Effect of Harvest Time on the Seed Yield and Quality of Kengyilia melanthera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Management
2.3. Experimental Design
2.4. Evaluations
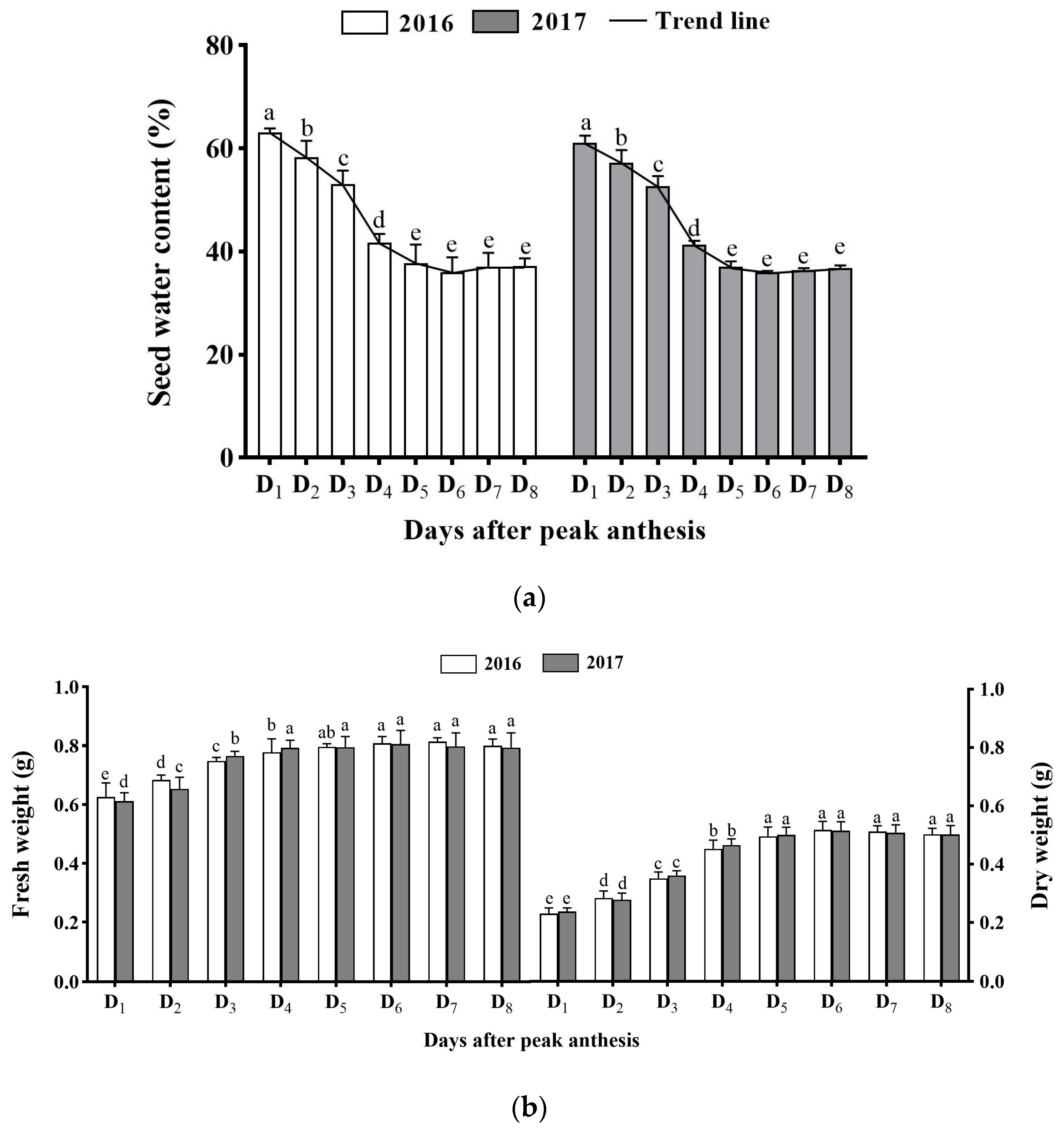
2.4.1. Seed Water Content, Fresh Weight, and Dry Weight
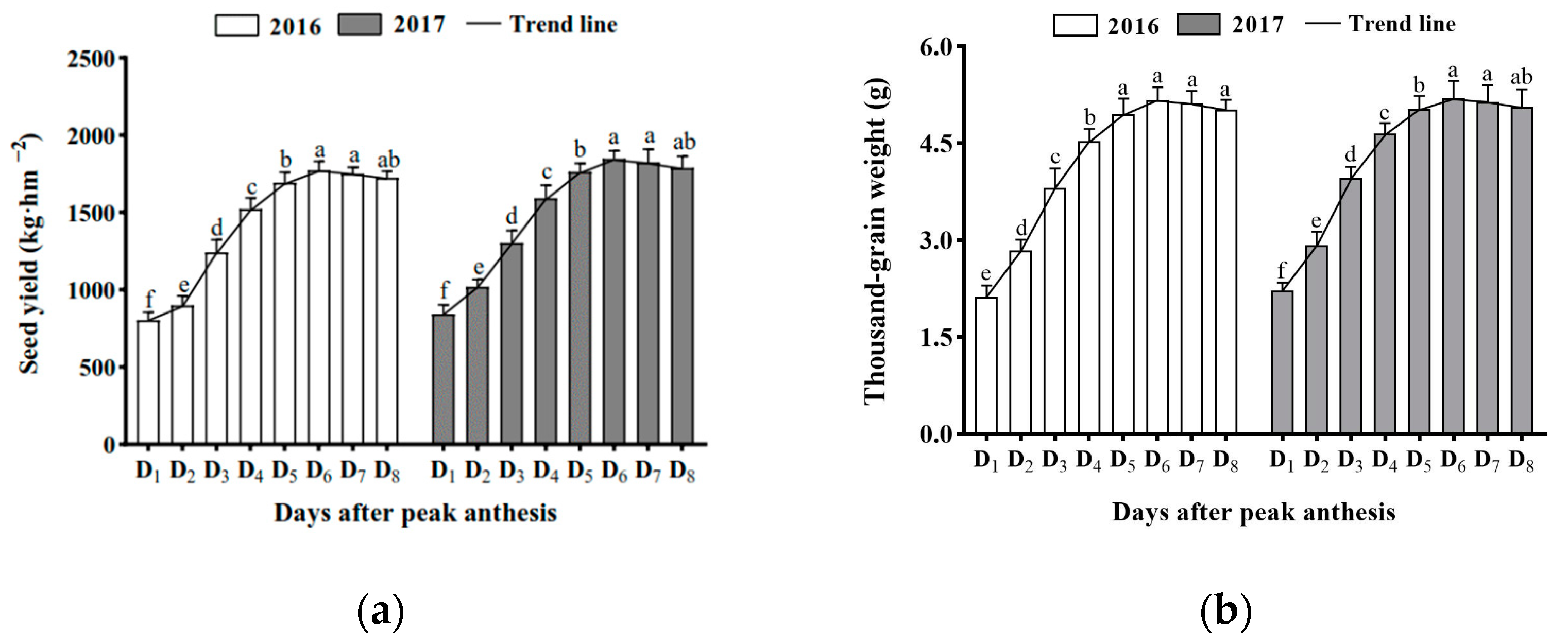
2.4.2. Thousand-Grain Weight
2.4.3. Seed Yield
2.4.4. Seed Germination Potential and Generation Percentage
2.4.5. Seed Accelerated Aging Germination Percentage
2.4.6. Dehydrogenase Activity and Acid Phosphoesterase Activity
2.5. Statistics Analysis
3. Results
3.1. Effects of Harvest Time on Seed Quality and Yield
3.2. Effect of Harvest Time on Seed Water Content, Fresh Weight, and Dry Seed Weight
3.3. Effect of Harvest Time on Seed Yield and Thousand-Grain Weight
3.4. Effect of Harvest Time on Seed Quality
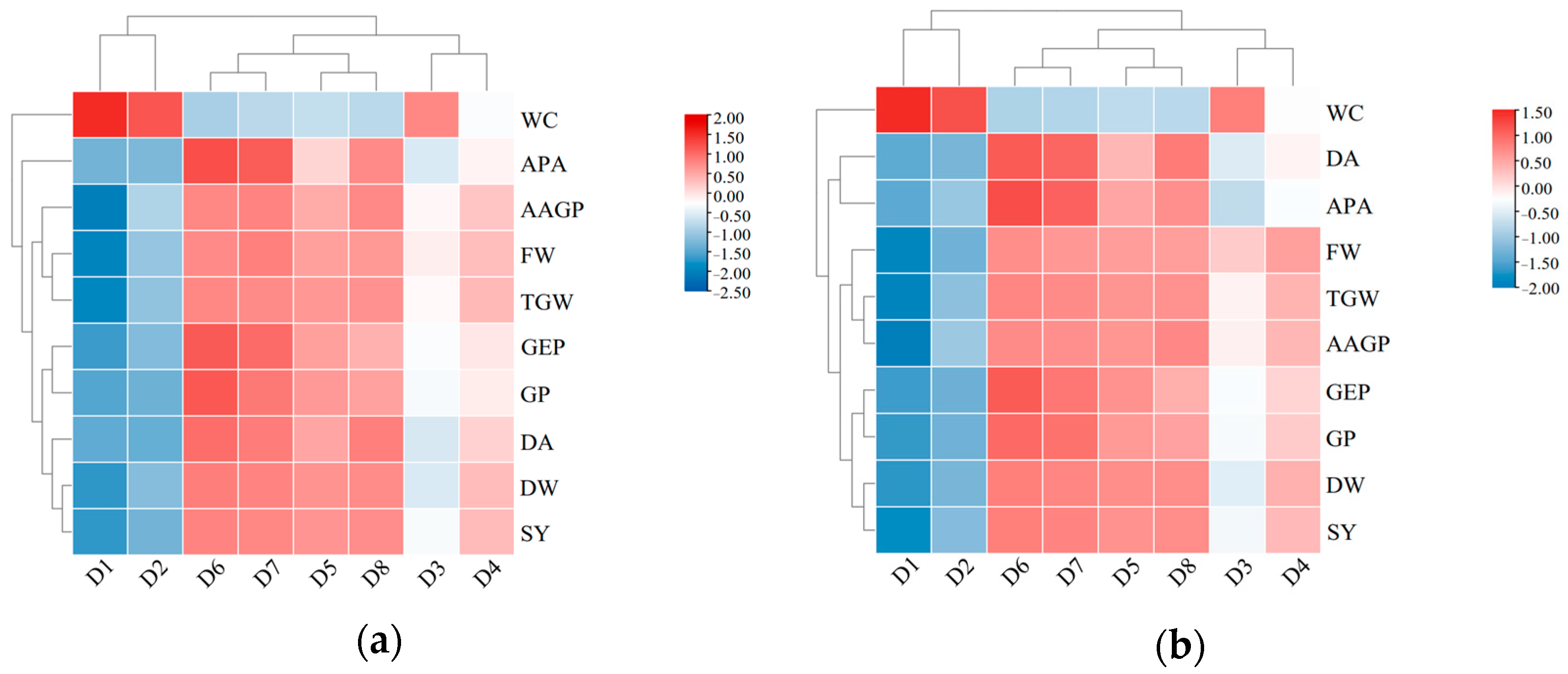
3.5. Analysis of Membership Function and Heatmap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, D.Y.; Chen, X.H.; Gu, H.R.; Zou, M.; Zou, Y.; Fang, J.; Tao, W.J.; Dai, X.Y.; Xiao, S.J.; Wang, Z.J. Chromosomal genome of Triplophysa bleekeri provides insights into its evolution and environmental adaptation. GigaScience 2020, 9, giaa132. [Google Scholar] [CrossRef]
- Liu, M.X.; Che, Y.D.; Jiao, J.; Li, L.R.; Jiang, X.X. Exploring the community phylogenetic structure along the slope aspect of subalpine meadows in the eastern Qinghai–Tibetan Plateau, China. Ecol. Evol. 2019, 9, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Wang, Z.F.; Zhang, Y.L. Crop cover reconstruction and its effects on sediment retention in the Tibetan Plateau for 1900–2000. J. Geogr. Sci. 2017, 27, 786–800. [Google Scholar] [CrossRef]
- Ma, T.; Deng, K.D.; Diao, Q.Y. Prediction of methane emission from sheep based on data measured in vivo from open-circuit respiratory studies. Asian-Australas J. Anim. Sci. 2019, 32, 1389–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atinkut, H.B.; Yan, T.W.; Zhang, F.Y.; Qin, S.Z.; Gai, H.; Liu, Q.Q. Cognition of agriculture waste and payments for a circular agriculture model in Central China. Sci. Rep. 2020, 10, 10826. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, C.C.; Xu, N.; Guo, L.; Wang, D. Quantifying the impact of grain for green program on ecosystem service management: A case study of Exibei region, China. Int. J. Environ. Res. Public Health 2019, 16, 2311. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Chen, J.L. Kengyilia, a new genus of tribe Triticeae of Gramineae from China. J. Sichuan Agr. Univ. 1990, 8, 75–76. [Google Scholar]
- Zeng, J.; Fan, X.; Sha, L.N.; Kang, H.Y.; Wang, Y.; Zhang, H.Q.; Zhou, Y.H. Molecular evolution and diversity of dimeric alpha-amylase inhibitor gene in Kengyilia species (Triticeae: Poaceae). Gene Ams. 2013, 7, 262–268. [Google Scholar] [CrossRef]
- Xiao, B.X.; Zhen, Q.Y.; Chen, Q.; Yang, M.Y.; Zhang, H.X.; Bai, S.Q. Effects of kuoji on broadleaf weeds in the seed field of Kengyilia rigidula. Pr. Sci. 2011, 28, 162–165. [Google Scholar]
- Yuan, S.W.; Wang, Y.T.; Zhang, C.P.; He, H.Z.; Yu, S.B. Genetic dissection of seed dormancy using chromosome segment substitution lines in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1344. [Google Scholar] [CrossRef] [Green Version]
- Ivleva, N.B.; Groat, J.; Staub, J.M.; Stephens, M. Expression of active subunit of nitrogenase via integration into plant organelle genome. PLoS ONE 2016, 11, e160951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eker, T.; Sari, D.; Sari, H.; Tosun, H.S.; Toker, C. A kabuli chickpea ideotype. Sci. Rep. 2022, 12, 1611. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Lamichaney, A.; Joshi, D.C.; Bajwa, A.; Subramanian, N.; Walsh, M.; Bagavathiannan, M. Seed shattering: A trait of evolutionary importance in plants. Front. Plant Sci. 2021, 12, 657773. [Google Scholar] [CrossRef] [PubMed]
- Rodrguez, J.P.; Aro, M.; Coarite, M.; Jacobsen, S.E.; Andreasen, C. Seed shattering of Cañahua (Chenopodium Pallidicaule aellen). J. Agr. Crop Sci. 2017, 203, 254–267. [Google Scholar] [CrossRef]
- Seepaul, R.; Marois, J.; Small, I.; George, S.; Wright, D.L. Optimizing swathing and chemical desiccant timing to accelerate winter Carinata maturation. Agronomy 2018, 110, 1379–1389. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, H.S.; Xiong, J.B.; Cai, H.; Liu, Y. Study on the seed shattering and suitable seed harvesting time of Bromus cartharticus Vahl. Hubei Agr. Sci. 2015, 54, 5969–5972. [Google Scholar]
- Xie, G.P.; Hu, T.M.; Wang, Q.Z.; Miao, Y.J.; Bian, B.Z.M.; Zhu, Y.; Xiong, X.R. A study on the impact of nitrogen application and harvest time on the seed yield of Tibetan wild Elymus nutans in Lhasa valley, Tibet. Acta Pr. Sinica 2010, 2, 89–96. [Google Scholar]
- Mao, P.S.; Han, J.G.; Wu, X.C. Effects of harvest time on seed yield of Siberian wildrye. Acta Agr. Si. 2003, 11, 33–37. [Google Scholar]
- Meehan, P.; Donnell, K.M.; Grant, J.; Finnan, J. The effect of harvest time and pre harvest treatment on the moisture content of Miscanthus × giganteus. Eur. J. Agron. 2014, 56, 37–44. [Google Scholar] [CrossRef]
- Yuan, S.; Ling, Y.; Xiong, Y.; Zhang, C.L.; Sha, L.N.; You, M.H.; Lei, X.; Bai, S.Q.; Ma, X. Effect of nitrogen fertilizer on seed yield and quality of Kengyilia melanthera (Triticeae, Poaceae). PeerJ 2022, 10, e14101. [Google Scholar] [CrossRef]
- Xiao, B.X.; Zhen, Q.Y.; Chen, Q. Experimental study on suitable planting time of Kengyilia rigidula ‘Aba’. Pr. Anim. Husb. 2013, 6, 26–28. [Google Scholar]
- Li, X.; Baskin, J.M.; Baskin, C.C. Seed morphology and physical dormancy of several North American Rhus species (Anacardiaceae). Seed Sci. Res. 1999, 9, 247–258. [Google Scholar] [CrossRef]
- Sinda, B.S.; Jon, G.T.; Collar, C.; Concha, C.; Iker, A.; Fermin, M. Durum wheat grain yield and quality under low and high nitrogen conditions: Insights into natural variation in low- and high-yielding genotypes. Plants 2020, 9, 1636. [Google Scholar]
- ISTA [International Seed Testing Association]. Available online: https://www.seedtest.org/en/publications/handbooks-1170.html (accessed on 19 December 2022).
- Kaewkham, T.; Hynes, R.K.; Suri, B. The effect of accelerated seed ageing on cucumber germination following seed treatment with fungicides and microbial biocontrol agents for managing gummy stem blight by didymella bryoniae. Bio. Sci. Tech. 2016, 26, 1048–1061. [Google Scholar] [CrossRef]
- Adhikari, D. Chromium induces genotoxicity in root tip cells of grass pea (Lathyrus sativus L., Variety Nirmal): A ROS-mediated acute toxicity study. J. Str. Phys. Bio. 2021, 2, 98–120. [Google Scholar]
- Stephen, M.G.D.; Daniel, D.; William, C.P. Purification and characterization of a phosphoenolpyruvate phosphatase from Brassica nigra suspension cells. Plant Phys. 1989, 2, 734–741. [Google Scholar]
- Wang, L.L.; Yang, L.C.; Xiong, F.; Nie, X.Q.; Li, C.B.; Xiao, Y.M.; Zhou, G.Y. Nitrogen fertilizer levels affect the growth and quality parameters of Astragalus mongolica. Molecules 2020, 25, 381. [Google Scholar] [CrossRef] [Green Version]
- Orihara, K.; Akiyama, K. The proper harvest time for ensiling the immature silage corn (Zea mays L.) seeded in summer. Jpn. J. Grassl. Sci. 2018, 64, 108–111. [Google Scholar]
- Dudziak, K.; Bulak, P.; Zapalska, M.; Borner, A.; Szczerba, H.; Lesniowska-Nowak, J.; Nowak, M. Using intervarietal substitution lines for the identification of wheat chromosomes involved in early responses to water-deficit stress. PLoS ONE 2019, 14, e221849. [Google Scholar] [CrossRef]
- Jat, R.S.; Gajbhiye, N.A. Herbage and phytochemicals yield of mandukaparni (Centella asiatica) influenced by time of harvest. Indian. J. Agr. 2016, 2, 242–247. [Google Scholar]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Mineral concentrations of fresh herbage from mixed grassland as influenced by botanical composition, harvest time and growth stage. Anim. Feed Sci. Tech. 2016, 219, 226–233. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N.W. Effects of inorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 2020, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Iris, L.; John, C.B.; Trindade, L.M.; Van, G.C.; Kai-Uwe, S.; Karl, M.S.; Alexander, A.; Chen, C.L.; Oene, D.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Fron. Plant Sci. 2016, 7, 1620. [Google Scholar]
- Su, F.Y.; He, M.D.; Niu, Y.H.; Guo, S.A. Effects of nitrogen fertilizer on seed yield and yield components in artificial Leymus chinensis grassland. J. Plant Nutri. Fert. 2016, 22, 1393–1401. [Google Scholar]
- Sun, T.J.; Han, J.G. Effects of fertilizer application on grass seed yield and physiological and biochemical characters during the seed developing period. Acta Agr. Sinica 2005, 2, 84–85. [Google Scholar]
- Foster, A.; Biligetu, B.; Malhi, S.S.; Gill, K.S.; Mollison, B.; Leach, D. Harvest time and fertility effects on yield and quality of forage from Alfalfa, Hybrid Bromegrass and their mixture. Agr. Sci. 2021, 12, 325–338. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.M.; Han, J.W.; Zhang, L.; Bian, C.S.; Jin, L.P.; Liu, J.G. The estimation of crop emergence in potatoes by UAV RGB imagery. Plant Methods 2019, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Shi, Z.G.; Wan, R.; Li, Y.X.; Wang, Y.J.; An, W.; Qin, K.F.; Cao, Y.L.; Chen, X.Y.; Wang, X.Y.; et al. Impact of phosphorus fertilizer level on the yield and metabolome of goji fruit. Sci. Rep. 2020, 1, 14656. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xue, S.M.; Cui, L.Y.; Xu, C.; Kuang, C.Y.; Huang, M.F. Effect of harvest time and harvest method on seed yield and quality of Brachiariadecumbens ‘basilisk’. Acta Pr. Sinica 2014, 23, 351–356. [Google Scholar]
- Pan, D.F.; Zhang, R.B.; Li, D.M.; Gao, C.; Wang, J.L.; Shen, Z.B.; Li, J.K.; Zhang, R.; Zhang, J.M. Effects of nitrogen application time and harvest time on seed yield and quality of Leymus chinensis. Pr. Sci. 2019, 36, 2078–2086. [Google Scholar]
- Lin, D.R.; Huang, Y.C.; Zhao, J.J.; Wu, Z.J.; Liu, S.L.; Qin, W.; Wu, D.T.; Chen, H.; Zhang, Q. Evaluation of seed nitrate assimilation and stimulation of phenolic-linked antioxidant on pentose phosphate pathway and nitrate reduction in three feed-plant species. BMC Plant Bio. 2020, 20, 267. [Google Scholar] [CrossRef]
- Chen, L.T.; Sun, A.Q.; Yang, M.; Chen, L.L.; Ma, X.L.; Li, M.L.; Yin, Y.P. Relationships of wheat seed vigor with enzyme activities and gene expression related to seed germination under stress conditions. Chi. J. Appl. Ecol. 2017, 2, 609–619. [Google Scholar]
- Wang, Z.F.; Wang, J.F.; Bao, Y.M.; Wang, F.H.; Zhang, H.S. Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. Bio. 2010, 11, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Maryam, S.; Abeer, H.; Ahanger, M.A.; Abd-Allah, E.F.; Alqarawi, A.A.; Alyemeni, M.N.; Ahmad, P.; Gucel, S. Mitigation of NaCl stress by arbuscular mycorrhizal fungi through the modulation of osmolytes, antioxidants and secondary metabolites in mustard (Brassica juncea L.) plants. Fron. Plant Sci. 2016, 7, 869. [Google Scholar]

| Index | Source of Variance | Df | F-Ratio | p-Value |
|---|---|---|---|---|
| WC (%) | Year (Y) | 1 | 0.074 | 0.787 |
| Days after peak anthesis (D) | 7 | 658.684 | 0.000 | |
| Interaction Y × D | 7 | 0.505 | 0.826 | |
| SY (kg·hm−2) | Year (Y) | 1 | 0.0380 | 0.845 |
| Days after peak anthesis (D) | 7 | 2315.693 | 0.000 | |
| Interaction Y × D | 7 | 0.173 | 0.990 | |
| TGW (g) | Year (Y) | 1 | 0.075 | 0.785 |
| Days after peak anthesis (D) | 7 | 1646.262 | 0.000 | |
| Interaction Y × D | 7 | 0.795 | 0.595 | |
| GEP (%) | Year (Y) | 1 | 0.004 | 0.952 |
| Days after peak anthesis (D) | 7 | 73.023 | 0.000 | |
| Interaction Y × D | 7 | 0.148 | 0.994 | |
| GP (%) | Year (Y) | 1 | 0.065 | 0.800 |
| Days after peak anthesis (D) | 7 | 63.310 | 0.000 | |
| Interaction Y × D | 7 | 0.293 | 0.953 | |
| AAGP (%) | Year (Y) | 1 | 0.064 | 0.832 |
| Days after peak anthesis (D) | 7 | 70.345 | 0.000 | |
| Interaction Y × D | 7 | 0.253 | 0.729 | |
| DA (µg·mL−1) | Year (Y) | 1 | 0.055 | 0.815 |
| Days after peak anthesis (D) | 7 | 131.304 | 0.000 | |
| Interaction Y × D | 7 | 0.588 | 0.369 | |
| APA (nmol·min−1. 50 seeds) | Year (Y) | 1 | 0.027 | 0.870 |
| Days after peak anthesis (D) | 7 | 355.659 | 0.000 | |
| Interaction Y × D | 7 | 0.688 | 0.681 |
| Year | Days after Peak Anthesis | GEP (%) | GP (%) | AAGP (%) | DA (µg·mL−1) | APA (nmol·min−1. 50 seeds) |
|---|---|---|---|---|---|---|
| 2016 | D1 | 69.50 e | 67.00 e | 21.50 f | 9.49 e | 1.24 e |
| D2 | 72.00 d | 68.00 e | 34.50 e | 9.59 e | 1.27 e | |
| D3 | 77.50 c | 74.50 d | 45.00 d | 11.51 d | 1.56 d | |
| D4 | 79.00 c | 76.00 d | 53.50 c | 13.46 c | 1.74 cd | |
| D5 | 83.00 b | 81.50 bc | 58.50 b | 14.66 b | 1.87 c | |
| D6 | 87.00 a | 85.00 a | 66.00 a | 16.23 a | 2.50 a | |
| D7 | 86.00 a | 83.00 b | 67.00 a | 15.83 a | 2.43 a | |
| D8 | 82.00 b | 80.50 c | 65.50 a | 15.73 ab | 2.21 b | |
| 2017 | D1 | 70.00 f | 65.50 f | 20.00 f | 9.40 f | 1.36 d |
| D2 | 71.50 e | 67.50 e | 33.50 e | 9.72 f | 1.51 d | |
| D3 | 77.50 d | 74.00 d | 45.50 d | 11.47 e | 1.62 cd | |
| D4 | 80.00 c | 77.50 c | 55.50 c | 12.44 d | 1.82 c | |
| D5 | 83.50 ab | 80.50 b | 62.50 b | 13.97 c | 2.18 b | |
| D6 | 86.50 a | 83.50 a | 65.00 a | 16.52 a | 2.55 a | |
| D7 | 85.00 a | 83.00 a | 64.00 ab | 16.19 a | 2.46 a | |
| D8 | 82.00 b | 80.00 b | 65.50 a | 15.60 b | 2.27 b |
| Parameter | Days after Peak Anthesis | |||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | |
| WC (%) | 0.00 | 0.17 | 0.37 | 0.79 | 0.94 | 1.00 | 0.96 | 0.96 |
| FW (g) | 0.00 | 0.31 | 0.65 | 0.80 | 0.90 | 0.97 | 1.00 | 0.92 |
| DW (g) | 0.00 | 0.19 | 0.42 | 0.77 | 0.92 | 1.00 | 0.98 | 0.95 |
| SY (kg·hm−2) | 0.00 | 0.10 | 0.45 | 0.74 | 0.92 | 1.00 | 0.98 | 0.95 |
| TGW (g) | 0.00 | 0.24 | 0.55 | 0.79 | 0.93 | 1.00 | 0.98 | 0.95 |
| GEP (%) | 0.00 | 0.14 | 0.46 | 0.54 | 0.77 | 1.00 | 0.94 | 0.71 |
| GP (%) | 0.00 | 0.06 | 0.42 | 0.50 | 0.78 | 1.00 | 0.89 | 0.75 |
| AAGP (%) | 0.00 | 0.29 | 0.52 | 0.70 | 0.81 | 0.98 | 1.00 | 0.97 |
| DA (µg·mL−1) | 0.00 | 0.01 | 0.30 | 0.59 | 0.77 | 1.00 | 0.94 | 0.93 |
| APA (nmol·min−1. 50 seeds) | 0.00 | 0.02 | 0.25 | 0.40 | 0.50 | 1.00 | 0.94 | 0.77 |
| Rank | 8 | 7 | 6 | 5 | 4 | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Yuan, S.; Xiong, Y.; Chen, S.; Feng, J.; Zhao, J.; Zhang, C.; Lei, X.; You, M.; Bai, S.; et al. Effect of Harvest Time on the Seed Yield and Quality of Kengyilia melanthera. Agronomy 2023, 13, 55. https://doi.org/10.3390/agronomy13010055
Ling Y, Yuan S, Xiong Y, Chen S, Feng J, Zhao J, Zhang C, Lei X, You M, Bai S, et al. Effect of Harvest Time on the Seed Yield and Quality of Kengyilia melanthera. Agronomy. 2023; 13(1):55. https://doi.org/10.3390/agronomy13010055
Chicago/Turabian StyleLing, Yao, Shuai Yuan, Yanli Xiong, Shuming Chen, Junjie Feng, Junming Zhao, Chenglin Zhang, Xiong Lei, Minghong You, Shiqie Bai, and et al. 2023. "Effect of Harvest Time on the Seed Yield and Quality of Kengyilia melanthera" Agronomy 13, no. 1: 55. https://doi.org/10.3390/agronomy13010055





