Compatible Graft Establishment in Fruit Trees and Its Potential Markers
Abstract
1. Introduction
2. Grafting Practice Generates Potential Varieties and Benefits
3. Grafting Skill: Crucial External Factor behind Uniform Graft-Take
4. Choice of Graft Partners: Demands vs. Plant Physiology
5. Graft Establishment: Basic Requirements
5.1. Wounding Response: A Necessary Nuisance
5.2. Mechanical Adhesion: A Necessary First Step towards Compatible Graft-Establishment
5.3. Callus-Bridge: A Necessary Physiological Embrace of the Graft Partners
5.4. Cellular Redifferentiation and Functional Connection Establishment: The Main Road towards Graft-Compatibility
6. Potential Factors behind Graft Compatibility
6.1. Compartmentalization Efficiency of a Tree
6.2. Cell Wall Modifications
6.3. Phytohormones
6.4. Balanced Photosynthate and Metabolites Translocation
6.5. Polyphenols, Peroxidases, and Lignification
7. Perspective and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adhikari, P.B.; Liu, X.; Wu, X.; Zhu, S.; Kasahara, R.D. Fertilization in flowering plants: An odyssey of sperm cell delivery. Plant Mol. Biol. 2020, 103, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr. Plant Propagation. Principles and Practices, 8th ed.; Pearson Education Limited: Edinburgh Gate, Harlow, UK, 2014. [Google Scholar]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 437–493. [Google Scholar]
- Maiti, R.; Rodríguez, H.G.; Ac, P.; Kumari, C.A.; Sarkar, N.C. A comparative wood anatomy of 15 woody species in north-eastern mexico. For. Res. 2016, 5, 166. [Google Scholar]
- Herrero, J. Studies of compatible and incompatible graft combinations with special reference to hardy fruit trees. J. Hortic. Sci. 1951, 26, 186–237. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.Á.; Pinochet, J. Graft compatibility between peach cultivars and Prunus rootstocks. Hortscience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- Moing, A.; Carde, J.-P. Growth, cambial activity and phloem structure in compatible and incompatible peach/plum grafts. Tree Physiol. 1988, 4, 347–359. [Google Scholar] [CrossRef]
- Moing, A.; Salesses, G.; Saglio, P.H. Growth and the composition and transport of carbohydrate in compatible and incompatible peach/plum grafts. Tree Physiol. 1987, 3, 345–354. [Google Scholar] [CrossRef]
- Zarrouk, O.; Testillano, P.S.; Risueño, M.D.C.; Moreno, M.Á.; Gogorcena, Y. Changes in cell/tissue organization and peroxidase activity as markers for early detection of graft incompatibility in peach/plum combinations. J. Am. Soc. Hortic. Sci. 2010, 135, 9–17. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Errea, P. Implications of phenolic compounds in graft incompatibility in fruit tree species. Sci. Hortic. 1998, 74, 195–205. [Google Scholar] [CrossRef]
- Loupit, G.; Cookson, S.J. Identifying molecular markers of successful graft union formation and compatibility. Front. Plant Sci. 2020, 11, 610352. [Google Scholar] [CrossRef]
- Čolić, S.; Fotirić-Akšić, M.; Rakonjac, V.; Nikolić, D. Predicting delayed graft incompatibility in sweet cherry by peroxidase isoenzyme analysis. Voćarstvo 2015, 49, 107–113. [Google Scholar]
- Habibi, F.; Liu, T.; Folta, K.M.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Darwin’s pangenesis and graft hybridization. In Advances in Genetics; Kumar, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 102, pp. 27–66. [Google Scholar]
- Tubbs, F.R. Growth relations of rootstock and scion in apples. J. Hortic. Sci. 1980, 55, 181–189. [Google Scholar] [CrossRef]
- Castle, W.S. A career perspective on citrus rootstocks, their development, and commercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef]
- Iacono, F.; Peterlunger, E. Rootstock-scion interaction may affect drought tolerance in vitis vinifera cultivars. Implications in selection programs. Acta Hortic. 2000, 528, 543–549. [Google Scholar] [CrossRef]
- Okudai, N.; Yoshinaga, K.; Takahara, T.; Ishiuchi, D.; Oiyama, I. Studies on the promotion of flowering and fruiting of juvenile citrus seedlings. II: Effect of grafting. Bull. Fruit Tree Res. Stn. D 1980, 2, 15–28. [Google Scholar]
- de Oliveira Castro, C.A.; dos Santos, G.A.; Takahashi, E.K.; Pires Nunes, A.C.; Souza, G.A.; de Resende, M.D.V. Accelerating Eucalyptus breeding strategies through top grafting applied to young seedlings. Ind. Crop. Prod. 2021, 171, 113906. [Google Scholar] [CrossRef]
- Cerruti, E.; Gisbert, C.; Drost, H.-G.; Valentino, D.; Portis, E.; Barchi, L.; Prohens, J.; Lanteri, S.; Comino, C.; Catoni, M. Grafting vigour is associated with DNA de-methylation in eggplant. Hortic. Res. 2021, 8, 241. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Y.; Jiang, X.; Yu, H.; Jia, H.; Tan, C.; Hu, G.; Hu, Y.; Rao, M.J.; Deng, X.; et al. Genome of a citrus rootstock and global DNA demethylation caused by heterografting. Hortic. Res. 2021, 8, 69. [Google Scholar] [CrossRef]
- Amos, J.; Hatton, R.G.; Hoblyn, T.N.; Knight, R.C. The effect of scion on root.: II. Stem-worked apples. J. Pomol. Hortic. Sci. 1930, 8, 248–258. [Google Scholar] [CrossRef]
- Tandonnet, J.P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Gautier, A.T.; Merlin, I.; Doumas, P.; Cochetel, N.; Mollier, A.; Vivin, P.; Lauvergeat, V.; Péret, B.; Cookson, S.J. Identifying roles of the scion and the rootstock in regulating plant development and functioning under different phosphorus supplies in grapevine. Environ. Exp. Bot. 2021, 185, 104405. [Google Scholar] [CrossRef]
- Gautier, A.T.; Cochetel, N.; Merlin, I.; Hevin, C.; Lauvergeat, V.; Vivin, P.; Mollier, A.; Ollat, N.; Cookson, S.J. Scion genotypes exert long distance control over rootstock transcriptome responses to low phosphate in grafted grapevine. BMC Plant Biol. 2020, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Tandonnet, J.-P.; Marguerit, E.; Cookson, S.J.; Ollat, N. Genetic architecture of aerial and root traits in field-grown grafted grapevines is largely independent. Theor. Appl. Genet. 2018, 131, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2018, 70, 747–755. [Google Scholar] [CrossRef]
- Wooldridge, T.J.S. Cross-Compatibility, Graft-Compatibility, and Phylogenetic Relationships in the Aurantioideae: New Data from the Balsamocitrinae; University of California: Riverside, CA, USA, 2016. [Google Scholar]
- Andrews, P.K.; Marquez, C.S. Graft incompatibility. Hortic. Rev. 1993, 15, 183–232. [Google Scholar]
- Copes, D. Graft union formation in douglas-fir. Am. J. Bot. 1969, 56, 285–289. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P. A review of new advances in mechanism of graft compatibility–incompatibility. Sci. Hortic. 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Asante, A.K.; Barnett, J.R. Graft union formation in mango (Mangifera indica L.). J. Hortic. Sci. 1997, 72, 781–790. [Google Scholar] [CrossRef]
- Ermel, F.F.; Poëssel, J.L.; Faurobert, M.; Catesson, A.M. Early scion/stock junction in compatible and incompatible pear/pear and pear/quince grafts: A histo-cytological study. Ann. Bot. 1997, 79, 505–515. [Google Scholar] [CrossRef]
- Yeoman, M.M. Cellular recognition systems in grafting. In Cellular Interactions; Linskens, H.F., Heslop-Harrison, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 453–472. [Google Scholar]
- Wulf, K.E.; Reid, J.B.; Foo, E. What drives interspecies graft union success? Exploring the role of phylogenetic relatedness and stem anatomy. Physiol. Plant. 2020, 170, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Notaguchi, M.; Kurotani, K.-I.; Sato, Y.; Tabata, R.; Kawakatsu, Y.; Okayasu, K.; Sawai, Y.; Okada, R.; Asahina, M.; Ichihashi, Y.; et al. Cell-cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 2020, 369, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Santamour, F.S. Graft incompatibility related to cambial peroxidase isozymes in chinese chestnut. J. Environ. Hortic. 1988, 6, 33–39. [Google Scholar] [CrossRef]
- Gulen, H.; Arora, R.; Kuden, A.; Krebs, S.L.; Postman, J. Peroxidase isozyme profiles in compatible and incompatible pear-quince graft combinations. J. Am. Soc. Hortic. Sci. 2002, 127, 152–157. [Google Scholar] [CrossRef]
- Irisarri, P.; Binczycki, P.; Errea, P.; Martens, H.J.; Pina, A. Oxidative stress associated with rootstock–scion interactions in pear/quince combinations during early stages of graft development. J. Plant Physiol. 2015, 176, 25–35. [Google Scholar] [CrossRef]
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Waite, H.; Armengol, J.; Billones-Baaijens, R.; Gramaje, D.; Hallen, F.; Marco, S.D.; Smart, R. A protocol for the management of grapevine rootstock mother vines to reduce latent infections by grapevine trunk pathogens in cuttings. Phytopathol. Mediterr. 2018, 57, 384–398. [Google Scholar]
- Swarbrick, T. The healing of wounds in woody stems. J. Pomol. Hortic. Sci. 1925, 5, 98–114. [Google Scholar] [CrossRef]
- Levy, A.; Epel, B.L. Chapter 4.4.2—cytology of the (1-3)-β-glucan (callose) in plasmodesmata and sieve plate pores. In Chemistry, Biochemistry, and Biology of 1–3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 439–463. [Google Scholar]
- Denaxa, N.-K.; Vemmos, S.N.; Roussos, P.A. Shoot girdling improves rooting performance of kalamata olive cuttings by upregulating carbohydrates, polyamines and phenolic compounds. Agriculture 2021, 11, 71. [Google Scholar] [CrossRef]
- Basak, U.C.; Das, A.B.; Das, P. Rooting response in stem cuttings from five species of mangrove trees: Effect of auxins and enzyme activities. Mar. Biol. 2000, 136, 185–189. [Google Scholar] [CrossRef]
- Howard, B.H. Manipulating rooting potential in stockplants before collecting cuttings. In Biology of Adventitious Root Formation; Davis, T.D., Haissig, B.E., Eds.; Springer: Boston, MA, USA, 1994; pp. 123–142. [Google Scholar]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock-scion interface in graft combinations between Cucurbita rootstocks and a melon scion. J. Hortic. Sci. Biotechnol. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Beveridge, C.A. Apical dominance. Curr. Biol. 2017, 27, R864–R865. [Google Scholar] [CrossRef] [PubMed]
- Sanbagavalli, S.; Bhavana, J.; Pavithra, S. Nipping—A simple strategy to boost the yield—Review. Ann. Res. Rev. Biol. 2020, 35, 45–51. [Google Scholar] [CrossRef]
- Jeffree, C.E.; Yeoman, M.M. Development of intercellular connections between opposing cells in a graft union. New Phytol. 1983, 93, 491–509. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Studies of vegetative compatibility-incompatibility in higher plants. I. A structural study of a compatible autograft in Sedum telephoides (crassulaceae). Am. J. Bot. 1981, 68, 820–830. [Google Scholar] [CrossRef]
- Trinchera, A.; Pandozy, G.; Rinaldi, S.; Crinò, P.; Temperini, O.; Rea, E. Graft union formation in artichoke grafting onto wild and cultivated cardoon: An anatomical study. J. Plant Physiol. 2013, 170, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Asante, A. Compatibility studies on cashew-mango graft combinations. Ghana J. Agric. Sci. 2001, 34, 3–9. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Studies of vegetative compatibility-incompatibility in higher plants. II. A structural study of an incompatible heterograft between Sedum telephoides (crassulaceae) and Solanum pennellii (solanaceae). Am. J. Bot. 1981, 68, 831–842. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Zhang, A.; Matsuoka, K.; Kareem, A.; Robert, M.; Roszak, P.; Blob, B.; Bisht, A.; De Veylder, L.; Voiniciuc, C.; Asahina, M.; et al. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Curr. Biol. 2022, 32, 1883–1894. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef] [PubMed]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef] [PubMed]
- Atakhani, A.; Bogdziewiez, L.; Verger, S. Characterising the mechanics of cell–cell adhesion in plants. Quant. Plant Biol. 2022, 3, E2. [Google Scholar] [CrossRef]
- Kollmann, R.; Glockmann, C. Studies on graft unions. I. Plasmodesmata between cells of plants belonging to different unrelated taxa. Protoplasma 1985, 124, 224–235. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P. Cellular viability and pectin localisation in apricot (P. armeniaca)/plum combinations related to graft response. In XIII International Symposium on Apricot Breeding and Culture 717; International Society for Horticultural Science: Leuven, Belgium, 2006; pp. 185–188. [Google Scholar]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Chen, N.; Chen, J.; Mu, C.; Yin, K.; He, Y.; Liu, H. Plant grafting relieves asymmetry of jasmonic acid response induced by wounding between scion and rootstock in tomato hypocotyl. PLoS ONE 2020, 15, e0241317. [Google Scholar] [CrossRef]
- Bao, W.-W.; Zhang, X.-C.; Zhang, A.L.; Zhao, L.; Wang, Q.-C.; Liu, Z.-D. Validation of micrografting to analyze compatibility, shoot growth, and root formation in micrografts of kiwifruit (Actinidia spp.). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 140, 209–214. [Google Scholar] [CrossRef]
- Errea, P.; Felipe, A.; Herrero, M. Graft establishment between compatible and incompatible Prunus spp. J. Exp. Bot. 1994, 45, 393–401. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Wagner, D. Ultrastructure of the bud graft union in Malus. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1968. [Google Scholar]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Nat. Acad. Sci. USA 2018, 115, E2447–E2456. [Google Scholar] [CrossRef]
- Hall, P.; Badenoch-Jones, J.; Parker, C.; Letham, D.; Barlow, B. Identification and quantification of cytokinins in the xylem sap of mistletoes and their hosts in relation to leaf mimicry. Funct. Plant Biol. 1987, 14, 429–438. [Google Scholar] [CrossRef]
- Bogoeva, V.; Ivanov, I.; Kulina, H.; Russev, G.; Atanasova, L. A novel cytokinin-binding property of mistletoe lectin I from Viscum album. Biotechnol. Biotechnol. Equip. 2013, 27, 3583–3585. [Google Scholar] [CrossRef]
- Zhang, X.; Teixeira da Silva, J.A.; Duan, J.; Deng, R.; Xu, X.; Ma, G. Endogenous hormone levels and anatomical characters of haustoria in Santalum album L. seedlings before and after attachment to the host. J. Plant Physiol. 2012, 169, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Moore, R. A model for graft compatibility-incompatibility in higher plants. Am. J. Bot. 1984, 71, 752–758. [Google Scholar] [CrossRef]
- Santamour, F.S. Graft compatibility in woody plants: An expanded perspective. J. Environ. Hortic. 1988, 6, 27–32. [Google Scholar] [CrossRef]
- Morris, H.; Hietala, A.M.; Jansen, S.; Ribera, J.; Rosner, S.; Salmeia, K.A.; Schwarze, F.W.M.R. Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay fungi. Ann. Bot. 2019, 125, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Physiological and morphological effects of size-controlling rootstocks on ‘Fuji’ apple scions. Acta Hortic. 2011, 903, 865–872. [Google Scholar] [CrossRef]
- Kamboj, J.S.; Browning, G.; Blake, P.S.; Quinlan, J.D.; Baker, D.A. GC-MS-SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple rootstocks. Plant Growth Regul. 1999, 28, 21–27. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, J.; Kumar, A.; Chaudhury, A.; Singh, S.P. Grafting triggers differential responses between scion and rootstock. PLoS ONE 2015, 10, e0124438. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xie, R.; Wang, Y.; Chen, Q.; Wang, H.; Yang, S.; Luo, Y.; Zhang, Y.; Tang, H.; Gmitter, F.G., Jr.; et al. Comparative transcriptomic analysis on compatible/incompatible grafts in Citrus. Hortic. Res. 2022, 9, uhab072. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, M.K.; Das, B. Standardization of grafting technique in litchi. Indian J. Hortic. 2017, 74, 16–19. [Google Scholar] [CrossRef]
- Smith, D.D.; Sperry, J.S. Coordination between water transport capacity, biomass growth, metabolic scaling and species stature in co-occurring shrub and tree species. Plant Cell Environ. 2014, 37, 2679–2690. [Google Scholar] [CrossRef]
- Eleftheriou, E.P. A decline-like disorder of pear in Greece: Pear decline or scion—Rootstock incompatibility? Sci. Hortic. 1985, 27, 241–250. [Google Scholar] [CrossRef]
- Gebhardt, K.; Feucht, W. Polyphenol changes at the union of Prunus avium/Prunus cerasus grafts. J. Hortic. Sci. 1982, 57, 253–258. [Google Scholar] [CrossRef]
- Guclu, S.F. Identification of polyphenols in homogenetic and heterogenetic combination of cherry grafting. Pak. J. Bot. 2019, 51, 2067–2072. [Google Scholar] [CrossRef]
- Errea, P.; Garay, L.; Marín, J.A. Early detection of graft incompatibility in apricot (Prunus armeniaca) using in vitro techniques. Physiol. Plant. 2001, 112, 135–141. [Google Scholar] [CrossRef]
- Ermel, F.F.; Kervella, J.; Catesson, A.M.; Poëssel, J.L. Localized graft incompatibility in pear/quince (Pyrus communis/Cydonia oblonga) combinations: Multivariate analysis of histological data from 5-month-old grafts. Tree Physiol. 1999, 19, 645–654. [Google Scholar] [CrossRef]
- Schmid, P.P.S.; Feucht, W. Compatibility in Prunus avium/Prunus cerasus graftings during the initial phase. III. Isoelectrofocusing of proteins, peroxidases and acid phosphatases during union formation. J. Hortic. Sci. 1985, 60, 311–318. [Google Scholar] [CrossRef]
- Deloire, A.; Hebant, C. Peroxidase activity and lignification at the interface between stock and scion of compatible and incompatible grafts of Capsicum on Lycopersicum. Ann. Bot. 1982, 49, 887–891. [Google Scholar] [CrossRef]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Esteves Amaro, A.C.; Pina, A.; Ferreira, G. An overview of grafting re-establishment in woody fruit species. Sci. Hortic. 2019, 243, 84–91. [Google Scholar] [CrossRef]
- Dunand, C.; De Meyer, M.; Crèvecoeur, M.; Penel, C. Expression of a peroxidase gene in zucchini in relation with hypocotyl growth. Plant Physiol. Biochem. 2003, 41, 805–811. [Google Scholar] [CrossRef]
- Sasaki, S.; Baba, K.i.; Nishida, T.; Tsutsumi, Y.; Kondo, R. The cationic cell-wall-peroxidase having oxidation ability for polymeric substrate participates in the late stage of lignification of Populus alba L. Plant Mol. Biol. 2006, 62, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Reimers, P.J.; Guo, A.; Leach, J.E. Increased activity of a cationic peroxidase associated with an incompatible interaction between Xanthomonas oryzae pv oryzae and rice (Oryza sativa). Plant Physiol. 1992, 99, 1044–1050. [Google Scholar] [CrossRef]
- Milinović, B.; Dragović-Uzelac, V.; Kazija, D.H.; Jelačić, T.; Vujević, P.; Čiček, D.; Biško, A.; Čmelik, Z. Influence of four different dwarfing rootstocks on phenolic acids and anthocyanin composition of sweet cherry ( Prunus avium L) cvs ‘Kordia’ and ‘Regina’. J. Appl. Bot Food Qual. 2016, 89, 29–37. [Google Scholar]
- Mng’omba, S.A.; du Toit, E.S.; Akinnifesi, F.K. The relationship between graft incompatibility and phenols in Uapaca kirkiana Müell Arg. Sci. Hortic. 2008, 117, 212–218. [Google Scholar] [CrossRef]
- Gamba, G.; Cisse, V.; Donno, D.; Razafindrakoto, Z.R.; Beccaro, G.L. Quali-quantitative study on phenol compounds as early predictive markers of graft incompatibility: A case study on chestnut (Castanea spp.). Horticulturae 2022, 8, 32. [Google Scholar] [CrossRef]
- Treutter, D.; Feucht, W. Accumulation of the flavonoid prunin in Prunus avium/P. cerasus grafts and its possible involvement in the process of incompatibility. Acta Hortic. 1988, 227, 74–78. [Google Scholar] [CrossRef]
- Lochard, R.G.; Schneider, G.W. Stock and scion growth relationships and the dwarfing mechanism in apple. In Horticultural Reviews; Janick, J., Ed.; AVI Publishing Company, Inc.: Westport, CT, USA, 1981; Volume 3, pp. 315–375. [Google Scholar]
- Zanardo, D.I.L.; Lima, R.B.; Ferrarese, M.d.L.L.; Bubna, G.A.; Ferrarese-Filho, O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ. Exp. Bot. 2009, 66, 25–30. [Google Scholar] [CrossRef]
- Kamisaka, S.; Takeda, S.; Takahashi, K.; Shibata, K. Diferulic and ferulic acid in the cell wall of Avena coleoptiles—Their relationships to mechanical properties of the cell wall. Physiol. Plant. 1990, 78, 1–7. [Google Scholar] [CrossRef]
- Tan, K.-S.; Hoson, T.; Masuda, Y.; Kamisaka, S. Correlation between cell wall extensibility and the content of diferulic and ferulic acids in cell walls of Oryza sativa coleoptiles grown under water and in air. Physiol. Plant. 1991, 83, 397–403. [Google Scholar] [CrossRef]
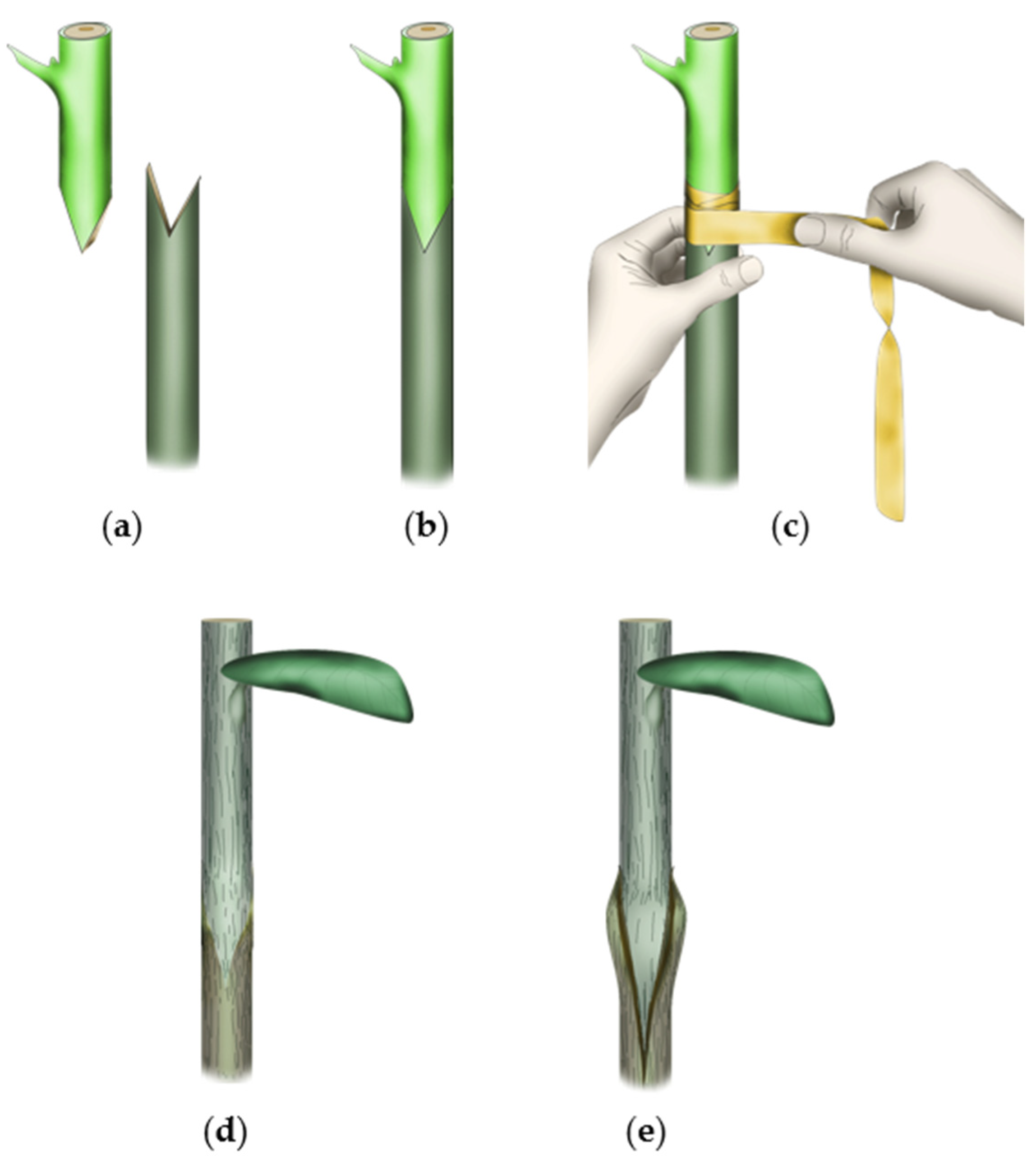
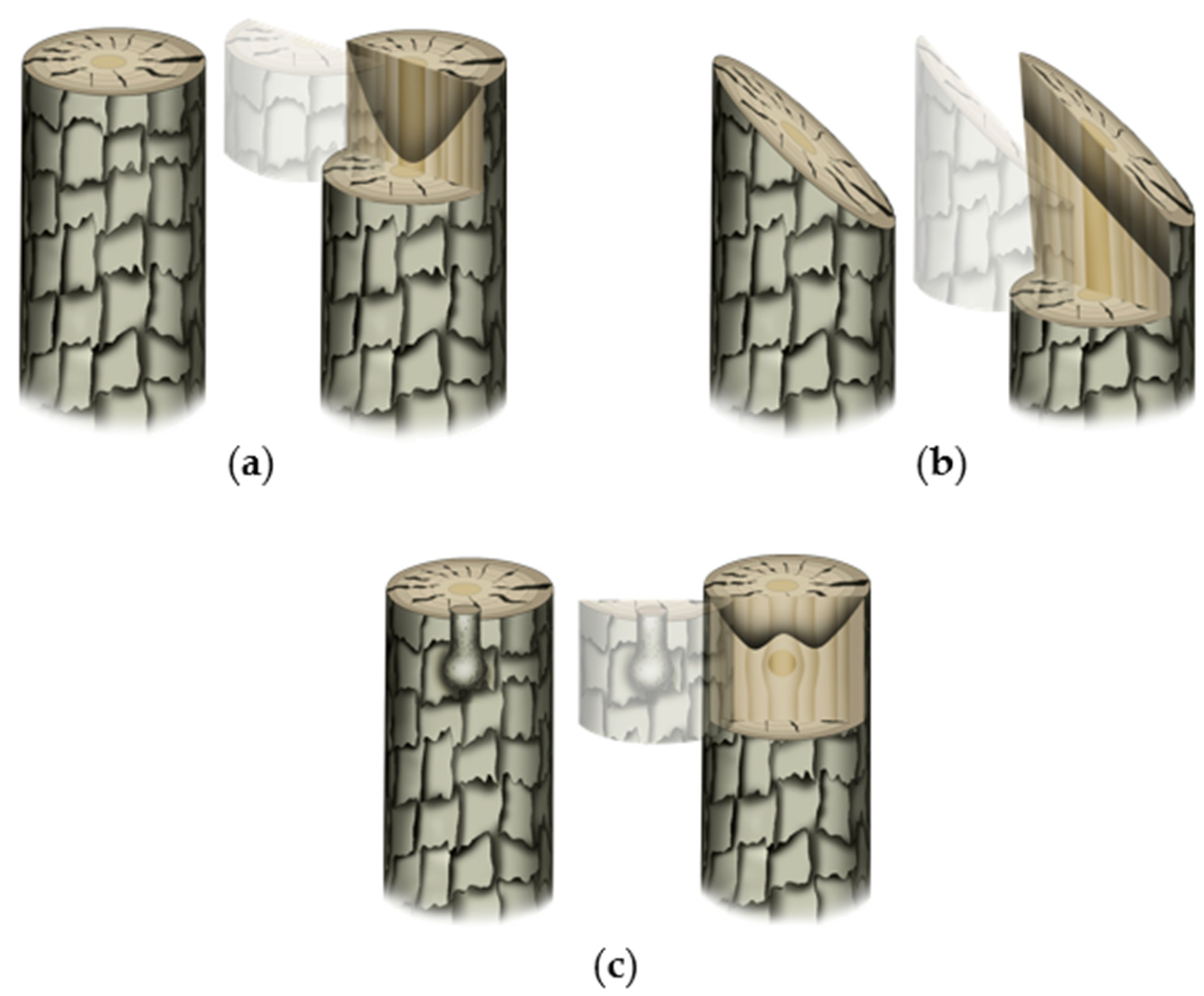

| Serial No. | Graft Progress | Physiological Characteristics | Metabolic Changes | Compatibility Features | Incompatibility Features |
|---|---|---|---|---|---|
| 1 | Incision |
|
| Reduction of metabolite leakage. |
|
| 2 | Mechanical adhesion of graft partners; necrotic layer formation at the graft interface |
|
| Mechanical graft-union establishment. |
|
| 3 | Callogenesis and bridge establishment |
|
| Initial physiological graft-union formation. |
|
| 4 | Cellular redifferentiation and vascular continuity |
|
| Functional graft-union establishment. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, P.B.; Xu, Q.; Notaguchi, M. Compatible Graft Establishment in Fruit Trees and Its Potential Markers. Agronomy 2022, 12, 1981. https://doi.org/10.3390/agronomy12081981
Adhikari PB, Xu Q, Notaguchi M. Compatible Graft Establishment in Fruit Trees and Its Potential Markers. Agronomy. 2022; 12(8):1981. https://doi.org/10.3390/agronomy12081981
Chicago/Turabian StyleAdhikari, Prakash Babu, Qiang Xu, and Michitaka Notaguchi. 2022. "Compatible Graft Establishment in Fruit Trees and Its Potential Markers" Agronomy 12, no. 8: 1981. https://doi.org/10.3390/agronomy12081981
APA StyleAdhikari, P. B., Xu, Q., & Notaguchi, M. (2022). Compatible Graft Establishment in Fruit Trees and Its Potential Markers. Agronomy, 12(8), 1981. https://doi.org/10.3390/agronomy12081981







