Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation–Animal Cell Interaction
Abstract
1. Introduction
2. Results
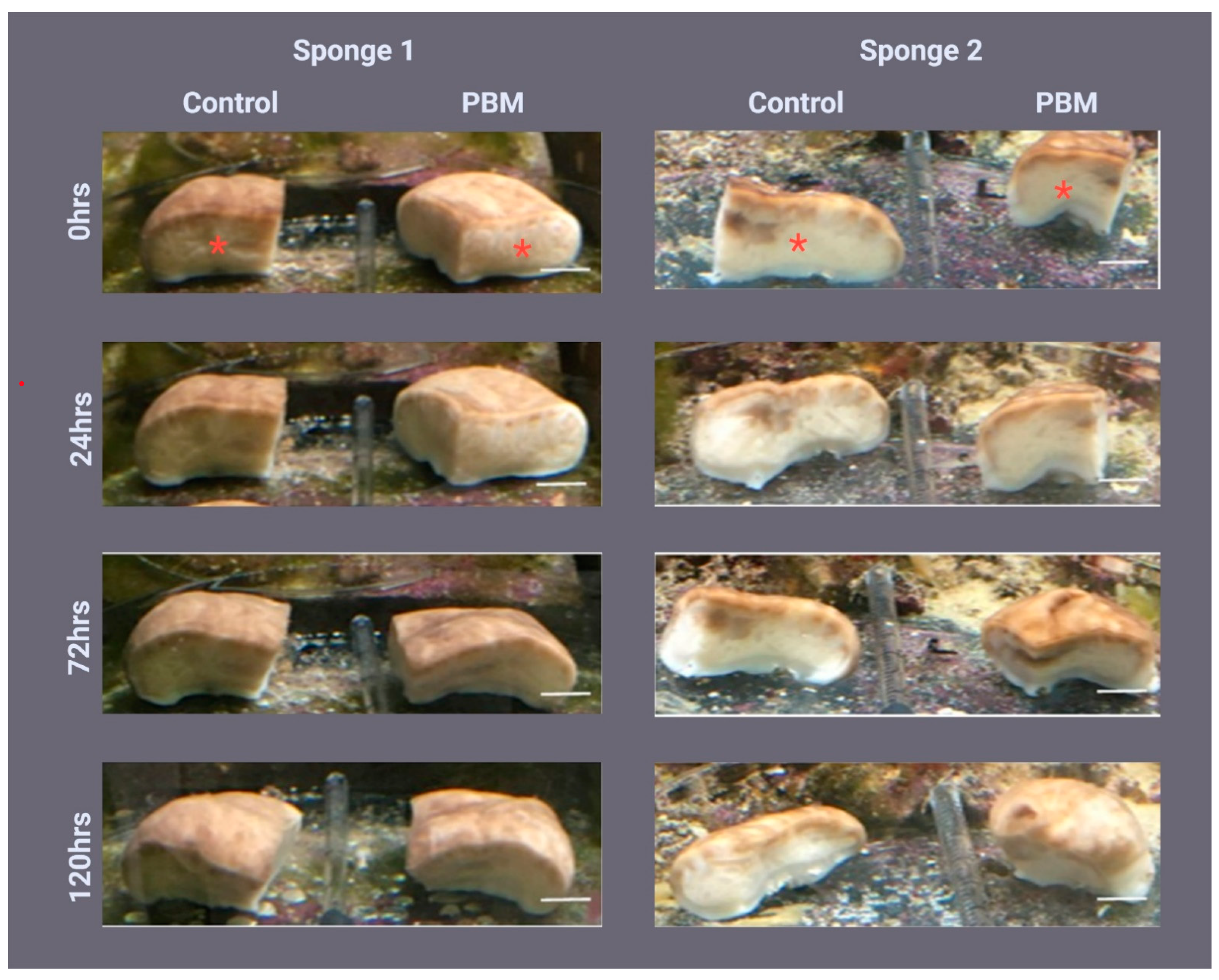
2.1. Sponge Morphology during Regeneration
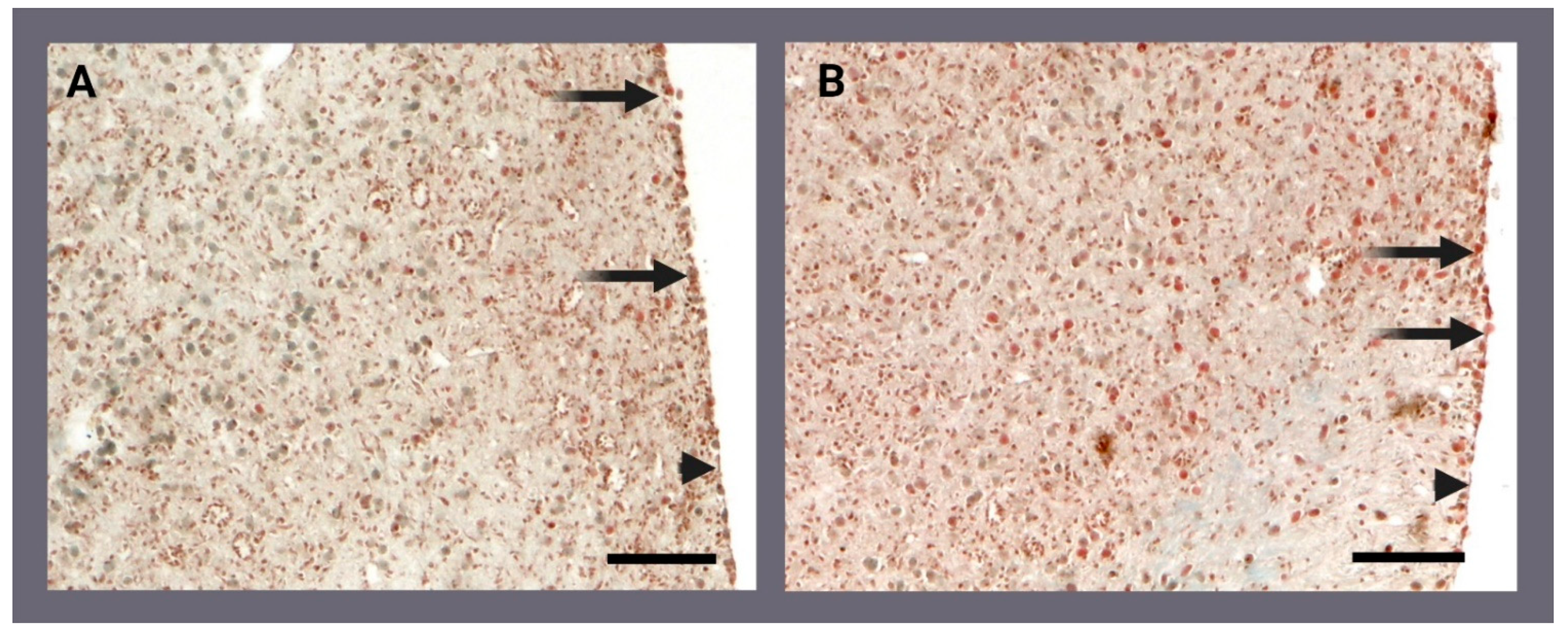
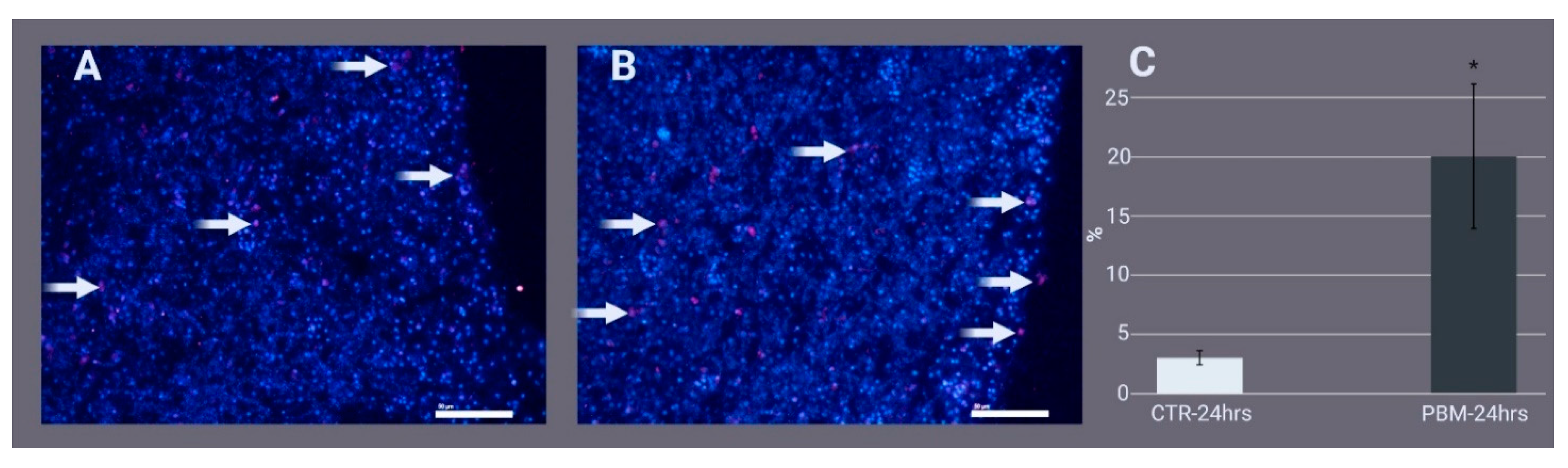
2.2. Histology and Archaeocyte Identification by Immunostaining
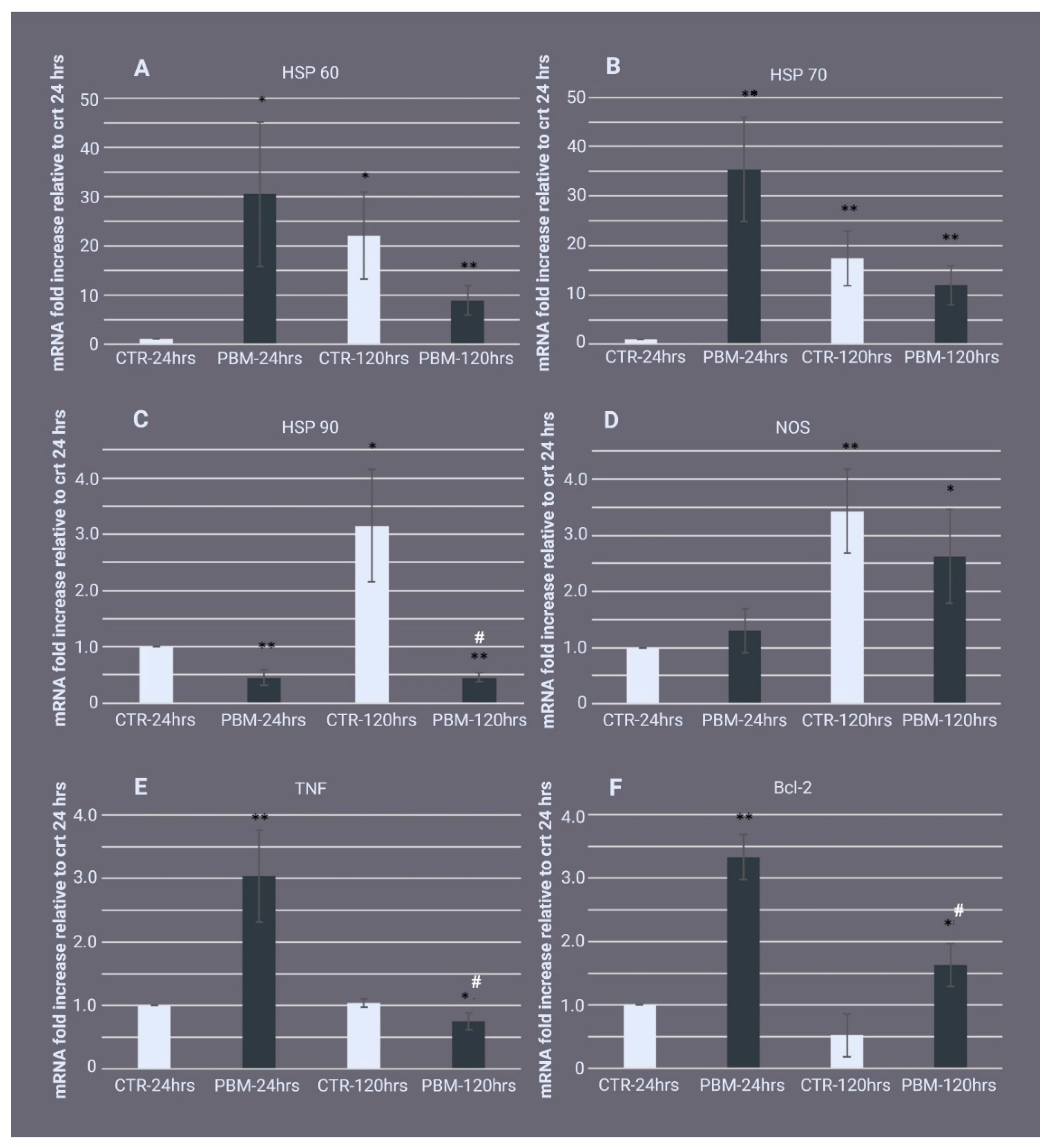
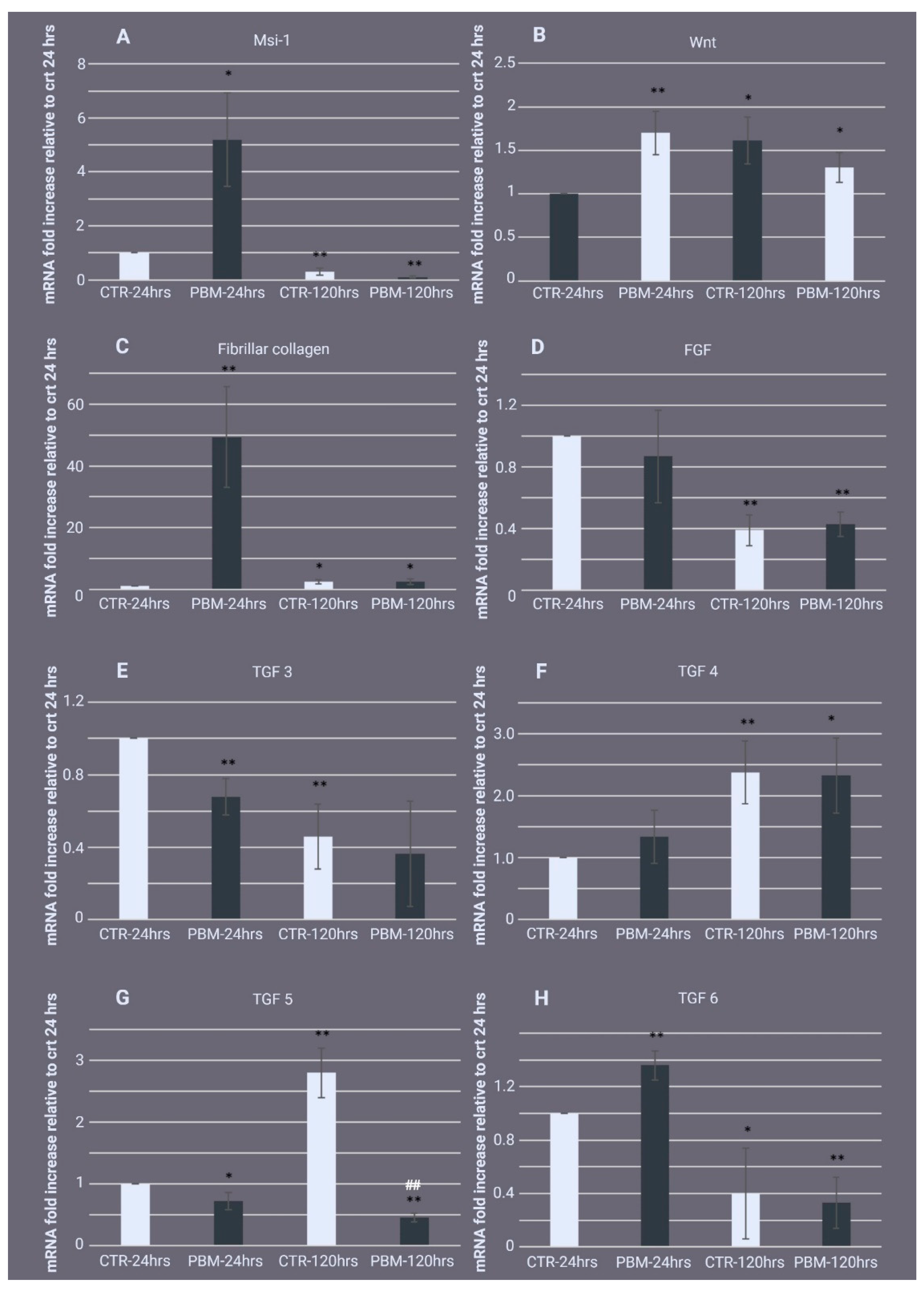
2.3. Gene Expression Profile
2.4. Sample Temperature Analysis
2.5. Oxidative Stress Evaluation
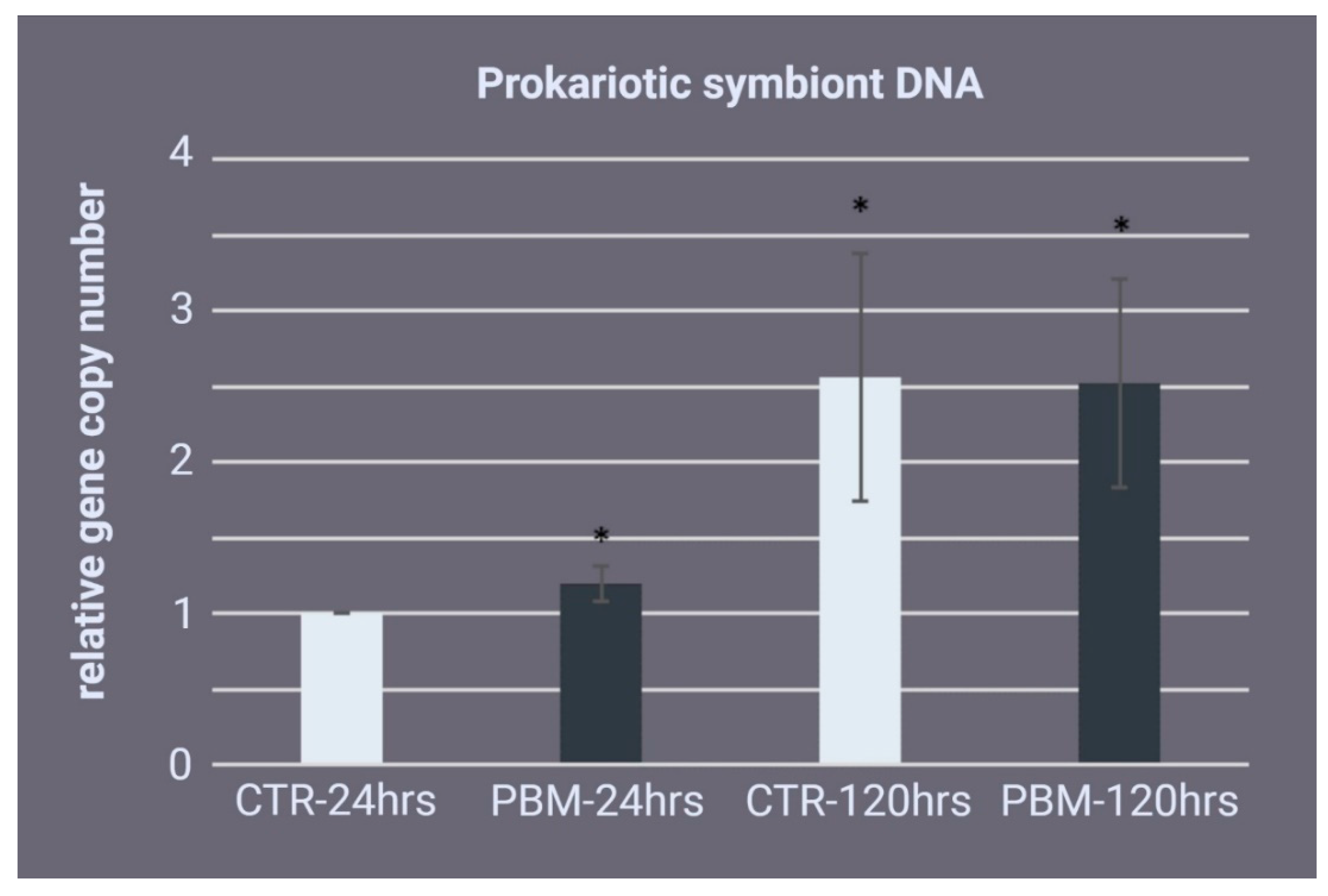
2.6. Prokaryotic Symbiont Quantification
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Animals
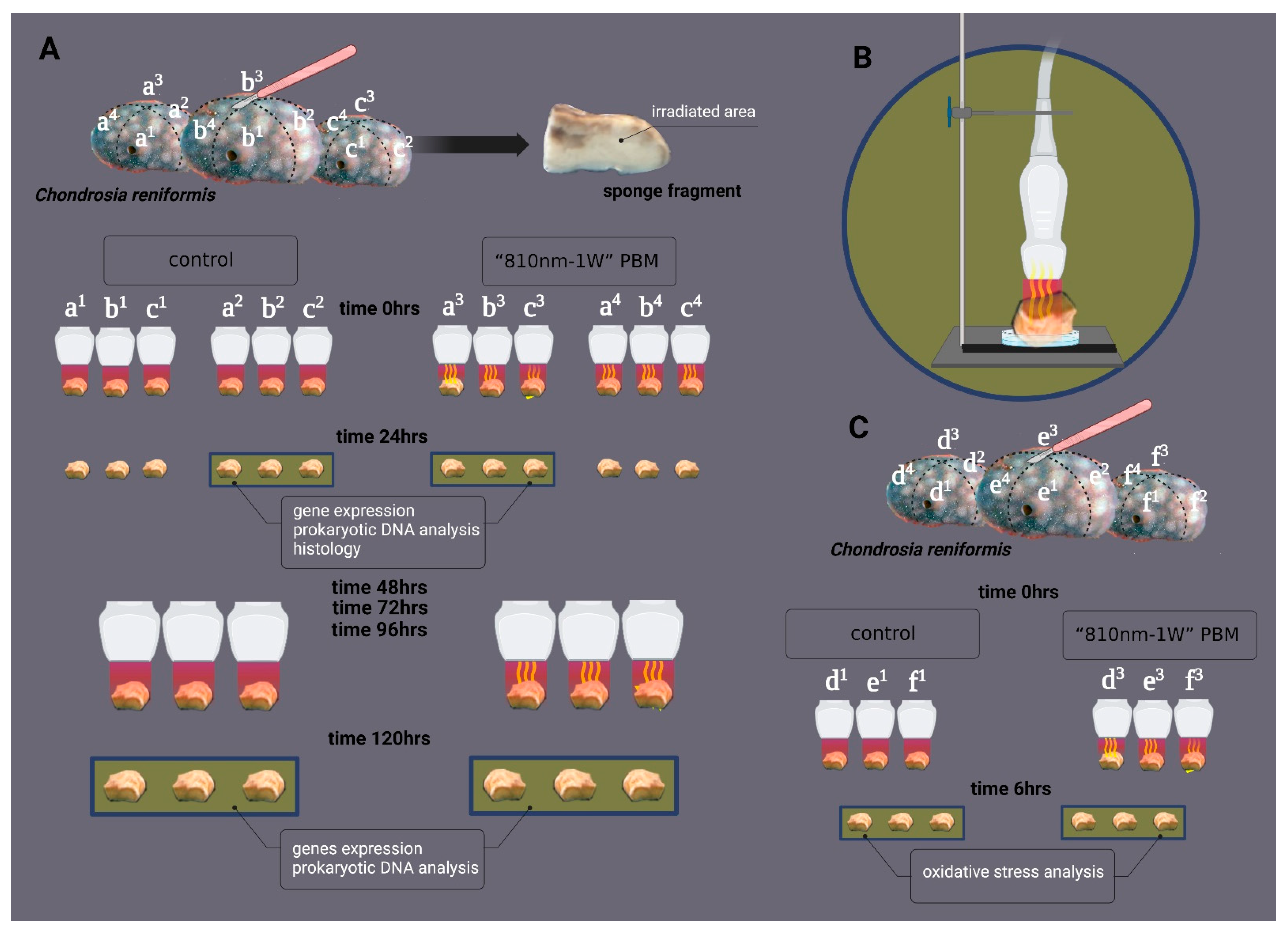
4.3. Experimental Design and Setting
4.4. Histology and Immunofluorescence
4.5. Gene Expression Evaluation
4.6. Oxidative Stress Enzyme Assays
4.6.1. Catalase Activity
4.6.2. GST Activity
4.6.3. Malondialdehyde (MDA) Content Determination
4.7. Symbiont Bacteria Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trevors, J.T. Origin of Life: Hypothesized Roles of High-Energy Electrical Discharges, Infrared Radiation, Thermosynthesis and Pre-Photosynthesis. Theory Biosci. 2012, 131, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Evolution under the Sun: Optimizing Light Harvesting in Photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Smol’yaninova, N.K.; Karu, T.I.; Fedoseeva, G.E.; Zelenin, A.V. Effects of He-Ne Laser Irradiation on Chromatin Properties and Synthesis of Nucleic Acids in Human Peripheral Blood Lymphocytes. Biomed. Sci. 1991, 2, 121–126. [Google Scholar] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; Gonzalez-Lima, F. Low-Level Light Therapy of the Eye and Brain. Eye Brain 2011, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y.Y.; Heiskanen, V. Non-Mammalian Hosts and Photobiomodulation: Do All Life-Forms Respond to Light? Photochem. Photobiol. 2019, 95, 126–139. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of All Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Amaroli, A.; Parker, S.; Dorigo, G.; Benedicenti, A.; Benedicenti, S. Paramecium: A Promising Non-Animal Bioassay to Study the Effect of 808 Nm Infrared Diode Laser Photobiomodulation. Photomed. Laser Surg. 2015, 33, 35–40. [Google Scholar] [CrossRef]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-Nm Laser Therapy with a Flat-Top Handpiece Photobiomodulates Mitochondria Activities of Paramecium Primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. Effect of 808 Nm Diode Laser on Swimming Behavior, Food Vacuole Formation and Endogenous ATP Production of Paramecium Primaurelia (Protozoa). Photochem. Photobiol. 2015, 91, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Andrea, A.; Silvia, R.; Steven, P.; Isabella, P.; Alberico, B.; Stefano, B. The Protozoan, Paramecium Primaurelia, as a Non-Sentient Model to Test Laser Light Irradiation: The Effects of an 808nm Infrared Laser Diode on Cellular Respiration. Altern. Lab. Anim. 2015, 43, 155–162. [Google Scholar]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 Nm and 980 Nm Infrared Laser Irradiation Affects Spore Germination and Stored Calcium Homeostasis: A Comparative Study Using Delivery Hand-Pieces with Standard (Gaussian) or Flat-Top Profile. J. Photochem. Photobiol. B Biol. 2019, 199, 111627. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ferrando, S.; Pozzolini, M.; Gallus, L.; Parker, S.; Benedicenti, S. The Earthworm Dendrobaena Veneta (Annelida): A New Experimental-Organism for Photobiomodulation and Wound Healing. Eur. J. Histochem. 2018, 62, 51–61. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Hanna, R.; Gallus, L.; Benedicenti, A.; Scarfì, S.; Pozzolini, M.; Benedicenti, S. The Photobiomodulation Effect of Higher-Fluence 808-Nm Laser Therapy with a Flat-Top Handpiece on the Wound Healing of the Earthworm Dendrobaena Veneta: A Brief Report. Lasers Med. Sci. 2017, 33, 221–225. [Google Scholar] [CrossRef]
- Amaroli, A.; Gambardella, C.; Ferrando, S.; Hanna, R.; Benedicenti, A.; Gallus, L.; Faimali, M.; Benedicenti, S. The Effect of Photobiomodulation on the Sea Urchin Paracentrotus Lividus (Echinodermata) Using Higher-Fluence on Fertilization, Embryogenesis, and Larval Development: An in Vitro Study. Photomed. Laser Surg. 2017, 35, 127–135. [Google Scholar] [CrossRef]
- Bozzo, M.; Pasquale, C.; Cuccaro, F.; Ferrando, S.; Zekiy, A.; Candiani, S.; Amaroli, A. Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma Lanceolatum): Proposal of an Ethical Model for Preclinical Screening. Photonics 2022, 9, 503. [Google Scholar] [CrossRef]
- Amaroli, A.; Sabbieti, M.G.; Marchetti, L.; Zekiy, A.O.; Utyuzh, A.S.; Marchegiani, A.; Laus, F.; Cuteri, V.; Benedicenti, S.; Agas, D. The Effects of 808-Nm near-Infrared Laser Light Irradiation on Actin Cytoskeleton Reorganization in Bone Marrow Mesenchymal Stem Cells. Cell Tissue Res. 2021, 383, 1003–1016. [Google Scholar] [CrossRef]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 Nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-Nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, C.; Colombo, E.; Benedicenti, S.; Signore, A.; Amaroli, A. 808-Nm Near-Infrared Laser Photobiomodulation versus Switched-Off Laser Placebo in Major Aphthae Management: A Randomized Double-Blind Controlled Trial. Appl. Sci. 2021, 11, 4717. [Google Scholar] [CrossRef]
- Pasquale, C.; Utyuzh, A.; Mikhailova, M.V.; Colombo, E.; Amaroli, A. Recovery from Idiopathic Facial Paralysis (Bell’s Palsy) Using Photobiomodulation in Patients Non-Responsive to Standard Treatment: A Case Series Study. Photonics 2021, 8, 341. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-Nm Diode Laser Light Promotes Wound Healing of Human Endothelial Cells through Increased Reactive Oxygen Species Production Stimulating Mitochondrial Oxidative Phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bertola, N.; Pasquale, C.; Bruno, S.; Benedicenti, S.; Ferrando, S.; Zekiy, A.; Arany, P.; Amaroli, A. 808-Nm Photobiomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Acting on Energy Metabolism and Oxidative Stress Production. Biomedicines 2021, 9, 1717. [Google Scholar] [CrossRef]
- Feuda, R.; Dohrmann, M.; Pett, W.; Philippe, H.; Rota-Stabelli, O.; Lartillot, N.; Wörheide, G.; Pisani, D. Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals. Curr. Biol. 2017, 27, 3864–3870.e4. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G. The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integr. Comp. Biol. 2003, 43, 3–10. [Google Scholar] [CrossRef]
- Müller, W.E.G. Review: How Was Metazoan Threshold Crossed? The Hypothetical Urmetazoa. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 433–460. [Google Scholar] [CrossRef]
- Müller, W.E.; Wiens, M.; Müller, I.M.; Schröder, H.C. The Chemokine Networks in Sponges: Potential Roles in Morphogenesis, Immunity and Stem Cell Formation. Prog. Mol. Subcell Biol. 2004, 34, 103–143. [Google Scholar] [CrossRef]
- Ereskovsky, A.V.; Tokina, D.B.; Saidov, D.M.; Baghdiguian, S.; Le Goff, E.; Lavrov, A.I. Transdifferentiation and Mesenchymal-to-Epithelial Transition during Regeneration in Demospongiae (Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2020, 334, 37–58. [Google Scholar] [CrossRef]
- Pozzolini, M.; Gallus, L.; Ghignone, S.; Ferrando, S.; Candiani, S.; Bozzo, M.; Bertolino, M.; Costa, G.; Bavestrello, G.; Scarfì, S. Insights into the Evolution of Metazoan Regenerative Mechanisms: Roles of TGF Superfamily Members in Tissue Regeneration of the Marine Sponge Chondrosia Reniformis. J. Exp. Biol. 2019, 222, jeb207894. [Google Scholar] [CrossRef] [PubMed]
- Funayama, N. The Stem Cell System in Demosponges: Insights into the Origin of Somatic Stem Cells. Dev. Growth Differ. 2010, 52, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Funayama, N. The Cellular and Molecular Bases of the Sponge Stem Cell Systems Underlying Reproduction, Homeostasis and Regeneration. Int. J. Dev. Biol. 2018, 62, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Ghignone, S.; Mussino, F.; Vezzulli, L.; Cerrano, C.; Giovine, M. Molecular Characterization and Expression Analysis of the First Porifera Tumor Necrosis Factor Superfamily Member and of Its Putative Receptor in the Marine Sponge Chondrosia Reniformis. Dev. Comp. Immunol. 2016, 57, 88–98. [Google Scholar] [CrossRef]
- Lazoski, C.; Solé-Cava, A.M.; Boury-Esnault, N.; Klautau, M.; Russo, C.A.M. Cryptic Speciation in a High Gene Flow Scenario in the Oviparous Marine Sponge Chondrosia Reniformis. Mar. Biol. 2001, 139, 421–429. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Vacelet, J. Transplantation of Marine Sponges to Different Conditions of Light and Current. J. Exp. Mar. Biol. Ecol. 1979, 37, 91–104. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfi, S.; Gallus, L.; Ferrando, S.; Cerrano, C.; Giovine, M. Silica-Induced Fibrosis: An Ancient Response from the Early Metazoans. J. Exp. Biol. 2017, 220, 4007–4015. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia Reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef]
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and Productive Source of Biopolymer Inspiration: Marine Collagens. Biomacromolecules 2021, 22, 1815–1834. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.S.; Cossarizza, A. Stress Proteins in Inflammation. EXS 1996, 77, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Giovine, M.; Pozzolini, M.; Favre, A.; Bavestrello, G.; Cerrano, C.; Ottaviani, F.; Chiarantini, L.; Cerasi, A.; Cangiotti, M.; Zocchi, E.; et al. Heat Stress-Activated, Calcium-Dependent Nitric Oxide Synthase in Sponges. Nitric Oxide 2001, 5, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Schütze, J.; Krasko, A.; Custodio, M.R.; Efremova, S.M.; Müller, I.M.; Müller, W.E.G. Evolutionary Relationships of Metazoa within the Eukaryotes Based on Molecular Data from Porifera. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Funayama, N.; Nakatsukasa, M.; Mohri, K.; Masuda, Y.; Agata, K. Piwi Expression in Archeocytes and Choanocytes in Demosponges: Insights into the Stem Cell System in Demosponges. Evol. Dev. 2010, 12, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt Signaling and Injury Repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Microbiota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 23, 1372. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E. Molecular Phylogeny of Eumetazoa: Genes in Sponges (Porifera) Give Evidence for Monophyly of Animals. Prog. Mol. Subcell Biol. 1998, 19, 89–132. [Google Scholar] [CrossRef] [PubMed]
- Colgren, J.; Nichols, S.A. Evolution as a Guide for Experimental Cell Biology. PLoS Genet. 2019, 15, e1007937. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Inge, M.M.; Mansfield, K.M.; Rasmussen, A.; Afghani, J.; Agrba, M.; Albert, C.; Andersson, C.; Babaei, M.; Babaei, M.; et al. Transcription Factor NF-ΚB in a Basal Metazoan, the Sponge, Has Conserved and Unique Sequences, Activities, and Regulation. Dev. Comp. Immunol. 2020, 104, 103559. [Google Scholar] [CrossRef]
- Adamska, M.; Degnan, S.M.; Green, K.M.; Adamski, M.; Craigie, A.; Larroux, C.; Degnan, B.M. Wnt and TGF-β Expression in the Sponge Amphimedon Queenslandica and the Origin of Metazoan Embryonic Patterning. PLoS ONE 2007, 2, e1031. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, I.; Bolshakov, F.V.; Ereskovsky, A.; Lavrov, A.I. Expression of Wnt and TGF-Beta Pathway Components during Whole-Body Regeneration from Cell Aggregates in Demosponge Halisarca Dujardinii. Genes 2021, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Tarashansky, A.J.; Musser, J.M.; Khariton, M.; Li, P.; Arendt, D.; Quake, S.R.; Wang, B. Mapping Single-Cell Atlases throughout Metazoa Unravels Cell Type Evolution. Elife 2021, 10, e66747. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective Photothermolysis: Precise Microsurgery by Selective Absorption of Pulsed Radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Martine, P.; Rébé, C. Heat Shock Proteins and Inflammasomes. Int. J. Mol. Sci. 2019, 20, 4508. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.D. Heat Shock Proteins and the Skin. Clin. Exp. Dermatol. 2002, 27, 220–224. [Google Scholar] [CrossRef]
- Evangelista, A.N.; Dos Santos, F.F.; De Oliveira Martins, L.P.; Gaiad, T.P.; Machado, A.S.D.; Rocha-Vieira, E.; Costa, K.B.; Santos, A.P.; Oliveira, M.X. Photobiomodulation Therapy on Expression of HSP70 Protein and Tissue Repair in Experimental Acute Achilles Tendinitis. Lasers Med. Sci. 2021, 36, 1201–1208. [Google Scholar] [CrossRef]
- Musser, J.M.; Schippers, K.J.; Nickel, M.; Mizzon, G.; Kohn, A.B.; Pape, C.; Ronchi, P.; Papadopoulos, N.; Tarashansky, A.J.; Hammel, J.U.; et al. Profiling Cellular Diversity in Sponges Informs Animal Cell Type and Nervous System Evolution. Science 2021, 374, 717–723. [Google Scholar] [CrossRef]
- Kozlov, M. Sponge Cells Hint at Origins of Nervous System. Nature 2021, 599, 193. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Webster, N.S.; Thomas, T. The Sponge Hologenome. MBio 2016, 7, e00135-16. [Google Scholar] [CrossRef]
- Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 22, 7788. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study between the Effectiveness of 980 Nm Photobiomodulation Delivered by Hand-Piece with Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–447. [Google Scholar] [CrossRef]
- Friedberg, T.; Milbert, U.; Bentley, P.; Guenther, T.M.; Oesch, F. Purification and Characterization of a New Cytosolic Glutathione S-Transferase (Glutathione S-Transferase X) from Rat Liver. Biochem. J. 1983, 215, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid Isolation of High Molecular Weight Plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]

| Sample | Catalase Activity (μmol H2O2/min/mg Protein) | GST Activity (μmol GST-CDNB/min/mg Protein) | Malondialdehyde Content (nmol/mg Protein) |
|---|---|---|---|
| CTR | 6.03 ± 0.1 | 0.46 ± 0.19 | 172.8 ± 75.37 |
| PBM | 5.41 ± 0.14 | 0.52 ± 0.12 | 225.61 ± 88.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaroli, A.; Tassara, E.; Ferrando, S.; Aicardi, S.; Pasquale, C.; Giovine, M.; Bertolino, M.; Zekiy, A.; Pozzolini, M. Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation–Animal Cell Interaction. Int. J. Mol. Sci. 2023, 24, 226. https://doi.org/10.3390/ijms24010226
Amaroli A, Tassara E, Ferrando S, Aicardi S, Pasquale C, Giovine M, Bertolino M, Zekiy A, Pozzolini M. Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation–Animal Cell Interaction. International Journal of Molecular Sciences. 2023; 24(1):226. https://doi.org/10.3390/ijms24010226
Chicago/Turabian StyleAmaroli, Andrea, Eleonora Tassara, Sara Ferrando, Stefano Aicardi, Claudio Pasquale, Marco Giovine, Marco Bertolino, Angelina Zekiy, and Marina Pozzolini. 2023. "Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation–Animal Cell Interaction" International Journal of Molecular Sciences 24, no. 1: 226. https://doi.org/10.3390/ijms24010226
APA StyleAmaroli, A., Tassara, E., Ferrando, S., Aicardi, S., Pasquale, C., Giovine, M., Bertolino, M., Zekiy, A., & Pozzolini, M. (2023). Near-Infrared 810 nm Light Affects Porifera Chondrosia reniformis (Nardo, 1847) Regeneration: Molecular Implications and Evolutionary Considerations of Photobiomodulation–Animal Cell Interaction. International Journal of Molecular Sciences, 24(1), 226. https://doi.org/10.3390/ijms24010226









