Abstract
Mung bean residues stimulate the hatching of soybean cyst nematode (SCN). In our previous study, combined incorporation of mung bean residues and biochar into soil can be effective in suppression of the soybean cyst nematode (SCN), Heterodera glycines, in the upper layer soil. However, there are no data available as to whether such effects are transmissible, and could for example be manifest in subsoil zones where such incorporation is confined to topsoils, via water-based pathways. We evaluated the effects of leachate passage from a biochar-amended soil in an upper soil zone to a lower zone in a microcosm-based system, upon a range of physicochemical properties and density of SCN. Disturbed soil was filled in a total of 9 cylindrical cores with two layers. The upper layer (0–15 cm) was amended with biochar at rates equivalent to 0, 0.3% or 1.8%, with bulk density set at of 1.1 g cm−3. The lower layer (15–25 cm) without biochar amendment was compacted to 1.2 g cm−3. Mung beans were grown for two weeks and incorporated into the upper layer. Water was surface-applied to the cores 4, 6, and 8 weeks after mung bean incorporation. After 16 weeks, the upper and lower layer soils were separately collected and assayed. The presence of biochar in the upper layer reduced the abundance of free-living nematodes, mainly bacterivorous, but increased that of a predator genus Ecumenicus in this zone. In the lower layer of soil under a biochar-amended upper layer, available P and soluble cations were increased as were abundances of total nematodes including Ecumenicus, resulting in greater maturity index, basal and structure indices. Notably, SCN density was decreased in lower zones by more than 90% compared to zero-biochar controls. This demonstrates that the effects of biochar upon soil properties, including impacts on biota and plant pathogens, are transmissible.
1. Introduction
Biochar is often vaunted—and is being increasingly applied as a soil amendment for use in agriculture, such as the remediation of saline soils [1] and carbon sequestration [2,3], as well as increased crop productivity [4,5], and as an agent for biological control of plant diseases [6]. In terms of the environment, biochar amendment to soil can mitigate global warming potential via C-sequestration and modulation of the nitrogen cycle, via associated soil microbiomes [7,8] to reduce emissions of NH3, N2O [9,10] and CH4 [11]. Importantly, biochar can reduce losses of minerals including N, P and K [12,13] and hence minimize environmental pollution [14,15]. Furthermore, biochar can act as a fertilizer source of these elements.
Plant-parasitic nematodes are a serious threat to a diverse range of crops and food security [16]. Soybean cyst nematode (SCN), Heterodera glycines, is a major pest of soybean that causes significant yield losses worldwide [17]. The concern is that it is difficult to control SCN because of the nature of their life cycle, range of host plants, and apparently inherent ability to adapt to changes in climatic conditions [18,19]. Hence, an improved understanding of the stimulation and inhibition phenomenon which govern propagation—especially hatching of cysts of SCN is requited [20]. Integrated practices that could suppress SCN have been proposed, including the combination of chemical and biological control. For instance, cover crops and double-cropping systems can be efficient practices to suppress the abundance of SCN [21,22]. It is considered that control strategies, which target the induction of hatching of cysts in the absence of main (crop) hosts such that emergent juveniles (so-called J2 stage) starve, offer strong potential. We recently reported that mung bean cultivation and its incorporation to soil decreased the abundance of SCN, improved soil properties, promoted free-living nematode communities [23,24]. Therefore, the use of green manure to control the infestation of SCN may be an effective practice in wider terms.
SCN can form a dormant stage under extreme environmental conditions, but can rapidly become active and highly prolific when the conditions become suitable [18,19]. In the subsoil zone, especially when compacted to produce a hardpan layer induced by intensive trafficking, mono-cropping and fertilization [25], root distribution [26], plant residue incorporation and nematode movement [27] are often restricted. The subsoil zone may therefore be relatively hostile to SCN colonization. Our recent study found that the application of rice husk biochar in combination with mung bean cultivation as green manure markedly decreased SCN populations via induced suppressive mechanisms related to the persistence of hatching-stimulants [23], but it is unclear what effects such treatment has upon the subsoil system. Given that the top- and sub-soil compartments are spatially separated, it is arguable that there may be little impact. However, under conditions where water is transmitted from upper to lesser layers, energy-containing substrate (particulate and dissolved organic carbon), mobile mineral nutrients, and biologically active molecules (including hatching stimulants) may induce microbial activity and affect SCN populations. We tested this hypothesis experimentally in a controlled column-based study utilizing a rice-husk biochar and light-clay soil supporting mung bean plants.
2. Materials and Methods
2.1. Biochar Preparation
Biochar was prepared from rice husk as previously described [23]. Briefly, rice husk (17 g) was filled into a steel core of 100 cm3, pressed to remove inside air as much as possible, and closed by lids. Cores containing rice husk were placed in an electric furnace for the pyrolysis process with a temperature of 300oC and a combustion time of 2 h. The yield of biochar was 9.0 g. The basic properties of biochar are reported in our previous study [23]: pH (H2O) (1:5) 7.1; total C 406 g kg−1; total N 5.73 g kg−1; C/N ratio 70.8; available phosphorus (Bray II) 72.6 mg kg−1. Exchangeable cations of Na, K, and Ca were 2.83, 0.83, 28.7 meq 100 g−1, respectively.
2.2. Soil Preparation
An infested soil with SCN was collected from a commercially managed field in Saitama Prefecture, Japan (35°50′ N, 139°51′ E) and evenly mixed through a 2 mm sieve. Soil was a low humic Andosol and light clay of soil texture (42% sand, 28% silt, and 30% clay) [24]. Basic soil properties were pH (H2O) (1:5) 6.6; EC (1:5) 1.1 mS cm−1; NH4-N 4.6 mg kg−1; NO3-N 162 mg kg−1; total C 22.2 g kg−1; total N 2.13 g kg−1, C/N ratio 10.4, available phosphorus (Bray II) 49.5 mg kg−1, exchangeable cations of Na, K, Ca were 0.05, 2.83, 1.6 meq 100 g−1, respectively, and CEC 6.1 meq 100 g−1 [23].
2.3. Soil Columns
A transparent polyvinyl chloride (PVC) column with 30 cm in length, 4.8 cm in diameter and closed bottom was used to simulate a soil profile. Each PVC column was filled with the same amount of disturbed soil without biochar amendment at the bottom (15 to 25 cm depths) and compacted to a bulk density of 1.2 g cm3. Deionized water (DI) was added to adjust to 60% maximum water holding capacity (MWHC) of soil, a circle of 2 cm diameter sieve was placed, and a piece of filter paper was placed on the sieve to separate with the upper layer. The upper layer was created by adding biochar-amended and unamended soils to reach a bulk density of 1.1 g cm−3 up to the surface of 15 cm depth (0 to 15 cm), with the amount of biochar at 0 (B0), 0.3% (B5), and 1.8% (B30), which are approximately equivalent to field rates of 0, 5 and 30 Mg ha−1 (a soil depth of 15 cm, bulk density: 1.10 g cm−3) in the upper layer. The maximum water holding capacity of B0, B5, and B30 were 0.80, 0.84, and 0.91 g g−1, respectively. The upper layer was added with DI water to adjust to 60% MWHC. Three soil columns for each treatment and a total of 9 soil columns were prepared.
2.4. Mung Bean Cultivation
Two seeds of mung bean (Vigna radiata (L.) Wilczek ‘Greenmappe’, Nakahara Seed Co., Fukuoka, Japan) were sown in each column and grown for 2 weeks in a Biotron (NK System (Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan), 1500 to 1700 lux) at 25 °C. Whole mung bean residue (shoots and roots) was cut into pieces with a few cm length and incorporated into 0 to 10 cm depth in the soil column. Soil columns were placed in room conditions at 25 °C and watered by DI to keep the initial water content until the leaching processes started.
2.5. Leaching
At 20 days after mung bean residue incorporation, the first leaching was performed by adding DI water to produce saturated condition in the upper layer, and an extra amount of 20 mL DI water was added to the soil surface, by which leached water from the upper layer were percolated to the lower layer to give the MWHC of 0.80 g g−1. The second and the third leaching processes were repeated with the same amount of DI water 6 and 8 weeks after mung bean residue incorporation. All soil columns were kept at room conditions (around 25 °C) with an aluminum cover on top until soil collection at 16 weeks and without further water addition.
2.6. Soil Sampling and Analysis
Soil was separately collected from depths of 0 to 15 cm and 15 to 25 cm after 16 weeks of incorporation. In upper layer, chemical properties, such as NH4-N, NO3-N, and soluble cations were analyzed. The nematode community of the main genus and bacterivorous feeders were separately counted. In the lower layer, available P, NH4-N, NO3-N and soluble cations were measured. The nematode communities, SCN density and microbial activity and some enzyme activities were also measured. The moist soils were used to analyze the chemical properties and microbial activity and a part of soil was air dried for enzyme activities.
2.6.1. Chemical Properties
Available P was determined by the Bray-II method [28]. Inorganic nitrogen (NH4+-N, NO3−-N) was extracted by using 2M KCl solution in a ratio of 1:10, sharking for 1 h, centrifuged at 7800 rpm for 5 min, and filtering through ADVANTEC 5C filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Ammonium was analyzed with the indophenol-blue method [29] and nitrate was measured with a spectrophotometer at a wavelength of 220 nm and subtracted the absorbance at a wavelength of 260 nm [30]. Soluble cations (K, Ca, Na) were analyzed by extracting a soil sample (2.0 g) with 20 mL deionized water, and shaking for 1 h at 120 rpm. The mixture was centrifuged at 7800 rpm for 5 min and passed through a filter paper (Advantec No. 5C) and ions in the filtrate were determined with a flame photometry (Flame Photometers, BWB, Newbury, UK).
2.6.2. Microbial Activity and Enzyme Activities
Microbial activity was measured as the CO2 respired from 10.0 g moist soil. Soil was put into a vial of 30 mL volume. An amount of 0.5 mL air in the headspace was taken immediately after closing the lid and injected into a TCD-GC (GC-8A, Shimadzu, Kyoto, Japan). Then, the soil was incubated at 25 °C for 3 h in dark conditions and 0.5 mL of the headspace was taken again. A 0.5 mL gas of pure CO2 was used as a standard. The differences in CO2 concentrations between 0 and 3 h were considered as the microbial activity.
The method of enzyme activities was followed by the method of Tabatabai [31] by using soil after 16 weeks of incubation. To determine β-glucosidase activity, 1.0 g of air-dry soil was placed into a 50 mL Erlenmeyer flask and incubated at 37 °C for 1 h with 0.25 mL toluene, 4 mL of modified universal buffer (MUB) with pH 6.0, and 1 mL of p-nitrophenyl-β-D-glucosidase solution. Subsequently, 0.1 M calcium chloride solution and 0.1 M of tris (hydroxymethyl) aminomethane solution (pH = 12) were added, and the soil suspension in the flask was passed through filter paper (Advantec No. 5C). The supernatants were measured at 410 nm. For acid phosphatase (ACP) and alkaline phosphatase (ALP) activities, 0.2 mL of toluene, 4 mL MUB (pH = 6.5 for assay of ACP and pH = 11 for assay of ALP), 1 mL PNP (p-nitrophenyl phosphate) solution was added to 1.0 g of air-dry and incubated at 37 °C. After 1 h, 1 mL of 0.5 M CaCl2 and 4 mL of 0.5 M NaOH were added and the mixtures were filtered through filter paper (Advantec No. 5C). The produced PNP was determined at 410 nm.
2.6.3. Nematode Extraction
Nematodes were extracted with the Baermann’s funnel method [32]. Briefly, 20 g of moist soil from 9 columns (three replicate columns each treatment) was placed in triplicate on one-layer tissue paper (Kimwipes S-200, Nippon Paper Crecia Co., Ltd., Tokyo, Japan) supported by a sieve (65 mm diameter and 1 mm mesh size). Then, the sieve was placed in a plastic funnel filled with tap water and kept at room temperature (ca. 25 °C) for 72 h. Total nematodes were counted and at least 100 individuals were randomly picked and mounted in a drop of glycerol on a glass slide, and sealed with a paraffin ring for nematode identification [33]. Nematodes were identified up to a genus level by using the significant books for terrestrial and freshwater nematodes [34,35,36] at a x400 or x1000 magnification by using microscopes (Carton-CS model, Nikon Eclipse TS2 (Nikon Corporation, Tokyo, Japan), and Olympus BX50 (Olympus Corporation, Tokyo, Japan) with the support of camera systems: Olympus U-TV0.5XC-3, Olympus DS21). Metabolic footprints were calculated using the NINJA online program at https://sieriebriennikov.shinyapps.io/ninja/, accessed on 28 July 2022 [37]. Nematode metabolic footprints quantify carbon utilization by different food web components and provide information on energy flow through various trophic groups, which gives additional descriptive information on the food web form and soil functions [38].
2.6.4. DNA Assay for Quantifying the Density of Soybean Cyst Nematode
Soils collected from columns were dried immediately at 60 °C for 24 h. The dry soil (20 g) was pulverised for 2 min at 45 m s−1 using a ball mill (Fast Prep; MP Biomedicals, Tokyo, Japan). Then, DNA was extracted in duplicates using the pulverized soil (0.5 g) following the method by Sato et al. [39]. The extracted DNA including all forms of SCN, that is adults, eggs and J2, was suspended in 100 μL of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) and used as a template after ten-fold dilution. Real-time PCR was performed using a Step One System (Life Technologies Japan) in a final volume of 10 μL [5.0 μL of a Fast SYBR Green Master Mix (Thermo Fisher Scientific, Yokohama, Japan); 0.4 μL of each primer, SCNnew-f (5′-CTGCACATGTGAAAGCCTGTGTA-3′)] and SCNnew-r (5′-GAGCGTGCATCCCACATTG -3′) [40]; 2.2 μL sterilized deionized water; and 2.0 μL of template DNA and amplified under the manufacturer’s recommended conditions [(95 °C for 10 s; 95 °C for 5 s; and 60 °C for 20 s) × 40 cycles].
2.7. Statistical Analysis
To test the effects of biochar application rates on soil parameters of each layer separately, one-way ANOVA was performed. The assumption of Levene’s test was performed for homogeneity of variance. Post hoc test was used to statics the significant differences among treatments by Tukey HSD at p < 0.05. To demonstrate the association of nematodes community composition, trophic structure, and functional guilds with soil properties, a principal component analysis (PCA) was performed based on the entire abundance of nematodes genera, trophic structure, and functional guilds with soil properties. PCA using PRIMER version 6 [41] was run on a full set (normalized data) of nematodes abundance or soil properties to get the same metric for all variables. The correlation of the abundance of each nematode genus and trophic structures to soil properties was performed. All statistical analyses were run by using the statistical package STATISTICA version 7 and Minitab version 16.
3. Results
3.1. Effects of Biochar Amendment to Upper Layer on the Chemical Properties in Upper and Lower Layer Soils
In the upper layer, biochar reduced significantly NH4-N by 76% at B5 and by 75% at B30 (Figure 1A), and reduced NO3-N by 54% at B30 compared to B0 (Figure 1B). The concentration of K in B30 was greater by 26% than that in B0 (Figure 1C), while soluble Ca was lesser in B30 by 20% to 30% than in B5 and B0 (Figure 1D). Biochar did not affect soluble Na in the upper layer (Figure 1E). In the lower layer, the concentration of NO3-N was lesser by 30% to 60% in B30 than in B5 and B0 (Figure 1B). There was no difference in NH4-N among treatments in the lower layer. In the lower soil, the available P was 1.5 times greater in B30 than in B0 (Figure 2).
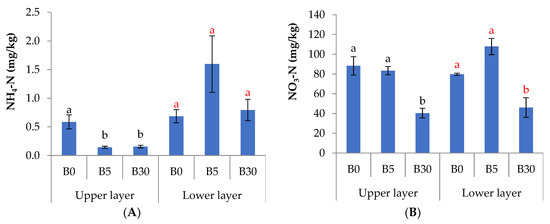
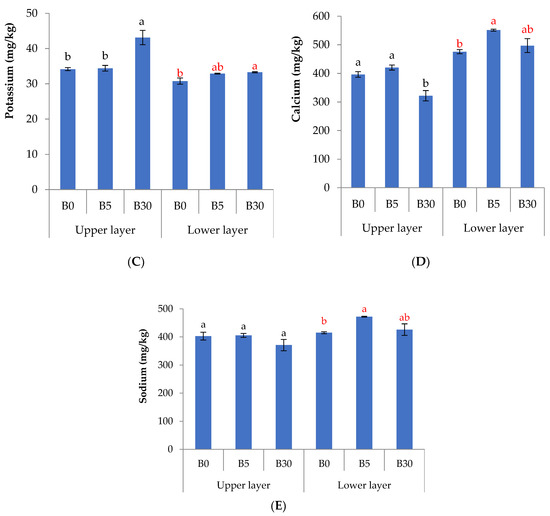
Figure 1.
Soil chemical properties in upper and lower layers with 0.3% (B5), 1.8% (B30) or without (B0) biochar amendment to the upper layer; ammonium nitrogen (N-NH4) (A), nitrate nitrogen (N-NO3) (B), soluble K (C), soluble Ca (D), and soluble Na (E). Bars present the means and standard errors (n = 3). Different letters indicate the significant differences (p < 0.05) among treatments of each upper layer and lower layer by Tukey’s HSD test.
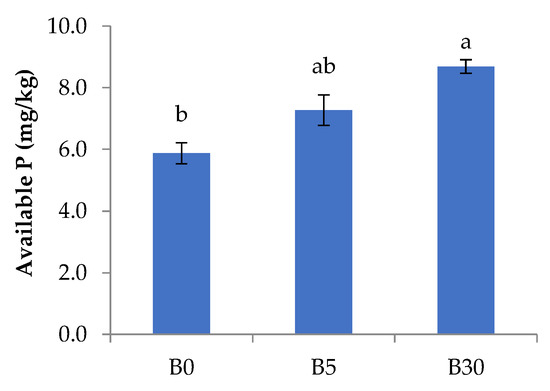
Figure 2.
Available phosphorus in soil of lower layer under biochar amendment to the upper layer. Bars indicates the means and standard errors (n = 3). Different letters indicate the significant difference (p < 0.05) among treatments by Tukey’s HSD test.
3.2. Effects of Biochar Amendment to Upper Layer on the Nematode Abundance and Composition in Upper Layer Soil
In upper layer, the abundances of total nematodes and bacterivorous feeders were significantly two times lesser in B5 and B30 than in B0 (Table 1). Those of Ecumenicus and Heterodera glycines J2 tended to be greater in B5 and B30, while abundance of Aphelenchus tended to be lesser in B5 and B30 than in B0.

Table 1.
Total abundance of total nematodes, bacterivorous feeders and dominant genera in upper layer with 0.3% (B5), 1.8% (B30) or without (B0) biochar amendment and corresponding lower soils layer received having no biochar amendment after 16 weeks of mung bean residue incorporation.
3.3. Effects of Biochar Amendment to Upper Layer on the Nematode Abundance and Composition in Lower Layer Soil
3.3.1. Nematode Community, Nematode Indices, and Metabolic Footprint
The total abundance of nematodes varied from 60 to 80 individuals per 100 g of dry soil, and there was no significant difference among treatments (Figure 3A). A total of 8 genera were recorded including Acrobeloides, Aphelenchus, Aporcelaimellus, Cephalobus, Ecumenicus, Mesodorylaimus, Mesorhabditis, and Heterodera glycines (Figure 3A). Among them, Aphelenchus and Ecumenicus were the most dominant genera, accounting for 36% and 33% of total abundance of nematodes in the lower layer, respectively. The abundance of free-living nematodes was greater (p < 0.05) in B5 and B30 than in B0, and Ecumenicus showed greater abundance (p < 0.01) in B5 and B30 than in B0 (Figure 3A). The abundance of Heterodera glycines J2 ranged from 5 ± 6 in B5 to 20 ± 9 individuals per 100 g of dry soil in B0 and it tended to be greater in B0 (Figure 3A). The maturity and structure indices were greater in B5 and B30 than in B0, while the basal index was greater in B0 than in B5 and B30 (Figure 3B). Metabolic footprint was greater in B5 and B30 than in B0 (Figure 3C).
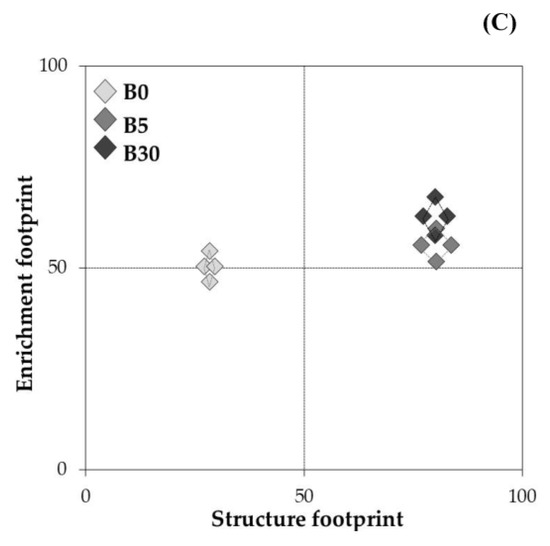
Figure 3.
Abundance of total nematodes and nematode-related ecological indicators in the lower layer under biochar amended 0.3% (B5), 1.8% (B30) and not-amended upper layer (B0): total nematodes and community composition (A), nematode indices (B), and metabolic footprint (C) after 16 weeks of mung bean residue incorporation. Bars present the average and standard error (n = 3). Different letters present the significant differences (p < 0.05) among treatments by Tukey’s HSD test. The metabolic footprint of nematodes presents the C sources that use for assimilation and respiration through nematodes community [38]. Bigger area size indicates the more energy transform through nematode.
3.3.2. Density of Soybean Cyst Nematode
PCR assay showed that the SCN density per 20 g dry soil was markedly lesser in B5 and B30 than in B0 (Figure 4).
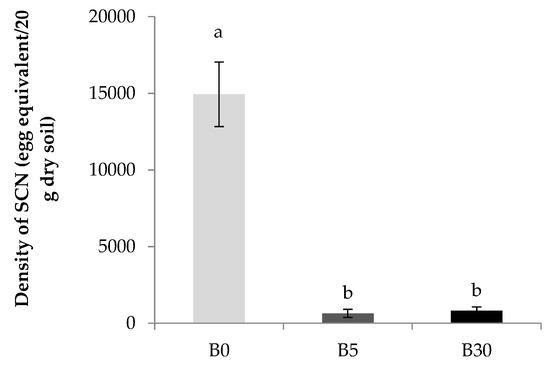
Figure 4.
Density of soybean cyst nematode (SCN) (egg equivalent based on the Ct value in real-time PCR assay 1 in the lower layer soil under biochar amended 0.3% (B5), 1.8% (B30) and not-amended upper layer (B0) after 16 weeks of mung bean residue incorporation. Bar indicates average and standard error (n = 3). Different letters present the significant differences among treatments from Tukey HSD test at p < 0.05 by one-way ANOVA. 1 Calibration equation: y = −2.82x + 34.5, R2 = 0.9782; y: Ct value; x: log10SCN 20 g−1) [40].
3.3.3. Microbial Activity and Enzyme Activity
Biochar tended to increase microbial activity, while there were no differences in glucosidase, acid phosphatase and alkaline phosphatase among treatments (Table 2).

Table 2.
Microbial activity, and enzyme activity of glucosidase, phosphatase of lower layer under biochar amended 0.3% (B5), 1.8% (B30) and not-amended in the upper layer (B0) after 16 weeks of mung bean residue incorporation.
3.4. Principal Component Analysis
PC1 strongly separated upper from lower soil layers, a phenomenon distinctly associated with chemical versus nematode population related loading factors (Figure 5). There was a consistent trajectory in PC2 with respect to biochar concentration in the upper soil zone, but less so in the lower zone. In the former, this was driven by three specific nematode genera—notably Ecumenicus—and potassium versus general nematode population attributes. The trend in PC2 for biochar-containing treatments was consistent, but PC2 was quite distinct for zero-biochar systems.
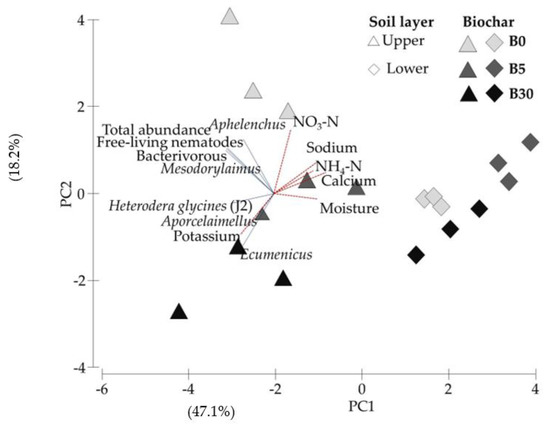
Figure 5.
Ordination of first (PC1) and second (PC2) principal components derived from analysis of whole soil properties (combined data of upper and lower layer soils) subject to biochar amendment in the upper layer at 0 (B0), 0.3% (B5) and 1.8% (B30). Percent variations accounted for by PC are given in parentheses on axis labels.
4. Discussion
4.1. Effects of Biochar Amendment to Upper Layer on the Chemical Properties in Upper and Lower Layer Soils
In the present study, rice husk biochar increased K in B30 in both upper and lower layers. This result can be explained that K in biochar could be easily released into the soil as an available form. Biochar is a good soil conditioner and is recommended to apply to the soil for enhancing soil properties and crop productivity due to its effects on soil properties [42,43] including total C [44], K [45], soil porosity [46], and water availability [47]. By contrast, the addition of biochar decreased NH4-N, NO3-N, and Ca in B30 in the lower layer. One possible reason is that biochar addition reduced the NO3-N concentration in the upper layer, therefore it may result in the reduction of inorganic N leaching to the lower layer. A previous study reported that the application of biochar reduced NO3-N concentration by 13% and up to 26% [10]. In the present study, biochar reduced inorganic N leaching, particularly in NO3-N, and inorganic N in the lower layer, and the result can be explained by various possible mechanisms. Firstly, biochar enhanced soil water content at 60% MWHC, it may be a factor that might decrease nitrification due to low oxygen conditions [13]. This tendency is also supported by previous studies that biochar increased saturated hydraulic conductivity [45,48] and water retention [49]. Another reason is that biochar absorbs ammonium [50], resulting in less nitrification. Another factor may be due to biochar-induced modulation of microbial communities related to N cycling. For instance, biochar enriched the main group of the nitrifier community such as Nitrobacter alkalicus and Nitrobacter vulgaris [51,52]. In our study, biochar was produced at 300oC and contained labile organic C of 195 mg kg−1, a source that can increase C mineralization and thereby may increase the immobilization of inorganic N in soil. Zimmerman et al. [53] reported that biochar produced at lower temperatures (250–400 °C) increases C mineralization, and Abell et al. [54] revealed a positive correlation between C mineralization and the reduction of accumulative NO3-N in soil. A greater concentration of labile C in the soil can stimulate microbial activity and affect nutrient dynamics that lead to enhanced N immobilization [55]. PCA confirmed that the most substantial effect was between layers, which would be expected given the substrate and biochar addition to the upper layer. However, PC2 revealed that biochar concentration effects were essentially consistent between layers, suggesting transmissible phenomena.
4.2. Biochar Reduced Bacterivorous in the Upper Layer but Stimulated Greater Trophic Guilds in the Lower Layer
Biochar can have negative and positive effects on soil quality, expressed as biological properties, resulting in the modification of communities [2,7,56], including the potential adverse risks [57]. In terms of positive effects, in the present study, biochar enhanced the quality of the lower layer, i.e., abundances of free-living nematodes and a dominant predator Ecumenicus, in spite that biochar was not directly applied to the lower layer. Many papers have already reported positive effects on the top soil where biochar was applied, including nematodes. For instance, rice husk biochar increased bacterial activity [43,58], and microbial biomass C and N [58], and promoted the omnivorous nematodes at a low amount of biochar [59].
In terms of negative effects; in the present study; biochar decreased the abundance of bacterivorous nematodes in the upper layer; a most dominant group; resulting in a lower total abundance of nematodes in biochar-amended soil. This result may be explained that biochar can contain toxic compounds; such as PAHs; among the carbonized materials [60] that may inhibit the growth of Caenorhabditis elegans; a bacterivorous nematode by reducing their offspring [61]. On the other hand; the adverse effects of biochar on soil organism communities have been reported as contrasting effects on the trophic structure of nematodes [62] and other soil organisms. For instance; biochar reduced the total abundance of nematodes (bacterivorous; fungivorous; and herbivorous feeders); the abundance of a springtail Folsomia candida and amoebae [58], and the abundance of nematodes in high functional guilds [23], while opposite results were reported in the abundance of other soil organisms such as an enchytraied Enchytraeus crypticus [63] and flagellates [58]. The effects of biochar on the nematode community may be altered by the modification of food resources; like microbial community under biochar amendment [64], a preference behavior of nematodes in soil habitats [65,66]. In contrast to bacterivorous nematodes; the abundance of a predator species; Ecumenicus increased in the soil treated with the highest biochar addition. It is consistent with our previous study in which biochar and mung bean incorporation stimulated the abundance of greater trophic levels of predator and omnivorous nematode species such as Ecumenicus and Aporcelaimellus; respectively [23]. Soil compaction reduced the abundance of omnivores/predator nematodes [67]. Biochar could improve soil physical properties such as porosity [68], total pore volume [48], and water content and capillary suction [1] that may support the survival and growth of Ecumenicus by inducing suitable conditions of their movement. The considerably greater abundance of nematodes in the upper layer demonstrated the extent to which nematodes were substrate-limited in the basal soil.
4.3. Effect of Biochar Amendment to Upper Layer on the Density of Soybean Cyst Nematode (SCN) in the Lower Layer Soil
It is well reported that hatching of SCN is stimulated by natural biological and artificial synthesized compounds [69], mung bean residue [23,70] as well as glycinoeclepin A [71] and zinc [72]. Most studies have tested the direct effects on hatching in soil or solution to which hatching stimulus was added. However, whether or not hatching stimulus added to upper layer affects SCN in the lower layer is unknown. In the present study, the biochar addition in the upper layer reduced the SCN density in the lower layer soil, indicating indirect effects through leachate from the upper layer to the lower layer. For mechanisms why the SCN density in the lower layer decreased, we hypothesized the modification of lower layer properties by the effect of biochar amendment to the upper layer. Firstly, in terms of soil chemical properties, biochar addition to upper layer increased K and P in the lower layer. The modified soil properties may stimulate the hatching of SCN and hatched J2 starve to death in the lower layer under conditions of no host plants, resulted in the reduction of the SCN density. The correlation results revealed that the SCN density in the lower soil corresponded to moisture contents, available K and P and abundance of Ecumenicus in the lower soil (Tables S1 and S2). In general, Ca content contributes to the stimulation of hatching of cyst nematodes because it is a major component of egg cells [73,74,75]. Moreover, the greater concentration of K in the lower layer could also be a factor involved in hatching. For instance, a previous study found that the application of K fertilizer reduced the infestation of SCN in field conditions [76]. Because hatching depends on many factors, like types of biochar, addition amount, and the age of cyst, the effects of biochar on the hatching may have contrasting effects. Li et al. [77] reported that the concentration of Na, K, and Ca did not affect the hatching of SCN, but it has indirect influences from the osmotic mechanisms [19]. Secondly, in terms of biological interactions, our previous study revealed that the incorporation of green manure suppressed SCN partly because it improved soil properties by increasing the abundances of a predator nematode Ecumenicus and an omnivorous Aporcelaimellus [23]. In the present study, we found that biochar amendment to the upper layer increased the abundance of Ecumenicus in both upper and lower layers. We can suggest that the increase of the predator Ecumenicus might be a factor that reduces the abundance of SCN due to top-down effects. Ecumenicus feeds by piercing prey using a hollow stylet similar to Aporcelaimellus and differs from other predators as it possesses an ingester that feeds by taking prey into the mouth and using teeth and/or denticles to access the contents [78]. We hypothesized that Ecumenicus may feed eggs or hatched-J2 inside cyst by directly piercing through cyst wall. However, the further tests should be done to clarify whether or not the feeding behavior of Ecumenicus and other pierce predators to unhatched cyst. The fact that an increase in predator and omnivorous nematodes could suppress the population of plant-parasitic nematodes via the prey-predators interaction [79,80] supports this hypothesis like our previous study [59]. Biochar has been reported as an amendment to control PPNs because it increases the diversity of nematode community, particularly fungivorous nematodes, resulting in a reduction of the abundance of Hirschmanniella, Coslenchus, Tylenchus, and Rotylenchus [6,81]. Furthermore, biochar enhances green manure growth, which may provide a larger amount of residue to increase hatching stimulus in the soils [23]. A part of hatching stimulus might leach and increase the hatching capacity of SCN in the lower layer soil, resulting in further suppression in a combination with biochar. In the lower layer, there were no biochar and no mung bean residue incorporation, thereby the leachate coming from the biochar-amended upper layer may play a role in the suppression of SCN. On the other hand, biochar tended to increase microbial activity in the lower layer, and their improvement might also be involved in the suppression of SCN.
5. Conclusions
Biochar addition in the upper layer increased available P and soluble cations and decreased nitrate soil quality in the lower layer soil. Biochar and mung bean residues incorporation in the upper layer decreased the number density of SCN and increased the densities of free-living nematodes, in particular a predator Ecumenicus in the subsoil, which resulted in the increased maturity index and structure index of nematodes. Hence, the effects of biochar upon soil systems may be transmissible via water-mediated pathways, and our data suggest this could have implications for management strategies to control SCN. Further tests should be addressed for optimal water management and biochar application rate in the field conditions to suppress SCN in lower layer soil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010053/s1, Table S1: The correlations of soil properties with the abundances of hatched Heterodera glycines J2, Aphelenchus and Ecumenicus based on the entire data along soil profile. Pearson’s r value and p-value; Table S2: The correlations of soil properties with the density of the soybean cyst nematode (SCN) Heterodera glycines estimated with real-time PCR, abundance of hatched Heterodera glycines J2 or abundance of Aphelenchus and Ecumenicus in the lower layer soil. Pearson’s r value and p-value.
Author Contributions
N.V.S. conceptualized the study, conducted the experiments and collected samples, performed sample analysis; D.T.T.L. performed sample analysis; N.V.S. and K.T. data curation; N.V.S. wrote the paper—original draft; N.V.S., N.T.K.P., K.R. and K.T. reviewed, edited, and finalized the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to the farmer who provided soil from his soybean field for use in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, X.; Yang, F.; Xing, Y.; Huang, Y.; Xu, L.; Liu, Z.; Holtzman, R.; Kan, I.; Li, Y.; Zhang, L.; et al. Use of biochar to manage soil salts and water: Effects and mechanisms. Catena 2022, 211, 106018. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Lu, T.; Wang, X.; Du, Z.; Wu, L. Impacts of continuous biochar application on major carbon fractions in soil profile of North China plain’s cropland: In comparison with straw incorporation. Agric. Ecosyst. Environ. 2021, 315, 107445. [Google Scholar] [CrossRef]
- Adebajo, S.O.; Oluwatobi, F.; Akintokun, P.O.; Ojo, A.E.; Akintokun, A.K.; Gbodope, I.S. Impacts of rice-husk biochar on soil microbial biomass and agronomic performances of tomato (Solanum lycopersicum L.). Sci. Rep. 2022, 12, 1787. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 2022, 4, 36. [Google Scholar] [CrossRef]
- Poveda, J.; Martínez-Gómez, Á.; Fenoll, C.; Escobar, C. The use of biochar for plant pathogen control. Phytopathology 2021, 111, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Dawar, K.; Fahad, S.; Jahangir, M.M.R.; Munir, I.; Alam, S.S.; Khan, S.A.; Mian, I.A.; Datta, R.; Saud, S.; Banout, J.; et al. Biochar and urease inhibitor mitigate NH3 and N2O emissions and improve wheat yield in a urea fertilized alkaline soil. Sci. Rep. 2021, 11, 17413. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Tran Sy, N.; Huynh Van, T.; Nguyen Huu, C.; Nguyen Van, C.; Mitsunori, T. Rice husk and melaleuca biochar additions reduce soil CH4 and N2O emissions and increase soil physicochemical properties. F1000Research 2022, 10, 1128. [Google Scholar] [CrossRef]
- Karhu, K.; Kalu, S.; Seppanen, A.; Kitzler, B.; Virtanen, E. Potential of biochar soil amendments to reduce N leaching in boreal field conditions estimated using the resin bag method. Agric. Ecosyst. Environ. 2021, 316, 107452. [Google Scholar] [CrossRef]
- Gelardi, D.L.; Ainuddin, I.H.; Rippner, D.A.; Patiño, J.E.; Abou Najm, M.; Parikh, S.J. Biochar alters hydraulic conductivity and impacts nutrient leaching in two agricultural soils. SOIL 2021, 7, 811–825. [Google Scholar] [CrossRef]
- An, X.; Wu, Z.; Shi, W.; Qi, H.; Zhang, L.; Xu, X.; Yu, B. Biochar for simultaneously enhancing the slow-release performance of fertilizers and minimizing the pollution of pesticides. J. Hazard. Mater. 2021, 407, 124865. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Savla, N.; Pandit, C.; Pandit, S.; Gupta, P.K.; Pant, M.; Khilari, S.; Kumar, Y.; Agarwal, D.; Nair, R.R.; et al. Use of biomass-derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment. Sci. Total Environ. 2022, 825, 153892. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M. Plant-parasitic nematode threats to global food security. J. Nematol. 2014, 46, 130. [Google Scholar]
- Fábia, S.O.L.; Valdir, R.C.; Sônia, R.N.; Patrícia, R.R.S. Nematodes affecting soybean and sustainable practices for their management. In Soybean—The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Gendron St-Marseille, A.-F.; Bourgeois, G.; Brodeur, J.; Mimee, B. Simulating the impacts of climate change on soybean cyst nematode and the distribution of soybean. Agric. For. Meteorol. 2019, 264, 178–187. [Google Scholar] [CrossRef]
- Tefft, P.M.; Rende, J.F.; Bone, L.W. Factors influencing egg hatching of the soybean cyst nematode Heterodera glycines race-3. Proc. Helminthol. Soc. Wash. 1982, 49, 258–265. [Google Scholar]
- Ochola, J.; Coyne, D.; Cortada, L.; Haukeland, S.; Ng’ang’a, M.; Hassanali, A.; Opperman, C.; Torto, B. Cyst nematode bio-communication with plants: Implications for novel management approaches. Pest Manage. Sci. 2021, 77, 1150–1159. [Google Scholar] [CrossRef]
- Acharya, K.; Yan, G.; Plaisance, A. Effects of cover crops on population reduction of soybean cyst nematode (Heterodera glycines). Plant Dis. 2021, 105, 764–769. [Google Scholar] [CrossRef]
- Rocha, L.F.; Pimentel, M.F.; Bailey, J.; Wyciskalla, T.; Davidson, D.; Fakhoury, A.M.; Bond, J.P. Impact of wheat on soybean cyst nematode population density in double-cropping soybean production. Front. Plant Sci. 2021, 12, 640714. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.S.; Chikamatsu, S.; Kato, R.; Chau, M.K.; Nguyen, T.K.P.; Ritz, K.; Toyota, K. A biochar improves the efficacy of green manure-based strategies to suppress soybean cyst nematode (Heterodera glycines) and promotes free-living nematode populations. J. Soil Sci. Plant Nutr. 2022, 22, 3414–3427. [Google Scholar] [CrossRef]
- Chikamatsu, S.; Takeda, A.; Ohta, K.; Imura, T.; Perry, R.N.; Toyota, K. Suppression of the soybean cyst nematode, Heterodera glycines, by short-term field cultivation and soil incorporation of mung bean. Nematology 2021, 23, 305–315. [Google Scholar] [CrossRef]
- Linh, T.B.; Van, K.L.; Van Elsacker, S.; Cornelis, W.M. Effect of cropping system on physical properties of clay soil under intensive rice cultivation. Land. Degrad. Dev. 2016, 27, 973–982. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Knox, O.; Polain, K.; Fortescue, E.; Griffiths, B. Distribution and restricted vertical movement of nematodes in a heavy clay soil. Agronomy 2020, 10, 221. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Vanderlee, J.J.; Novozamsky, I. Soil and plant analysis: A series of syllabi. In Part 5b Soil Analysis Procedures Other Procedures, 6th ed.; Wageningen Agricultural University, Department of Soil Science and Plant Nutrition: Wageningen, The Netherlands, 1995. [Google Scholar]
- Kaneko, S.; Inagaki, M.; Morishita, T. A simple method for the determination of nitrate in potassium chloride extracts from forest soils. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Published on DVD: Brisbane, Australia. [Google Scholar]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Soil Science Society of America: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006. [Google Scholar]
- De Grisse, A. Redescription ou modifications de quelques techniques utililisées dans l’etude des nematodes phytoparasitaires. Meded. Rijksfac. Landbouwwet. Gent 1969, 34, 351–369. [Google Scholar]
- Eyualem-Abebe; Andrássy, I.; Traunspurger, W. Freshwater Nematodes: Ecology and Taxonomy; CAB International Publishing: Surrey, UK, 2006. [Google Scholar]
- Andrassy, I. A Taxonomic Review of the Suborder Rhabditina (Nematode: Secernnentia); Zoosystematica and Ecologica Institute, Eôtvôs Lorand University: Budapest, Hungary, 1983. [Google Scholar]
- Zulini, A. Identification Manual for Freshwater Nematode Genera; Lecture Book for Msc in Nematology Ghent University: Ghent, Belgium, 2010; p. 112. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. Ninja: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Sato, E.; Min, Y.Y.; Toyota, K.; Takada, A. Relationships between the damage to radish caused by the root-lesion nematode Pratylenchus penetrans, its density prior to cultivation and the soil nematode community structure evaluated by polymerase chain reaction-denaturing gradient gel electrophoresis. Soil Sci. Plant Nutr. 2009, 55, 478–484. [Google Scholar] [CrossRef]
- Shirai, S.; Toyota, K. Optimisation of a species-specific primer set to quantify the soybean cyst nematode, Heterodera glycines, in soil using real-time PCR. Nematology 2019, 21, 1037–1042. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Liu, S.N.; Meng, J.; Jiang, L.L.; Yang, X.; Lan, Y.; Cheng, X.Y.; Chen, W.F. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Munda, S.; Bhaduri, D.; Mohanty, S.; Chatterjee, D.; Tripathi, R.; Shahid, M.; Kumar, U.; Bhattacharyya, P.; Kumar, A.; Adak, T.; et al. Dynamics of soil organic carbon mineralization and C fractions in paddy soil on application of rice husk biochar. Biomass Bioenergy 2018, 115, 1–9. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Linh, T.B.; Minh, D.D.; Duc, T.A.; Sinh, N.V.; Linh, T.T.; Toyota, K. Influence of rice husk biochar and compost amendments on salt contents and hydraulic properties of soil and rice yield in salt-affected fields. Agronomy 2020, 10, 1101. [Google Scholar] [CrossRef]
- Gluba, Ł.; Rafalska-Przysucha, A.; Szewczak, K.; Łukowski, M.; Szlązak, R.; Vitková, J.; Kobyłecki, R.; Bis, Z.; Wichliński, M.; Zarzycki, R.; et al. Effect of fine size-fractionated sunflower husk biochar on water retention properties of arable sandy soil. Materials 2021, 14, 1335. [Google Scholar] [CrossRef]
- Ahmed, R.; Li, Y.; Mao, L.; Xu, C.; Lin, W.; Ahmed, S.; Ahmed, W. Biochar effects on mineral nitrogen leaching, moisture content, and evapotranspiration after 15N urea fertilization for vegetable crop. Agronomy 2019, 9, 331. [Google Scholar] [CrossRef]
- Pratiwi, E.P.A.; Shinogi, Y. Rice husk biochar application to paddy soil and its effects on soil physical properties, plant growth, and methane emission. Paddy. Water Environ. 2016, 14, 521–532. [Google Scholar] [CrossRef]
- Huang, H.; Reddy, N.G.; Huang, X.; Chen, P.; Wang, P.; Zhang, Y.; Huang, Y.; Lin, P.; Garg, A. Effects of pyrolysis temperature, feedstock type and compaction on water retention of biochar amended soil. Sci. Rep. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Sinh, N.V.; Tarao, M.; Toyota, K. Potential use of rice husk biochar and compost to improve P availability and reduce GHG emissions in acid sulfate soil. Agronomy 2020, 10, 685. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Roig, A.; Sánchez-Monedero, M.A.; Cayuela, M.L. Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front. Environ. Sci. 2014, 2, 25. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, J.; Li, G.; Zhang, G.-L.; Wang, Z.-W.; Zhi, Y.-C.; Wu, M.-L.; Lai, X.; Yang, D.-L.; Ren, T.-Z. Biochar application affects Nitrobacter rather than Nitrospira in plastic greenhouse vegetable soil. Appl. Soil Ecol. 2022, 175, 104449. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Abell, J.; Laverman, A.M.; Van Cappellen, P. Bioavailability of organic matter in a freshwater estuarine sediment: Long-term degradation experiments with and without nitrate supply. Biogeochemistry 2009, 94, 13–28. [Google Scholar] [CrossRef]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Vedove, G.D.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial mineralization of biochar and wheat straw mixture in soil: A short-term study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sed. 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.H.; Hu, Z.K.; Xue, J.R.; Lu, Y.Y.; Chen, X.Y.; Griffiths, B.S.; Whalen, J.K.; Liu, M.Q. Biochar exerts negative effects on soil fauna across multiple trophic levels in a cultivated acidic soil. Biol. Fertil. Soils 2020, 56, 597–606. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Kato, R.; Doan, T.T.L.; Nguyen, T.K.P.; Toyota, K. Influence of rice husk biochar on soil nematode community under upland and flooded conditions: A microcosm experiment. Agronomy 2022, 12, 378. [Google Scholar]
- Wang, C.Y.; Wang, Y.D.; Herath, H. Polycyclic aromatic hydrocarbons (PAHs) in biochar—Their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.X.; Zhang, G.L.; Ruan, W.B.; Shan, S.J.; Lai, X.; Yang, D.L.; Yu, Z.G. Integration of behavioural tests and transcriptome sequencing of C. elegans reveals how the nematode responds to peanut shell biochar amendment. Sci. Total Environ. 2020, 707, 136024. [Google Scholar] [CrossRef] [PubMed]
- Domene, X.; Mattana, S.; Sanchez-Moreno, S. Biochar addition rate determines contrasting shifts in soil nematode trophic groups in outdoor mesocosms: An appraisal of underlying mechanisms. Appl. Soil Ecol. 2021, 158, 103788. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Yang, K.; Wang, P.; Wang, H.; Guo, L.; Zhu, S.; Zhu, Y.; He, X. Biochar application alleviated negative plant-soil feedback by modifying soil microbiome. Front. Microbiol. 2020, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Eyre, M.; Venette, R.C.; Lau, S.S. Population energetics of bacterial-feeding nematodes: Stage-specific development and fecundity rates. Soil Biol. Biochem. 1996, 28, 271–280. [Google Scholar] [CrossRef]
- Venette, R.C.; Ferris, H. Influence of bacterial type and density on population growth of bacterial-feeding nematodes. Soil Biol. Biochem. 1998, 30, 949–960. [Google Scholar] [CrossRef]
- Bouwman, L.A.; Arts, W.B.M. Effects of soil compaction on the relationships between nematodes, grass production and soil physical properties. Appl. Soil Ecol. 2000, 14, 213–222. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Charlson, D.V.; Tylka, G.L. Heterodera glycines cyst components and surface disinfestants affect H. glycines hatching. J. Nematol. 2003, 35, 458–464. [Google Scholar]
- Chikamatsu, S.; Xiaoman, W.; Ito, D.; Yamada, E.; Toyota, K. Effect of short-term growth of mung bean and its soil incorporation on the density of the soybean cyst nematode, Heterodera glycines, in pot experiments. Nematology 2017, 19, 1147–1155. [Google Scholar] [CrossRef]
- Fukuzawa, A.; Furusaki, A.; Ikura, M.; Masamune, T. Glycinoeclepin A, a natural hatching stimulus for the soybean cyst nematode. J. Chem. Soc. Chem. Commun. 1985, 4, 222–224. [Google Scholar] [CrossRef]
- Tefft, P.M.; Bone, L.W. Zinc-mediated hatching of eggs of soybean cyst nematode, Heterodera glycines. J. Chem. Ecol. 1984, 10, 361–372. [Google Scholar] [CrossRef]
- Clarke, A.J.; Perry, R.N. The role of egg-shell calcium in the hatching of Heterodera schachtii. Nematologica 1985, 31, 151–158. [Google Scholar] [CrossRef]
- Atkinson, H.J.; Ballantyne, A.J. Evidence for the involvement of calcium in the hatching of Globodera rostochiensis. Ann. Appl. Biol. 1979, 93, 191–198. [Google Scholar] [CrossRef]
- Clarke, A.J.; Perry, R.N. Egg-shell calcium and the hatching of Globodera rostochiensis. Int. J. Parasitol. 1985, 15, 511–516. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Zhao, X.; Wu, Q. Potassium-induced plant resistance against soybean cyst nematode via root exudation of phenolic acids and plant pathogen-related genes. PLoS ONE 2018, 13, e0200903. [Google Scholar] [CrossRef] [PubMed]
- Li, T.J.; Wang, D.Y.; Li, B.X.; Wu, H.Y. The hatching of cereal cyst nematode (Heterodera avenae) in response to different inorganic ions. Sci. Agric. 2022, 79, e20200072. [Google Scholar] [CrossRef]
- Hodda, M. Phylum nematoda: Feeding habits for all valid genera using a new, universal scheme encompassing the entire phylum, with descriptions of morphological characteristics of the stoma, a key, and discussion of the evidence for trophic relationships. Zootaxa 2022, 5114, 318–451. [Google Scholar] [CrossRef]
- Moore, J.C.; McCann, K.; Setälä, H.; De Ruiter, P.C. Top-down is bottom-up: Does predation in the rhizosphere regulate aboveground dynamics? Ecology 2003, 84, 846–857. [Google Scholar] [CrossRef]
- Khan, Z.; Kim, Y.H. A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl. Soil Ecol. 2007, 35, 370–379. [Google Scholar] [CrossRef]
- Zhang, X.K.; Li, Q.; Liang, W.J.; Zhang, M.; Bao, X.L.; Xie, Z.B. Soil nematode response to biochar addition in a Chinese wheat field. Pedosphere 2013, 23, 98–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).