The Utility of High Intensity Interval Training to Improve Cognitive Aging in Heart Disease Patients
Abstract
1. Introduction
2. Cerebral Blood Flow Regulation
3. Cardiovascular Disease and Brain Health
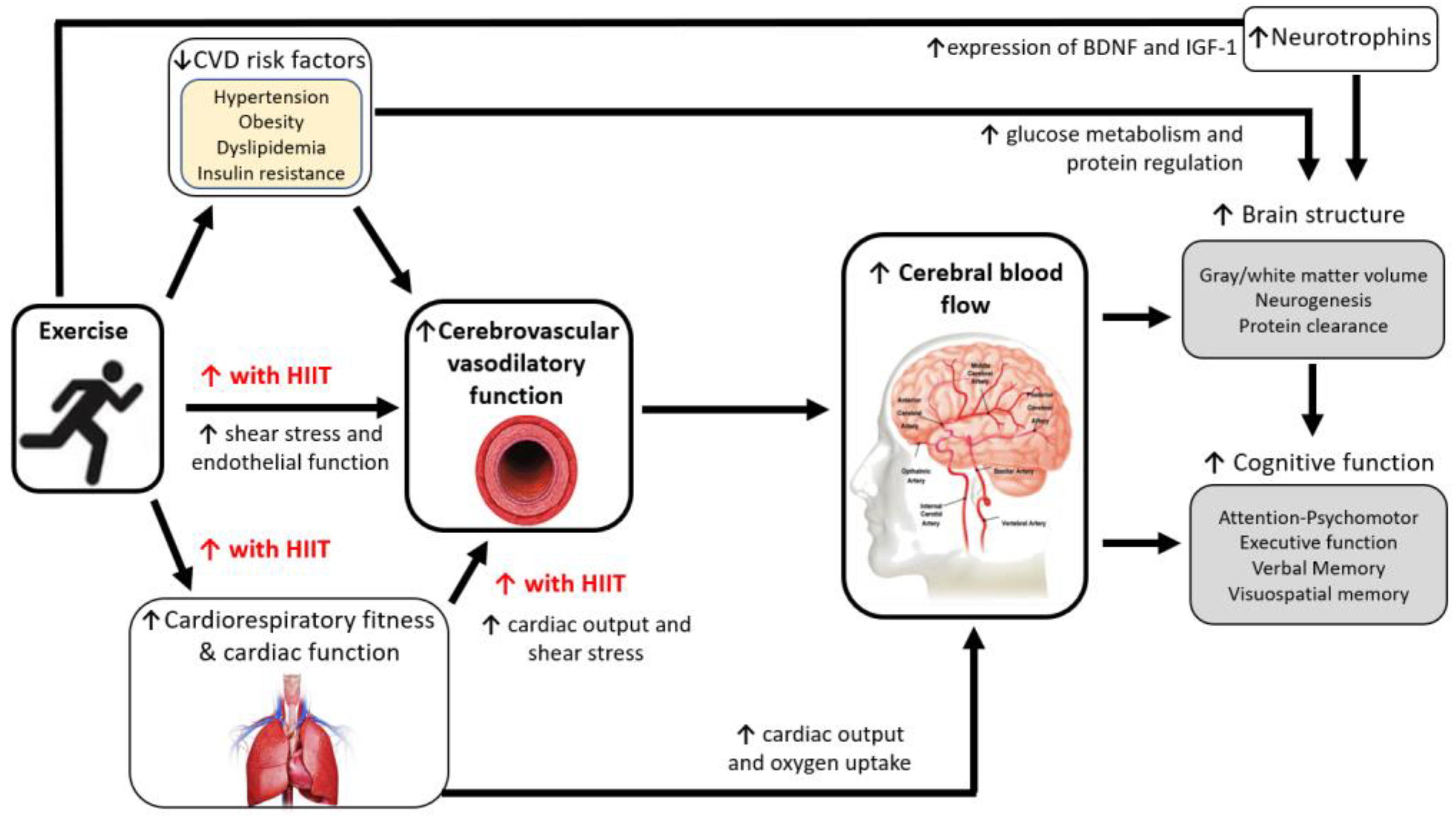
4. Mechanisms for Improving Brain Health with Exercise
5. Effect of Exercise Training on Brain Health in CVD
5.1. Cognitive Function
5.2. Brain Structure and Cerebrovascular Function
6. Is There Rationale for Higher Intensity Exercise?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prima, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Virani Salim, S.; Alonso, A.; Benjamin Emelia, J.; Bittencourt Marcio, S.; Callaway Clifton, W.; Carson April, P.; Chamberlain Alanna, M.; Chang Alexander, R.; Cheng, S.; Delling Francesca, N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Deckers, K.; Schievink, S.H.J.; Rodriquez, M.M.F.; van Oostenbrugge, R.J.; van Boxtel, M.P.J.; Verhey, F.R.J.; Köhler, S. Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0184244. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Cha, R.H.; Pankratz, V.S.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Mielke, M.M.; Petersen, R.C. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: Stronger effect on women. JAMA Neurol. 2013, 70, 374–382. [Google Scholar] [CrossRef]
- Xie, W.; Zheng, F.; Yan, L.; Zhong, B. Cognitive Decline Before and After Incident Coronary Events. J. Am. Coll. Cardiol. 2019, 73, 3041. [Google Scholar] [CrossRef] [PubMed]
- Hammond Christa, A.; Blades Natalie, J.; Chaudhry Sarwat, I.; Dodson John, A.; Longstreth, W.T.; Heckbert Susan, R.; Psaty Bruce, M.; Arnold Alice, M.; Dublin, S.; Sitlani Colleen, M.; et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure. Circ. Heart Fail. 2018, 11, e004476. [Google Scholar] [CrossRef]
- Wolters Frank, J.; Zonneveld Hazel, I.; Hofman, A.; van der Lugt, A.; Koudstaal Peter, J.; Vernooij Meike, W.; Ikram, M.A. Cerebral Perfusion and the Risk of Dementia. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef]
- Lu, H.; Xu, F.; Rodrigue, K.M.; Kennedy, K.M.; Cheng, Y.; Flicker, B.; Hebrank, A.C.; Uh, J.; Park, D.C. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb. Cortex 2011, 21, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N.; Schmidt, J.E.; Nicholson, W.T.; Joyner, M.J. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J. Appl. Physiol. 2012, 112, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, U.C.; Shoemaker, J.K.; Suskin, N.; Ssali, T.; Wang, D.J.J.; St Lawrence, K.S. Impaired Cerebrovascular Function in Coronary Artery Disease Patients and Recovery Following Cardiac Rehabilitation. Front. Aging Neurosci. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, U.C.; Shoemaker, J.K.; Suskin, N.; St Lawrence, K.S. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. Neuroimage Clin. 2013, 3, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Leip, E.P.; Larson, M.G.; D’Agostino, R.B.; Beiser, A.; Wilson, P.W.; Wolf, P.A.; Levy, D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006, 113, 791–798. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Smith, E.C.; Pizzey, F.K.; Askew, C.D.; Mielke, G.I.; Ainslie, P.N.; Coombes, J.S.; Bailey, T.G. Effects of cardiorespiratory fitness and exercise training on cerebrovascular blood flow and reactivity: A systematic review with meta-analyses. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H59–H76. [Google Scholar] [CrossRef]
- Willie, C.K.; Colino, F.L.; Bailey, D.M.; Tzeng, Y.C.; Binsted, G.; Jones, L.W.; Haykowsky, M.J.; Bellapart, J.; Ogoh, S.; Smith, K.J.; et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J. Neurosci. Methods 2011, 196, 221–237. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Phillips, A.A.; Chan, F.H.; Zheng, M.M.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow. Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef]
- Claassen, J.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Labrecque, L.; Smirl, J.D.; Tymko, M.M.; Caldwell, H.G.; Hoiland, R.L.; Lucas, S.J.E.; Denault, A.Y.; Couture, E.J.; Ainslie, P.N. Losing the dogmatic view of cerebral autoregulation. Physiol. Rep. 2021, 9, e14982. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, P.N.; Duffin, J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1473–R1495. [Google Scholar] [CrossRef] [PubMed]
- Secher, N.H.; Seifert, T.; Lieshout, J.J.V. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef]
- Ogoh, S.; Brothers, R.M.; Barnes, Q.; Eubank, W.L.; Hawkins, M.N.; Purkayastha, S.; O-Yurvati, A.; Raven, P.B. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J. Physiol. 2005, 569, 697–704. [Google Scholar] [CrossRef]
- Zhang, R.; Levine, B.D. Autonomic Ganglionic Blockade Does Not Prevent Reduction in Cerebral Blood Flow Velocity during Orthostasis in Humans. Stroke 2007, 38, 1238–1244. [Google Scholar] [CrossRef]
- Vogels, R.L.C.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef]
- Haring, B.; Leng, X.; Robinson, J.; Johnson, K.C.; Jackson, R.D.; Beyth, R.; Wactawski-Wende, J.; von Ballmoos, M.W.; Goveas, J.S.; Kuller, L.H.; et al. Cardiovascular disease and cognitive decline in postmenopausal women: Results from the Women’s Health Initiative Memory Study. J. Am. Heart Assoc. 2013, 2, e000369. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Bennet, A.M.; Gatz, M.; Dickman, P.W.; Pedersen, N.L. Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 213–219. [Google Scholar] [CrossRef]
- Barnes, J.N. Exercise, cognitive function, and aging. Adv. Physiol. Educ. 2015, 39, 55–62. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. The Autonomic Nervous System and Hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp. Physiol. 2008, 93, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.M.; Ghiadoni, L.; Seravalle, G.; Dell’oro, R.; Taddei, S.; Grassi, G. Sympathetic regulation of vascular function in health and disease. Front. Physiol. 2012, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Weinstein, A.M.; Marsland, A.L.; Gianaros, P.J.; Erickson, K.I. Body–Brain Connections: The Effects of Obesity and Behavioral Interventions on Neurocognitive Aging. Front. Aging Neurosci. 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Alberti, K.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Takeda, S.; Sato, N.; Morishita, R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front. Aging Neurosci. 2014, 6, 171. [Google Scholar] [CrossRef]
- de la Torre, J.C. Critical threshold cerebral hypoperfusion causes Alzheimer’s disease? Acta Neuropathol. 1999, 98, 1–8. [Google Scholar] [CrossRef]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Fraser, K.S.; Heckman, G.A.; McKelvie, R.S.; Harkness, K.; Middleton, L.E.; Hughson, R.L. Cerebral Hypoperfusion Is Exaggerated With an Upright Posture in Heart Failure: Impact of Depressed Cardiac Output. JACC Heart Fail. 2015, 3, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hou, W.; Chui, J.; Han, R.; Gelb, A.W. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology 2015, 123, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, J.S.; Yang, Y.J.; Park, K.M.; Lee, C.W.; Kim, Y.H.; Hong, M.K.; Song, J.K.; Park, S.W.; Park, S.J.; et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2006, 97, 1365–1369. [Google Scholar] [CrossRef]
- van Bommel, R.J.; Marsan, N.A.; Koppen, H.; Delgado, V.; Borleffs, C.J.W.; Ypenburg, C.; Bertini, M.; Schalij, M.J.; Bax, J.J. Effect of Cardiac Resynchronization Therapy on Cerebral Blood Flow. Am. J. Cardiol. 2010, 106, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, D.; Wang, Y.; Yan, M.; Zheng, J.; Ren, J. Cognitive Impairment in Heart Failure: Landscape, Challenges, and Future Directions. Front. Cardiovasc. Med. 2022, 8, 831734. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Hashimoto, J. Mechanical Factors in Arterial Aging: A Clinical Perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef]
- Roos, A.d.; Grond, J.v.d.; Mitchell, G.; Westenberg, J. Magnetic Resonance Imaging of Cardiovascular Function and the Brain. Circulation 2017, 135, 2178–2195. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Qiu, C.; Fratiglioni, L. A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 2015, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Mah, D.; Gyenes, G. Cardiac rehabilitation and its effects on cognition in patients with coronary artery disease and heart failure. Expert Rev. Cardiovasc. Ther. 2018, 16, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Sweet, J.J.; Finnin, E.; Wolfe, P.L.; Beaumont, J.L.; Hahn, E.; Marymont, J.; Sanborn, T.; Rosengart, T.K. Absence of cognitive decline one year after coronary bypass surgery: Comparison to nonsurgical and healthy controls. Ann. Thorac. Surg. 2008, 85, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Selnes, O.A.; Gottesman, R.F.; Grega, M.A.; Baumgartner, W.A.; Zeger, S.L.; McKhann, G.M. Cognitive and Neurologic Outcomes after Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2012, 366, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Arena, R.; Ellingsen, Ø.; Harber, M.P.; Myers, J.; Ozemek, C.; Ross, R. Cardiorespiratory fitness and cardiovascular disease—The past, present, and future. Prog. Cardiovasc. Dis. 2019, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Green, D.J. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J. Physiol. 2009, 587, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Thompson Paul, D.; Buchner, D.; Piña Ileana, L.; Balady Gary, J.; Williams Mark, A.; Marcus Bess, H.; Berra, K.; Blair Steven, N.; Costa, F.; Franklin, B.; et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease. Circulation 2003, 107, 3109–3116. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154. [Google Scholar] [CrossRef]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef]
- Wittfeld, K.; Jochem, C.; Dörr, M.; Schminke, U.; Gläser, S.; Bahls, M.; Markus, M.R.P.; Felix, S.B.; Leitzmann, M.F.; Ewert, R.; et al. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin. Proc. 2020, 95, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Tomoto, T.; Repshas, J.; Wang, C.; Hynan, L.S.; Cullum, C.M.; Zhu, D.C.; Zhang, R. Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness. Neuroimage 2021, 225, 117512. [Google Scholar] [CrossRef] [PubMed]
- Bailey Damian, M.; Marley Christopher, J.; Brugniaux Julien, V.; Hodson, D.; New Karl, J.; Ogoh, S.; Ainslie Philip, N. Elevated Aerobic Fitness Sustained Throughout the Adult Lifespan Is Associated With Improved Cerebral Hemodynamics. Stroke 2013, 44, 3235–3238. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.A.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef]
- Tari, A.R.; Nauman, J.; Zisko, N.; Skjellegrind, H.K.; Bosnes, I.; Bergh, S.; Stensvold, D.; Selbæk, G.; Wisløff, U. Temporal changes in cardiorespiratory fitness and risk of dementia incidence and mortality: A population-based prospective cohort study. Lancet Public Health 2019, 4, e565–e574. [Google Scholar] [CrossRef]
- Davenport, M.H.; Hogan, D.B.; Eskes, G.A.; Longman, R.S.; Poulin, M.J. Cerebrovascular Reserve: The Link Between Fitness and Cognitive Function? Exerc. Sport Sci. Rev. 2012, 40, 153–158. [Google Scholar] [CrossRef]
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020, 94, e2245–e2257. [Google Scholar] [CrossRef]
- Tomoto, T.; Liu, J.; Tseng, B.Y.; Pasha, E.P.; Cardim, D.; Tarumi, T.; Hynan, L.S.; Munro Cullum, C.; Zhang, R. One-Year Aerobic Exercise Reduced Carotid Arterial Stiffness and Increased Cerebral Blood Flow in Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 80, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N.; Taylor, J.L.; Kluck, B.N.; Johnson, C.P.; Joyner, M.J. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J. Appl. Physiol. 2013, 114, 1383–1387. [Google Scholar] [CrossRef]
- Miller, K.B.; Howery, A.J.; Harvey, R.E.; Eldridge, M.W.; Barnes, J.N. Cerebrovascular Reactivity and Central Arterial Stiffness in Habitually Exercising Healthy Adults. Front. Physiol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Campos, D.; Mora, J.; Castro-Piñero, J.; González-Montesinos, J.L.; Conde-Caveda, J.; Chicharro, J.L. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J. Sports Med. Phys. Fitness 2012, 52, 537–544. [Google Scholar] [PubMed]
- Ivey, F.M.; Ryan, A.S.; Hafer-Macko, C.E.; Macko, R.F. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011, 42, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Pumpa, K.L.; Quinlan, C.; Ikin, A.; Toohey, K.; Smee, D.J.; Rattray, B. Cognition in breast cancer survivors: A pilot study of interval and continuous exercise. J. Sci. Med. Sport. 2019, 22, 580–585. [Google Scholar] [CrossRef]
- Thomas, B.P.; Yezhuvath, U.S.; Tseng, B.Y.; Liu, P.; Levine, B.D.; Zhang, R.; Lu, H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J. Magn. Reson. Imaging 2013, 38, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Lavey, J.A.; Carlson, A.R.; Barnes, J.N.; Johnson, B.D. A Pilot Study to Investigate the Effect of Hypercapnia Training on Cerebrovascular Reactivity in Healthy Adults. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Human Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Dabbaghipour, N.; Javaherian, M.; Moghadam, B.A. Effects of cardiac rehabilitation on cognitive impairments in patients with cardiovascular diseases: A systematic review. Int. J. Neurosci. 2021, 131, 1124–1132. [Google Scholar] [CrossRef]
- Taylor, J.L. Exercise and the Brain in Cardiovascular Disease: A Narrative Review. Heart Mind, 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Balady Gary, J.; Williams Mark, A.; Ades Philip, A.; Bittner, V.; Comoss, P.; Foody JoAnne, M.; Franklin, B.; Sanderson, B.; Southard, D. Core Components of Cardiac Rehabilitation/Secondary Prevention Programs: 2007 Update. Circulation 2007, 115, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.-P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 460–495. [Google Scholar] [CrossRef] [PubMed]
- Tanne, D.; Freimark, D.; Poreh, A.; Merzeliak, O.; Bruck, B.; Schwammenthal, Y.; Schwammenthal, E.; Motro, M.; Adler, Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int. J. Cardiol. 2005, 103, 145–149. [Google Scholar] [CrossRef]
- Gunstad, J.; Macgregor, K.L.; Paul, R.H.; Poppas, A.; Jefferson, A.L.; Todaro, J.F.; Cohen, R.A. Cardiac rehabilitation improves cognitive performance in older adults with cardiovascular disease. J. Cardiopulm. Rehabil. 2005, 25, 173–176. [Google Scholar] [CrossRef]
- Stanek, K.M.; Gunstad, J.; Spitznagel, M.B.; Waechter, D.; Hughes, J.W.; Luyster, F.; Josephson, R.; Rosneck, J. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. Int. J. Neurosci. 2011, 121, 86–93. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Cardiac rehabilitation is associated with lasting improvements in cognitive function in older adults with heart failure. Acta Cardiol. 2014, 69, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Herrmann, N.; Swardfager, W.; Saleem, M.; Oh, P.I.; Black, S.E.; Bradley, J.; Lanctôt, K.L. Subcortical hyperintensities in the cholinergic system are associated with improvements in executive function in older adults with coronary artery disease undergoing cardiac rehabilitation. Int. J. Geriatr. Psychiatry 2018, 33, 279–287. [Google Scholar] [CrossRef]
- Salzwedel, A.; Heidler, M.D.; Meng, K.; Schikora, M.; Wegscheider, K.; Reibis, R.; Völler, H. Impact of cognitive performance on disease-related knowledge six months after multi-component rehabilitation in patients after an acute cardiac event. Eur. J. Prev. Cardiol. 2019, 26, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Tsai, M.-C.; Brooks, D.; Oh, P.I. Randomised controlled trial in women with coronary artery disease investigating the effects of aerobic interval training versus moderate intensity continuous exercise in cardiac rehabilitation: CAT versus MICE study. BMJ Open Sport Exerc. Med. 2019, 5, e000589. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Minami, Y.; Yamaoka-Tojo, M.; Kutsuna, T.; Obara, S.; Aoyama, A.; Ako, J. Effect of cardiac rehabilitation on cognitive function in elderly patients with cardiovascular diseases. PLoS ONE 2020, 15, e0233688. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.A.; Bourbeau, K.; Mermier, C.; Kravitz, L.; Gibson, A.; Beltz, N.; Negrete, O.; Zuhl, M. Exercise-Based Cardiac Rehabilitation Improves Cognitive Function Among Patients With Cardiovascular Disease. J. Cardiopulm. Rehabil. Prev. 2020, 40, 407–413. [Google Scholar] [CrossRef]
- Sumida, H.; Yasunaga, Y.; Takasawa, K.; Tanaka, A.; Ida, S.; Saito, T.; Sugiyama, S.; Matsui, K.; Nakao, K.; Tsujita, K.; et al. Cognitive function in post-cardiac intensive care: Patient characteristics and impact of multidisciplinary cardiac rehabilitation. Heart Vessels. 2020, 35, 946–956. [Google Scholar] [CrossRef]
- Redwine, L.S.; Pung, M.A.; Wilson, K.; Bangen, K.J.; Delano-Wood, L.; Hurwitz, B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J. Psychosom. Res. 2020, 128, 109883. [Google Scholar] [CrossRef]
- Smith, K.; Moreno-Suarez, I.; Scheer, A.; Dembo, L.; Naylor, L.; Maiorana, A.; Green, D. Cerebral blood flow responses to exercise are enhanced in left ventricular assist device patients following an exercise rehabilitation program. J. Appl. Physiol. 2020, 128, 108–116. [Google Scholar] [CrossRef]
- Allman, J.M.; Hakeem, A.; Erwin, J.M.; Nimchinsky, E.; Hof, P. The Anterior Cingulate Cortex. Ann. N. Y. Acad. Sci. 2001, 935, 107–117. [Google Scholar] [CrossRef]
- Cilliers, K.; Page, B.J. Review of the Anatomy of the Distal Anterior Cerebral Artery and Its Anomalies. Turk. Neurosurg. 2016, 26, 653–661. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Mazereeuw, G.; Oh, P.I.; Goldstein, B.I.; Kiss, A.; Shammi, P.; Lanctôt, K.L. Association between Endothelial Function and Cognitive Performance in Patients with Coronary Artery Disease during Cardiac Rehabilitation. Psychosom. Med. 2019, 81, 184–191. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Gurd, B.J.; Bonafiglia, J.T.; Voisin, S.; Li, Z.; Harvey, N.; Croci, I.; Taylor, J.L.; Gajanand, T.; Ramos, J.S.; et al. A Multi-Center Comparison of VO2peak Trainability Between Interval Training and Moderate Intensity Continuous Training. Front. Physiol. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, N.; Beulque, R.; Cornelissen, V. Aerobic Interval vs. Continuous Training in Patients with Coronary Artery Disease or Heart Failure: An Updated Systematic Review and Meta-Analysis with a Focus on Secondary Outcomes. Sports Med. 2018, 48, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Kumar Bundhun, P.; Yuan, Z.L.; Chen, M.H. The effect of high-intensity interval training on exercise capacity in post-myocardial infarction patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 475–484. [Google Scholar] [CrossRef]
- Taylor, J.L.; Keating, S.E.; Holland, D.J.; Green, D.J.; Coombes, J.S.; Bailey, T.G. Comparison of high intensity interval training with standard cardiac rehabilitation on vascular function. Scand. J. Med. Sci. Sports 2022, 32, 512–520. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Taylor, J.L.; Holland, D.J.; Keating, S.E.; Leveritt, M.D.; Gomersall, S.R.; Rowlands, A.V.; Bailey, T.G.; Coombes, J.S. Short-term and Long-term Feasibility, Safety, and Efficacy of High-Intensity Interval Training in Cardiac Rehabilitation: The FITR Heart Study Randomized Controlled Trial. JAMA Cardiol. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Rognmo, Ø.; Moholdt, T.; Bakken, H.; Hole, T.; Mølstad, P.; Myhr, N.E.; Grimsmo, J.; Wisløff, U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012, 126, 1436–1440. [Google Scholar] [CrossRef]
- Wewege Michael, A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients With Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef]
- Cornelis, J.; Beckers, P.; Taeymans, J.; Vrints, C.; Vissers, D. Comparing exercise training modalities in heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 221, 867–876. [Google Scholar] [CrossRef]
- Gayda, M.; Ribeiro, P.A.; Juneau, M.; Nigam, A. Comparison of Different Forms of Exercise Training in Patients With Cardiac Disease: Where Does High-Intensity Interval Training Fit? Can. J. Cardiol. 2016, 32, 485–494. [Google Scholar] [CrossRef]
- Taylor, J.L.; Bonikowske, A.R.; Olson, T.P. Optimizing Outcomes in Cardiac Rehabilitation: The Importance of Exercise Intensity. Front. Cardiovasc. Med. 2021, 8, 734278. [Google Scholar] [CrossRef]
- Taylor, J.L.; Holland, D.J.; Spathis, J.G.; Beetham, K.S.; Wisløff, U.; Keating, S.E.; Coombes, J.S. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog. Cardiovasc. Dis. 2019, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rognmo, O.; Hetland, E.; Helgerud, J.; Hoff, J.; Slordahl, S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.; Aamot, I.-L.; Haykowsky, M.; Rognmo, Ø. High Intensity Interval Training for Maximizing Health Outcomes. Prog. Cardiovasc Dis. 2017, 60, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Holland, D.J.; Keating, S.E.; Bonikowske, A.R.; Coombes, J.S. Adherence to High-Intensity Interval Training in Cardiac Rehabilitation: A Review and Recommendations. J. Cardiopulm. Rehabil. Prev. 2021, 41, 61–77. [Google Scholar] [CrossRef]
- Calverley, T.A.; Ogoh, S.; Marley, C.J.; Steggall, M.; Marchi, N.; Brassard, P.; Lucas, S.J.E.; Cotter, J.D.; Roig, M.; Ainslie, P.N.; et al. HIITing the brain with exercise; mechanisms, consequences and practical recommendations. J. Physiol. 2020, 598, 2513–2530. [Google Scholar] [CrossRef]
- Ogoh, S.; Washio, T.; Suzuki, K.; Iemitsu, M.; Hashimoto, T.; Iwamoto, E.; Bailey, D.M. Greater increase in internal carotid artery shear rate during aerobic interval compared to continuous exercise in healthy adult men. Physiol. Rep. 2021, 9, e14705. [Google Scholar] [CrossRef]
- Klein, T.; Bailey, T.G.; Abeln, V.; Schneider, S.; Askew, C.D. Cerebral Blood Flow during Interval and Continuous Exercise in Young and Old Men. Med. Sci. Sports Exerc. 2019, 51, 1523–1531. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N. Cerebral blood flow during exercise: Mechanisms of regulation. J. Appl. Physiol. 2009, 107, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Cotter, J.; Brassard, P.; Bailey, D. High-intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J. Cereb. Blood Flow Metab. 2015, 35, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Faull, O.K.; Cotter, J.D.; Lucas, S.J. Cerebrovascular responses during rowing: Do circadian rhythms explain morning and afternoon performance differences? Scand. J. Med. Sci. Sports 2015, 25, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Furlong, R.J.; Weaver, S.R.; Sutherland, R.; Burley, C.V.; Imi, G.M.; Lucas, R.A.I.; Lucas, S.J.E. Exercise-induced elevations in cerebral blood velocity are greater in running compared to cycling at higher intensities. Physiol. Rep. 2020, 8, e14539. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.A.; Alwatban, M.; Freemyer, A.; Perales-Puchalt, J.; Billinger, S.A. Effects of high intensity interval exercise on cerebrovascular function: A systematic review. PLoS ONE 2020, 15, e0241248. [Google Scholar] [CrossRef]
- Coetsee, C.; Terblanche, E. Cerebral oxygenation during cortical activation: The differential influence of three exercise training modalities. A randomized controlled trial. Eur. J. Appl. Physiol. 2017, 117, 1617–1627. [Google Scholar] [CrossRef]
- Drapeau, A.; Labrecque, L.; Imhoff, S.; Paquette, M.; Le Blanc, O.; Malenfant, S.; Brassard, P. Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol. Rep. 2019, 7, e14185. [Google Scholar] [CrossRef]
- Kovacevic, A.; Fenesi, B.; Paolucci, E.; Heisz, J.J. The effects of aerobic exercise intensity on memory in older adults. Appl. Physiol. Nutr. Metab. 2019, 45, 591–600. [Google Scholar] [CrossRef]
- Mekari, S.; Earle, M.; Martins, R.; Drisdelle, S.; Killen, M.; Bouffard-Levasseur, V.; Dupuy, O. Effect of High Intensity Interval Training Compared to Continuous Training on Cognitive Performance in Young Healthy Adults: A Pilot Study. Brain Sci. 2020, 10, 81. [Google Scholar] [CrossRef]
| Study, Year, Design, and Sample Size | Patient Characteristics | Exercise Intervention | Change in Exercise Capacity | Summary of Brain-Related Outcome Results |
|---|---|---|---|---|
| Tanne et al. [88] (2005) Controlled Trial; Ex = 20, Control = 5 | HF—NYHA III 63 ± 13 years; 20% F BMI: 26.7 EF: 26 ± 5% 6 MWT: 308 ± 87 m | 18-week CR; 2 d/w Aerobic; 50 min 60–70% HRmax | ↑ 6 MWT distance (MD = 115 m) ↑ Modified Bruce Test (MD = 4.2 min) | Cognition: (+) Improved Attention-Psychomotor (TMT-A) and Executive functions (TMT-B, Stroop-A) for Ex group only. (−) NSC in Global function (MMSE), Visuospatial memory (Rey-Osterrieth Complex Figure), Language (Verbal fluency), and Stroop B + C or Continuous performance test. MCAv (TCD): (−) NSC in MCAv & CVR breath-hold index Other Hemodynamics: (+) Improved CV hemodynamics (↑ cardiac index & ↓ systemic vascular resistance). |
| Gunstad et al. [89] (2005) Case-Series; Ex = 18, no Control | CAD/CABG/HF 68 ± 8 years; 28% F METs: 5.2 ± 2.0 | 12-week CR; 3 d/w Aerobic; 10–45 min Intensity NR | ↑ Peak METs on clinical stress test (MD = 2.2) | Cognition: (+) Improved Attention-Psychomotor function (TMT-A & DSC) (−) NSC in GP & Language (Animal fluency). |
| Stanek et al. [90] (2011) Case-Series; Ex = 51, no Control | CAD/CABG/HF 68 ± 9 years; 35% F BMI: 31.3 METs: 7.2 ± 2.7 | 12-week CR; 3 d/w Aerobic; 60 min Intensity NR | ↑ Peak METs (MD = 2.8) | Cognition: (+) Improved Attention-Psychomotor function (LNS, GP) & Verbal Memory (HVLT) > practice effects. (+) Improved Global function (Modified MMSE) and Visuospatial Memory (BVMT) = practice effects. (−) NSC Executive function (TMT, FAB) or Language (Animal fluency, Boston naming). MCAv and ACAv (TCD): (+) ↑ACAv. (−) NSC in MCAv or pulsatility index. |
| Anazodo et al. [15] (2013); Case-Series; Ex = 24, no Control Anazodo et al. [14] (2016); Ex = 17 | CAD 59 ± 6 years; 25% F BMI: 29.8 ± 4.7 EF: 64 ± 8% PeakVO2: 26 ± 2 | 6-month CR; 3 d/w Aerobic + RT 20–30 min RPE 11–14 40–70%HRR | NSC PeakVO2 (MD = 5% increase, 1.3 mL/kg/min) | Cognition: (−) NSC MoCA score. Brain structure: (+) ↑ Gray matter volume bilaterally in frontal lobe, middle temporal gyrus, supplementary motor area. CBF (ASL): (+) ↑ Regional gray matter CBF in bilateral Anterior Cingulate. (−) NSC in Global Gray Matter CBF. |
| Alosco et al. [91] (2014) Case-Series; Ex = 52, no Control | HF—NYHA II/III 67 ± 8 years; 25% F EF: 39 ± 12% 2-min step test: 72 ± 20 | 12-week CR; 3 d/w Aerobic; 40 min Intensity NR | ↑ 2-min step test over 12-wk (MD = 4) NSC 2-min step test over 12-months (MD = 5) | Cognition: (+) Improved Attention-Psychomotor function (DSC) at 12-wks & 12-months. (+) Improved Verbal Memory (CVLT-II) at 12-months but NSC at 12-wks. (−) NSC in Global function (MMSE), Executive function (TMT-B), Language (Animal Fluency, Boston Naming), or TMT-A. |
| Santiago et al. [92] (2018) Case-Series; Ex = 50, no Control | ACS 67 ± 7 years; 16% F BMI: 28.6 ± 4.2 PeakVO2: 19 ± 5 | 48-week CR; 1 d/w + 4 d/w at home Aerobic + RT Walk/jog; Intensity NR | ↑ PeakVO2 (MD = 28%, 5.3 mL/kg/min) | Cognition: (+) Improved Attention-Psychomotor function (TMT-A, DSC) & Verbal memory (CVLT-II). (−) NSC in Executive function (TMT-B), Visuospatial memory (BVMT-R), & Language (Animal fluency, FAS Verbal fluency). |
| Salzwedel et al. [93] (2019) Cohort Study; Ex = 401, no control | ACS/CABG 55 ± 6 years; 20% F BMI 28.7 ± 5.1 | 3-week CR Exercise details NR | ↑ 6 MWT distance (MD = 83 m) | Cognition: (+) Improved MoCA score. |
| Lee et al. [94] (2019) RCT; Ex1 = 7, Ex2 = 7, no control | CAD/PCI/CABG 68 ± 9 years; 100% F BMI 28 ± 6 EF: >35% PeakVO2: 19 ± 4 | 24-week CR; 5 d/w Aerobic walk/jog Ex1: 30–40 min 60–80% PeakVO2 Ex2: 4 × 4 min 90–95% PeakHR RPE > 17 3 d/w + MICE 2 d/w | ↑ PeakVO2 for Ex2 (MD = 7%; 1.3 mL/kg) NSC PeakVO2 for Ex1 (MD = 2% 0.4 mL/kg). NS difference between groups. | Cognition: (−) NSC in Attention-Psychomotor function (DSC), Executive function (TMT-B, Digit span test), and Verbal Memory (CVLT-II) for moderate intensity (Ex1) or aerobic interval training (Ex2) groups. |
| Fujiyoshi et al. [95] (2020) Controlled Trial; Ex1 = 27, Ex2 = 39 | CVD 77 ± 5 years; 44% F BMI: 24 ± 3 y 6 MWT: 465 ± 98 m | 6-month CR Aerobic + RT BORG 10–13 Ex1: 1/month Ex2: <1/month | ↑ 6 MWT distance for Ex1 (MD = 40 m) NSC 6 MWT distance for Ex2 (MD = −14 m) | Cognition: (+) Improvement in Global (MMSE) and Executive functions (FAB) was significantly greater monthly CR (Ex1) than <monthly CR (Ex2), specifically improved were temporal orientation, attention, calculation, No/No-Go task). Vascular function: (+) Improvement in vascular function (as reactive hyperemia peripheral arterial tonometry) was significantly greater with monthly CR (Ex1) than the <monthly CR (Ex2). |
| Moriarty et al. [96] (2020) Case-Series; Ex = 20, no Control | CVD 65 ± 12 years; 25% F BMI: 29 ± 6 y METs: 5.5 ± 2.5 | 6-week CR 3 d/w, 30–60 min 50–80%HRR RPE 3–5/10 | ↑ METs from submaximal treadmill test (MD = 1.4) | Cognition: (+) Improved Global function (NIH Toolbox Fluid Composite score), and specifically Attention, Processing Speed, Executive Function, Visuospatial Working Memory. (−) NSC in Episodic (Verbal) Memory. Cerebral oxygenation (NIRS): (+) Improved oxygenation of right and left pre-frontal cortex during cognitive testing |
| Sumida et al. [97] (2020); Cohort Study Ex = 111, no Control | ACS/CABG/HF Age: 77 years BMI: 22 | 2–6 week inpatient CR; 2–3 d/w; Intensity NR | ↑ FIM-Physical score | Cognition: (+) Improved Functional Independence Measure (FIM-Cognitive). |
| Redwine et al. [98] (2020); RCT; Ex1 = 24, Ex2 = 22, Control = 23 | Symptomatic HF 65 ± 10 years; 11% F LVEF: 46 ± 14% | 16-week; 2 d/w; 60 min; RPE 11–13 Ex1: Tai Chi; Ex2:RT | None reported | Cognition: (+) Improved MoCA score for Ex groups (Tai Chi & RT) compared with Control. |
| Smith et al. [99] (2020) Case-Series; Ex = 12, no Control | LVAD 54 ± 12 years; 42% F PeakVO2: 12 ± 3 | 12-week CR 3 d/w, 60 min Aerobic + RT 59–90% VO2R; RPE 11–15; 50–60%1 RM | ↑ PeakVO2 (MD = 25%; 2.9 mL/kg) | Cognition: Not assessed. MCAv and PCAv (TCD): (+) Improved PCAv regulation during maximal exercise following the training period. Improved ICA flow regulation during submaximal exercise following training period. NSC in MCAv regulation. (−) Decrease in resting PCAv. NSC in resting MCAv. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, J.L.; Barnes, J.N.; Johnson, B.D. The Utility of High Intensity Interval Training to Improve Cognitive Aging in Heart Disease Patients. Int. J. Environ. Res. Public Health 2022, 19, 16926. https://doi.org/10.3390/ijerph192416926
Taylor JL, Barnes JN, Johnson BD. The Utility of High Intensity Interval Training to Improve Cognitive Aging in Heart Disease Patients. International Journal of Environmental Research and Public Health. 2022; 19(24):16926. https://doi.org/10.3390/ijerph192416926
Chicago/Turabian StyleTaylor, Jenna L., Jill N. Barnes, and Bruce D. Johnson. 2022. "The Utility of High Intensity Interval Training to Improve Cognitive Aging in Heart Disease Patients" International Journal of Environmental Research and Public Health 19, no. 24: 16926. https://doi.org/10.3390/ijerph192416926
APA StyleTaylor, J. L., Barnes, J. N., & Johnson, B. D. (2022). The Utility of High Intensity Interval Training to Improve Cognitive Aging in Heart Disease Patients. International Journal of Environmental Research and Public Health, 19(24), 16926. https://doi.org/10.3390/ijerph192416926






