Abstract
Watermelon grafting is practiced in order to improve tolerance to poor environments such as low temperature. This study was conducted to investigate the effect of low-temperature tolerant bottle gourd (Lagenaria siceraria) rootstocks on the growth and fruit quality of watermelon in semi-forcing and retarding culture where plants were exposed to low or high temperature. Five bottle gourd accessions (FR79, IT207112, BG702, BG703, and FRD22) with low temperature tolerance were evaluated as rootstock for the watermelon scion ‘Sambokkul’. Non-grafted watermelon and watermelon grafted onto commercial rootstock ‘Shintozwa’ (Cucurbita maxima D. × C. moschata D.) or ‘Bullojangsaeng’ (L. siceraria) were used as controls. Watermelons were cultivated in spring (April to June, semi-forcing culture) and autumn (August to October, retarding culture). The responses to low-temperature, growth, yield, and fruit quality differed depending on the rootstocks and growing season. In semi-forcing culture, the monthly averages of daily and minimum temperature in April were, respectively, 13.4 and 1.5 °C. Although the low temperature of the early growth stage suppressed the initial growth of watermelons, grafting mitigated the low-temperature stress. The fruit quality of non-grafted watermelons was greater, but the fruit mass was the lowest (4.8 kg). Grafting onto ‘Shintozwa’ increased the fruit weight (7.0 kg) but reduced the fruit quality. Grafting onto bottle gourd rootstocks had high affinity, good root growth, tolerance to low temperature, and little effect on fruit quality. In retarding culture, the temperature conditions in early and late growth were very high and low, respectively. The growth and fruit quality of grafted watermelons were not superior to those of non-grafted watermelons. Accordingly, these results suggest that watermelon grafting onto the bottle gourd rootstocks may increase the low-temperature tolerance, especially in the early growth stage, and the marketable yield without a reduction in fruit quality. The most promising accession for this purpose was found to be ‘FR79’.
1. Introduction
Watermelon (Citullus lanatus) is one of the major cultivated vegetables. The minimum and maximum temperatures for growth are 35 and 18 °C, respectively. The optimum air and soil temperature for watermelon growth are in the range of 22 to 30 °C and 20 to 35 °C, respectively.
In Korea, watermelon is cultivated in not only open fields in the summer, but also in a greenhouse all year round. The purposes of watermelon production in a greenhouse are stable production, quality improvement, and harvest time control such as early harvest. The cropping pattern of watermelon in a greenhouse is divided into forcing (January through March or April), semi-forcing (March through May or June), and retarding culture (August through October or November) [1]. Watermelon native to the tropical and sub-tropical areas is particularly sensitive to cold stress [2]. When the temperature drops below 13 °C, plants do not flower. Young plants stop growing at temperatures below 10 °C and even die at temperatures below 1 °C [3]. Cold stress can cause stunted plant growth, wilting, necrotic lesions on leaves, and increased susceptibility to diseases and pathogens and can result in yield reduction [4].
Vegetable grafting has been widely practiced around the world for various purposes, including the enhancement of tolerance to abiotic and biotic stresses [5]. Grafting is mostly practiced to produce fruit and vegetables of Cucurbitaceae and Solanaceae [6,7]. The main purpose of watermelon grafting is to improve tolerance to abiotic and biotic stress such as low temperature [2,8,9], high salinity [10,11,12], and Fusarium wilt [13]. Most watermelon growers in Korea use grafted seedlings [14,15].
Cucurbita spp., squash interspecific hybrids, and bottle gourd (Lagenaria siceraria) are usually used as rootstocks for watermelon [16]. Even though Cucurbita spp. has many advantages as rootstock, such as a vigorous root system and high tolerance to abiotic and biotic stress, it often lowers fruit quality in terms of texture, flavor, and taste. On the other hand, bottle gourd is preferred for watermelon grafting on account of its resistance to high affinity, Fusarium wilt, and highly stable growth after grafting.
Watermelon grafted onto bottle gourd rootstocks increased fruit yield, flesh firmness, total sugar content, lycopene, and carotenoid in fruits compared to non-grafted plants [17]. Bottle gourd rootstocks also improve the tolerance to cold, drought, and salt stress by enhancing antioxidant activity and higher expression of enzymes related to the Calvin cycle [2]. Recently, the molecular mechanism of improvement of cold tolerance after grafting onto bottle gourd rootstocks have been studied [18].
Watermelon fruits are usually harvested three or four months after transplanting. Watermelons are exposed to low-temperature conditions at different growth stages depending on the cropping pattern. In semi-forcing culture, plants in the early growth stage are exposed to low temperatures from March to early April. However, in retarding culture, plants may be exposed to low temperatures at the maturity stage, nearing harvest in late October to November.
This study evaluated the effects of bottle gourd rootstocks with low-temperature tolerance on the growth, yield, and fruit quality of grafted watermelons under greenhouse conditions in semi-forcing and retarding culture.
2. Materials and Methods
2.1. Plant Materials
Five bottle gourd accessions (FR79, IT207112, BG702, BG703, FRD22) with low-temperature tolerance were evaluated as rootstocks (Table 1). Low-temperature tolerance was evaluated based on early seedling growth in a growth chamber set at 15/8 °C light/dark period) for three weeks and early growth in an unheated greenhouse during the low-temperature period from November to February of the following year [19]. These rootstocks were also screened for their resistance to both Fusarium wilt and Monosporascus root rot and vine decline [20].

Table 1.
The characteristics of bottle gourd accessions used as rootstocks.
The watermelon ‘Sambokkul’ (FarmHannong Co., Ltd., Seoul, Korea) was used as a scion. Non-grafted watermelon and watermelon grafted onto commercial rootstock ‘Shintozwa’ (Cucurbita maxima D. × C. moschata D.) or ‘Bullojangsaeng’ (L. siceraria, Syngenta Korea Co., Ltd., Seoul, Korea) were used as controls.
Rootstocks and scions were sown into 50- (W 280 × L 540 × H 46 mm, 73 cc/cell, Bumnong Co., Ltd., Jeongeup, Korea) and 128-cell plugtrays (W 280 × L 540 × H 41 mm, 17.5 cc/cell, Bumnong Co., Ltd., Jeongeup, Korea) filled with commercial growing media (Biomedia, FarmHannong Co., Ltd., Seoul, Korea), respectively. In semi-forcing culture, scions were sown four days after sowing of rootstocks. In retarding culture, scions and rootstock were sown on the same day. After sowing, plugtrays were over-irrigated and placed in a germination room set at 28 °C for two days to promote germination. Then, they were moved to a greenhouse and grown on the bench. Non-grafted watermelons were sown seven days after sowing scions.
Eight days after sowing scions, non-grafted watermelons were sown, and grafting was conducted using one cotyledon splice-grafting method described by Lee et al. [5]. Grafted watermelon seedlings were placed on a multi-stage bed (W 500 × L 2000 × H 1500 mm) with LED lights (Red:Blue = 2:1, PPF 80 μmol∙m−2∙s−1) in a healing room. They were healed and acclimatized for seven days. After six-day healing and acclimatization in a healing room, they were grown in a greenhouse before transplanting.
2.2. Cultivation of Grafted Watermelons in a Greenhouse
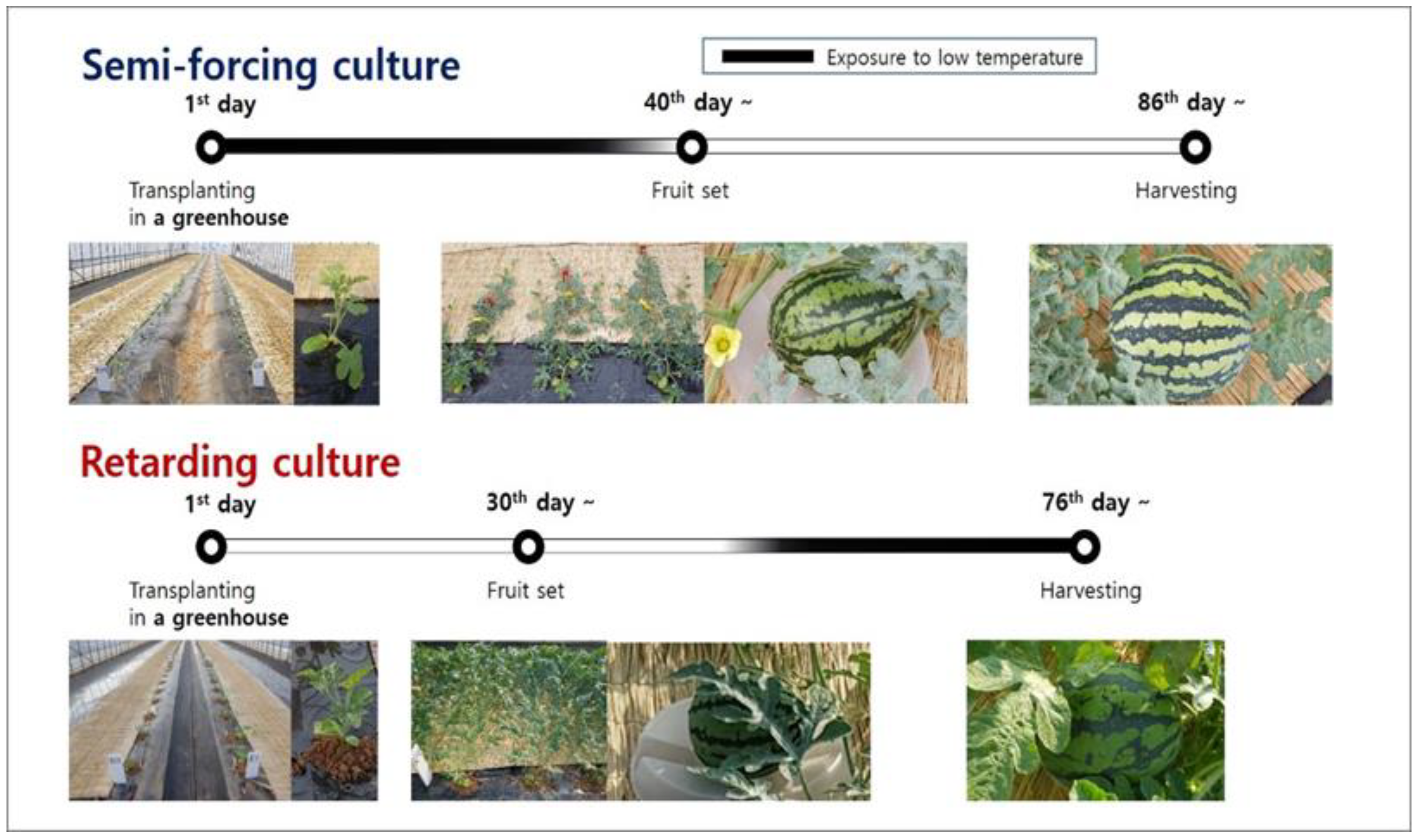
The research was carried out in the greenhouse of the National Institute of Horticultural and Herbal Science located in Wanju (35.8 °N, 127.1 °W, altitude 56 m), Jeollabuk-do, Korea. The annual mean temperature was 12.5 °C and the annual precipitation was 1326.8 mm. Watermelons were cultivated in spring (April to June, semi-forcing culture) and autumn (August to October, retarding culture) in order to evaluate the effects of bottle gourd rootstocks with low-temperature tolerance on the growth, yield, and fruit quality of grafted watermelons under greenhouse conditions without heating (Figure 1).
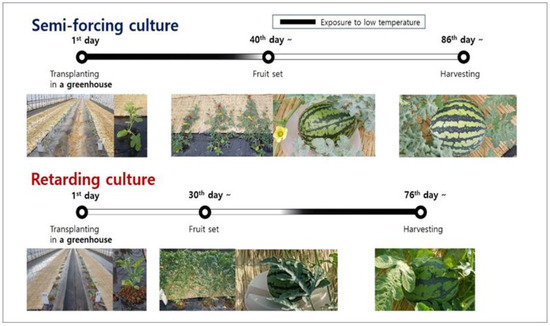
Figure 1.
Cultivation schedule in semi-forcing (April to June) and retarding culture (August to October).
In semi-forcing culture, 36-day-old grafted and 29-day-old non-grafted watermelon seedlings were transplanted in a greenhouse (W 7 × L 40 m, 280 m2) on 31 March 2020. In retarding culture, 26-day-old grafted and 19-day-old non-grafted watermelon seedlings were transplanted on 20 August 2019. Two rows (2 × 36 m) were made and each row was mulched with black plastic film before planting. Three rows of drip irrigation tapes were placed under the plastic mulching film in each row.
The distance between plants within a row was 0.5 m and the plant density was 1.94 m2. In both growing seasons, the experiment was arranged in a randomized complete block design with two blocks. Each block consisted of eight treatments (seven rootstocks, including two commercial rootstocks and non-grafted watermelon). The number of replicates for each treatment in the block was six plants in semi-forcing culture and ten plants in retarding culture, except rootstock with low emergence percentage or graft-take.
Irrigation and fertilizer application were carried out using standard procedures for watermelon cultivation [1]. After the fruit set, 200 g and 400 g of ‘Hanbang’ for vegetable cultivation (N-P-K-Ca-Mg = 15-3-6-8 me∙L−1, Coseal Co., Ltd., Seoul, Korea) were applied twice at weekly intervals.
Rice straw was laid on the plastic mulching film and watermelon vines were trained and fixed with pins. Watermelons were pruned to a primary vine and two secondary vines. Manual cross-pollination was conducted for the fruit set 37 days after transplanting in retarding culture and 17 days after transplanting in semi-forcing culture, respectively. The second and third female flowers on the primary vine were pollinated and one fruit per plant was set.
To measure photosynthetic photon flux (PPF), air and root temperature, and relative humidity data inside the greenhouse, the PAR (Photosynthetically active radiation) sensor (LightScout Quantum Light sensor, Range/Resolution 0∼2500 μmol·m−2·s−1, Accuracy ±5%, Spectrum Technologies, IncAurora, IL, USA) and the data logger embedded with the temperature–humidity sensor (WatchDog 1000 Series Micro Stations, Temperature range −40∼85 °C, Accuracy: ±0.6 °C at −20 to 50 °C, else ±1.2 °C, RH range 0∼100%, Accuracy: ±3% at 25 °C and 10∼90%, else ±5%, Spectrum Technologies, Inc., Aurora, IL, USA) were installed at the center of the house near plants (0.3 m high above the floor). The data were collected per 30 min during the cultivation period. Growing degree days (GDD) were calculated using the equation below [21,22].
where TMAX is the daily maximum air temperature, TMIN is the daily minimum air temperature, and TBASE is the temperature below which the growth does not progress. TBASE of watermelon was calculated as 10 °C.
GDD = [(TMAX + TMIN)/2] − TBASE
2.3. Measurements and Sensory Evaluation
At eight days after sowing and before grafting, hypocotyl length, cotyledon length, and the width of five rootstock and scion plants were measured. Before transplanting, the growth parameters such as shoot length, number of true leaves, leaf area (by LI-3100 area meter, Li-Cor Bioscience, Lincoln, NE, USA), SPAD value (by SPAD 502 Plus Chlorophyll meter, Konica Minolta, Tokyo, Japan), and fresh mass of three grafted and non-grafted watermelon transplants were measured. Dry mass was measured after drying the samples at 80 °C for at least three days using the dry oven (DS-80-3, Dasol Scientific Co., Ltd., Hwaseong-si, Gyeonggi-do, Korea). The length of the primary vine was measured to evaluate the early growth at 27 days after transplanting. The stem diameter of the graft junction usually related to graft compatibility was measured before harvesting [23].
Mature fruits were harvested on 25 June (86 days after transplanting in the semi-forcing culture) and 4 November (76 days after transplanting in the retarding culture) according to the date of pollination. Six mature fruits per treatment were harvested, except for the treatments in which the fruit could not be harvested due to the failure of the fruit set. The length, width, and weight of the fruit were measured. Three fruits of each treatment were longitudinally sliced into two, and the rind thickness at the middle of the slice was measured. From the center of the slice, a piece of fruit flesh was cut off and squeezed to extract the juice for measuring total soluble solids (TSS) using a refractometer (ABBE refractometer, PAL-1, Atago, Tokyo, Japan).
Referring to the sensory evaluation method of Lee et al., the sensory evaluation of watermelon fruits was conducted by 59 and 30 evaluators with experience in evaluation in semi-forcing and retarding cultures, respectively [24]. The sample evaluation was conducted using a 10-point hedonic sensory evaluation scale anchored in the middle and extreme regions of the scale (1 = disliked extremely, 5 = neither liked nor disliked, 10 = liked extremely) for the following attributes: sweetness, texture, flavor, and overall acceptance.
2.4. Statistical Analysis
All data were statistically analyzed by analysis of variance using SAS version 9.4 175 (SAS Institute Inc., Cary, NC, USA) software. The data were subjected to one and two-way analyses of variance. Levene’s test was used for homogeneity of variances. The Kruskal–Wallis test was used for the analysis of sensory evaluation. Duncan’s multiple range test was performed at p ≤ 0.05 on each of the variables measured.
3. Results
3.1. Environment in Semi-Forcing and Retarding Culture
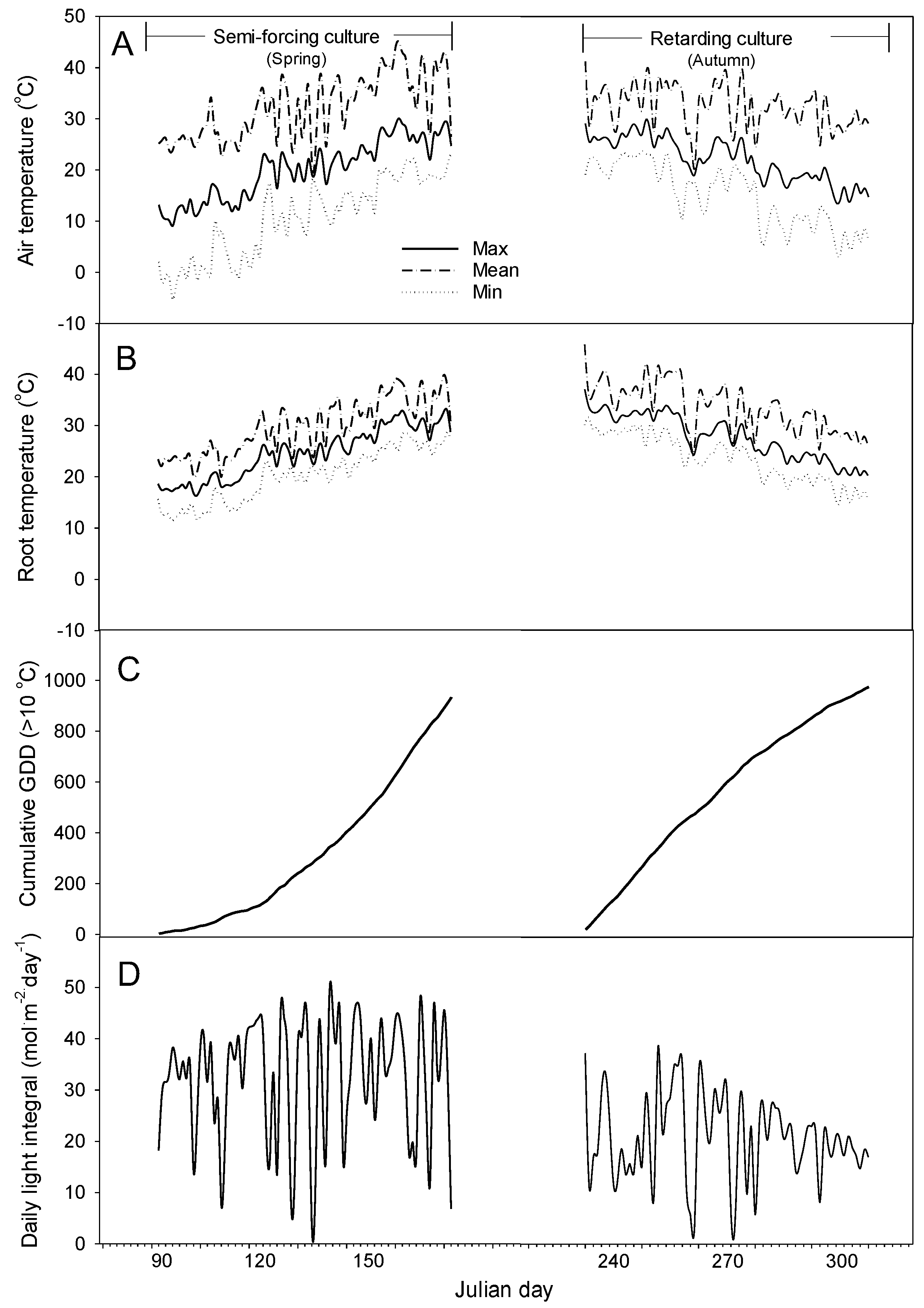
In semi-forcing culture, the average daily minimum, mean, and maximum air temperatures were 10.3, 19.8, and 32.0 °C, respectively (Figure 2). The monthly averages of daily minimum and mean temperature in April of the early growth stage were, respectively, 1.5 and 13.4 °C. As time went by, the temperature rose and the monthly average minimum temperature in May and June of the middle and late growth stages was 12.6 and 19.1 °C, respectively. The average daily minimum, mean, and maximum root temperatures in May and June were 21.2, 25.4, and 29.7 °C, respectively. The root temperature was stable, with less variation than the air temperature. The average daily light integral (DLI) during the cultivation was 32 mol∙m−2∙day−1.
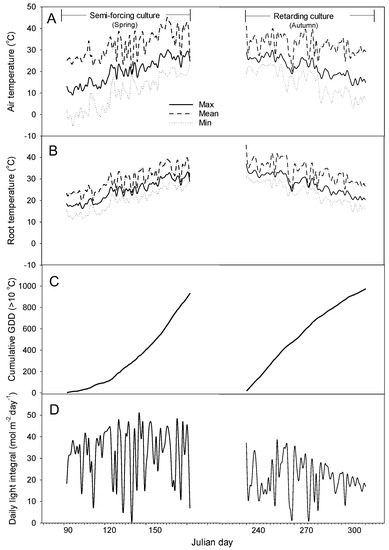
Figure 2.
Changes in daily minimum (min), mean, and minimum (max) air (A) and root temperature (B), cumulative GDD (C), and daily light integral (D) during semi-forcing (March to June) and retarding culture (August to October).
In retarding culture, the average daily minimum, mean, and maximum air temperatures were 16.9, 23.6, and 33.3 ℃, respectively. The monthly averages of daily minimum and mean temperature in August of the early growth stage were, respectively, 20.8 and 26.6 ℃. As time went by, the temperature dropped and the monthly average minimum temperature in September and October of the middle and late growth stages was 19.0 and 11.0℃, respectively. The average daily minimum, mean, and maximum root temperatures were 25.6, 29.8, and 33.1 ℃, respectively.
The cumulative GDD from transplanting to harvesting in semi-forcing and retarding culture were 934 and 975, respectively. In semi-forcing culture, the cumulative GDD increased slowly in the early period but rapidly increased in the later period. On the other hand, the cumulative GDD in retarding culture increased rapidly in the early stage and gradually increased in the later stage. The average DLI during the cultivation was 22 mol∙m−2∙day−1.
3.2. The Growth of Bottle Gourd Rootstock Accessions and Grafted Watermelon Transplants
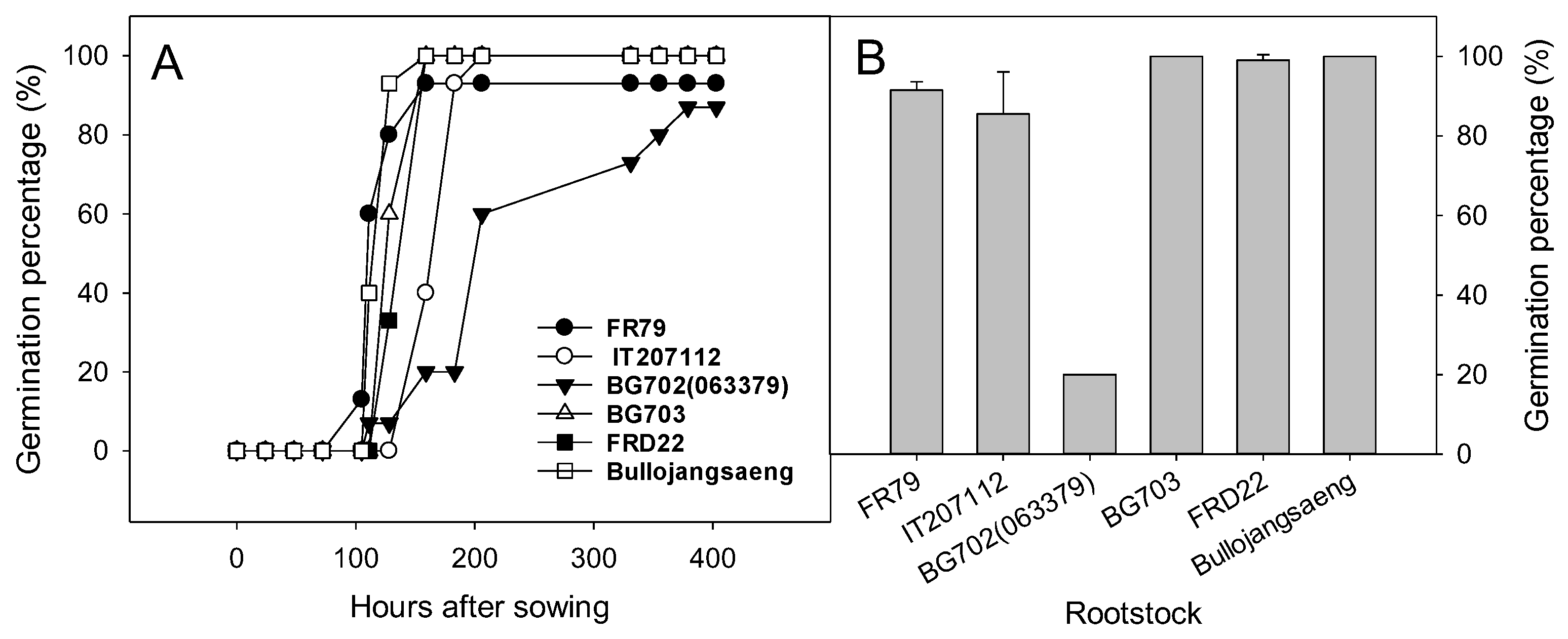
Bottle gourd rootstock accessions except ‘BG702’ germinated between the fourth and sixth days after sowing (Figure 3). The germination of ‘BG702′ was slower than that of other rootstock and the germination percentage of ‘BG702’ was only 20% on the seventh day after transplanting (Figure 3 and Figure 4). Thus, ‘BG702’ was excluded from the greenhouse evaluation.
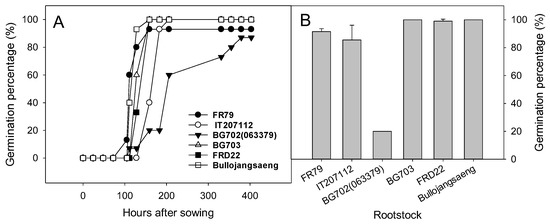
Figure 3.
Germination percentage of Lagenaria rootstock accessions after sowing (A) and at seven days after sowing (B).

Figure 4.
The seeds and seedlings of Lagenaria accessions at seven days after sowing.
The hypocotyl length of bottle gourd rootstock accessions at 11 days after sowing in semi-forcing culture was around 3 cm (Table 2). The size of the cotyledon was 4.3–5.9 cm in length, 2.3–3.2 cm in width, and 17.1–29.4 cm2 in area, smaller than that of the commercial bottle gourd rootstock ‘Bullojangsaeng’ and pumpkin rootstock ‘Shintozwa’.

Table 2.
The growth of bottle gourd rootstock accessions at 11 days after sowing in semi-forcing culture.
The fresh mass of bottle gourd rootstock accessions was 1048–1744 mg in the shoot, 169–346 mg in the root and the dry mass was 1048–1744 mg in the shoot, 169–346 mg in the root. Among bottle gourd rootstock accessions, the dry mass of ‘FRD22’ was 130.7 mg and greatest. That of ‘IT207112’ was 90.0 mg, which was the smallest.
The watermelon transplants before transplanting had 4–6 true leaves irrespective of cropping season (Table 3). The shoot length in the retarding culture was longer than that in the semi-forcing culture. The non-grafted watermelon seedlings in the retarding culture were 26.1 cm and 2.4 times longer than in the semi-forcing culture. The grafted watermelon transplants had a thicker stem diameter and greater root mass than non-grafted transplants. The watermelon seedlings grafted onto commercial bottle gourd rootstock ‘Bullojangsaeng’ had the greatest growth, regardless of cropping season. Among bottle gourd rootstock accessions, ‘FR703’ exhibited higher growth in both seasons. The non-grafted watermelon seedlings in the semi-forcing culture and ‘FRD22’ in retarding culture showed the lowest growth.

Table 3.
The growth of watermelon transplants influenced by bottle gourd rootstock accessions before transplanting.
3.3. The Growth of Watermelons in the Greenhouse
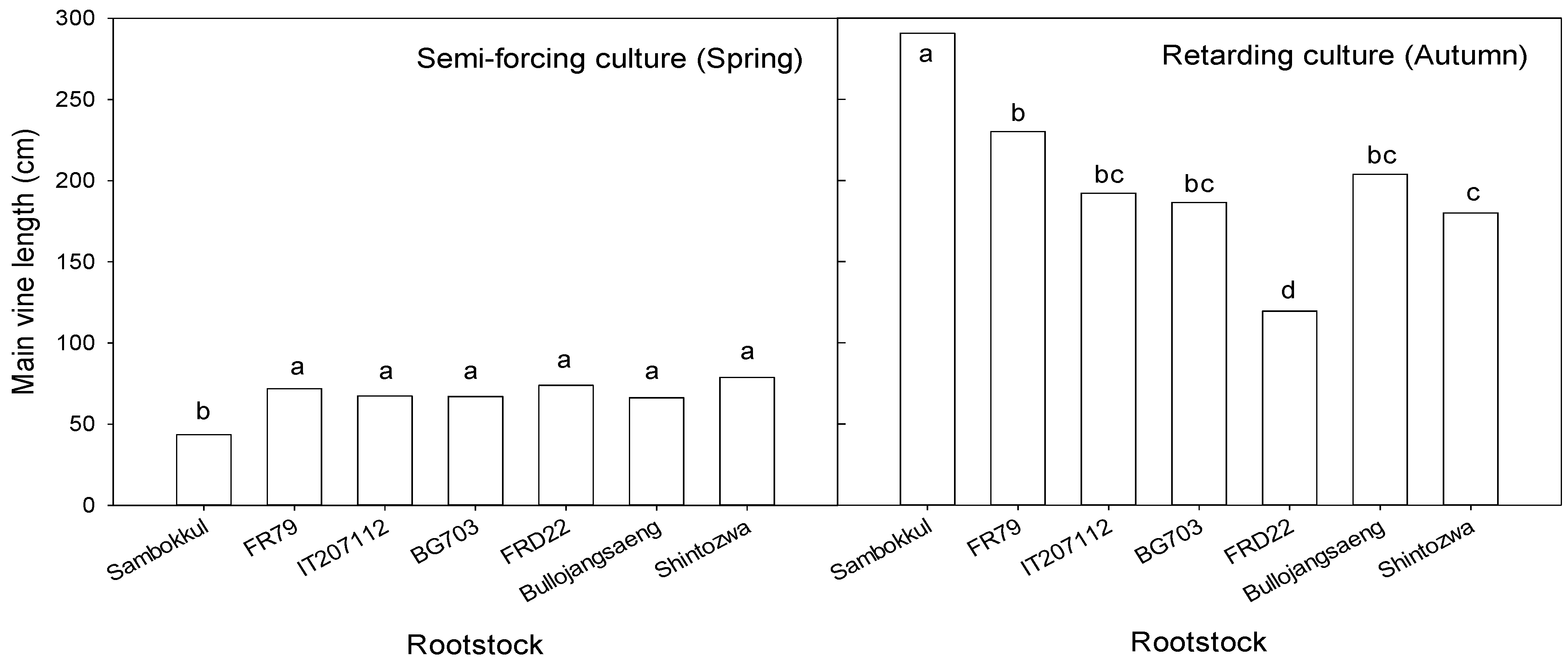
In semi-forcing culture, watermelons were grown under low-temperature conditions in the early stages of cultivation in spring (April to June). The low temperature in April delayed the early growth. The mean main vine length of grafted watermelons at 27 days after transplanting was 70 cm and about 2 times that of non-grafted ones in semi-forcing culture (Figure 5).
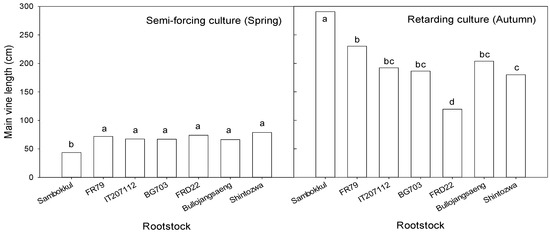
Figure 5.
Main vine length influenced by bottle gourd rootstocks at 27 days after transplanting in semi-forcing and retarding culture. Different letters indicated a significant difference within the column at the p ≤ 0.05, according to Duncan’s multiple range test.
Conversely, the watermelon plants in retarding culture were exposed to high temperatures at the early growth stage and low temperatures at the late growth stage. High temperature in August, an average of 13 ℃ higher than in April, stimulated the early growth. The main vine length of grafted watermelons was 120–230 cm and was shorter than the non-grafted one in retarding culture. Among bottle gourd rootstock accessions, ‘FR79’ was the longest, and ‘FRD22’ was the shortest. The watermelon plants grafted onto ‘FRD22’ exhibited delayed growth, and some plants died during the growth.
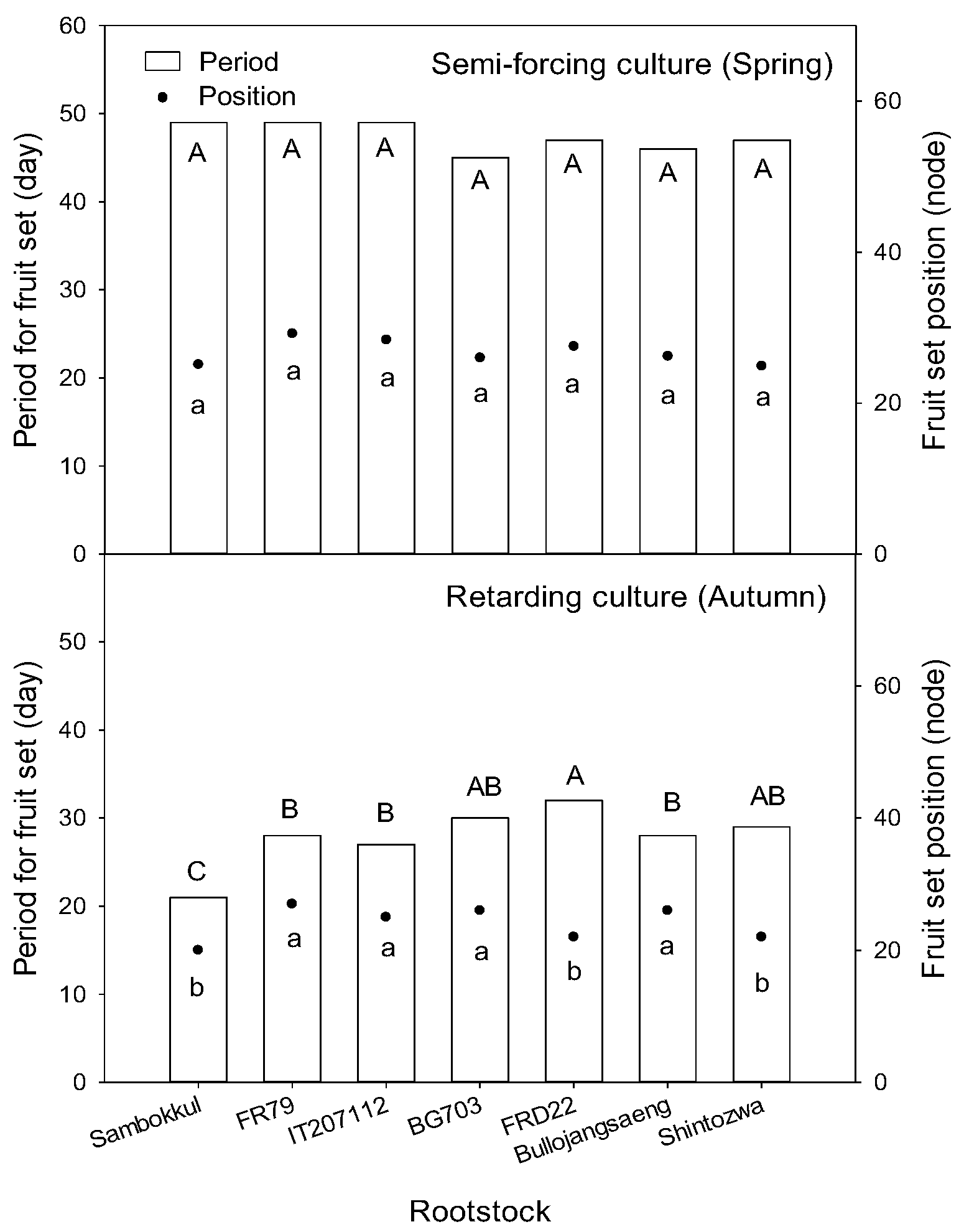
In semi-forcing culture, the fruit was set on the main vines between the 25th and 29th node from 45 to 49 days after transplanting. There was no significant difference between rootstock (Figure 6). However, in retarding culture, the non-grafted watermelon with early vigorous growth set the fruit on the 21st day after transplanting. This was about ten days earlier than the grafted watermelons. Among rootstocks, ‘FR79’, ‘IT207112’, and ‘Bullojangseang’ had earlier fruit sets.
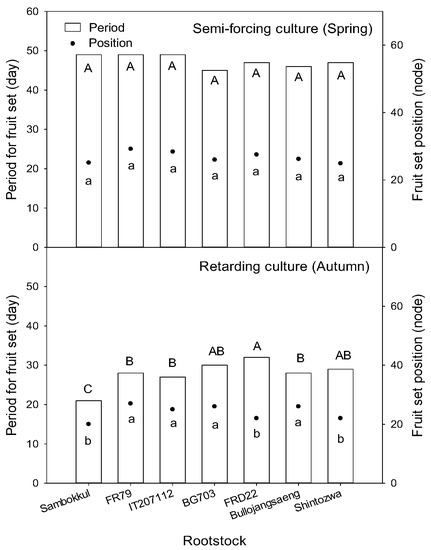
Figure 6.
The fruit set position and period for fruit set after transplanting influenced by bottle gourd rootstocks. Different letters indicated a significant difference within the column at the p ≤ 0.05 according to Duncan’s multiple range test.
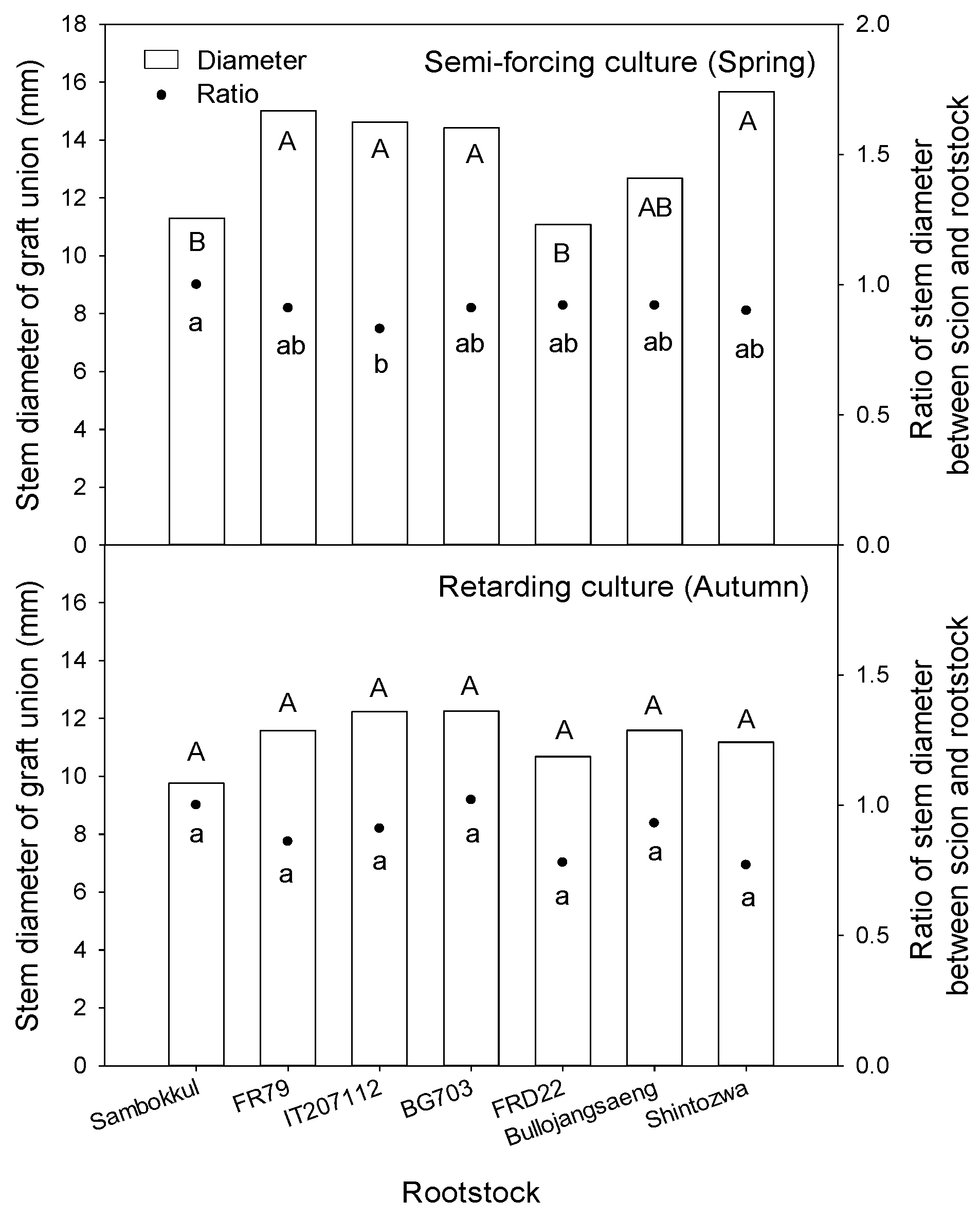
The stem diameter of graft union in semi-forcing culture ranged from 11 mm to 16 mm (Figure 7). The watermelon plants grafted onto ‘Shintozwa’, ‘FR79’, ‘IT207112’, ‘BG703’ had thicker stems than non-grafted watermelon or watermelon grafted onto ‘FRD22’. The ratio of stem diameter between scion and rootstock (scion stem diameter/rootstock stem diameter) was over 0.90 in all bottle gourd rootstock accessions except ‘IT207112’ (0.83). In retarding culture, the stem diameter of graft union and the ratio of stem diameter between scion and rootstock ranged from 9 mm to 12 mm and from 0.77 to 1.02, respectively. There was no significant difference between rootstocks concerning the stem diameter and the ratio of stem diameter between scion and rootstock.
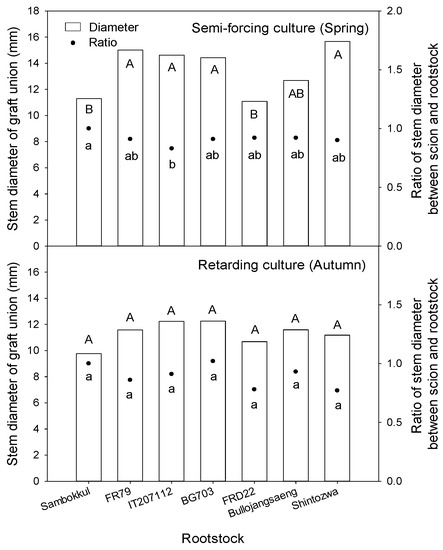
Figure 7.
The stem diameter of graft union and ratio of stem diameter between scion and rootstock influenced by bottle gourd rootstocks before harvesting. Different letters indicated a significant difference within the column at the p ≤ 0.05 according to Duncan’s multiple range test.
After harvesting, the grafted watermelons exhibited thicker and greater root growth than non-grafted ones (Figure 8). ‘Shintozwa’ produced the greatest quantity of root and well-developed fine root.

Figure 8.
The root of bottle gourd rootstocks after planting in semi-forcing and retarding culture.
In the semi-forcing culture, the average fruit mass was 4.8–7.0 kg (Table 4). The watermelon fruits grafted onto ‘Shintozwa’ were the heaviest, and those of the non-grafted watermelon and those grafted onto ‘IT207112’ were the lowest. TSS of ‘Sambokkul’ was the highest at 15.3 °Brix, followed by the watermelon grafted onto ‘Bullojangsaeng’ at 12.6 °Brix. The fruits grafted onto the other rootstocks were as low as 11 °Brix. In retarding culture, the average fruit mass was 3.4–4.9 kg, which was smaller than those in the semi-forcing culture. The TSS of fruits was in the range of 13–14 °Brix. Among them, the watermelon grafted onto the ‘BG703’ was heavy and had a high TSS of 14.9 °Brix.

Table 4.
Fruit mass, fruit length and width, flesh thickness, and total soluble solids (TSS) of watermelon influenced by bottle gourd rootstocks.
In the sensory evaluation, the non-grafted watermelon fruit had the best evaluation in taste, texture, aroma, and overall taste, irrespective of the growing season (Table 5). The evaluation of the fruit grafted onto ‘FR79’ was close to the non-grafted fruit of ‘Sambokkul’. In semi-forcing culture, the fruit grafted onto the ‘Shintozwa’ had the lowest evaluation value. In retarding culture, the evaluation value of the fruit grafted onto the ‘FRD22’ with bad growth and plant death was the lowest, and the fruit grafted to the ‘Shintozwa’ was the next lowest.

Table 5.
The sensory characteristics of watermelon influenced by bottle gourd rootstocks.
4. Discussion
Watermelon is a thermophilic crop with an optimum temperature range of 22 to 30 °C. However, watermelon is often cultivated under non-optimal stressful conditions in order to extend the production period. In the experiments, watermelon was exposed to seasonal temperature variations ranging from below zero to more than 40 °C. Depending on the growing season, the watermelon plants were exposed to low temperatures at the different growth stages. In semi-forcing culture, the watermelon plants in the early growth stage were exposed to low temperatures below 0 °C, but the plants in the late growth stage were exposed to high temperatures over 40 °C. On the other hand, in the retarding culture, the watermelon plants at early and late growth stages were exposed to high temperatures over 40 °C and low temperature near 0 °C, respectively.
Low temperature negatively impacts cold-sensitive plants and restricts their growth and yield [25]. Sub-optimal temperature causes serious damages such as the increase in the production of reactive oxygen species (ROS), cell membrane stiffness, ion leakage and unbalance, cell death, chlorosis, a change in hormone production, reduction in photosynthesis, wilting, and growth retardation [25,26,27].
The impact of temperature on plant growth depends on the growth stage, as well as the exposure intensity and length. Watermelons are highly temperature sensitive and the optimal temperature in their early growth stage is 25 °C. Young watermelon plants stop growing at 10 °C and cannot survive below 1 °C. However, mature plants can survive at an extremely high temperature above 45 °C [3,28]. Chilling damage is also affected by the light conditions, and the water status of plants before and after exposure to low temperatures [29].
The impact of low temperature was greater in the semi-forcing culture than in the retarding culture. In the semi-forcing culture, the growth of non-grafted watermelons was inhibited by the low temperature at the early stage. The main vine length of non-grafted watermelons one month after transplanting was about 30 cm. On the other hand, the watermelons grafted onto low-temperature rootstocks were twice as long. Delayed growth in the early stage inhibited the vegetative growth of non-grafted watermelons. The fruit mass was lower than that of grafted watermelons, even though the fruit quality of non-grafted watermelons was greater.
Grafting onto low-temperature tolerant rootstocks has many advantages, such as the prevention of plant and fruit damage from low temperatures, the extension of the growing season and area, better use of greenhouse facilities, and the saving fossil fuel, water, and fertilizer. Low-temperature-tolerant rootstocks maintain higher root growth (sink strength) and absorption of nutrients and water via an increase in the root hydraulic conductance and photosynthesis and a decrease in lipid peroxidation [27].
Fast and uniform germination and growth of scions and rootstocks are very important for grafted transplant production [30]. The seeds of Largenaria species usually germinate slowly. Largenaria species are reported to be very sensitive and require longer times to germinate at low temperatures [31]. Slow and uneven germination and growth restrict the availability of a rootstock. Bottle gourd rootstock accessions except ‘BG702’ germinated within seven days after sowing. However, ‘BG702’ required over a week for germination and showed low uniformity in germination and growth.
The scion and rootstock of grafted plants sometimes grow at different growth rates, causing disproportionate overgrowth above or below the graft union [32]. This symptom is reported especially frequently in fruit tree grafting [33]. This is attributed mainly to poor scion–rootstock compatibility, which may result in blocking the transport of photoassimilates, sugars, amino acids, organic acids, hormones, nutrients, and water between scions and rootstocks [32,34,35]. In the experiment, the stem diameter below the graft union was slightly thicker than that above the graft union, and the ratio of stem diameter between scion and rootstock ranged from 0.77 to 1.02.
Grafting also affects the flowering and harvesting of fruits. The watermelons grafted onto bottle gourd rootstocks formed female flowers earlier than other rootstocks. However, under suboptimal conditions, grafting onto bottle gourd rootstocks delays flowering and harvesting for up to one week [16].
The vigorous root growth of rootstock has been reported to promote the growth of grafted seedlings [16,36,37,38]. ‘Shintozwa’ rootstock has a vigorous root system [39]. The root mass of ‘Shintozwa’ rootstock at 11 days after sowing and of the watermelon transplants grafted onto ‘Shintozwa’ before transplanting were greater than those of bottle gourd rootstock accessions and non-grafted seedlings. The watermelon grafted onto ‘Shintozwa’ after fruit harvesting had well-developed roots with high fine-root density. Grafting onto ‘Shintozwa’ improves resistance to Fusarium wilt and tolerance to low temperature, drought, and salinity especially under stressful conditions, resulting in greater growth and yield [16,36,40,41,42]. However, grafting onto ‘Shintozwa’ adversely affects watermelon fruit quality, leading to a decrease in taste, TSS, and sugar content [38].
The effect of grafting on fruit quality depends on the combination of scion and rootstock, and it is positive or negative [16,43]. Watermelon fruits grafted onto ‘Shintozwa’ are reported to have lower sugar content and poor taste [44]. This deterioration of watermelon fruit quality is attributed to vigorous nutrient uptake [16]. In particular, the grafting of mini-watermelon onto vigorous Cucurbita spp. rootstocks resulted in severe deterioration in quality, leading to the occurrence of fruit enlargement, shape deformation, and the appearance of white fibers at the heart of fruits [45]. In semi-forcing culture, grafting onto ‘Shintozwa’ increased the fruit mass but reduced the fruit quality. The fruits grafted onto ‘Shintozwa’ obtained the lowest score in sensory evaluation.
Bottle gourd is a vine crop of the Cucurbitaceae that originated from sub-Saharan Africa and has been cultivated in arid and semi-arid tropics. Bottle gourd is widely used as a rootstock, as well as in food, medicine, decoration, and household utensils. Bottle gourd has been reported to have tolerance to biotic and abiotic stress such as soil-borne diseases, low temperature, drought, and salt stress without the deterioration of watermelon fruit quality [46,47,48,49]. In the experiment, BG703 had excellent growth and the greatest fruit mass among bottle gourd rootstocks. However, the fruit quality grafted onto BG703 was poor, similar to those grafted onto pumpkin rootstock. Watermelons grafted onto bottle gourd rootstock ‘FR79’ had the highest grafting affinity, good root growth, low-temperature tolerance, and had little effect on fruit quality.
However, in retarding culture, when watermelons were exposed to low temperature at the late growth stage, grafting onto low-temperature-tolerant rootstocks merely affected the growth of watermelons. The main vine of non-grafted watermelons in the early growth stage when plants were exposed to high temperature was the longest. The reciprocal grafting of a stress-tolerant scion onto a stress-sensitive rootstock has been reported to reveal that the stress-sensitive rootstock weakened the stress tolerance of scions [50,51,52]. In the experiment, it is considered that the high-temperature tolerance of watermelons was greater than that of bottle gourd or pumpkin rootstocks. Grafting watermelons onto bottle gourd or pumpkin rootstocks caused an adverse effect, reducing the early growth of watermelons under high-temperature conditions. In grafting cultivation using low-temperature tolerant rootstocks, it is necessary to select a rootstock in consideration of the cultivation season so that positive effects can be obtained without adverse effects caused by grafting.
5. Conclusions
The low-temperature-tolerant bottle gourd accessions as the rootstock could maintain growth under cold stress conditions. Although the growth of the watermelon was improved by grafting onto low-temperature rootstocks, it depended on the growing season and growth stage. The grafting was more effective in semi-forcing culture exposed to low temperature in the early growth stage. In retarding culture, when watermelon plants were exposed to low temperatures in the late growth stage, the effect of grafting onto low-temperature tolerant rootstock was not significant. Accordingly, it is necessary to select a rootstock considering the cultivation environmental characteristics according to cropping season. Furthermore, it is required to develop a multi-stress-tolerant rootstock for year-round stable production.
Author Contributions
Conceptualization, Y.J.; methodology, J.-H.M.; formal analysis, T.K.; investigation, S.-G.K. and O.-J.L.; writing—original draft preparation, Y.J.; writing—review and editing, J.-H.M.; supervision, H.-J.L. and S.-H.W.; project administration, Y.J. and T.K.; funding acquisition, J.-H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Administration (PJ01262202) “Breeding of disease resistant Cucurbitaceae rootstocks for high quality watermelon”.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rural Development Administration (RDA). Watermelon Cultivation; The Textbook for Farming No. 104; RDA: Jeonju, Republic of Korea, 2021. [Google Scholar]
- Lu, K.; Sun, J.; Li, Q.; Li, X.; Jin, S. Effect of cold stress on growth, physiological characteristics, and Calvin-cycle-related gene expression of grafted watermelon seedlings of different gourd rootstocks. Horticulturae 2021, 7, 391. [Google Scholar] [CrossRef]
- Noh, J.; Kim, J.M.; Sheikh, S.; Lee, S.G.; Lim, J.H.; Seong, M.H.; Jung, G.T. Effect of heat treatment around the fruit set region on growth and yield of watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai]. Physiol. Mol. Biol. Plants 2013, 19, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Dufault, R.J. Short-term cyclic cold temperature stress on watermelon yield. Hort. Sci. 2002, 37, 487–489. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hort. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Jang, Y.; Mun, B.; Do, K.; Um, Y.; Chun, C. Effects of photosynthetic photon flux and carbon dioxide concentration on the photosynthesis and growth of grafted pepper transplants during healing and acclimatization. Hort. Environ. Biotechnol. 2014, 55, 387–396. [Google Scholar] [CrossRef]
- Pérez-Grajales, M.; Pérez-Reyes, T.Q.; Cruz-Álvarez, O.; Castro-Brindis, R.; Martínez-Damián, M.T. Compatibility of the rootstock CM-334 and its response on the yield, physicochemical quality and content of capsaicinoids in Capsicum pubescens. ITEA-Inf. Técnica Económica Agrar. 2021, 117, 332–346. [Google Scholar]
- Suárez-Hernández, Á.M.; Grimaldo-Juárez, O.; García-López, A.M.; González-Mendoza, D.; Huitrón-Ramírez, M.V. Influence of rootstock on postharvest watermelon quality. Rev. Chapingo. Ser. Hortic. 2017, 23, 49–58. [Google Scholar] [CrossRef]
- Devi, P.; Perkins-Veazie, P.; Miles, C. Impact of grafting on watermelon fruit maturity and quality. Horticulturae 2020, 6, 97. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. Hort. Sci. 2006, 41, 622–627. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ. Exp. Bot. 2010, 68, 283–291. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, S.; Wei, M.; Gong, B.; Shi, Q. Effect of different rootstocks on the salt stress tolerance in watermelon seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of different rootstock on plant growth, yield and quality of watermelon. Aust. J. Exp. Agri. 2003, 43, 1269–1274. [Google Scholar] [CrossRef]
- Jang, Y. Current Status of Grafted Fruit Vegetable Transplants Production and Cultivation; National Institute of Horticultural and Herbal Science: Suwon, Republic of Korea, 2013; pp. 86–102. [Google Scholar]
- An, S.; Bae, J.H.; Kim, H.C.; Kwack, Y. Production of grafted vegetable seedlings in the Republic of Korea: Achievements, challenges and perspectives. Hort. Sci. Tech. 2021, 39, 547–559. [Google Scholar]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S.G.; Huh, Y.C.; Sun, Z.; Miguel, A.; King, S.R.; et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Mkhize, P.; Mashilo, J.; Shimelis, H. Progress on genetic improvement and analysis of bottle gourd [Lagenaria siceraria (Molina) Standl.] for agronomic traits, nutrient compositions, and stress tolerance: A review. Front. Sustain. Food Syst. 2021, 5, 683635. [Google Scholar] [CrossRef]
- Wanog, Y.; Wang, L.; Xing, N.; Wu, X.; Wu, X.; Wang, B.; Lu, Z.; Xu, P.; Tao, Y.; Li, G.; et al. A universal pipeline for mobile mRNA detection and insights into heterografting advantages under chilling stress. Hortic. Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- Kim, T.B.; Kim, E.J.; Nam, S.C. Standardized Production and Rootstock Development of Small and Medium-Sized Cucurbitaceae vegetables for Export; Research reports for 2021 (PJ012608); Rural Development Administration: Jeonju, Republic of Korea, 2022. [Google Scholar]
- Moon, J.H.; An, Y.G.; An, S.W. Breeding and Utilization of Rootstock Varieties to Reduce the Injury by Continuous Cropping in Cucurbitaceae vegetables; Research reports for 2017 (PJ008638); Rural Development Administration: Jeonju, Republic of Korea, 2018. [Google Scholar]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agri. Forest Meteor. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Begna, S.H.; Angadi, S.V. Effects of planting date on winter canola growth and yield in the Southwestern U.S. Am. J. Plant Sci. 2016, 7, 201–217. [Google Scholar] [CrossRef]
- Xiong, M.; Liu, C.; Guo, L.; Wang, J.; Wu, X.; Li, L.; Bie, Z.; Huang, Y. Compatibility evaluation and anatomical observation of melon grafted onto eight Cucurbitaceae. Front. Plant Sci. 2021, 12, 762889. [Google Scholar] [CrossRef]
- Lee, D.U.; Bae, J.H.; Lim, J.H.; Choi, J.H. Prediction of consumer acceptance of oriental melon based on physicochemical and sensory characteristics. Hort. Sci. Tech. 2017, 35, 446–455. [Google Scholar]
- Ntatsi, G.; Savvas, D.; Papasotiropoulos, V.; Katsileros, A.; Zrenner, R.M.; Hincha, D.K.; Zuther, E.; Schwarz, D. Rootstock sub-optimal temperature tolerance determines transcriptomic responses after long-term root cooling in rootstocks and scions of grafted tomato plants. Front. Plant Sci. 2017, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Duan, X.; Liu, T.; Qi, H. Short-term suboptimal low temperature has short- and long-term effects on melon seedlings. Sci. Hort. 2022, 297, 110967. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hort. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Nabiwire, S.; Wakholi, C.; Faqeerzada, M.A.; Arief, M.A.A.; Kim, M.S.; Cho, B. Estimation of cold stress, plant age, and number of leaves in watermelon plants using image analysis. Front. Plant Sci. 2022, 13, 847225. [Google Scholar] [CrossRef] [PubMed]
- Kozik, E.U.; Wehner, T.C. Tolerance of watermelon seedlings to low-temperature chilling injury. Hort. Sci. 2014, 49, 240–243. [Google Scholar] [CrossRef]
- An, S.; Park, S.W.; Kwack, Y. Growth of cucumber scions, rootstocks, and grafted seedlings as affected by different irrigation regimes during cultivation of ‘Joenbaekdadagi’ and ‘Heukjong’ seedlings in a plant factory with artificial lighting. Agronomy 2020, 10, 1943. [Google Scholar] [CrossRef]
- Kenanoğlu, B.; Demir, I.; Mavi, K.; Yetisir, E. Effect of Priming on Germination of Lagenaria siceraria Genotypes at Low Temperatures. Tarim Bilim. Derg. 2007, 13, 169–175. [Google Scholar]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hort. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Ko, K.D. Response of Cucurbitaceous Rootstock Species to Biological and Environmental Stresses. Ph.D. Dissertation, Seoul National University, Seoul, Republic of Korea, 1999. [Google Scholar]
- Camalle, M.D.; Sikron, N.; Zurgil, U.; Khadka, J.; Pivonia, S.; Pěnčík, A.; Novák, O.; Fait, A.; Tel-Zur, N. Does scion–rootstock compatibility modulate photoassimilate and hormone trafficking through the graft junction in melon–pumpkin graft combinations? Plant Sci. 2021, 306, 110852. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Jang, Y.; Yang, E.; Cho, M.; Um, Y.; Ko, K.; Chun, C. Effect of grafting on growth and incidence of Phytophthora blight and bacterial wilt of pepper (Capsicum annuum L.). Hort. Envrion. Biotechnol. 2012, 53, 9–19. [Google Scholar] [CrossRef]
- Jang, Y.; Huh, Y.C.; Park, D.K.; Mun, B.; Lee, S.; Um, Y. Greenhouse evaluation of melon rootstock resistance to Monosporascus root rot and vine decline as well as yield and fruit quality in grafted ‘Inodorus’ melons. Korean J. Hort. Sci. Technol. 2014, 32, 614–622. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Khah, E.M.; Passam, H.C. Evaluation of rootstocks for watermelon grafting with reference to plant development, yield and fruit quality. Int. J. Plant Prod. 2012, 6, 481–491. [Google Scholar]
- Jang, Y.; Moon, J.H.; An, S.; Kim, S.G.; Huh, Y.C.; Lee, H.J.; Wi, S.H.; Chun, H. Improvement of the growth and fruit quality of mini watermelons grafted onto rootstocks of the wild watermelon accessions. Prot. Hort. Plant Fact. 2019, 28, 438–446. [Google Scholar] [CrossRef]
- Parsafar, A.; Panahandeh, J.; Zarehaghi, D. Assessment of Iranian rainfed and seedy watermelon landraced as potential rootstocks for enhancing drought tolerance. Hortic. Sci. Tech. 2019, 37, 354–364. [Google Scholar]
- Yuan, H.; Zhao, L.; Kong, Q.; Cheng, F.; Niu, M.; Xie, J.; Muhammad, A.N.; Bie, Z. Comprehensive mineral nutrition analysis of watermelon grafted onto two different rootstocks. Hortic. Plant J. 2016, 2, 105–113. [Google Scholar]
- Miguel, A.; Maroto, J.V.; Bautista, A.S.; Baixauli, C.; Cebolla, V.; Pascual, B.; López, S.; Guardiola, J.L. The grafting of triploid watermelon is an advantageous alternative to soil fumigation by methyl bromide for control of Fusarium wilt. Sci. Hort. 2004, 103, 9–17. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting effects on vegetable quality. Hort. Sci. 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Huh, Y.C.; Woo, Y.H.; Lee, J.M.; Om, Y.H. Growth and fruit characteristics of watermelon grafted onto Citrullus rootstocks selected for disease resistance. J. Kor. Soc. Hort. Sci. 2003, 44, 649–654. [Google Scholar]
- Edelstein, M.; Tyutyunik, J.; Fallik, E.; Meir, A.; Yadmor, Y.; Cohen, R. Horticultural evaluation of exotic watermelon germplasm as potential rootstocks. Sci. Hort. 2014, 165, 196–202. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, L.; Wang, L.; Guo, S. Bottle gourd rootstock-grafting promotes photosynthesis by regulating the stomat and non-stomata performances in leaves of watermelon seedlings under NaCl stress. J. Plant Physiol. 2015, 186–187, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.; Silverman, E.; Reiland, D.; Wehner, T.C. Effects of cold durations on chilling injury in Lagenaria germplasm. Hort. Sci. 2020, 55, 1551–1557. [Google Scholar] [CrossRef]
- Kim, S.; Huh, Y.; Park, T.; Yang, E.; Chae, S.; An, S.; Park, D.; Moon, J. Symptoms of infected seedlings and screening of breeding lines and F1 hybrids for resistance to Fusarium wilt in bottle gourd (Lagenaria siceraria Standl.). J. Korean Soc. Int. Agric. 2016, 28, 553–557. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.d.M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci. 2012, 188–189, 89–96. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, W.B.; Yao, X.D.; Wang, C.L.; Cao, Y.Q.; Zhang, L.J.; Zhang, H.J.; Xie, F.T.; Song, S.H. Photosynthesis in reciprocal grafts of drought-tolerant and drought-sensitive cultivars of soybean under water stress. Photosynthetica 2019, 57, 945–949. [Google Scholar] [CrossRef]
- Spiral, J.; Ouazzani, S.; Vial, N.H.; Michaux, S.; Barro, L.; Darracq, O.; Arigoni, F. Reciprocal grafting reveals differential metabolic responses between robusta clones with contrasting tolerance to drought. Agric. Res. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).