Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis
Abstract
:1. Introduction
2. Results
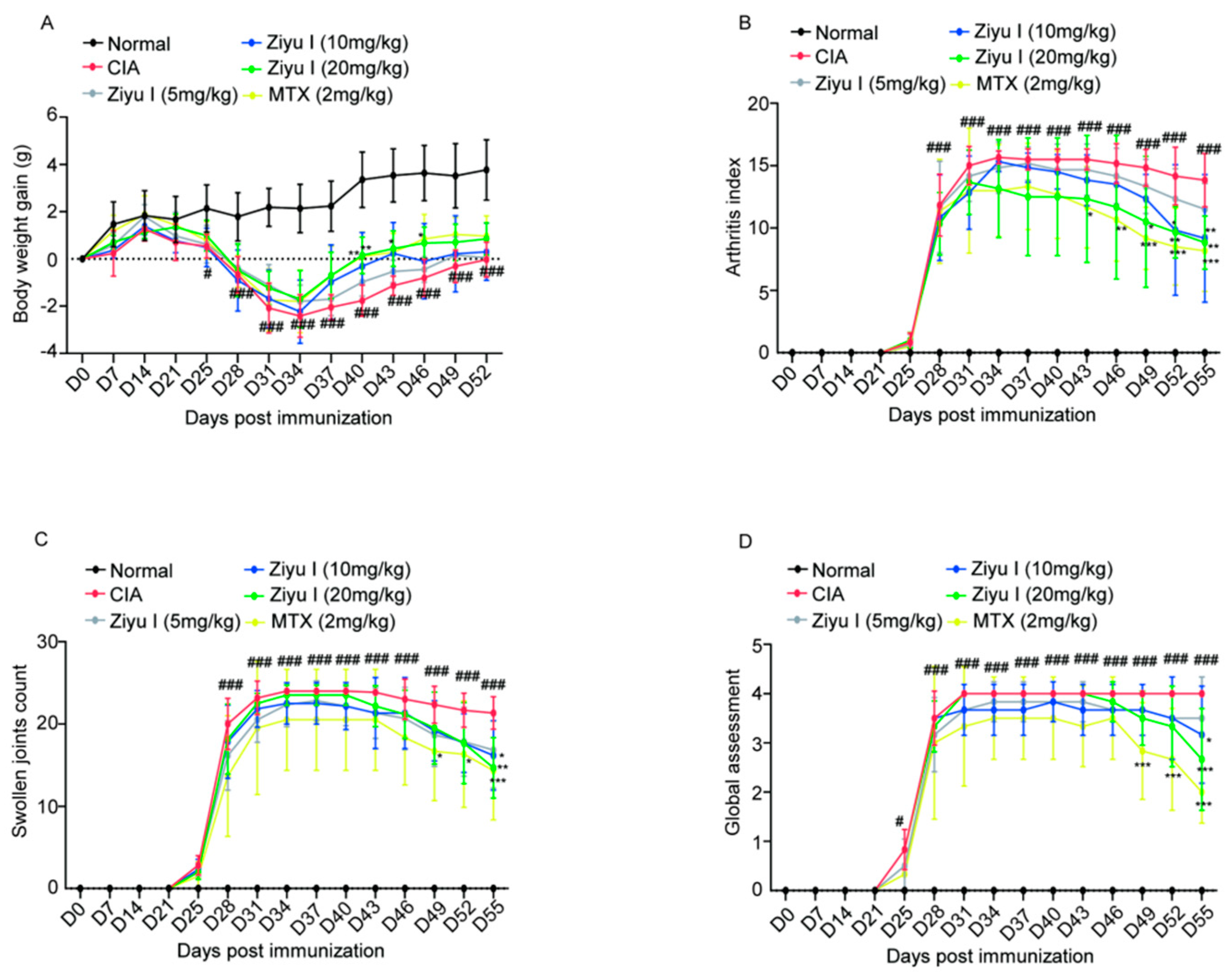
2.1. Ziyu I Ameliorates Arthritis Manifestations of CIA Mice
2.2. Ziyu I Improves the Pathologic Change of Joints and Spleens of CIA Mice
2.3. Ziyu I Restores the Th17/Treg Balance of CIA Mice
2.4. Ziyu I Restores Serum Levels of Cytokines Transforming Growth Factor-beta (TGF-β) and Interleukin-17 (IL-17) as Well as the Gene Expression of Retinoid-Related Orphan Receptor-γt (RORγt) and Foxp3
2.5. In Silico Docking Analysis Indicates Ziyu I Interacts with Akt1
2.6. Ziyu I Reduces the Activation of mTOR in T Cells of CIA Mice
3. Discussion
4. Materials and Methods
4.1. Induction and Treatment of Collagen-Induced Arthritis in Mice
4.2. Histopathological Examination of the Spleen and Joints
4.3. Serum Cytokine Detection with ELISA
4.4. Viability of T Lymphocytes
4.5. Flow Cytometry
4.6. RT-qPCR
4.7. Molecular Docking Analysis
4.8. Western Blotting
4.9. Immunofluorescence
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.J.; Wang, D.D.; Tao, J.; Tai, Y.; Zhou, Z.W.; Wang, Z.; Guo, P.P.; Sun, W.Y.; Chen, J.Y.; Wu, H.X.; et al. Deficiency of beta-arrestin2 exacerbates inflammatory arthritis by facilitating plasma cell formation. Acta Pharmacol. Sin. 2021, 42, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, X.Z.; Wang, C.; Tang, X.Y.; Zhu, Y.; Cai, X.Y.; Wu, Y.J.; Shu, J.L.; Wang, Q.T.; Chen, J.Y.; et al. IgD-Fc-Ig fusion protein, a new biological agent, inhibits T cell function in CIA rats by inhibiting IgD-IgDR-Lck-NF-kappaB signaling pathways. Acta Pharmacol. Sin. 2020, 41, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Boissier, M.C.; Assier, E.; Falgarone, G.; Bessis, N. Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm. Jt. Bone Spine Rev. Du Rhum. 2008, 75, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhong, J.; Xiong, Y.; Song, X.; Li, C.; He, Z. Development of Broad-Spectrum Antiviral Agents-Inspiration from Immunomodulatory Natural Products. Viruses 2021, 13, 1257. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Nanjundaiah, S.M.; Venkatesha, S.H.; Astry, B.; Yu, H.; Moudgil, K.D. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin. Immunol. 2014, 155, 220–230. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmianski, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. Determined with the LC-DAD-ESI-QTOF-MS/MS Analysis and Their In Vitro Antioxidant, Antidiabetic, Antiproliferative Potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- Yu, T.; Lee, Y.J.; Yang, H.M.; Han, S.; Kim, J.H.; Lee, Y.; Kim, C.; Han, M.H.; Kim, M.Y.; Lee, J.; et al. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE(2) production is mediated by suppression of NF-kappaB and AP-1 activation signaling cascade. J. Ethnopharmacol. 2011, 134, 11–17. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Hwang, Y.H.; Gu, M.J.; Cho, W.K.; Ma, J.Y. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-alpha/IFN-gamma-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine 2015, 22, 1262–1268. [Google Scholar] [CrossRef]
- Elekofehinti, O.O. Saponins: Anti-diabetic principles from medicinal plants—A review. Pathophysiology 2015, 22, 95–103. [Google Scholar] [CrossRef]
- Sun, A.; Xu, X.; Lin, J.; Cui, X.; Xu, R. Neuroprotection by saponins. Phytother. Res. 2015, 29, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. Correction to: The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keerthivasan, S.; Suleiman, R.; Lawlor, R.; Roderick, J.; Bates, T.; Minter, L.; Anguita, J.; Juncadella, I.; Nickoloff, B.J.; Le Poole, I.C.; et al. Notch signaling regulates mouse and human Th17 differentiation. J. Immunol. 2011, 187, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Wang, Y.; Liu, X.; Li, L.; Opejin, A.; Hsueh, E.C.; Luo, H.; Wang, T.; Hawiger, D.; Peng, G. TLR7 Signaling Regulates Th17 Cells and Autoimmunity: Novel Potential for Autoimmune Therapy. J. Immunol. 2017, 199, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Essig, K.; Hu, D.; Guimaraes, J.C.; Alterauge, D.; Edelmann, S.; Raj, T.; Kranich, J.; Behrens, G.; Heiseke, A.; Floess, S.; et al. Roquin Suppresses the PI3K-mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity 2017, 47, 1067–1082.e1012. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.F.; Zhou, M.Y.; Tan, T.; Zhong, C.C.; Wang, Q.; Pan, L.L.; Luo, Y.Y.; Yang, S.L.; Feng, Y.L.; Ouyang, H. A Sample and Sensitive HPLC-MS/MS Method for Simultaneous Determination of Ziyuglycoside I and Its Metabolite Ziyuglycoside II in Rat Pharmacokinetics. Molecules 2018, 23, 543. [Google Scholar] [CrossRef] [Green Version]
- Toh, M.L.; Miossec, P. The role of T cells in rheumatoid arthritis: New subsets and new targets. Curr. Opin. Rheumatol. 2007, 19, 284–288. [Google Scholar] [CrossRef]
- Fox, D.A. Citrullination: A Specific Target for the Autoimmune Response in Rheumatoid Arthritis. J. Immunol. 2015, 195, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Nagai, S.; Kurebayashi, Y.; Koyasu, S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann. N. Y. Acad. Sci. 2013, 1280, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhang, Z.; Zhou, L.; Zhang, H.Y.; Chen, Y.; Tang, Y. Repurposing Ziyuglycoside II Against Colorectal Cancer via Orchestrating Apoptosis and Autophagy. Front. Pharmacol. 2020, 11, 576547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Wu, L.; Zhang, M.; Hu, S.; Wang, R.; Han, Y.; Wu, Y.; Zhang, L.; Wang, X.; et al. Paroxetine alleviates T lymphocyte activation and infiltration to joints of collagen-induced arthritis. Sci. Rep. 2017, 7, 45364. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, M.; Hu, S.; Liu, K.; Tai, Y.; Tao, J.; Zhou, W.; Zhao, Z.; Wang, Q.; Wei, W. Ginsenoside metabolite compound-K regulates macrophage function through inhibition of beta-arrestin2. Biomed. Pharmacother. 2019, 115, 108909. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Q.T.; Song, S.S.; Wu, Y.J.; Ma, Y.K.; Zhang, L.L.; Chen, J.Y.; Wu, H.X.; Jiang, L.; Wei, W. Combined use of etanercept and MTX restores CD4+/CD8+ ratio and Tregs in spleen and thymus in collagen-induced arthritis. Inflamm. Res. 2012, 61, 1229–1239. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Tao, J.; Zhou, W.; Wang, R.; Tai, Y.; Xiao, F.; Wang, Q.; Wei, W. Ginsenoside compound-K inhibits the activity of B cells through inducing IgD-B cell receptor endocytosis in mice with collagen-induced arthritis. Inflammopharmacology 2019, 27, 845–856. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Luo, X.; Zhang, Y.; Si, M.; Wu, H.; Yan, C.; Wei, W. Ginsenoside metabolite compound K exerts joint-protective effect by interfering with synoviocyte function mediated by TNF-alpha and Tumor necrosis factor receptor type 2. Eur. J. Pharmacol. 2016, 771, 48–55. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Zhang, Y.; Wang, Y.; Cui, D.; Luo, T.; Zhang, Y.; Liu, Q.; Li, H.; Wang, C.; et al. Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats. Acta Pharm. Sin. B 2021, 11, 1835–1852. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Jia, X.; Wang, C.; Wu, Y.; Zhang, L.; Chang, Y.; Wei, W. CP-25 combined with MTX/ LEF ameliorates the progression of adjuvant-induced arthritis by the inhibition on GRK2 translocation. Biomed. Pharmacother. 2019, 110, 834–843. [Google Scholar] [CrossRef]






| Genes | Forward Sequence | Reverse Sequence |
|---|---|---|
| RORγt | 5′-CCGCTGAGAGGGCTTCAC-3′ | 5′-TGCAGGAGTAGGCCACATTACA-3′ |
| Foxp3 | 5′-ATGTTCGCCTACTTCAGAA-3′ | 5′-TCATCTACGGTCCACACT-3′ |
| GAPDH | 5′-AAATGGTGAAGGTCGGTGTGAAC-3′ | 5′-CGACATACTCAGCACCAGCACACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Su, T.; Sun, H.; Cheng, H.; Jiang, C.; Guo, P.; Zhu, Z.; Fang, R.; He, F.; Ge, M.; et al. Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis. Int. J. Mol. Sci. 2022, 23, 16105. https://doi.org/10.3390/ijms232416105
Wang M, Su T, Sun H, Cheng H, Jiang C, Guo P, Zhu Z, Fang R, He F, Ge M, et al. Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis. International Journal of Molecular Sciences. 2022; 23(24):16105. https://doi.org/10.3390/ijms232416105
Chicago/Turabian StyleWang, Manman, Tiantian Su, Hanfei Sun, Huijuan Cheng, Chunru Jiang, Paipai Guo, Zhenduo Zhu, Ruhong Fang, Feng He, Mingli Ge, and et al. 2022. "Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis" International Journal of Molecular Sciences 23, no. 24: 16105. https://doi.org/10.3390/ijms232416105
APA StyleWang, M., Su, T., Sun, H., Cheng, H., Jiang, C., Guo, P., Zhu, Z., Fang, R., He, F., Ge, M., Guan, Q., Wei, W., & Wang, Q. (2022). Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis. International Journal of Molecular Sciences, 23(24), 16105. https://doi.org/10.3390/ijms232416105





