Spectrum of Clinical Features and Genetic Profile of Left Ventricular Noncompaction Cardiomyopathy in Children
Abstract
:1. Introduction
2. What Is Missing?
3. Materials and Methods
4. Results
5. Discussion
6. What Are the Clinical Implications?
Limitations of the Study
7. Conclusions
- Although heart failure and arrhythmias were very frequent in our study group, thromboembolic events and genetic syndromes were rare.
- Echocardiographic examination is the gold standard for the diagnosis of LVNC. However, cardiac CMR is recommended to confirm the diagnosis, especially in uncertain cases.
- Our results indicate that CMR has a good correlation with echocardiography and a high sensitivity and specificity in detecting non-compacted segments.
- For the accurate and reliable assessment of children with LVNC, it is necessary to get to know their family history and detailed clinical profile.
- The high genetic yield resulted in the explanation of molecular etiology in over half (53%) of the studied children.
- Identifying the genetic cause allows for risk stratification and may help in the clinical management and counseling of patients and their relatives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neudorf, U.E.; Hussein, A.; Trowitzsch, E.; Schmaltz, A.A. Clinical features of isolated noncompaction of the myocardium in children. Cardiol. Young 2001, 11, 439–442. [Google Scholar] [CrossRef]
- Dong, X.; Fan, P.; Tian, T.; Yang, Y.; Xiao, Y.; Yang, K.; Liu, Y.; Zhou, X. Recent advancements in the molecular genetics of left ventricular noncompaction cardiomyopathy. Clin. Chim. Acta 2017, 465, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Ergul, Y.; Nisli, K.; Demirel, A.; Varkal, M.A.; Oner, N.; Dursun, M.; Dindar, A.; Aydogan, U.; Omeroglu, R.E. Left ventricular non-compaction in children and adolescents: Clinical features, treatment and follow-up. Cardiol. J. 2011, 18, 176–184. [Google Scholar]
- Jefferies, J.L.; Wilkinson, J.D.; Sleeper, L.A.; Colan, S.D.; Lu, M.; Pahl, E.; Kantor, P.F.; Everitt, M.D.; Webber, S.A.; Kaufman, B.D.; et al. Pediatric cardiomyopathy registry investigators. Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: Results from the pediatric cardiomyopathy registry. J. Card. Fail. 2015, 21, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Kubik, M.; Dąbrowska-Kugacka, A.; Lewicka, E.; Daniłowicz-Szymanowicz, L.; Raczak, G. Predictors of poor outcome in patients with left ventricular noncompaction: Review of the literature. Adv. Clin. Exp. Med. 2018, 27, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Weisz, S.H.; Limongelli, G.; Pacileo, G.; Calabro, P.; Russo, M.G.; Calabro, R.; Vatta, M. Left ventricular non compaction in children. Congenit. Heart Dis. 2010, 5, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Makhija, P. Left ventricular noncompaction cardiomyopathy in pediatric patients: A case series of a clinically heterogeneous disease. Pediatr. Cardiol. 2017, 38, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, J.C.; Deshmukh, C.T.; Hajela, S.A. Left ventricular noncompaction: A cardiomyopathy often mistaken. Indian J. Med. Sci. 2009, 63, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Dursun, M.; Agayev, A.; Nisli, K.; Ertugrul, T.; Onur, I.; Oflaz, H.; Yekeler, E. MR imaging features of ventricular noncompaction: Emphasis on distribution and pattern of fibrosis. Eur. J. Radiol. 2010, 74, 147–151. [Google Scholar] [CrossRef]
- Pignatelli, R.H.; McMahon, C.J.; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.W.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical characterization of left ventricular noncompaction in children: A relatively common form of cardiomyopathy. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef]
- Elshershari, H.; Okutan, V.; Celiker, A. Isolated noncompaction of ventricular myocardium. Cardiol. Young 2001, 11, 472–475. [Google Scholar] [CrossRef]
- Negri, F.; De Luca, A.; Fabris, E.; Korcova, R.; Cernetti, C.; Grigoratos, C.; Aquaro, G.D.; Nucifora, G.; Camici, P.G.; Sinagra, G. Left ventricular noncompaction, morphological, and clinical features for an integrated diagnosis. Heart Fail. Rev. 2019, 24, 315–323. [Google Scholar] [CrossRef]
- Hirono, K.; Hata, Y.; Miyao, N.; Okabe, M.; Takarada, S.; Nakaoka, H.; Ibuki, K.; Ozawa, S.; Yoshimura, N.; Nishida, N.; et al. Left ventricular noncompaction and congenital heart disease increases the risk of congestive heart failure. J. Clin. Med. 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichida, F.; Tsubata, S.; Bowles, K.R.; Haneda, N.; Uese, K.; Miyawaki, T.; Dreyer, W.J.; Messina, J.; Li, H.; Bowles, N.E.; et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 2001, 103, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarotto, F.; Hawley, M.H.; Beltrami, M.; Beekman, L.; de Marvao, A.; McGurk, K.A.; Statton, B.; Boschi, B.; Girolami, F.; Roberts, A.M.; et al. Systematic large-scale assessment of the genetic architecture of left ventricular noncompaction reveals diverse etiologies. Genet. Med. 2021, 23, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Alehan, D. Clinical features of isolated left ventricular noncompaction in children. Int. J. Cardiol. 2004, 97, 233–237. [Google Scholar] [CrossRef]
- Lilje, C.; Razek, V.; Joyce, J.J.; Rau, T.; Finckh, B.F.; Weiss, F.; Habermann, C.R.; Rice, J.C.; Weil, J. Complications of non-compaction of the left ventricular myocardium in a paediatric population: A prospective study. Eur. Heart J. 2006, 27, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Wengrofsky, P.; Armenia, C.; Oleszak, F.; Kupferstein, E.; Rednam, C.; Mitre, C.A.; McFarlane, S.I. Left ventricular trabeculation and noncompaction cardiomyopathy: A review. EC Clin. Exp. Anat. 2019, 2, 267–283. [Google Scholar]
- Tsai, S.F.; Ebenroth, E.S.; Hurwitz, R.A.; Cordes, T.M.; Schamberger, M.S.; Batra, A.S. Is left ventricular noncompaction in children truly an isolated lesion? Pediatr. Cardiol. 2009, 30, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Ergul, Y.; Nisli, K.; Varkal, M.A.; Oner, N.; Dursun, M.; Dindar, A.; Aydogan, U.; Omeroglu, R.E. Electrocardiographic findings at initial diagnosis in children with isolated left ventricular noncompaction. Ann. Noninvasive Electrocardiol. 2011, 16, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gungor, B.; Alper, A.T.; Celebi, A.; Bolca, O. Sinus node dysfunction as the first manifestation of left ventricular noncompaction with multiple cardiac abnormalities. Indian Pacing Electrophysiol. J. 2013, 13, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Glancy, D.L.; Helmcke, F.R.; Hoang, A.P. Bradycardia, syncope, and left ventricular noncompaction cardiomyopathy. Am. J. Cardiol. 2017, 120, 716–717. [Google Scholar] [CrossRef]
- Miyake, C.Y.; Kim, J.J. Arrhythmias in left ventricular noncompaction. Card. Electrophysiol. Clin. 2015, 7, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Proce, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 4, 2202–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, T.S.; Valdes, S.O.; Hope, K.D.; Morris, S.A.; Landstrom, A.P.; Schneider, A.E.; Miyake, C.Y.; Denfield, S.W.; Pignatelli, R.H.; Wang, Y.; et al. Association of Wolff-Parkinson-White with left ventricular noncompaction cardiomyopathy in children. J. Card. Fail. 2019, 25, 1004–1008. [Google Scholar] [CrossRef]
- Muser, D.; Liang, J.J.; Witschey, W.R.; Pathak, R.K.; Castro, S.; Magnani, S.; Zado, E.S.; Garcia, F.C.; Desjardins, B.; Callans, D.J.; et al. Ventricular arrhythmias associated with left ventricular noncompaction: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm 2017, 14, 166–175. [Google Scholar] [CrossRef]
- Xiaoxiao, Z.; Li, Y.; Linli, Q.; Yang, Y.; Lv, Q.; Li, L.; Wang, J.; He, L.; Zhang, L.; Wang, Y.; et al. Incremental value of contrast echocardiography in the diagnosis of left ventricular noncompaction. Front. Med. 2016, 10, 499–506. [Google Scholar]
- Chebrolu, L.H.; Mehta, A.M.; Nanda, N.C. Noncompaction cardiomyopathy: The role of advanced multimodality imaging techniques in diagnosis and assessment. Echocardiography 2017, 34, 279–289. [Google Scholar] [CrossRef]
- Daimon, Y.; Watanabe, S.; Takeda, S.; Hijikata, Y.; Komuro, I. Two-layered appearance of noncompaction of the ventricular myocardium on magnetic resonance imaging. Circ. J. 2002, 66, 619–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, D.; Sharma, N.; Imundo, J.R. Pediatric noncompaction patients with high spatial QRS-T angles are at increased risk for ventricular tachycardia. Ann. Noninvasive Electrocardiol. 2019, 24, e12588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziółkowska, L.; Petryka, J.; Boruc, A.; Kawalec, W. Comparison of echocardiography with tissue Doppler imaging and magnetic resonance imaging with delayed enhancement in the assessment of children with hypertrophic cardiomyopathy. Arch. Med. Sci. 2017, 13, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Stankala, S.; Zielińska, D.; Mrowiec, J.; Juszczyk, Z. Zastosowanie ergospirometrii w chorobach układu krazenia [Ergospirometry use in cardiovascular diseases]. Kardiol. Pol. 2008, 66, 1135–1139. [Google Scholar] [PubMed]
- Adwani, S.S.; Whitehead, B.F.; Rees, P.G.; Morris, A.; Turnball, D.M.; Elliott, M.J.; de Level, M.R. Heart transplantation for Barth syndrome. Pediatr. Cardiol. 1997, 18, 143–145. [Google Scholar] [CrossRef]

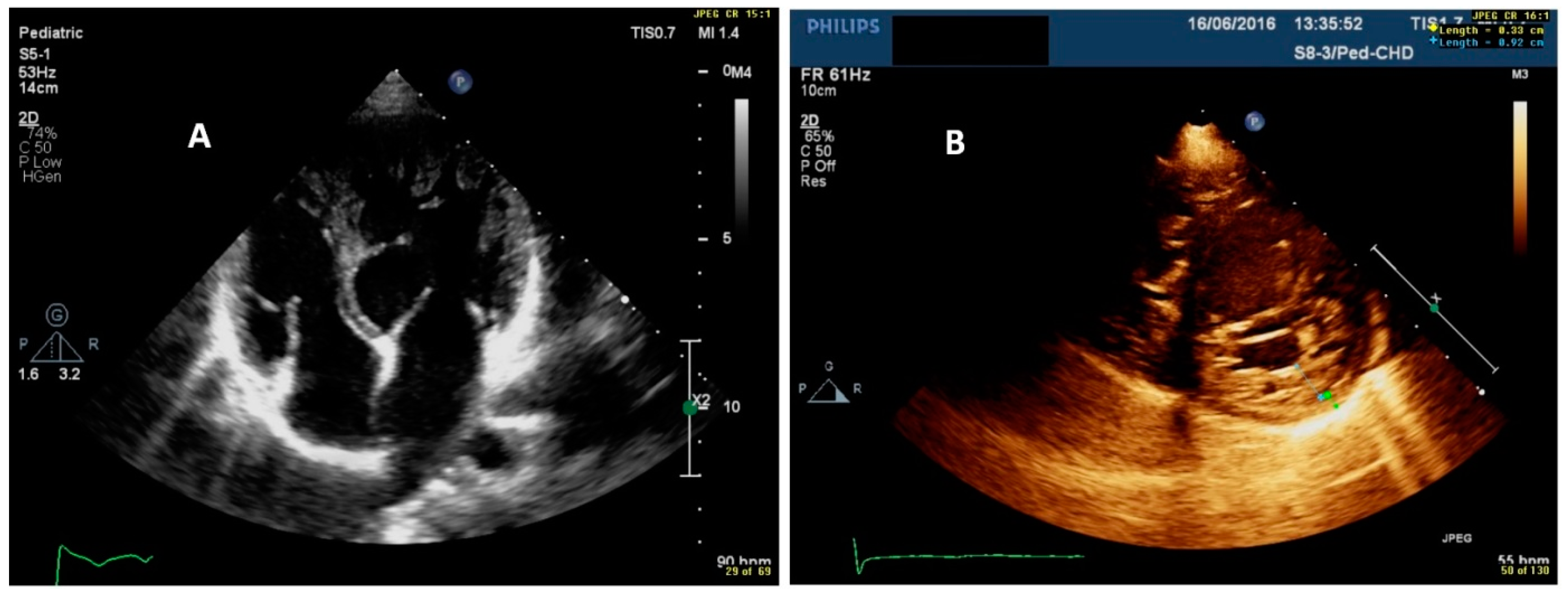
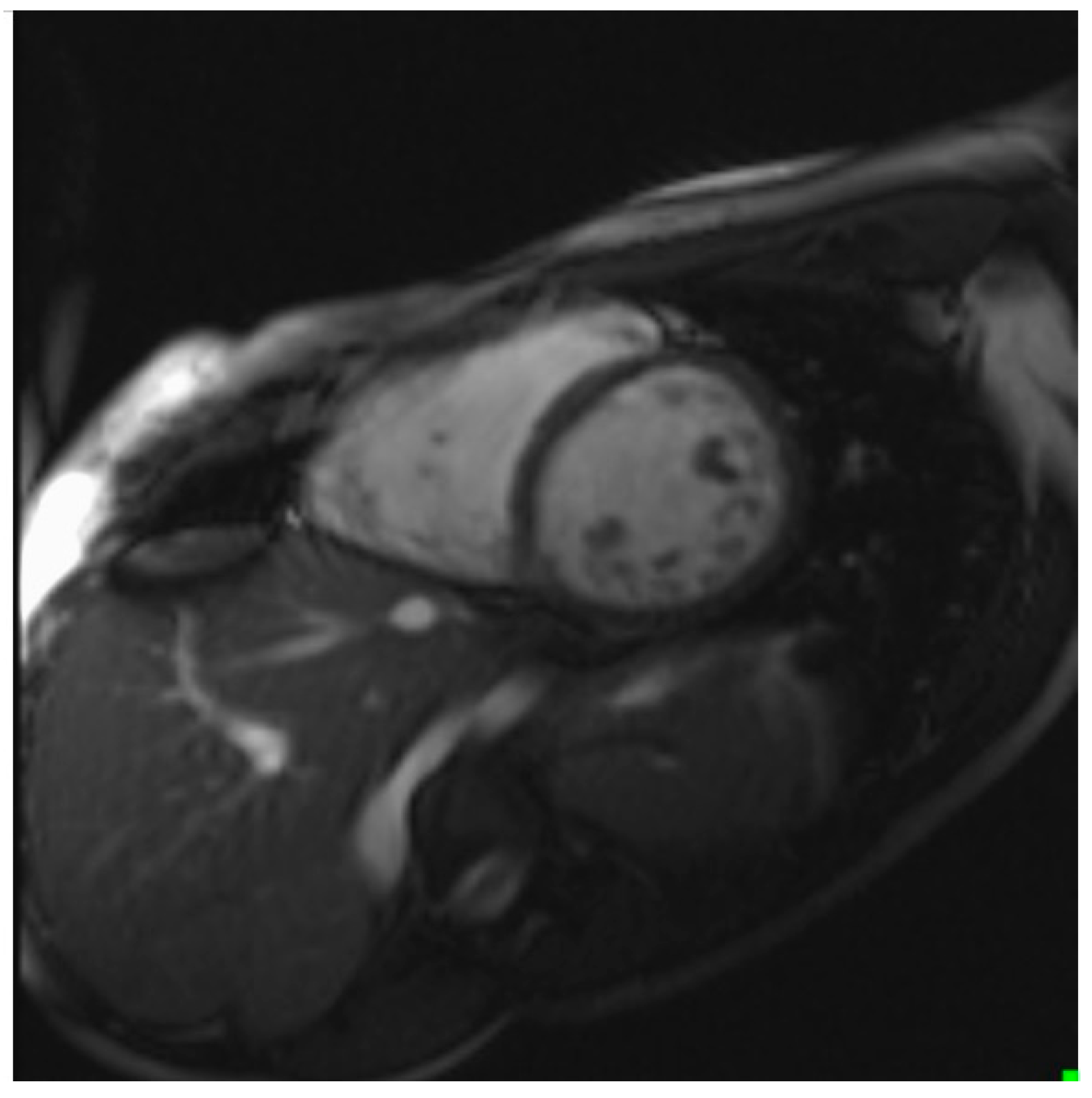
| Clinical Parameters | Total n = 32 |
|---|---|
| Age, cohort median, yrs (IQR) | 11.5 (6–15) |
| Age ≤1 yr, n (%) | 1 (3%) |
| Age >1 and ≤10 yrs, n (%) | 10 (31%) |
| Age >10 and <18 yrs, n (%) | 21 (66%) |
| Positive family history, n (%) | 17 (53%) |
| LVNC, n (%) | 10 (31%) |
| HCM, n (%) | 2 (6%) |
| DCM, n (%) | 2 (6%) |
| CHD, n (%) | 1 (3%) |
| Bradycardia, n (%) | 4 (13%) |
| WPW, n (%) | 2 (6%) |
| AVNRT, n (%) | 1 (3%) |
| SCD, n (%) | 3 (9%) |
| Clinical symptoms, n (%) | 11 (34%) |
| Chest pain, n (%) | 3 (9%) |
| Palpitations, n (%) | 2 (6%) |
| Syncope, n (%) | 3 (9%) |
| Pre-syncope, n (%) | 3 (9%) |
| Thromboembolic episodes, n (%) | 0 (0%) |
| NYHA functional class, n (%) | |
| I | 7 (22%) |
| II | 24 (77%) |
| III | 0 (0%) |
| IV | 1 (3%) |
| Genetic syndrome, n (%) | 2 (6%) |
| Increased NTproBNP value, n (%) | 5 (16%) |
| NTproBNP value, median (IQR) | 349.40–27,057.00, 66.24 (25.71–105.35) |
| Chest X-ray | |
| CTR value, median (IQR) | 0.55–0.69, 0.56 (0.55–0.64) |
| Pulmonary congestion | 1 (3%) |
| ECG, n (%) | 32 (100%) |
| ECG changes, n (%) | 18 (56%) |
| Sinus bradycardia, n (%) | 7 (22%) |
| Nodal rhythm, n (%) | 2 (6%) |
| WPW, n (%) | 1 (3%) |
| RBBB, n (%) | 1 (3%) |
| LBBB, n (%) | 0 (0%) |
| LV overload, n (%) | 4 (13%) |
| ST-T changes, n (%) | 12 (38%) |
| Permanent cardiac pacing, n (%) | 1 (3%) |
| Echocardiography, n (%) | 32 (100%) |
| NC/C = 2.06–5.14, n (%) | 30 (94%) |
| NC/C = 1.9, n (%) | 1 (3%) |
| NC/C = 1.8, n (%) | 1 (3%) |
| Reduced LVEF acc. Simpson formula | 10 (31%) |
| LVEF 51–55%, n (%) | 7 (22%) |
| LVEF 46–50, n (%) | 2 (6%) |
| LVEF 40–45, n (%) | 1 (3%) |
| CMR, n (%) | 29 (91%) |
| NC/C = 2.3–6.24, n (%) | 24 (82%) |
| NC/C = 1.2–2.1, n (%) | 5 (17%) |
| Pharmacological treatment, n (%) | 22 (69%) |
| Beta-blockers, n (%) | 9 (28%) |
| ACE-I, n (%) | 19 (59%) |
| Furosemide, n (%) | 1 (3%) |
| Spironolaktone, n (%) | 16 (50%) |
| Acetylsalicylic acid, n (%) | 3 (9%) |
| Acenokumarol, n (%) | 1 (3%) |
| Salbutamol, n (%) | 4 (13%) |
| Other procedures, n (%) | 6 (19%) |
| Electrophysiological study, n (%) | 1 (3%) |
| RF ablation, n (%) | 1 (3%) |
| Pacemaker, n (%) | 2 (6%) |
| LVAD, n (%) | 1 (3%) |
| W/L for HTx, n (%) | 1 (3%) |
| Death, n (%) | 1 (3%) |
| Heart Rhythm and Conduction Disturbances | Number of Patients, n = 32 (100%) |
|---|---|
| Supraventricular premature beats, n (%) | 5 (16%) |
| Ventricular premature beats, n (%) | 8 (25%) |
| Nodal rhythm, n (%) | 3 (9%) |
| Sinus bradycardia, n (%) | 7 (22%) |
| Sick sinus syndrome, n (%) | 2 (6%) |
| WPW syndrome, n (%) | 2 (6%) |
| Second degree paroxysmal block a-v, n (%) | 1 (3%) |
| Third degree paroxysmal block a-v, n (%) | 4 (13%) |
| VT, n (%) | 3 (9%) |
| Supraventricular tachycardia, n (%) | 1 (3%) |
| Atrioventricular tachycardia, n (%) | 1 (3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkowska, A.; Mirecka-Rola, A.; Piekutowska-Abramczuk, D.; Ciara, E.; Mazurkiewicz, Ł.; Bieganowska, K.; Ziółkowska, L. Spectrum of Clinical Features and Genetic Profile of Left Ventricular Noncompaction Cardiomyopathy in Children. Cardiogenetics 2021, 11, 191-203. https://doi.org/10.3390/cardiogenetics11040020
Paszkowska A, Mirecka-Rola A, Piekutowska-Abramczuk D, Ciara E, Mazurkiewicz Ł, Bieganowska K, Ziółkowska L. Spectrum of Clinical Features and Genetic Profile of Left Ventricular Noncompaction Cardiomyopathy in Children. Cardiogenetics. 2021; 11(4):191-203. https://doi.org/10.3390/cardiogenetics11040020
Chicago/Turabian StylePaszkowska, Agata, Alicja Mirecka-Rola, Dorota Piekutowska-Abramczuk, Elżbieta Ciara, Łukasz Mazurkiewicz, Katarzyna Bieganowska, and Lidia Ziółkowska. 2021. "Spectrum of Clinical Features and Genetic Profile of Left Ventricular Noncompaction Cardiomyopathy in Children" Cardiogenetics 11, no. 4: 191-203. https://doi.org/10.3390/cardiogenetics11040020
APA StylePaszkowska, A., Mirecka-Rola, A., Piekutowska-Abramczuk, D., Ciara, E., Mazurkiewicz, Ł., Bieganowska, K., & Ziółkowska, L. (2021). Spectrum of Clinical Features and Genetic Profile of Left Ventricular Noncompaction Cardiomyopathy in Children. Cardiogenetics, 11(4), 191-203. https://doi.org/10.3390/cardiogenetics11040020






