Modified Body Mass Index as a Novel Nutritional and Prognostic Marker in Patients with Cardiac Amyloidosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Clinical and Laboratory Assessment
2.3. Neurologic Assessment
2.4. Nutrition Assessment
2.5. Echocardiographic Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic Assessment
3.3. Nutritional Assessment
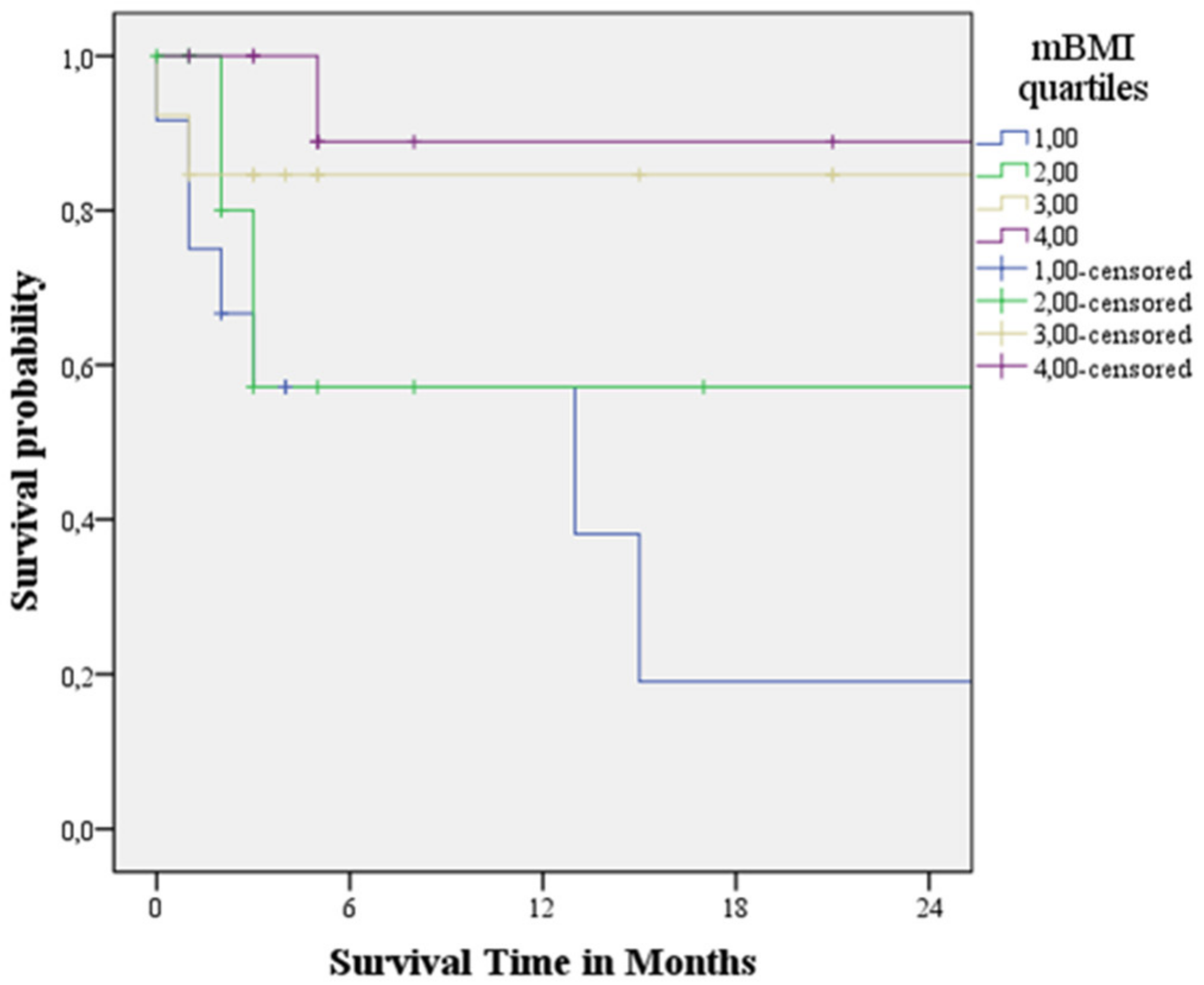
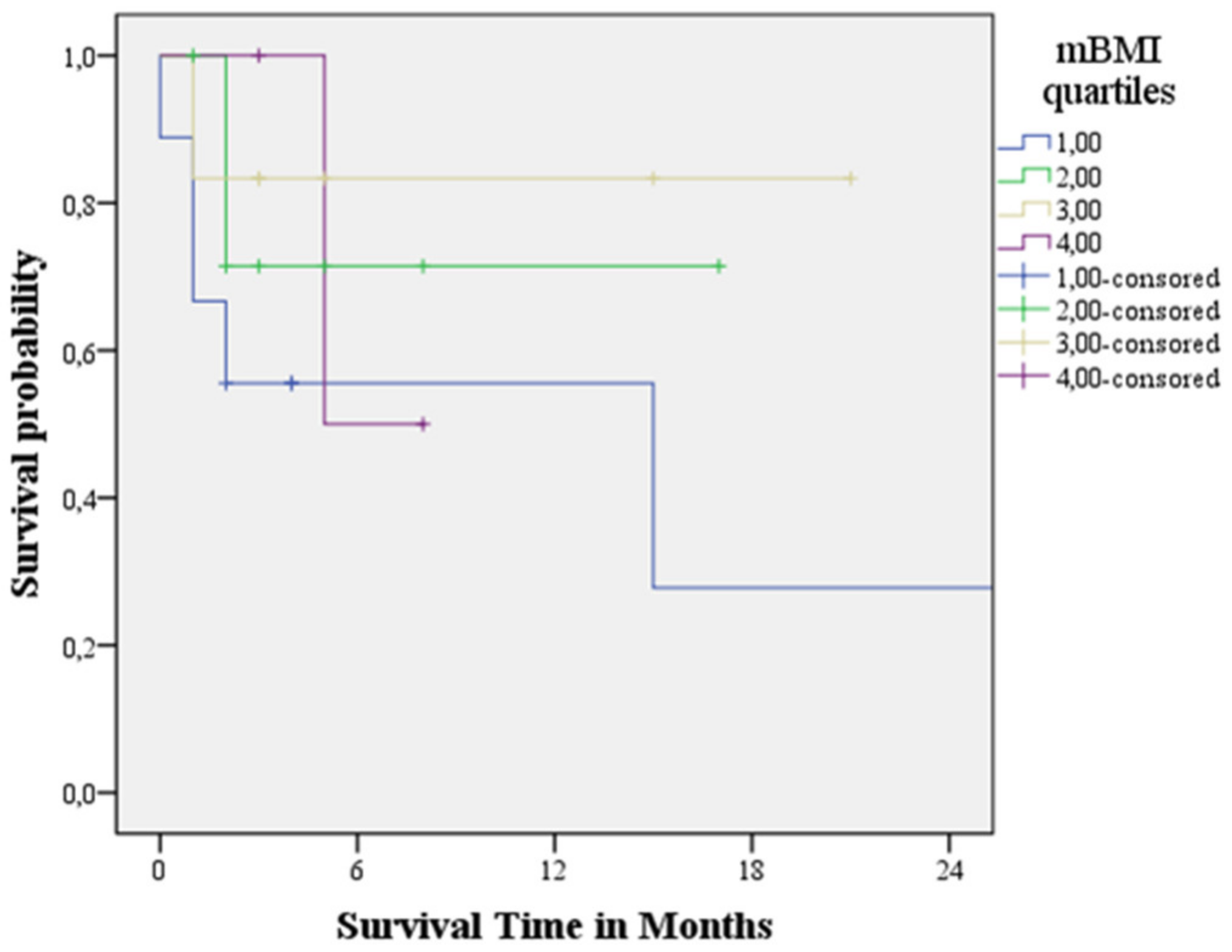
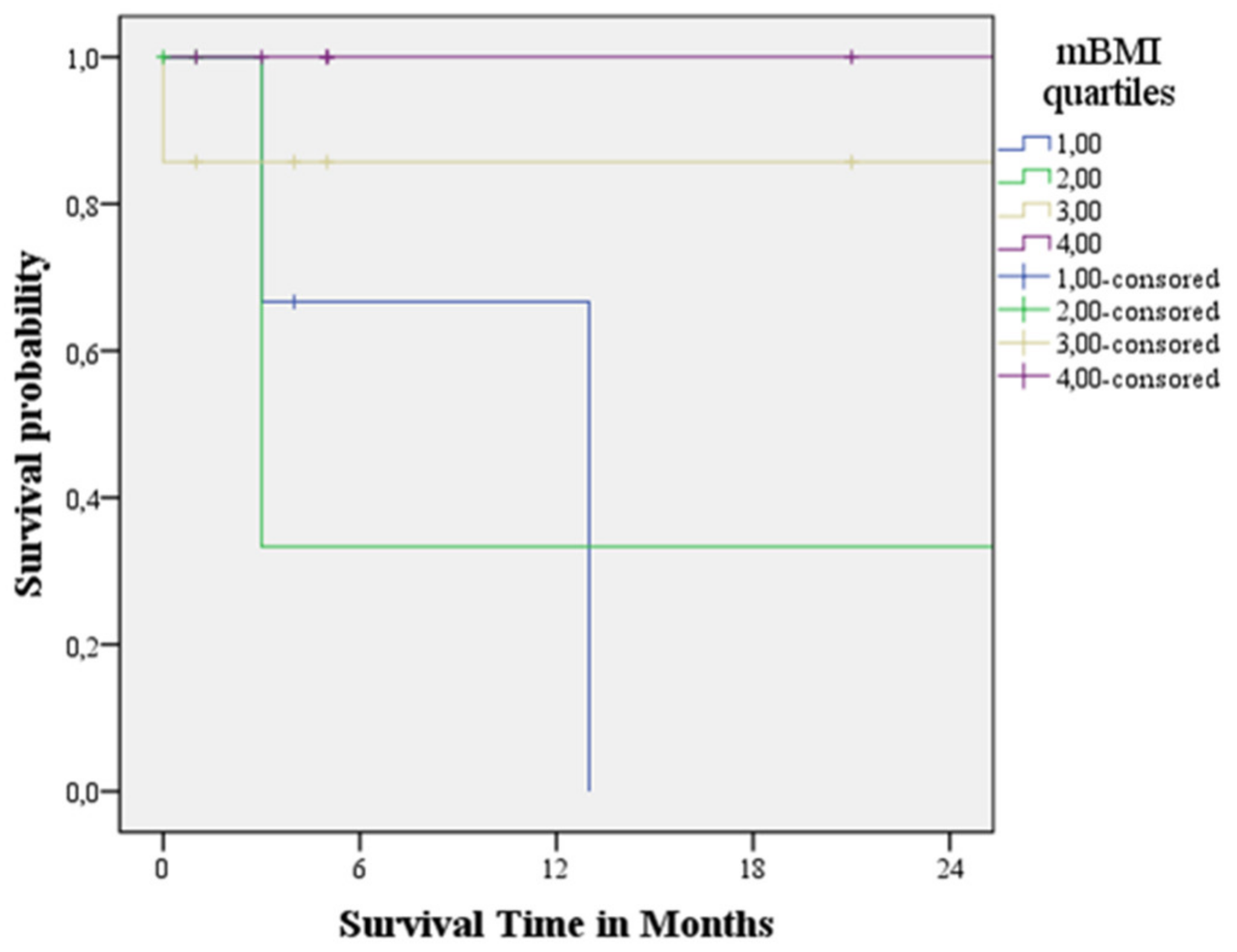
3.4. Survival Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rahman, A.; Jafry, S.; Jeejeebhoy, K.; Nagpal, A.D.; Pisani, B.; Agarwala, R. Malnutrition and Cachexia in Heart Failure. J. Parenter. Enter. Nutr. 2016, 40, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Palladini, G.; Klersy, C.; Cereda, E.; Bonardi, C.; Cameletti, B.; Montagna, E.; Russo, P.; Foli, A.; Milani, P.; et al. Nutritional status independently affects quality of life of patients with systemic immunoglobulin light-chain (AL) amyloidosis. Ann. Hematol. 2012, 91, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Block, G.; Horwich, T.; Fonarow, G.C. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 1439–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossniklaus, D.A.; O’Brien, M.C.; Clark, P.C.; Dunbar, S.B. Nutrient intake in heart failure patients. J. Cardiovasc. Nurs. 2008, 23, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Verri, M.; Boschi, F.; Febo, O.; Pasini, E.; Pastoris, O. Is nutritional intake adequate in chronic heart failure patients? J. Am. Coll. Cardiol. 2003, 42, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2018: Recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018, 25, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [Green Version]
- Muchtar, E.; Gertz, M.A.; Kyle, R.A.; Lacy, M.Q.; Dingli, D.; Leung, N.; Buadi, F.K.; Hayman, S.R.; Kapoor, P.; Hwa, Y.L.; et al. A Modern Primer on Light Chain Amyloidosis in 592 Patients with Mass Spectrometry-Verified Typing. Mayo Clin. Proc. 2019, 94, 472–483. [Google Scholar] [CrossRef]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; Macgillivray, T.E.; et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38a MAPK pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef] [Green Version]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin. Hematol. 1995, 32, 45–59. [Google Scholar]
- Dubrey, S.W.; Cha, K.; Anderson, J.; Chamarthi, B.; Reisinger, J.; Skinner, M.; Falk, R.H. The clinical features of immunoglobulin light-chain amyloidosis (AL) with heart involvement. Q. J. Med. 1998, 91, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Grigoletti, S.S.; Zuchinali, P.; Lemieux-Blanchard, É.; Béchard, S.; Lemieux, B.; Ribeiro, P.A.B.; Tournoux, F. Focused review on nutritional status of patients with immunoglobulin light chain amyloidosis. Curr. Probl. Cancer 2022, 46, 100833. [Google Scholar] [CrossRef]
- Hayman, S.R.; Lacy, M.Q.; Kyle, R.A.; Gertz, M.A. Primary systemic amyloidosis: A cause of malabsorption syndrome. Am. J. Med. 2001, 111, 535–540. [Google Scholar] [CrossRef]
- Merlini, G. AL amyloidosis: Therapeutic strategies 2004. Hematol. Am. Soc. Hematol. Educ. Program 2004, 1, 262–270. [Google Scholar]
- Wilson, M.M.; Vaswani, S.; Liu, D.; Morley, J.E.; Miller, D.K. Prevalence and causes of undernutrition in medical outpatients. Am. J. Med. 1998, 104, 56–63. [Google Scholar] [CrossRef]
- Campillo, B.; Paillaud, E.; Uzan, I.; Merlier, I.; Abdellaoui, M.; Perennec, J.; Louarn, F.; Bories, P. Value of body mass index in the detection of severe malnutrition: Influence of the pathology and changes in anthropometric parameters. Clin. Nutr. 2004, 23, 551–559. [Google Scholar] [CrossRef]
- Nishikido, T.; Oyama, J.-I.; Nagatomo, D.; Node, K. A reduction of BMI predicts the risk of rehospitalization and cardiac death in nonobese patients with heart failure. Int. J. Cardiol. 2019, 276, 166–170. [Google Scholar] [CrossRef]
- Van Haute, M.; Rondilla, E., II; Vitug, J.L.; Batin, K.D.; Abrugar, R.E.; Quitoriano, F.; Dela Merced, K.; Maaño, T.; Higa, J.; Almoro, J.G.; et al. Assessment of a proposed BMI formula in predicting body fat percentage among Filipino young adults. Sci. Rep. 2020, 10, 21988. [Google Scholar] [CrossRef]
- Russo, M.; Vita, G.L.; Stancanelli, C.; Mazzeo, A.; Vita, G.; Messina, S. Parenteral nutrition improves nutritional status, autonomic symptoms and quality of life in transthyretin amyloid polyneuropathy. Neuromuscul. Disord. 2016, 26, 374–377. [Google Scholar] [CrossRef]
- Driggin, E.; Helmke, S.; De Los Santos, J.; Teruya, S.; Guadalupe, S.; Goldsmith, J.; Maurer, M.S. Markers of nutritional status and inflammation in transthyretin cardiac amyloidosis: Association with outcomes and the clinical phenotype. Amyloid 2020, 27, 73–80. [Google Scholar] [CrossRef]
- Jeon, H.G.; Choi, D.K.; Sung, H.H.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Lee, H.M. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann. Surg. Oncol. 2016, 23, 321–327. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Sung, S.H.; Cheng, H.M.; Hsu, P.F.; Guo, C.Y.; Yu, W.C.; Chen, C.H. Prognostic Nutritional Index and the Risk of Mortality in Patients with Acute Heart Failure. J. Am. Heart Assoc. 2017, 6, e004876. [Google Scholar] [CrossRef]
- Sattianayagam, P.T.; Lane, T.; Fox, Z.; Petrie, A.; Gibbs, S.D.J.; Pinney, J.H.; Risom, S.S.; Rowczenio, D.M.; Wechalekar, A.D.; Lachmann, H.J.; et al. A prospective study of nutritional status in immunoglobulin light chain amyloidosis. Haematologica 2013, 98, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.K.; Gertz, M.A.; Dispenzieri, A. Validation of Mayo Clinic Staging System for Light Chain Amyloidosis with High-Sensitivity Troponin. J. Clin. Oncol. 2019, 37, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Yazaki, M.; Kametani, F.; Takei, Y.; Ikeda, S. Marked regression of abdominal fat amyloid in patients with familial amyloid polyneuropathy during long-term follow-up after liver transplantation. Liver Transpl. 2008, 14, 563–570. [Google Scholar] [CrossRef]
- Alvares-da-Silva, M.R.; da Silveira, T.R. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005, 21, 113–117. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Milani, P.; Dispenzieri, A.; Gertz, M.A.; Lacy, M.Q.; Baudi, F.K.; Kumar, S.K.; Maurer, M.S.; Klarich, K.; Hayman, S.R.; Leung, N.; et al. In patients with light-chain (AL) amyloidosis myocardial contraction fraction (MCF) is a simple, but powerful prognostic measure that can be calculated from a standard echocardiogram. Blood 2015, 126, 1774. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Senapati, A.; Sperry, B.W.; Grodin, J.L.; Kusunose, K.; Thavendiranathan, P.; Jaber, W.; Collier, P.; Hanna, M.; Popovic, Z.B.; Phelan, D. Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart 2016, 102, 748–754. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Ericzon, B.G.; Wilczek, H.E.; Larsson, M.; Wijayatunga, P.; Stangou, A.; Pena, J.R.; Furtado, E.; Barroso, E.; Daniel, J.; Samuel, D.; et al. Liver transplantation for hereditary transthyretin amyloidosis: After 20 years still the best therapeutic alternative? Transplantation 2015, 99, 1847–1854. [Google Scholar] [CrossRef]
- Sahutoglu, T. Relationship between Modified Body Mass Index and Prognosis of Renal Amyloid a Amyloidosis. Med. Bull. Sisli Etfal Hosp. 2018, 52, 103–108. [Google Scholar]
- Sundaram, V.; Fang, J.C. Gastrointestinal and liver issues in heart failure. Circulation 2016, 133, 1696–1703. [Google Scholar] [CrossRef]
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Valentova, M.; von Haehling, S.; Bauditz, J.; Doehner, W.; Ebner, N.; Bekfani, T.; Elsner, S.; Sliziuk, V.; Scherbakov, N.; Murin, J.; et al. Intestinal congestion and right ventricular dysfunction: A link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur. Heart J. 2016, 37, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- Valentova, M.; von Haehling, S.; Krause, C.; Ebner, N.; Steinbeck, L.; Cramer, L.; Doehner, W.; Murin, J.; Anker, S.D.; Sandek, A. Cardiac cachexia is associated with right ventricular failure and liver dysfunction. Int. J. Cardiol. 2013, 169, 219–224. [Google Scholar] [CrossRef]
- Wixner, J.; Mundayat, R.; Karayal, O.N.; Anan, I.; Karling, P.; Suhr, O.B. THAOS: Gastrointestinal manifestations of transthyretin amyloidosis-common complications of a rare disease. Orphanet J. Rare Dis 2014, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Den Braber-Ymker, M.; Heijker, S.; Lammens, M.; Croockewit, S.; Nagtegaal, I.D. Intestinal involvement in amyloidosis is a sequential process. Neurogastroenterol. Motil. 2018, 30, e13469. [Google Scholar] [CrossRef] [Green Version]
- Ebert, E.C.; Nagar, M. Gastrointestinal manifestations of amyloidosis. Am. J. Gastroenterol. 2008, 103, 776–787. [Google Scholar] [CrossRef]
- Wixner, J.; Tornblom, H.; Karling, P.; Anan, I.; Lindberg, G. Abnormal small bowel motility in patients with hereditary transthyretin amyloidosis. Neurogastroenterol. Motil. 2018, 30, e13354. [Google Scholar] [CrossRef]
| CA (n = 50) | AL CA (n = 26) | ATTRwt CA (n = 24) | p-Value | |
|---|---|---|---|---|
| Baseline clinical and functional parameters | ||||
| Age, years | 69.2 ± 14.0 | 60.6 ± 13.4 | 78.6 ± 7.1 | 0.0001 |
| Male sex | 34 (68%) | 13 (50%) | 21 (87.5%) | 0.005 |
| NYHA functional class | 0.260 | |||
| Class I | 5 (10%) | 3 (11.5%) | 2 (8.5%) | |
| Class II | 25 (50%) | 10 (38.5%) | 15 (62.5%) | |
| Class III | 18 (36%) | 11 (42%) | 7 (29%) | |
| Class IV | 2 (4%) | 2 (8%) | 0 (0%) | |
| Peripheral neuropathy | 22 (44%) | 6 (23%) | 16 (66%) | 0.002 |
| Cardiac dysautonomia | 18 (36%) | 7 (27%) | 11 (46%) | 0.136 |
| Cardiovascular death | 15 (30%) | 9 (30%) | 6 (25%) | 0.334 |
| Height, cm | 166.4 ± 7.9 | 166.9 ± 9.1 | 165.8 ± 6.6 | 0.646 |
| Weight, kg | 70.2 ± 12.1 | 67.9 ± 12.0 | 72.7 ± 11.9 | 0.156 |
| BSA, m2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | 0.363 |
| cBMI, kg/m2 | 25.3 ± 3.9 | 24.3 ± 3.7 | 26.4 ± 3.9 | 0.061 |
| nBMI, kg/m2 | 25.5 ± 4.0 | 24.5 ± 3.9 | 26.6 ± 3.9 | 0.062 |
| mBMI | 954.6 ± 227.9 | 874.7 ± 216.2 | 1041.1 ± 211.9 | 0.008 |
| PNI | 70.5 ± 12.6 | 69.9 ± 18.8 | 71.2 ± 8.1 | 0.739 |
| SBP, mmHg | 115.8 ± 22.4 | 111.9 ± 24.5 | 120.1 ± 19.6 | 0.198 |
| DBP, mmHg | 67.8 ± 12.1 | 66.3 ± 12.8 | 69.4 ± 11.4 | 0.377 |
| Stage | Revised Mayo Clinic | Gillmore | ||
| I | 3 (11.5%) | 11 (46%) | ||
| II | 5 (19%) | 7 (29%) | ||
| III | 8 (31%) | 6 (25%) | ||
| IV | 10 (38.5%) | |||
| Laboratory parameters | ||||
| RBC, millions/mm3 | 4.5 ± 0.6 | 4.4 ± 0.7 | 4.6 ± 0.6 | 0.342 |
| Haemoglobin, g/dL | 13.2 ± 1.9 | 12.8 ± 1.7 | 13.6 ± 2.1 | 0.199 |
| WBC, x103/μL | 6635.0 ± 2727.9 | 6862.1 ± 3387.4 | 6389.0 ± 1805.7 | 0.546 |
| Creatinine, mg/dL | 1.2 ± 1.1 | 1.2 ± 1.6 | 1.1 ± 0.3 | 0.687 |
| eGFR, mL/min/1.73 m2 | 69.7 ± 33.1 | 80.9 ± 38.6 | 57.7 ± 20.7 | 0.012 |
| Serum albumin, g/dL | 3.7 ± 0.6 | 3.6 ± 0.6 | 3.9 ± 0.6 | 0.036 |
| NT-proBNP, pg/mL, median (IQR) | 3153 (1306–9140) | 3617 (1039–) | 3153 (1565–4411) | 0.190 |
| HS cTnI, pg/mL, median (IQR) | 145 (63–308) | 142 (39–319) | 145 (78–303) | 0.301 |
| Medications | ||||
| Beta-blockers | 5 (10%) | 7 (27%) | 10 (41%) | 0.212 |
| Amiodaron | 17 (34%) | 1 (4%) | 4 (15%) | 0.150 |
| ACE-inhibitors | 10 (20%) | 2 (7%) | 8 (33%) | 0.070 |
| Sartans | 1 (2%) | 0 (0%) | 1 (4%) | 0.480 |
| Diuretics | 47 (94%) | 24 (92%) | 23 (96%) | 0.531 |
| Anti-mineralcorticoids | 18 (36%) | 10 (38%) | 8 (33%) | 0.468 |
| Calcium-antagonists | 1 (2%) | 1 (4%) | 0 (0%) | 0.520 |
| Antiplatelet agents | 8 (16%) | 4 (15%) | 1 (16%) | 0.601 |
| Vitamin K antagonists | 4 (8%) | 3 (11%) | 1 (4%) | 0.336 |
| Direct oral anticoagulants | 17 (34%) | 4 (15%) | 13 (54%) | 0.004 |
| CA (n = 50) | AL CA (n = 26) | ATTRwt CA (n = 24) | p-Value | |
|---|---|---|---|---|
| Echocardiographic parameters | ||||
| LVEDD, mm | 42.7 ± 5.9 | 40.9 ± 5.7 | 44.5 ± 5.6 | 0.030 |
| LVESD, mm | 28.6 ± 6.5 | 26.5 ± 6.7 | 30.9 ± 5.6 | 0.016 |
| IVSD, mm | 16.9 ± 3.5 | 16.5 ± 2.8 | 17.4 ± 4.1 | 0.360 |
| PWD, mm | 15.4 ± 3.4 | 14.7 ± 2.9 | 16.1 ± 3.7 | 0.172 |
| RWT ratio | 0.74 ± 0.22 | 0.74 ± 0.19 | 0.74 ± 0.25 | 0.926 |
| LVM, g | 297.6 ± 102.0 | 265.2 ± 85.4 | 332.7 ± 108.6 | 0.018 |
| LVM indexed, g/m2 | 166.7 ± 54.3 | 149.9 ± 42.5 | 184.9 ± 60.5 | 0.021 |
| LVEDV, mL | 73.7 ± 26.9 | 66.7 ± 28.3 | 81.4 ± 23.6 | 0.054 |
| LVESV, mL | 37.2 ± 18.5 | 33.4 ± 21.4 | 41.3 ± 14.2 | 0.136 |
| LVEF, % | 50.7 ± 8.7 | 51.6 ± 8.4 | 49.7 ± 8.9 | 0.449 |
| MCF, % | 13.8 ± 5.3 | 14.1 ± 5.7 | 13.6 ± 4.9 | 0.760 |
| LVGLS, % | −10.5 ± 3.3 | −10.5 ± 3.5 | −10.5 ± 3.1 | 0.961 |
| RRSR | 0.98 ± 0.41 | 0.99 ± 0.36 | 0.97 ± 0.47 | 0.840 |
| E wave, cm/s | 88.6 ± 23.6 | 88.9 ± 28.0 | 88.2 ± 18.2 | 0.916 |
| A wave, cm/s | 55.5 ± 26.9 | 58.6 ± 30.6 | 50.3 ± 19.3 | 0.406 |
| E/A ratio | 3.0 ± 5.9 | 3.6 ± 7.5 | 2.1 ± 0.9 | 0.505 |
| DecT, ms | 151.7 ± 48.3 | 146.1 ± 47.5 | 157.7 ± 49.4 | 0.407 |
| E/e’ ratio | 18.1 ± 8.4 | 19.7 ± 10.5 | 16.4 ± 5.0 | 0.175 |
| LAD, mm | 46.8 ± 8.7 | 44.6 ± 7.7 | 49.3 ± 9.3 | 0.055 |
| LAV, mL | 87.1 ± 32.3 | 73.0 ± 23.2 | 102.5 ± 34.1 | 0.001 |
| LAVI, mL/m2 | 48.5 ± 16.3 | 41.4 ± 13.1 | 56.2 ± 16.2 | 0.001 |
| TAPSE, mm | 14.9 ± 5.0 | 15.0 ± 5.4 | 14.9 ± 4.7 | 0.948 |
| St wave, cm/s | 10.9 ± 3.1 | 11.2 ± 3.2 | 10.7 ± 3.0 | 0.577 |
| sPAP, mmHg | 40.3 ± 9.9 | 38.1 ± 8.6 | 42.5 ± 10.9 | 0.118 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Age, years | 0.998 (0.961–1.036) | 0.908 | ||
| NYHA class | 2.550 (1.221–5.324) | 0.013 | ||
| cBMI, kg/m2 | 0.875 (0.748–1.024) | 0.097 | ||
| nBMI, kg/m2 | 0.862 (0.737–1.007) | 0.061 | ||
| mBMI | 0.996 (0.994–0.999) | 0.001 | 0.996 (0.994–0.999) | 0.006 |
| PNI | 0.995 (0.954–1.039) | 0.835 | ||
| eGFR, mL/min/1.73 m2 | 0.996 (0.979–1.014) | 0.678 | ||
| NT-proBNP, pg/mL | 1.000 (1.000–1.000) | 0.679 | ||
| HS-cTnI, pg/mL | 1.001 (1.000–1.002) | 0.002 | ||
| MCF, % | 0.972 (0.876–1.080) | 0.602 | ||
| LVEF, % | 0.995 (0.935–1.058) | 0.865 | ||
| LVGLS, % | 0.863 (0.715–1.042) | 0.126 | ||
| E/e’ ratio | 1.064 (1.014–1.116) | 0.011 | ||
| LAVI, mL/m2 | 1.007 (0.974–1.040) | 0.701 | ||
| TAPSE, mm | 0.912 (0.807–1.031) | 0.142 | ||
| St, cm/s | 0.874 (0.731–1.045) | 0.139 | ||
| sPAP, mmHg | 1.024 (0.978–1.073) | 0.308 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dongiglio, F.; Palmiero, G.; Monda, E.; Rubino, M.; Verrillo, F.; Caiazza, M.; Cirillo, A.; Fusco, A.; Vetrano, E.; Lioncino, M.; et al. Modified Body Mass Index as a Novel Nutritional and Prognostic Marker in Patients with Cardiac Amyloidosis. Cardiogenetics 2022, 12, 185-197. https://doi.org/10.3390/cardiogenetics12020017
Dongiglio F, Palmiero G, Monda E, Rubino M, Verrillo F, Caiazza M, Cirillo A, Fusco A, Vetrano E, Lioncino M, et al. Modified Body Mass Index as a Novel Nutritional and Prognostic Marker in Patients with Cardiac Amyloidosis. Cardiogenetics. 2022; 12(2):185-197. https://doi.org/10.3390/cardiogenetics12020017
Chicago/Turabian StyleDongiglio, Francesca, Giuseppe Palmiero, Emanuele Monda, Marta Rubino, Federica Verrillo, Martina Caiazza, Annapaola Cirillo, Adelaide Fusco, Erica Vetrano, Michele Lioncino, and et al. 2022. "Modified Body Mass Index as a Novel Nutritional and Prognostic Marker in Patients with Cardiac Amyloidosis" Cardiogenetics 12, no. 2: 185-197. https://doi.org/10.3390/cardiogenetics12020017
APA StyleDongiglio, F., Palmiero, G., Monda, E., Rubino, M., Verrillo, F., Caiazza, M., Cirillo, A., Fusco, A., Vetrano, E., Lioncino, M., Diana, G., Di Fraia, F., Cerciello, G., Manganelli, F., Vriz, O., & Limongelli, G. (2022). Modified Body Mass Index as a Novel Nutritional and Prognostic Marker in Patients with Cardiac Amyloidosis. Cardiogenetics, 12(2), 185-197. https://doi.org/10.3390/cardiogenetics12020017








