Genetic Diagnostics Contribute to the Risk Stratification for Major Arrhythmic Events in Pediatric Patients with Long QT Syndrome Type 1–3
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
3.2. Risk Factors for Major Arrhythmic Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moss, A.J.; Kass, R.S. Long QT syndrome: From channels to cardiac arrhythmias. J. Clin. Investig. 2005, 115, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M.; Kass, R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, M.S.; Peng, G.; Robey, S.H.; Terrenoire, C.; Iyer, V.; Sampson, K.J.; Kass, R.S. Molecular Pathophysiology of Congenital Long QT Syndrome. Physiol. Rev. 2017, 97, 89–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, A.; Novelli, V.; Amin, A.S.; Abiusi, E.; Care, M.; Nannenberg, E.A.; Feilotter, H.; Amenta, S.; Mazza, D.; Bikker, H.; et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation 2020, 141, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.R.; Winbo, A.; Abrams, D.; Vohra, J.; Wilde, A.A. Channelopathies That Lead to Sudden Cardiac Death: Clinical and Genetic Aspects. Heart Lung Circ. 2019, 28, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011, 8, 1308–1339. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Crotti, L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation 2011, 124, 2181–2184. [Google Scholar] [CrossRef] [Green Version]
- Vincent, G.M.; Timothy, K.; Fox, J.; Zhang, L. The inherited long QT syndrome: From ion channel to bedside. Cardiol. Rev. 1999, 7, 44–55. [Google Scholar] [CrossRef]
- Hobbs, J.B.; Peterson, D.R.; Moss, A.J.; McNitt, S.; Zareba, W.; Goldenberg, I.; Qi, M.; Robinson, J.L.; Sauer, A.J.; Ackerman, M.J.; et al. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA 2006, 296, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, I.; Moss, A.J.; Peterson, D.R.; McNitt, S.; Zareba, W.; Andrews, M.L.; Robinson, J.L.; Locati, E.H.; Ackerman, M.J.; Benhorin, J.; et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation 2008, 117, 2184–2191. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Jons, C.; Moss, A.J.; McNitt, S.; Peterson, D.R.; Qi, M.; Zareba, W.; Robinson, J.L.; Barsheshet, A.; Ackerman, M.J.; et al. Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long QT syndrome. J. Am. Coll. Cardiol. 2011, 57, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, I.; Bradley, J.; Moss, A.; McNitt, S.; Polonsky, S.; Robinson, J.L.; Andrews, M.; Zareba, W. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: Implications for patient management. J. Cardiovasc. Electrophysiol. 2010, 21, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Kutyifa, V.; Daimee, U.A.; McNitt, S.; Polonsky, B.; Lowenstein, C.; Cutter, K.; Lopes, C.; Zareba, W.; Moss, A.J. Clinical aspects of the three major genetic forms of long QT syndrome (LQT1, LQT2, LQT3). Ann. Noninvasive Electrocardiol. 2018, 23, e12537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzanti, A.; Maragna, R.; Vacanti, G.; Monteforte, N.; Bloise, R.; Marino, M.; Braghieri, L.; Gambelli, P.; Memmi, M.; Pagan, E.; et al. Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome. J. Am. Coll. Cardiol. 2018, 71, 1663–1671. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Schwartz, P.J.; Grillo, M.; Bloise, R.; Ronchetti, E.; Moncalvo, C.; Tulipani, C.; Veia, A.; Bottelli, G.; et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA 2004, 292, 1341–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zareba, W.; Moss, A.J.; Daubert, J.P.; Hall, W.J.; Robinson, J.L.; Andrews, M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J. Cardiovasc. Electrophysiol. 2003, 14, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar]
- Priori, S.G.; Schwartz, P.J.; Napolitano, C.; Bloise, R.; Ronchetti, E.; Grillo, M.; Vicentini, A.; Spazzolini, C.; Nastoli, J.; Bottelli, G.; et al. Risk stratification in the long-QT syndrome. N. Engl. J. Med. 2003, 348, 1866–1874. [Google Scholar] [CrossRef]
- Sauer, A.J.; Moss, A.J.; McNitt, S.; Peterson, D.R.; Zareba, W.; Robinson, J.L.; Qi, M.; Goldenberg, I.; Hobbs, J.B.; Ackerman, M.J.; et al. Long QT syndrome in adults. J. Am. Coll. Cardiol. 2007, 49, 329–337. [Google Scholar] [CrossRef]
- Wedekind, H.; Burde, D.; Zumhagen, S.; Debus, V.; Burkhardtsmaier, G.; Monnig, G.; Breithardt, G.; Schulze-Bahr, E. QT interval prolongation and risk for cardiac events in genotyped LQTS-index children. Eur. J. Pediatr. 2009, 168, 1107–1115. [Google Scholar] [CrossRef]
- Westphal, D.S.; Burkard, T.; Moscu-Gregor, A.; Gebauer, R.; Hessling, G.; Wolf, C.M. Reclassification of genetic variants in children with long QT syndrome. Mol. Genet. Genom. Med. 2020, 8, e1300. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS, 5th ed.; Sage Publications Ltd.: London, UK, 2013; p. 952. [Google Scholar]
- Koponen, M.; Marjamaa, A.; Hiippala, A.; Happonen, J.M.; Havulinna, A.S.; Salomaa, V.; Lahtinen, A.M.; Hintsa, T.; Viitasalo, M.; Toivonen, L.; et al. Follow-up of 316 molecularly defined pediatric long-QT syndrome patients: Clinical course, treatments, and side effects. Circ. Arrhythmia Electrophysiol. 2015, 8, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zareba, W.; Moss, A.J.; Schwartz, P.J.; Vincent, G.M.; Robinson, J.L.; Priori, S.G.; Benhorin, J.; Locati, E.H.; Towbin, J.A.; Keating, M.T.; et al. Influence of the genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N. Engl. J. Med. 1998, 339, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Bikker, H.; Christiaans, I. Long QT Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1129/ (accessed on 22 December 2021).
- Moss, A.J.; Zareba, W.; Kaufman, E.S.; Gartman, E.; Peterson, D.R.; Benhorin, J.; Towbin, J.A.; Keating, M.T.; Priori, S.G.; Schwartz, P.J.; et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation 2002, 105, 794–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, W.; Moss, A.J.; Wilde, A.A.; Towbin, J.A.; Ackerman, M.J.; January, C.T.; Tester, D.J.; Zareba, W.; Robinson, J.L.; Qi, M.; et al. Genotype-phenotype aspects of type 2 long QT syndrome. J. Am. Coll. Cardiol. 2009, 54, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Migdalovich, D.; Moss, A.J.; Lopes, C.M.; Costa, J.; Ouellet, G.; Barsheshet, A.; McNitt, S.; Polonsky, S.; Robinson, J.L.; Zareba, W.; et al. Mutation and gender-specific risk in type 2 long QT syndrome: Implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm 2011, 8, 1537–1543. [Google Scholar] [CrossRef] [Green Version]
- January, C.T.; Gong, Q.; Zhou, Z. Long QT syndrome: Cellular basis and arrhythmia mechanism in LQT2. J. Cardiovasc. Electrophysiol. 2000, 11, 1413–1418. [Google Scholar] [CrossRef]
- Han, L.; Liu, F.; Li, Q.; Qing, T.; Zhai, Z.; Xia, Z.; Li, J. The Efficacy of Beta-Blockers in Patients With Long QT Syndrome 1–3 According to Individuals’ Gender, Age, and QTc Intervals: A Network Meta-analysis. Front. Pharmacol. 2020, 11, 579525. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 101) | LQT1 (n = 54) | LQT2 (n = 40) | LQT3 (n = 7) | p-Value |
|---|---|---|---|---|---|
| Families, n | 82 | 46 | 30 | 6 | 0.436 a |
| Female, N/n (%) | 54/101 (53.5) | 32/54 (59.3) | 18/40 (45.0) | 4/7 (57.1) | 0.383 a |
| Age at diagnosis (years), median (min–max) | 7.7 (0.0–18.0) | 6.1 (0.0–17.8) | 8.4 (0.0–18.0) | 7.7 (0.0–13.2) | 0.265 b |
| Subjects with follow-up, N/n (%) | 83/101 (82.2) | 44/54 (81.5) | 33/40 (82.5) | 6/7 (85.7) | 0.961 a |
| Follow-up time c (years), median (min–max) | 4.6 (0.1–24.3) | 4.5 (0.1–24.3) | 4.6 (0.2–17.7) | 7.9 (0.8–10.4) | 0.809 b |
| QTc time (ms), median (min–max) | 470 (370–740) | 460 (370–554) | 490 (400–630) | 480 (417–740) | 0.047 b |
| QTc categories (1–4), N/n (%) | 0.163 a | ||||
| ≤449 ms | 23/101 (22.8) | 14/54 (25.9) | 8/40 (20.0) | 1/7 (14.3) | 0.682 a |
| 450–499 ms | 50/101 (49.5) | 30/54 (55.6) | 17/40 (42.5) | 3/7 (42.9) | 0.427 a |
| 500–549 ms | 15/101 (14.9) | 8/54 (14.8) | 6/40 (15.0) | 1/7 (14.3) | 0.999 a |
| ≥550 ms | 13/101 (12.9) | 2/54 (3.7) | 9/40 (22.5) | 2/7 (28.6) | 0.012 a |
| Syncope, N/n (%) | 29/101 (28.7) | 12/54 (22.2) | 16/40 (40.0) | 1/7 (14.3) | 0.116 a |
| Syncope at enrollment | 27/101 (26.7) | 10/54 (18.5) | 16/40 (40.0) | 1/7 (14.3) | 0.050 a |
| Syncope during follow-up | 16/83 (19.3) | 7/44 (15.9) | 9/33 (27.3) | 0/6 (0.0) | 0.211 a |
| Family history, N/n (%) | 83/101 (82.1) | 45/54 (83.3) | 33/40 (82.5) | 5/7 (71.4) | 0.739 a |
| Definite LQTS of relative | 61/83 (73.5) | 33/45 (73.3) | 26/33 (78.8) | 2/5 (40.0) | 0.187 a |
| SCD/ACA of relative | 28/83 (33.7) | 13/45 (28.9) | 15/33 (45.5) | 0/5 (0.0) | 0.080 a |
| MAE, N/n (%) | 18/101 (17.8) | 3/54 (5.6) | 13/40 (32.5) | 2/7 (28.6) | 0.002 a |
| Type of MAE, N/n (%) | 0.334 a | ||||
| SCD | 0/18 (0.0) | 0/3 (0.0) | 0/13 (0.0) | 0/2 (0.0) | n.a. |
| ACA | 8/18 (44.4) | 2/3 (66.7) | 4/13 (30.8) | 2/2 (100.0) | 0.130 a |
| Appropriate ICD discharge | 6/18 (33.3) | 1/3 (33.3) | 5/13 (38.5) | 0/2 (0.0) | 0.562 a |
| Sustained VT | 4/18 (22.2) | 0/3 (0.0) | 4/13 (30.8) | 0/2 (0.0) | 0.372 a |
| Age at first MAE (years), median (min–max) | 13.4 (0.0–23.3) | 13.6 (6.5–20.8) | 13.5 (0.0–23.3) | 0.0 (0.0–0.0) | 0.144 b |
| Schwartz score, median (min–max) | 4.0 (1.0–7.0) | 4.0 (1.0–6.0) | 4.0 (1.0–7.0) | 4.0 (3.0–5.0) | 0.005 b |
| Cardiac therapy d, N/n (%) | 87/101 (86.1) | 45/54 (83.3) | 36/40 (90.0) | 6/7 (85.7) | 0.652 a |
| ICD, N/n (%) | 25/101 (24.8) | 5/54 (9.3) | 18/40 (45.0) | 2/7 (28.6) | <0.001 a |
| Primary prevention | 16/25 (64.0) | 3/5 (60.0) | 12/18 (66.7) | 1/2 (50.0) | 0.878 a |
| Secondary prevention | 9/25 (36.0) | 2/5 (40.0) | 6/18 (33.3) | 1/2 (50.0) | 0.878 a |
| Beta-blockers at enrollment, N/n (%) | 72/101 (71.3) | 37/54 (68.5) | 31/40 (77.5) | 4/7 (57.1) | 0.440 a |
| Beta-blockers at follow-up, N/n (%) | 76/83 (91.6) | 40/44 (90.9) | 30/33 (90.9) | 6/6 (100.0) | 0.742 a |
| Type of beta-blockers, N/n (%), (mg/kg BW/day), median (min–max) | |||||
| Metoprolol | 36/76 (47.4), | 21/40 (52.5), | 13/30 (43.3), | 2/6 (47.4), | 0.579 a |
| 1.4 (0.0–2.7) | 1.3 (0.0–2.1) | 1.6 (0.5–2.7) | 1.4 (1.2–1.7) | 0.112 b | |
| Bisoprolol | 7/76 (9.2), | 5/40 (12.5), | 2/30 (6.7), | 0/6 (0.0), | 0.507 a |
| 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | n.a. | 0.699 b | |
| Atenolol | 3/76 (3.9), | 0/40 (0.0), | 2/30 (6.7), | 1/6 (16.7), | 0.091 a |
| 1.5 (0.6–2.5) | n.a. | 0.6 (0.6–0.6) | 2.5 (2.5–2.5) | 0.317 b | |
| Propranolol | 30/76 (39.5), | 14/40 (35.0), | 13/30 (43.3), | 3/6 (50.0), | 0.670 a |
| 1.8 (1.1–4.8) | 1.7 (1.2–3.5) | 1.8 (1.1–3.7) | 3.2 (2.1–4.8) | 0.176 b | |
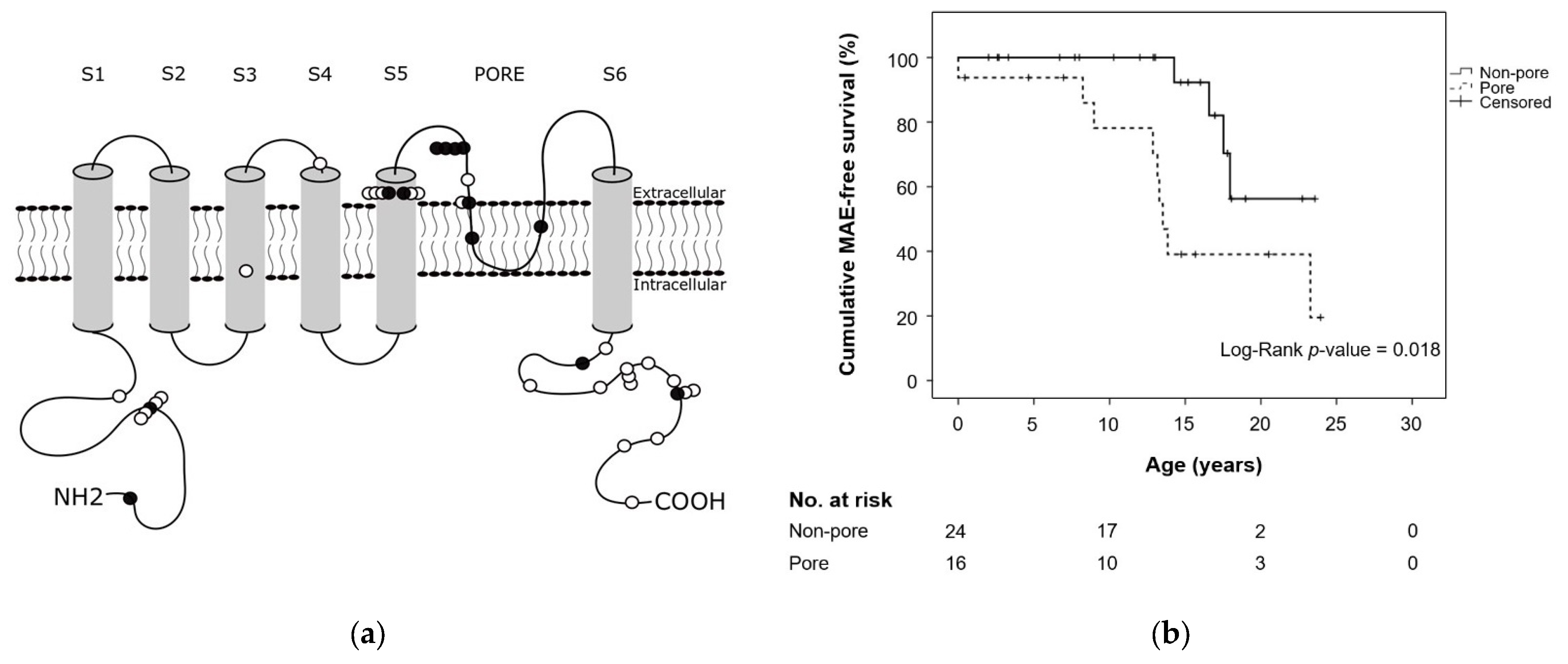
| Location variant, N/n (%) | 0.003 a | ||||
| N-term | 10/101 (9.9) | 3/54 (5.6) | 7/40 (17.5) | 0/7 (0.0) | 0.105 a |
| S1–S4 region | 26/101 (25.7) | 22/54 (40.7) | 2/40 (5.0) | 2/7 (28.6) | <0.001 a |
| S5-loop-S6 region e | 28/101 (27.7) | 12/54 (22.2) | 16/40 (40.0) | 0/7 (0.0) | 0.039 a |
| C-term | 36/101 (35.6) | 17/54 (31.5) | 15/40 (37.5) | 4/7 (57.1) | 0.391 a |
| Interdomain | 1/101 (1.0) | n.a. | n.a. | 1/7 (14.2) | n.a. |
| Type of variant, N/n (%) | 0.019 a | ||||
| Missense | 66/101 (65.3) | 39/54 (72.2) | 20/40 (50.0) | 7/7 (100.0) | 0.011 a |
| Frameshift | 19/101 (18.8) | 6/54 (11.1) | 13/40 (32.5) | 0/7 (0.0) | 0.013 a |
| Small in-frame deletion/duplication | 6/101 (5.9) | 6/54 (11.1) | 0/40 (0.0) | 0/7 (0.0) | 0.062 a |
| Nonsense | 4/101 (4.0) | 2/54 (3.7) | 2/20 (5.0) | 0/7 (0.0) | 0.814 a |
| Intragenic deletion | 3/101 (3.0) | 0/54 (0.0) | 3/20 (7.5) | 0/7 (0.0) | 0.095 a |
| Splice | 2/101 (2.0) | 0/54 (0.0) | 2/20 (5.0) | 0/7 (0.0) | 0.211 a |
| Nearsplice | 1/101 (1.0) | 1/54 (1.9) | 0/20 (0.0) | 0/7 (0.0) | 0.644 a |
| Characteristics | Total (n = 101) | MAE (n = 18) | Non-MAE (n = 83) | p-Value |
|---|---|---|---|---|
| Female, N/n (%) | 54/104 (53.5) | 11/18 (61.1) | 43/83 (51.8) | 0.473 a |
| Age at diagnosis (years), median (min–max) | 7.7 (0.0–18.0) | 8.5 (0.0–18.0) | 7.5 (0.0–17.8) | 0.289 b |
| Subjects with follow-up, N/n (%) | 83/101 (82.2) | 17/18 (94.4) | 66/83 (79.5) | 0.134 a |
| Follow-up time c (years), median (min–max) | 4.6 (0.1–24.3) | 8.4 (0.8–19.7) | 4.0 (0.1–24.3) | 0.023 b |
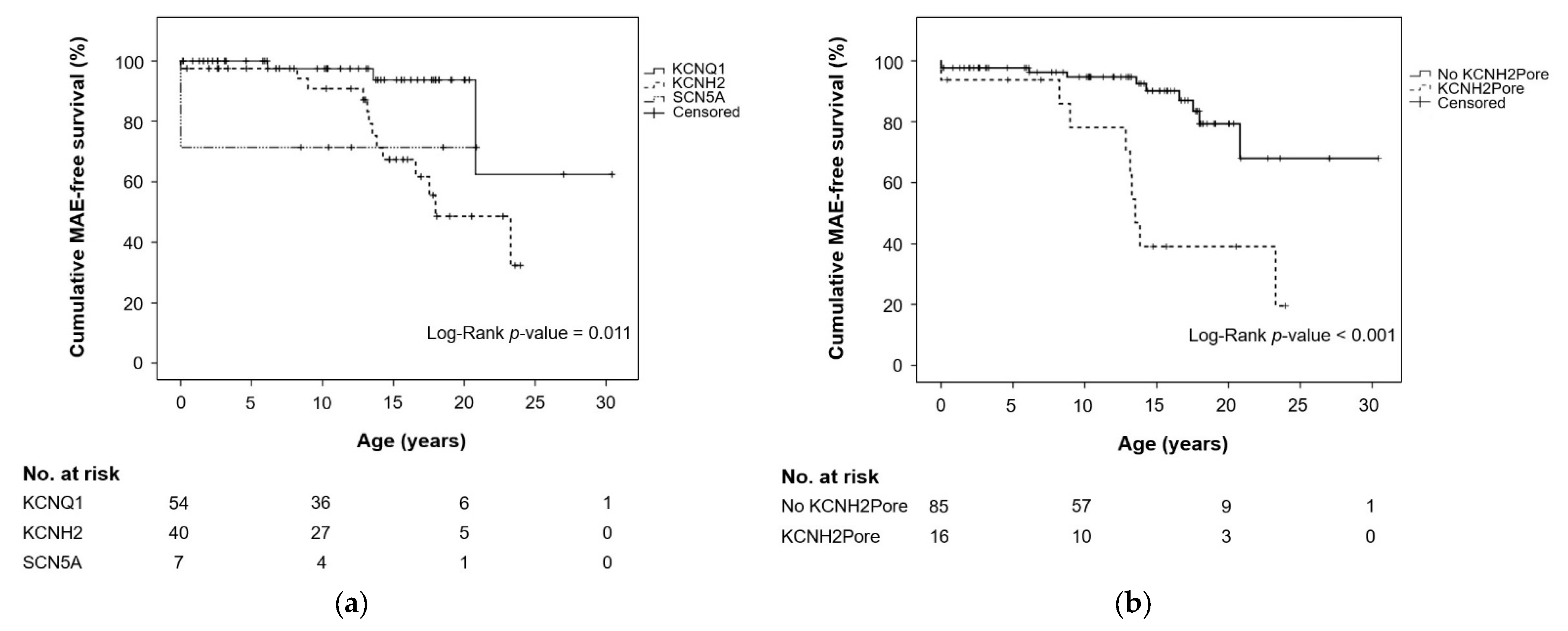
| Genetic findings, N/n (%) | 0.002 a | |||
| KCNQ1 | 54/101 (53.5) | 3/18 (16.7) | 51/83 (61.4) | |
| KCNH2 | 40/101 (39.6) | 13/18 (72.2) | 27/83 (32.5) | |
| SCN5A | 7/101 (6.9) | 2/18 (11.1) | 5/83 (6.0) | |
| QTc time (ms), median (min–max) | 470 (370–740) | 504 (417–740) | 470 (370–590) | 0.013 b |
| QTc categories (1–4), N/n (%) | 0.107 a | |||
| ≤449 ms | 23/101 (22.8) | 3/18 (16.7) | 20/83 (24.1) | 0.496 a |
| 450–499 ms | 50/101 (49.5) | 6/18 (33.3) | 44/83 (53.0) | 0.130 a |
| 500–549 ms | 15/101 (14.9) | 4/18 (22.2) | 11/83 (13.3) | 0.332 a |
| ≥550 ms | 13/101 (12.9) | 5/18 (27.8) | 8/83 (9.6) | 0.037 a |
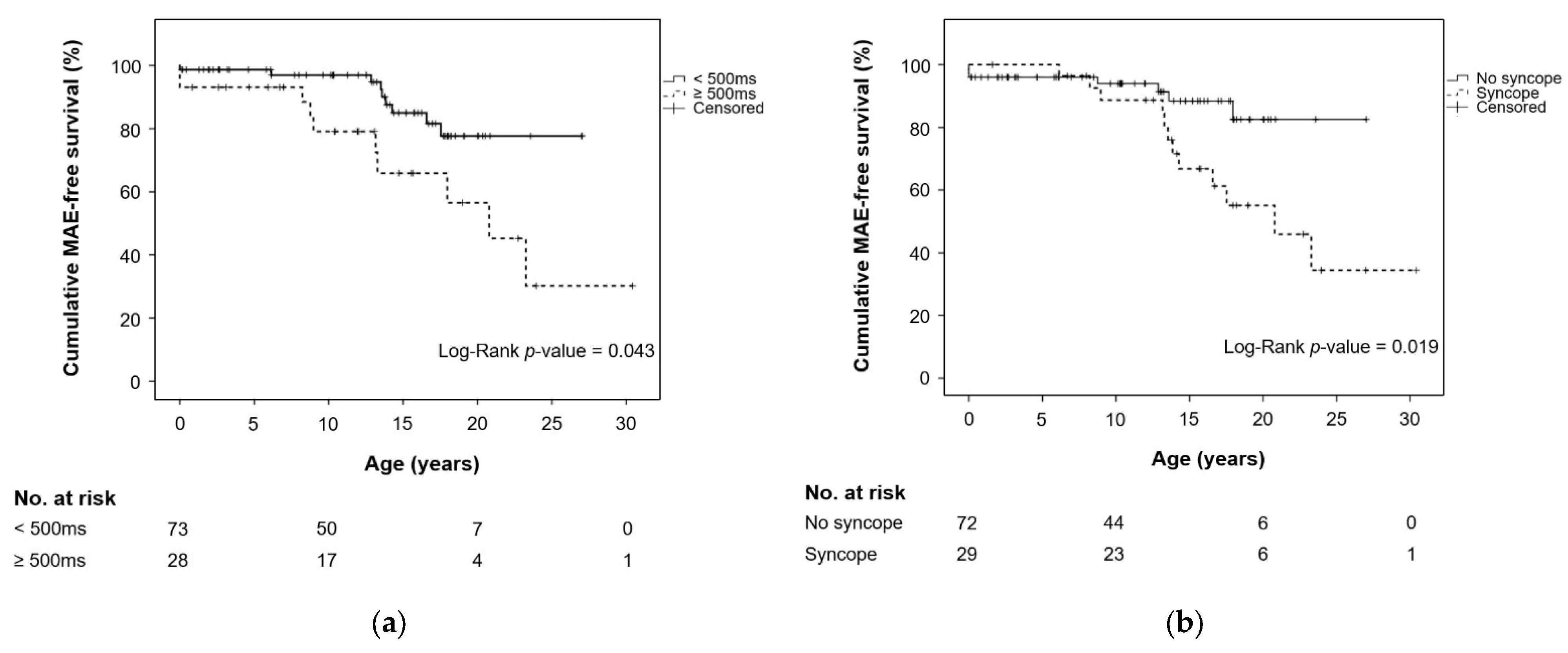
| Syncope, N/n (%) | 29/101 (28.7) | 12/18 (66.7) | 17/83 (20.5) | <0.001 a |
| Syncope at enrollment | 27/101 (26.7) | 11/18 (61.1) | 16/83 (19.3) | <0.001 a |
| Syncope during follow-up | 16/83 (19.3) | 9/17 (52.9) | 7/66 (10.6) | <0.001 a |
| Family history, N/n (%) | 83/101 (82.1) | 13/18 (72.2) | 70/83 (82.2) | 0.223 a |
| Definite LQTS of relative | 61/83 (73.5) | 7/13 (53.8) | 54/70 (77.1) | 0.081 a |
| SCD/ACA of relative | 28/83 (33.7) | 4/13 (30.8) | 24/70 (34.3) | 0.805 a |
| Schwartz score, median (min–max) | 4.0 (1.0–7.0) | 6.0 (4.0–7.0) | 4.0 (1.0–5.0) | <0.001 b |
| Cardiac therapy d, N/n (%) | 87/101 (86.1) | 18/18 (100.0) | 69/83 (82.2) | 0.060 a |
| ICD, N/n (%) | 25/101 (24.8) | 15/18 (83.3) | 10/83 (12.0) | <0.001 a |
| Primary prevention | 16/25 (64.0) | 6/15 (40.0) | 10/10 (100.0) | 0.002 a |
| Secondary prevention | 9/25 (36.0) | 9/9 (100.0) | 0/9 (0.0) | 0.002 a |
| Beta-blockers at enrollment, N/n (%) | 72/101 (71.3) | 18/18 (100.0) | 54/83 (65.1) | 0.003 a |
| Beta-blockers at follow-up, N/n (%) | 76/83 (91.6) | 16/17 (94.1) | 60/66 (90.9) | 0.671 a |
| Type of beta-blockers, N/n (%), (mg/kg BW/day), median (min–max) | ||||
| Metoprolol | 36/76 (47.4), | 7/16 (43.8), | 29/60 (48.3), | 0.744 a |
| 1.4 (0.0–2.7) | 1.5 (0.4–2.7) | 1.4 (0.0–2.5) | 0.845 b | |
| Bisoprolol | 7/76 (9.2), | 4/16 (25.0), | 3/60 (78.9), | 0.014 a |
| 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | 0.1 (0.1–0.1) | 0.629 b | |
| Atenolol | 3/76 (3.9), | 0/16 (0.0), | 3/60 (5.0), | 0.361 a |
| 1.5 (0.6–2.5) | n.a. | 1.5 (0.6–2.5) | n.a. | |
| Propranolol | 30/76 (39.5), | 5/16 (31.3), | 25/60 (41.7), | 0.449 a |
| 1.8 (1.1–4.8) | 3.1 (1.1–4.8) | 1.7 (1.2–3.5) | 0.186 b | |
| Location variant, N/n (%) | 0.037 a | |||
| N-term | 10/101 (9.9) | 2/18 (11.1) | 8/83 (9.6) | 0.850 a |
| S1–S4 region | 26/101 (25.7) | 2/18 (11.1) | 24/83 (28.9) | 1.117 a |
| S5-loop-S6 region e | 28/101 (27.7) | 10/18 (55.6) | 18/83 (21.7) | 0.004 a |
| C-term | 36/101 (35.6) | 4/18 (22.2) | 32/83 (38.6) | 0.190 a |
| Interdomain | 1/101 (1.0) | n.a. | 1/83 (1.2) | n.a. |
| Type of variant, N/n (%) | 0.398 a | |||
| Missense | 66/101 (65.3) | 15/18 (83.3) | 51/83 (61.4) | 0.077 a |
| Frameshift | 19/101 (18.8) | 2/18 (11.1) | 17/83 (20.5) | 0.356 a |
| Small in-frame deletion/duplication | 6/101 (5.9) | 0/18 (0.0) | 6/83 (7.2) | 0.240 a |
| Nonsense | 4/101 (4.0) | 0/18 (0.0) | 4/83 (4.8) | 0.342 a |
| Intragenic deletion | 3/101 (3.0) | 0/18 (0.0) | 3/83 (3.6) | 0.413 a |
| Splice | 2/101 (2.0) | 1/18 (5.6) | 1/83 (1.2) | 0.230 a |
| Nearsplice | 1/101 (1.0) | 0/18 (0.0) | 1/83 (1.2) | 0.640 a |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age at diagnosis (per additional year) | 0.948 | 0.865–1.040 | 0.262 | |||
| Female | 1.322 | 0.511–3.420 | 0.565 | |||
| QTc ≥ 500 ms | 2.588 | 1.002–6.681 | 0.049 | 0.870 | 0.262–2.891 | 0.820 |
| Time-dependent syncope a | 3.260 | 1.080–9.846 | 0.036 | 2.653 | 0.702–10.027 | 0.150 |
| SCD-FH | 0.764 | 0.234–2.492 | 0.656 | |||
| LQT-FH | 0.451 | 0.151–1.349 | 0.154 | |||
| Missense | 0.434 | 0.125–1.511 | 0.190 | |||
| LQTS genotype | ||||||
| LQTS2 vs. LQTS1 | 5.361 | 1.523–18.868 | 0.009 | 5.403 | 1.191–16.281 | 0.026 |
| LQTS3 vs. LQTS1 | 6.033 | 0.998–36.472 | 0.050 | 8.853 | 1.402–55.919 | 0.020 |
| LQTS2 vs. LQTS3 | 0.889 | 0.197–3.999 | 0.878 | |||
| Pore region b | 3.214 | 1.254–8.236 | 0.015 | 2.257 | 0.724–7.034 | 0.160 |
| Time-dependent beta-blocker use c | 1.294 | 0.433–3.867 | 0.644 | |||
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| QTc ≥ 500 ms | 2.588 | 1.002–6.681 | 0.049 | 0.870 | 0.262–2.891 | 0.820 |
| Time-dependent syncope a | 3.260 | 1.080–9.846 | 0.036 | 2.653 | 0.702–10.027 | 0.150 |
| KCNH2Pore b | 5.299 | 2.071–13.556 | 0.001 | 4.241 | 1.408–12.774 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkard, T.; Westphal, D.S.; Markel, F.; Gebauer, R.A.; Hessling, G.; Wolf, C.M. Genetic Diagnostics Contribute to the Risk Stratification for Major Arrhythmic Events in Pediatric Patients with Long QT Syndrome Type 1–3. Cardiogenetics 2022, 12, 90-101. https://doi.org/10.3390/cardiogenetics12010009
Burkard T, Westphal DS, Markel F, Gebauer RA, Hessling G, Wolf CM. Genetic Diagnostics Contribute to the Risk Stratification for Major Arrhythmic Events in Pediatric Patients with Long QT Syndrome Type 1–3. Cardiogenetics. 2022; 12(1):90-101. https://doi.org/10.3390/cardiogenetics12010009
Chicago/Turabian StyleBurkard, Tobias, Dominik Sebastian Westphal, Franziska Markel, Roman Antonin Gebauer, Gabriele Hessling, and Cordula Maria Wolf. 2022. "Genetic Diagnostics Contribute to the Risk Stratification for Major Arrhythmic Events in Pediatric Patients with Long QT Syndrome Type 1–3" Cardiogenetics 12, no. 1: 90-101. https://doi.org/10.3390/cardiogenetics12010009
APA StyleBurkard, T., Westphal, D. S., Markel, F., Gebauer, R. A., Hessling, G., & Wolf, C. M. (2022). Genetic Diagnostics Contribute to the Risk Stratification for Major Arrhythmic Events in Pediatric Patients with Long QT Syndrome Type 1–3. Cardiogenetics, 12(1), 90-101. https://doi.org/10.3390/cardiogenetics12010009






