Scholar] [CrossRef]
- Zemrak, F.; Ahlman, M.A.; Captur, G.; Mohiddin, S.; Kawel-Boehm, N.; Prince, M.; Moon, J.; Hundley, W.G.; Lima, J.A.; Bluemke, D.; et al. The Relationship of Left Ventricular Trabeculation to Ventricular Function and Structure Over a 9.5-Year Follow-Up. J. Am. Coll. Cardiol. 2014, 64, 1971–1980. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) Classification for a Phenotype–Genotype Nomenclature of Cardiomyopathy. J. Am. Coll. Cardiol. 2013, 62, 2046–2072. [Google Scholar] [CrossRef]
- Aras, D.; Tufekcioglu, O.; Ergun, K.; Özeke, Ö.; Yildiz, A.; Topaloglu, S.; Deveci, B.; Sahin, O.; Kisacik, H.L.; Korkmaz, S. Clinical Features of Isolated Ventricular Noncompaction in Adults Long-Term Clinical Course, Echocardiographic Properties, and Predictors of Left Ventricular Failure. J. Card. Fail. 2006, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ronderos, R.; Avegliano, G.; Borelli, E.; Kuschnir, P.; Castro, F.; Sanchez, G.; Perea, G.; Corneli, M.; Zanier, M.M.; Andres, S.; et al. Estimation of Prevalence of the Left Ventricular Noncompaction Among Adults. Am. J. Cardiol. 2016, 118, 901–905. [Google Scholar] [CrossRef]
- Kovacevic-Preradovic, T.; Jenni, R.; Oechslin, E.; Noll, G.; Seifert, B.; Jost, C.A. Isolated Left Ventricular Noncompaction as a Cause for Heart Failure and Heart Transplantation: A Single Center Experience. Cardiology 2009, 112, 158–164. [Google Scholar] [CrossRef]
- Lilje, C.; Porciani, M.C.; Lilli, A.; Macioce, R.; Cappelli, F.; Demarchi, G.; Pappone, A.; Ricciardi, G.; Padeletti, L. Complications of non-compaction of the left ventricular myocardium in a paediatric population: A prospective study. Eur. Hear. J. 2006, 27, 1855–1860. [Google Scholar] [CrossRef]
- Pignatelli, R.H.; McMahon, C.J.; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical Characterization of Left Ventricular Noncompaction in Children. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef]
- Shi, W.Y.; Moreno-Betancur, M.; Nugent, A.W.; Cheung, M.; Colan, S.; Turner, C.; Sholler, G.F.; Robertson, T.; Justo, R.; Bullock, A.; et al. Long-Term Outcomes of Childhood Left Ventricular Noncompaction Cardiomyopathy. Circulation 2018, 138, 367–376. [Google Scholar] [CrossRef]
- Marques, L.C.; Liguori, G.R.; Souza, A.C.A.A.; Aiello, V.D. Left Ventricular Noncompaction Is More Prevalent in Ventricular Septal Defect Than Other Congenital Heart Defects: A Morphological Study. J. Cardiovasc. Dev. Dis. 2020, 7, 39. [Google Scholar] [CrossRef]
- Choudhary, P.; Strugnell, W.; Puranik, R.; Hamilton-Craig, C.; Kutty, S.; Celermajer, D.S. LV non-compaction in patients with coarctation of the aorta: Prevalence and effects on cardiac function. Cardiol. Young 2021, 31, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Buechel, E.V.; Jost, C.H.A.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Hirono, K.; Hata, Y.; Miyao, N.; Okabe, M.; Takarada, S.; Nakaoka, H.; Ibuki, K.; Ozawa, S.; Yoshimura, N.; Nishida, N.; et al. Left Ventricular Noncompaction and Congenital Heart Disease Increases the Risk of Congestive Heart Failure. J. Clin. Med. 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Strugnell, W.; Puranik, R.; Hamilton-Craig, C.; Kutty, S.; Celermajer, D.S. Left ventricular non-compaction in patients with single ventricle heart disease. Cardiol. Young 2020, 30, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Khan, R.; Silverman, N.H.; Singh, G.K. Tricuspid Atresia with Non-compaction: An Early Experience with Implications for Surgical Palliation. Pediatr. Cardiol. 2016, 38, 495–505. [Google Scholar] [CrossRef]
- Tian, T.; Yang, Y.; Zhou, L.; Luo, F.; Li, Y.; Fan, P.; Dong, X.; Liu, Y.; Cui, J.; Zhou, X. Left Ventricular Non-Compaction: A Cardiomyopathy With Acceptable Prognosis in Children. Hear. Lung Circ. 2018, 27, 28–32. [Google Scholar] [CrossRef]
- Ramachandran, P.; Woo, J.G.; Ryan, T.D.; Bryant, R.; Heydarian, H.C.; Jefferies, J.L.; Towbin, J.A.; Lorts, A. The Impact of Concomitant Left Ventricular Non-compaction with Congenital Heart Disease on Perioperative Outcomes. Pediatr. Cardiol. 2016, 37, 1307–1312. [Google Scholar] [CrossRef]
- Habib, G.; Charron, P.; Eicher, J.C.; Giorgi, R.; Donal, E.; Laperche, T.; Boulmier, D.; Pascal, C.; Logeart, D.; et al.; Working Groups ‘Heart Failure and Cardiomyopathies’ and ‘Echocardiography’ of the French Society of Cardiology. Isolated left ventricular non-compaction in adults: Clinical and echocardiographic features in 105 patients. Results from a French registry. Eur. J. Heart Fail. 2011, 13, 177–185. [Google Scholar] [CrossRef]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekolglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Hear. J. 2005, 26, 187–192. [Google Scholar] [CrossRef]
- Lofiego, C.; Biagini, E.; Pasquale, F.; Ferlito, M.; Rocchi, G.; Perugini, E.; Reggiani, M.L.B.; Boriani, G.; Leone, O.; Caliskan, K.; et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 2007, 93, 65–71. [Google Scholar] [CrossRef]
- Peters, F.; Khandheria, B.K.; dos Santos, C.; Matioda, H.; Maharaj, N.; Libhaber, E.; Mamdoo, F.; Essop, M.R. Isolated Left Ventricular Noncompaction in Sub-Saharan Africa: A Clinical and Echocardiographic Perspective. Circ. Cardiovasc. Imaging 2012, 5, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, Y.; Gao, L.; Wang, J.; Sun, K.; Zou, Y.; Wang, L.; Zhang, L.; Li, Y.; Xiao, Y.; et al. Isolated left ventricular noncompaction: Clinical profile and prognosis in 106 adult patients. Hear. Vessel. 2014, 29, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.S.; Valdes, S.O.; Hope, K.D.; Morris, S.A.; Landstrom, A.P.; Schneider, A.E.; Miyake, C.Y.; Denfield, S.W.; Pignatelli, R.H.; Wang, Y.; et al. Association of Wolff-Parkinson-White With Left Ventricular Noncompaction Cardiomyopathy in Children. J. Card. Fail. 2019, 25, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, A.; Ganesan, G.; Sangareddi, V.; Pillai, A.P.; Ramasamy, A. Isolated Noncompaction of Right Ventricle-A Case Report. Echocardiography 2012, 29, E169–E172. [Google Scholar] [CrossRef]
- Maestrini, V.; Torlasco, C.; Hughes, R.; Moon, J.C. Cardiovascular Magnetic Resonance and Sport Cardiology: A Growing Role in Clinical Dilemmas. J. Cardiovasc. Transl. Res. 2020, 13, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Saglam, M.; Saygin, H.; Kozan, H.; Ozturk, E.; Mutlu, H. Noncompaction of Ventricular Myocardium Involving the Right Ventricle. Kor. Circ. J. 2015, 45, 439–441. [Google Scholar] [CrossRef]
- Ulusoy, R.E.; Kucukarslan, N.; Kirilmaz, A.; Demiralp, E. Noncompaction of ventricular myocardium involving both ventricles. Eur. J. Echocardiogr. 2006, 7, 457–460. [Google Scholar] [CrossRef][Green Version]
- Sirin, B.; Kurdal, A.; Iskesen, I.; Cerrahoglu, M. Right Ventricular Outflow Obstruction of the Patient with Biventricular Non-Compaction. Thorac. Cardiovasc. Surg. 2010, 58, 364–366. [Google Scholar] [CrossRef]
- Martinez, H.R.; Miller, E.; Mead, R.; Osher, J.; Almasri, M.; Parent, J.J. Biventricular noncompaction cardiomyopathy with severe dilated phenotype in a family with a novel MYH7 gene variant. Prog. Pediatr. Cardiol. 2020, 59, 101205. [Google Scholar] [CrossRef]
- Miura, F.; Shimada, J.; Kitagawa, Y.; Otani, K.; Sato, T.; Toki, T.; Takahashi, T.; Yonesaka, S.; Mizukami, H.; Ito, E. MYH7 mutation identified by next-generation sequencing in three infant siblings with bi-ventricular noncompaction presenting with restrictive hemodynamics. J. Cardiol. Cases 2019, 19, 140–143. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Tubio-Gómez, S.; Bellón, J.M.C.; Bautista-Rodríguez, C.; Sanchez-De-Toledo, J. Neonatal Non-compacted Cardiomyopathy: Predictors of Poor Outcome. Pediatr. Cardiol. 2019, 41, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Bruce, C.; Connolly, H.; Brady, P.; Syed, I.; Hodge, D.; Asirvatham, S.; Friedman, P. Isolated Left Ventricular Noncompaction Syndrome. Am. J. Cardiol. 2009, 104, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J.; Blazek, G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am. J. Cardiol. 2002, 90, 899–902. [Google Scholar] [CrossRef]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Hear. J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
- Dodd, J.D.; Holmvang, G.; Hoffmann, U.; Ferencik, M.; Abbara, S.; Brady, T.J.; Cury, R.C. Quantification of Left Ventricular Noncompaction and Trabecular Delayed Hyperenhancement with Cardiac MRI: Correlation with Clinical Severity. Am. J. Roentgenol. 2007, 189, 974–980. [Google Scholar] [CrossRef]
- Andreini, D.; Pontone, G.; Bogaert, J.; Roghi, A.; Barison, A.; Schwitter, J.; Mushtaq, S.; Vovas, G.; Sormani, P.; Aquaro, G.D.; et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction. J. Am. Coll. Cardiol. 2016, 68, 2166–2181. [Google Scholar] [CrossRef]
- Laat, L.E.D.G.-D.; Krenning, B.J.; Cate, F.J.T.; Roelandt, J.R. Usefulness of Contrast Echocardiography for Diagnosis of Left Ventricular Noncompaction. Am. J. Cardiol. 2005, 95, 1131–1134. [Google Scholar] [CrossRef]
- Ari, M.E.; Cetin, I.I.; Kocabas, A.; Ekici, F.; Ceylan, O.; Surucu, M. Decreased Deformation in Asymptomatic Children with Isolated Left Ventricular Non-compaction and Normal Ejection Fraction. Pediatr. Cardiol. 2016, 37, 201–207. [Google Scholar] [CrossRef]
- Bellavia, D.; Michelena, H.I.; Martinez, M.; Pellikka, P.A.; Bruce, C.J.; Connolly, H.M.; Villarraga, H.R.; Veress, G.; Oh, J.K.; Miller, F.A. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart 2009, 96, 440–447. [Google Scholar] [CrossRef]
- Arunamata, A.; Stringer, J.; Balasubramanian, S.; Tacy, T.A.; Silverman, N.H.; Punn, R. Cardiac Segmental Strain Analysis in Pediatric Left Ventricular Noncompaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 763–773.e1. [Google Scholar] [CrossRef] [PubMed]
- Huttin, O.; Venner, C.; Frikha, Z.; Voilliot, D.; Marie, P.-Y.; Aliot, E.; Sadoul, N.; Juillière, Y.; Brembilla-Perrot, B.; Selton-Suty, C. Myocardial deformation pattern in left ventricular non-compaction: Comparison with dilated cardiomyopathy. IJC Heart Vasc. 2014, 5, 9–14. [Google Scholar] [CrossRef][Green Version]
- Tarando, F.; Coisne, D.; Galli, E.; Rousseau, C.; Viera, F.; Bosseau, C.; Habib, G.; Lederlin, M.; Schnell, F.; Donal, E. Left ventricular non-compaction and idiopathic dilated cardiomyopathy: The significant diagnostic value of longitudinal strain. Int. J. Cardiovasc. Imaging 2016, 33, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Haland, T.F.; Saberniak, J.; Leren, I.S.; Edvardsen, T.; Haugaa, K.H. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int. J. Cardiol. 2016, 228, 900–905. [Google Scholar] [CrossRef]
- Peters, F.; Khandheria, B.K.; Libhaber, E.; Maharaj, N.; Dos Santos, C.; Matioda, H.; Essop, M.R. Left ventricular twist in left ventricular noncompaction. Eur. Hear. J. Cardiovasc. Imaging 2014, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, B.M.; Caliskan, K.; Soliman, O.I.; Kauer, F.; van der Zwaan, H.B.; Vletter, W.B.; van Vark, L.C.; Cate, F.J.T.; Geleijnse, M.L. Diagnostic Value of Rigid Body Rotation in Noncompaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2011, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Gebhard, C.; Stähli, B.E.; Greutmann, M.; Biaggi, P.; Jenni, R.; Tanner, F.C. Reduced Left Ventricular Compacta Thickness: A Novel Echocardiographic Criterion for Non-Compaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 1050–1057. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction: Insights From Cardiovascular Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass 52 using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Stacey, R.B.; Andersen, M.M.; Clair, M.S.; Hundley, W.G.; Thohan, V. Comparison of Systolic and Diastolic Criteria for Isolated LV Noncompaction in CMR. JACC Cardiovasc. Imaging 2013, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Muthurangu, V.; Cook, C.; Flett, A.S.; Wilson, R.; Barison, A.; Sado, D.M.; Anderson, S.; McKenna, W.J.; Mohun, T.J.; et al. Quantification of left ventricular trabeculae using fractal analysis. J. Cardiovasc. Magn. Reson. 2013, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, G.; Casanova, J.; González-Carrillo, J.; Gimeno-Blanes, J. Towards an Enhanced Tool for Quantifying the Degree of LV Hyper-Trabeculation. J. Clin. Med. 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, G.; Casanova, J.D.; Cuenca, J.; González-Carrillo, J. A self-optimized software tool for quantifying the degree of left ventricle hyper-trabeculation. J. Supercomput. 2018, 75, 1625–1640. [Google Scholar] [CrossRef]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006174. [Google Scholar] [CrossRef]
- Stöllberger, C.; Gerecke, B.; Engberding, R.; Grabner, B.; Wandaller, C.; Finsterer, J.; Gietzelt, M.; Balzereit, A. Interobserver Agreement of the Echocardiographic Diagnosis of LV Hypertrabeculation/Noncompaction. JACC Cardiovasc. Imaging 2015, 8, 1252–1257. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Thrombi in the left ventricular hypertrabeculation/noncompaction. Acta Cardiol. 2004, 59, 341–344. [Google Scholar] [CrossRef]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Noncompaction. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Lin, X.; Fang, L. Influence of Right Ventricular Dysfunction on Outcomes of Left Ventricular Non-compaction Cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 816404. [Google Scholar] [CrossRef]
- Crousillat, D.R.; Ghoshhajra, B.B.; Scott, N.S. Pregnancy in Familial Left Ventricular Noncompaction-Associated Cardiomyopathy. JACC Case Rep. 2020, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Subahi, A.; Hassan, A.I.; Abubakar, H.; Ibrahim, W. Isolated left ventricular non-compaction (LVNC) and recurrent strokes: To anticoagulate or not to anticoagulate, that is the question. BMJ Case Rep. 2017, 2017, bcr2017220954. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).