Enhanced Efinaconazole Permeation and Activity Against Trichophyton rubrum and Trichophyton mentagrophytes with a Self-Nanoemulsifying Drug Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Method for Sample Analysis
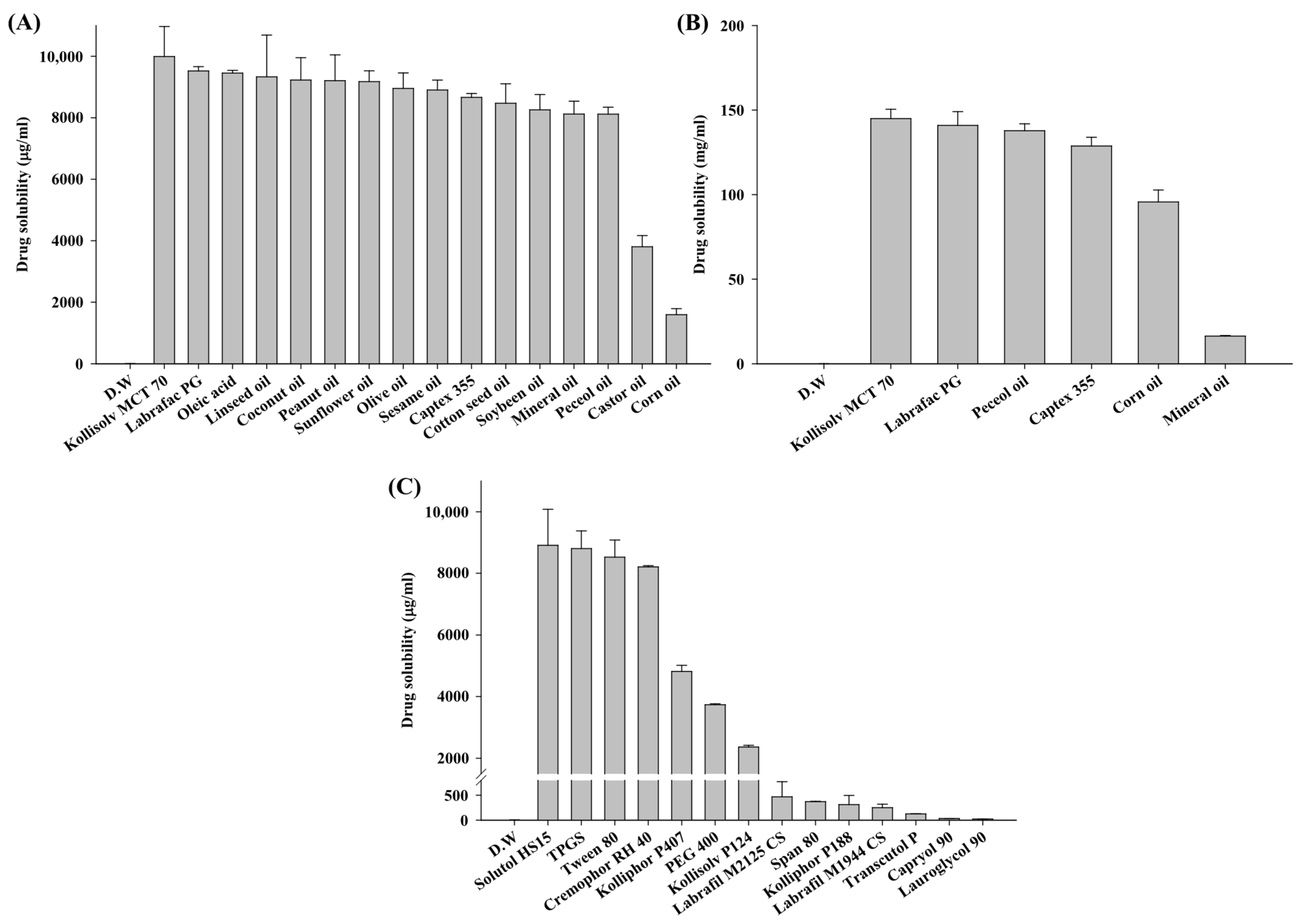
2.3. Solubility Study
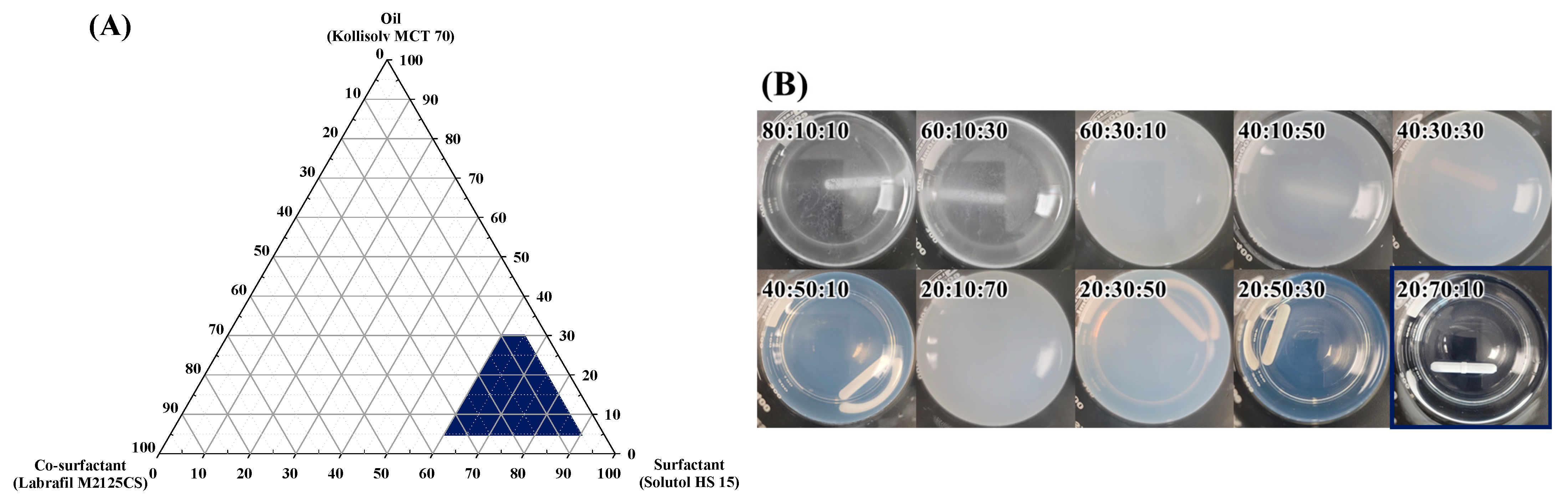
2.4. Construction of Pseudo-Ternary Phase Diagram
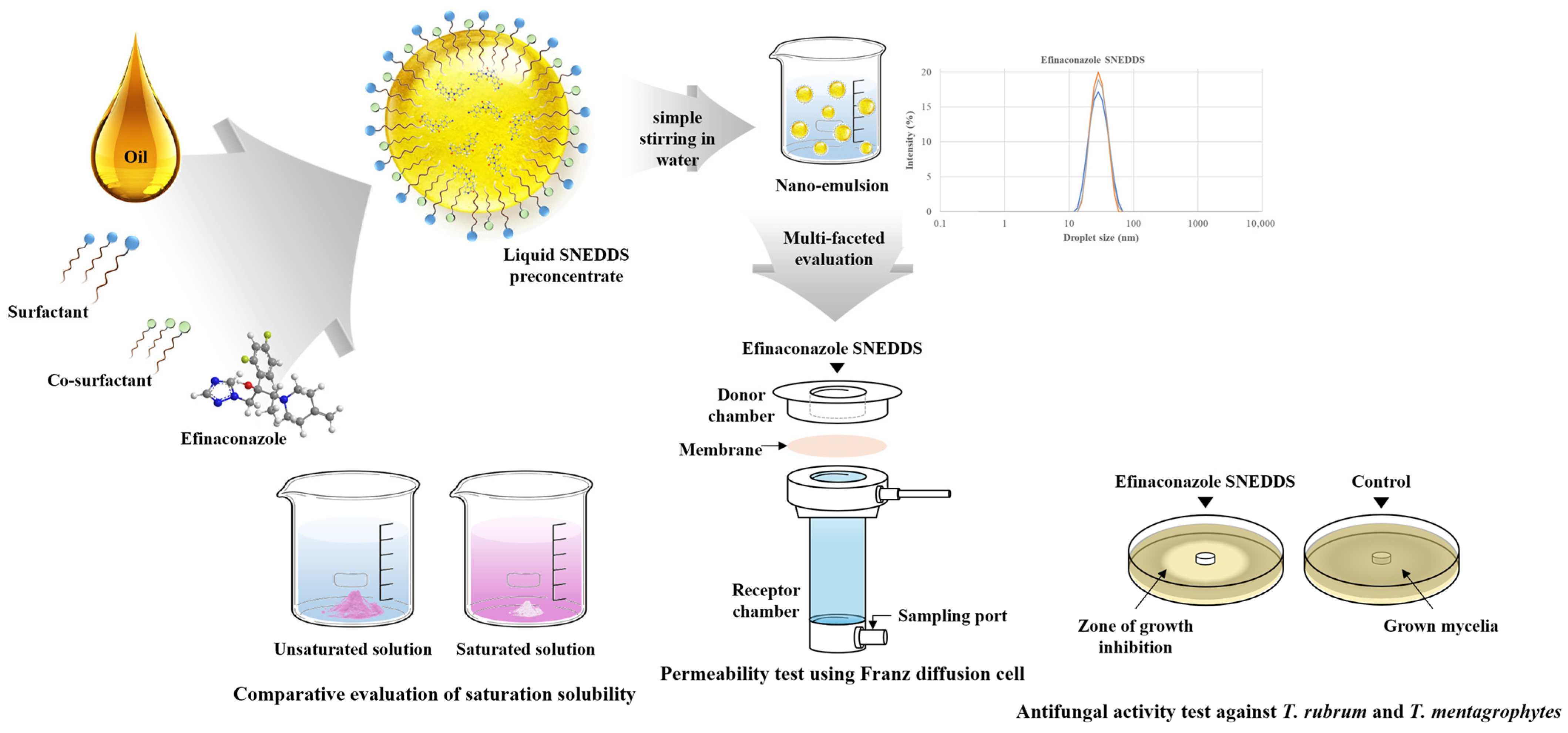
2.5. Preparation of SNEDDS
2.6. Droplet Size, Polydispersity Index, and Zeta Potential
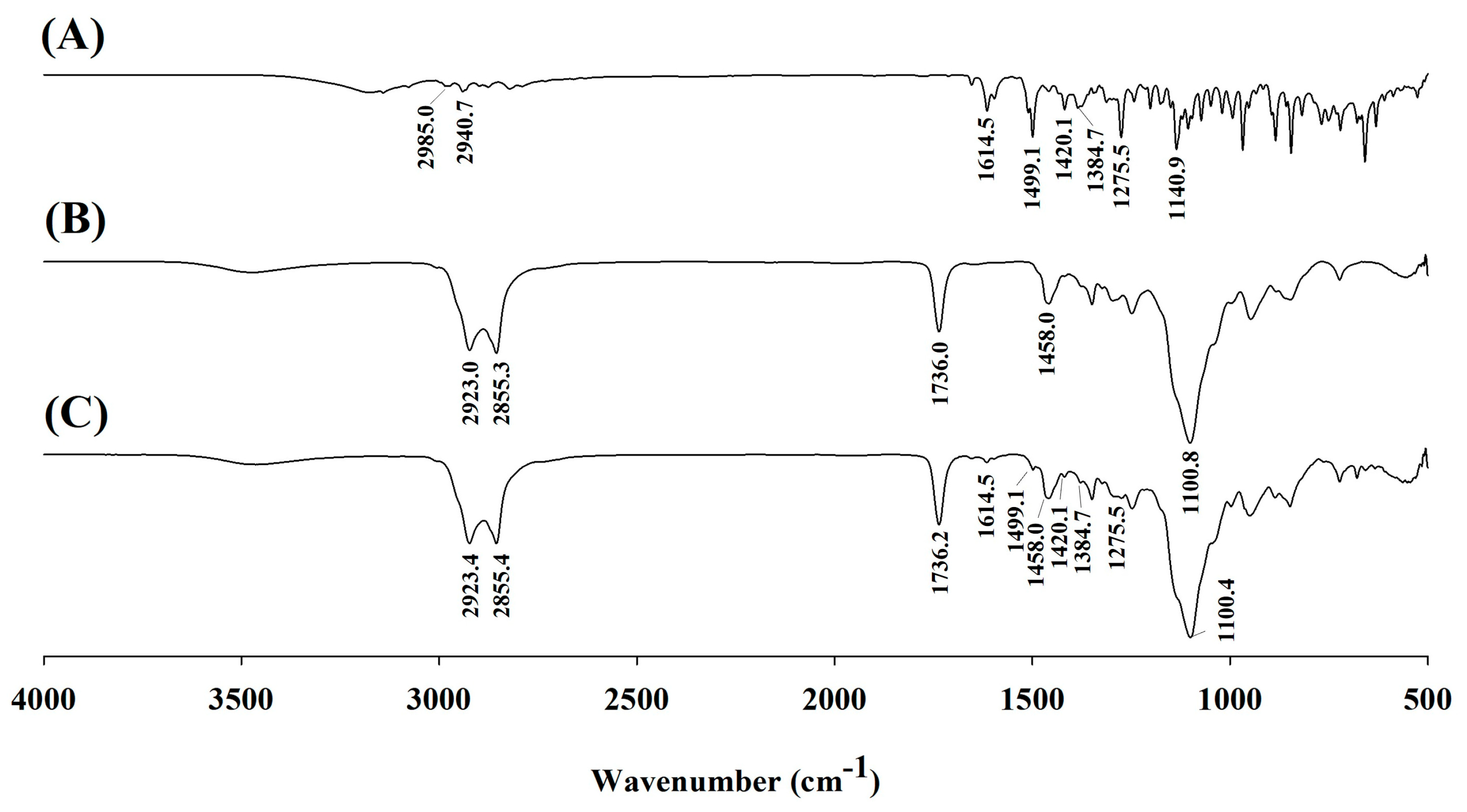
2.7. Fourier Transform Infrared Spectroscopy
2.8. Transmission Electron Microscopy Analysis
2.9. Saturated Solubility Study
2.10. In Vitro Artificial Transdermal Membrane Permeability Study
2.11. Stability Study
2.12. In Vitro Antifungal Activity—Paper Disc Diffusion Test
2.13. Scanning Electron Microscopy Analysis of Morphological Changes in Fungi After EFN SNEDDS Treatment
2.13.1. Morphological Changes in Fungi on Drug-Containing Media
2.13.2. Morphological Changes in Fungi on Human Nails
2.14. Statistical Evaluation
3. Results
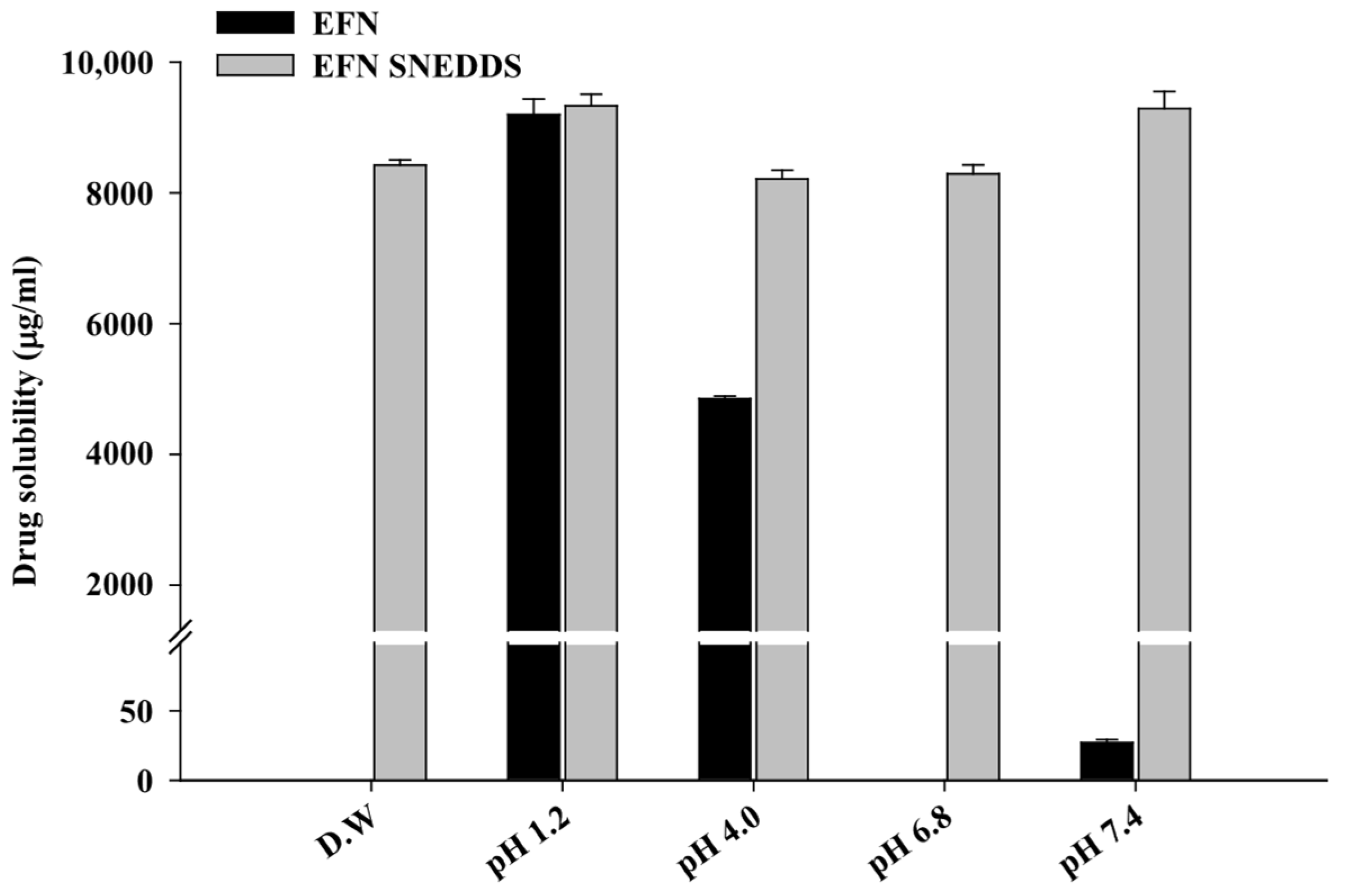
3.1. Solubility Study
3.2. Construction of Pseudo-Ternary Phase Diagrams
3.3. Characterization of EFN SNEDDS
3.3.1. Droplet Size, PDI, and ZP
3.3.2. FT-IR Analysis
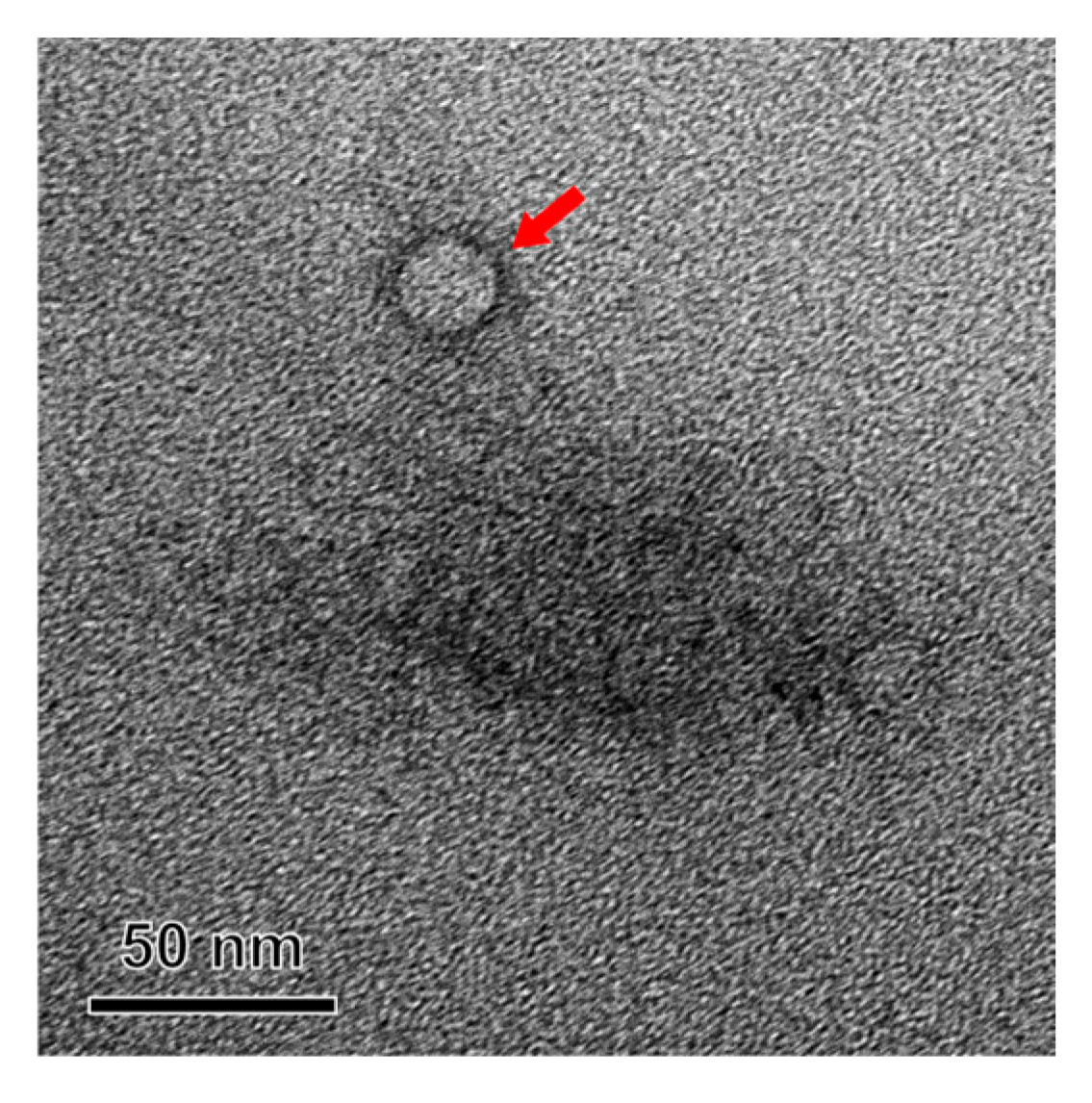
3.3.3. TEM Analysis
3.4. Saturated Solubility Study
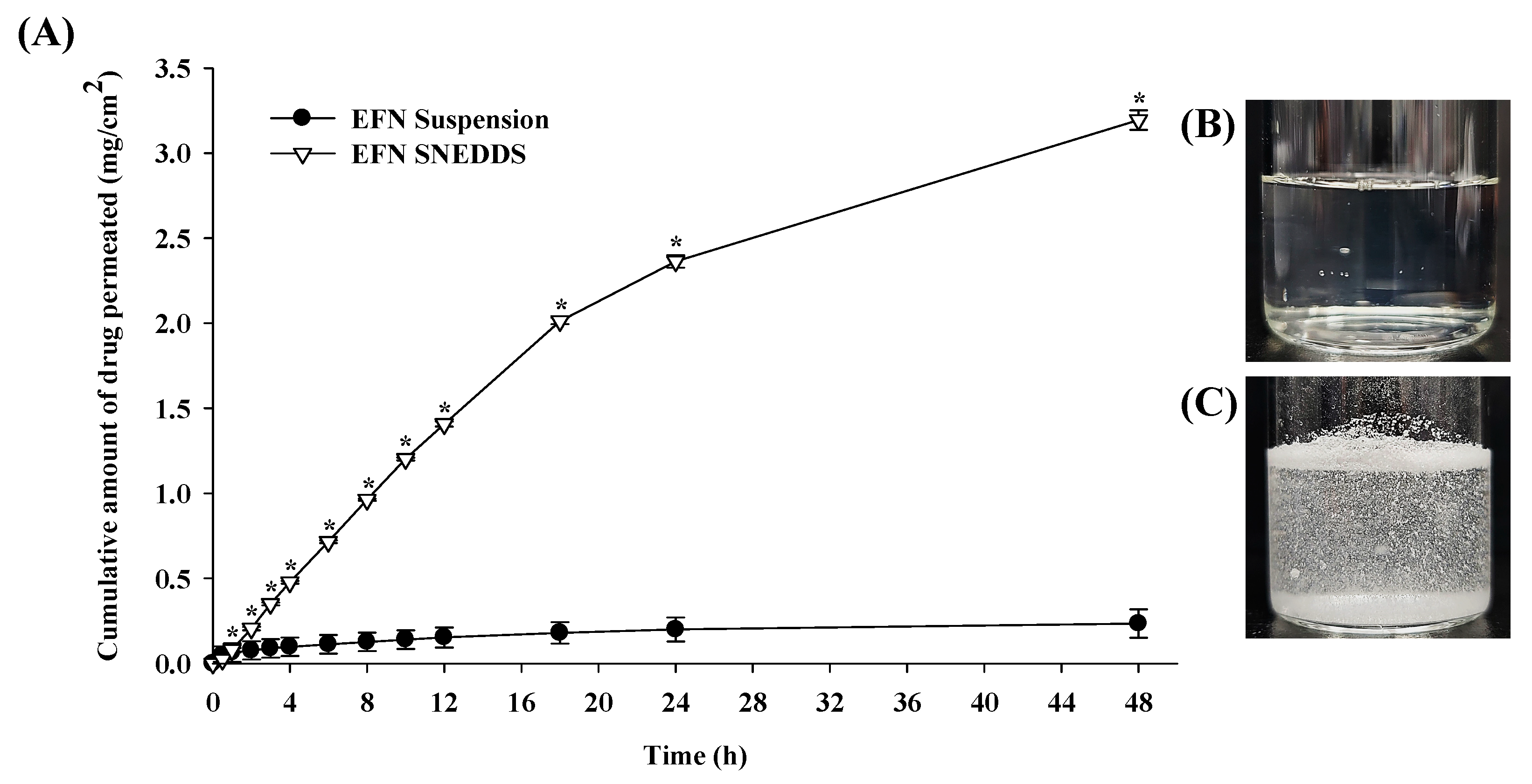
3.5. In Vitro Artificial Transdermal Membrane Permeability Study
3.6. Stability Study
3.7. In Vitro Antifungal Activity—Paper Disc Diffusion Test
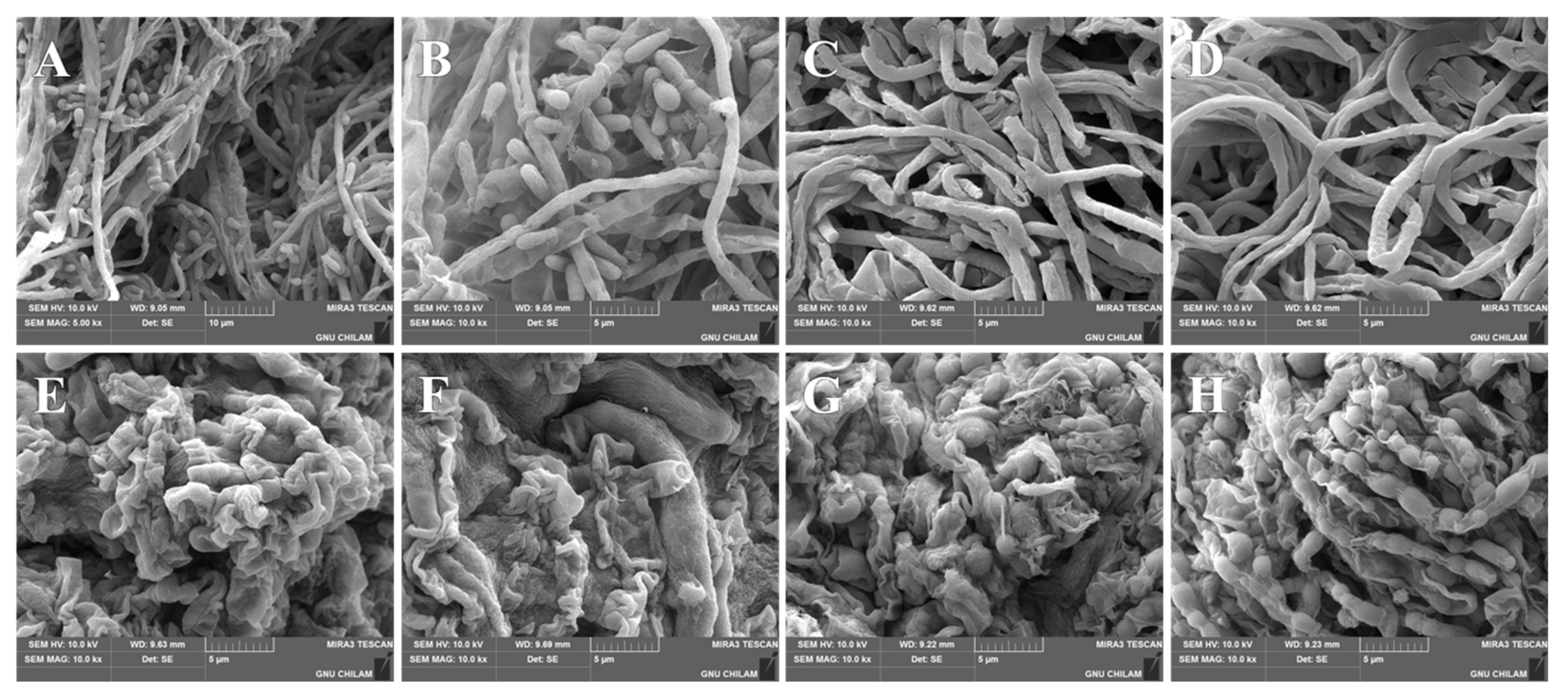
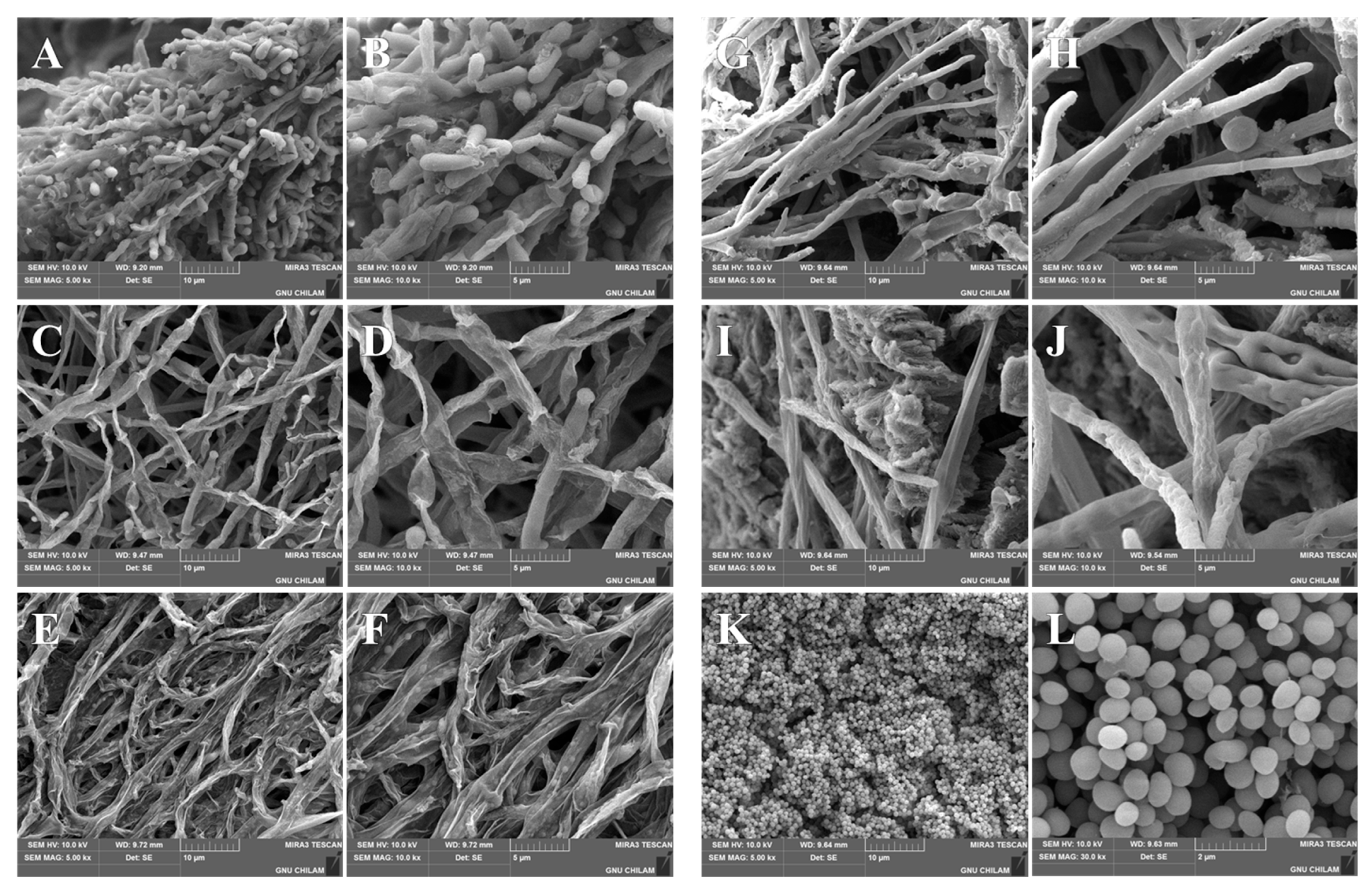
3.8. SEM Analysis of Morphological Changes in T. rubrum and T. mentagrophytes After EFN SNEDDS Treatment
3.8.1. Morphological Changes in Fungi on Drug-Containing Media
3.8.2. Morphological Changes in Fungi on Human Nails
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrawal, V.; Patel, R.; Patel, M.; Thanki, K.; Mishra, S. Design and evaluation of microemulsion-based efinaconazole formulations for targeted treatment of onychomycosis through transungual route: Ex vivo and nail clipping studies. Colloids Surf. B Biointerfaces 2021, 201, 111652. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Polla Ravi, S.; Mann, A.; Bamimore, M.A. Global prevalence of onychomycosis in general and special populations: An updated perspective. Mycoses 2024, 67, e13725. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Talukder, M. Onychomycosis in older adults: Prevalence, diagnosis, and management. Drugs Aging 2022, 39, 191–198. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Srinonprasert, V.; Kiratiwongwan, R.; Wongdama, S.; Leeyaphan, C. Onychomycosis in older adults: The age and associated factors affecting the complete cure rate. Australas. J. Dermatol. 2022, 63, 74–80. [Google Scholar] [CrossRef]
- Trovato, L.; Calvo, M.; De Pasquale, R.; Scalia, G.; Oliveri, S. Prevalence of onychomycosis in diabetic patients: A case-control study performed at university hospital Policlinico in Catania. J. Fungi 2022, 8, 922. [Google Scholar] [CrossRef]
- Vlahovic, T.C.; Sebag, J.A. Onychomycosis in Diabetics. In Onychomycosis: An Illustrated Guide to Diagnosis and Treatment; Tosti, A., Vlahovic, T.C., Arenas, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 169–174. [Google Scholar]
- Chang, P.; Ucelo, Z.M.Q.; del Pilar Garzaro Chávez, H.M. Onychomycosis and immunodepression. Curr. Fungal Infect. Rep. 2017, 11, 252–257. [Google Scholar] [CrossRef]
- Ramos-e-Silva, M.; Lima, C.M.O.; Schechtman, R.C.; Trope, B.M.; Carneiro, S. Superficial mycoses in immunodepressed patients (AIDS). Clin. Dermatol. 2010, 28, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Faergemann, J.; Baran, R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br. J. Dermatol. 2003, 149, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Taylor, W.; Boyle, J. Guidelines for treatment of onychomycosis. Br. J. Dermatol. 2003, 148, 402–410. [Google Scholar] [CrossRef]
- Christenson, J.K.; Peterson, G.M.; Naunton, M.; Bushell, M.; Kosari, S.; Baby, K.E.; Thomas, J. Challenges and Opportunities in the Management of Onychomycosis. J. Fungi 2018, 4, 87. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Carviel, J.L.; Cooper, E.A.; Piguet, V. Combatting antifungal resistance: Paradigm shift in the diagnosis and management of onychomycosis and dermatomycosis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, A.; Kamel, B.R.; Saffaei, A.; Tavakoli-Ardakani, M. Hepatotoxicity induced by azole antifungal agents: A review study. Iran. J. Pharm. Res. IJPR 2023, 22, e130336. [Google Scholar] [CrossRef]
- Adis Medical Writers. Long-term use of systemic azole antifungals can result in hepatotoxicity and other serious adverse effects. Drugs Ther. Perspect. 2020, 36, 112–115. [Google Scholar] [CrossRef]
- Childs-Kean, L.M.; Jourjy, J. Antifungal Penetration into the Nail and New Topicals for Onychomycosis. Curr. Fungal Infect. Rep. 2016, 10, 24–29. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Starace, M.; Rubin, A.I.; Di Chiacchio, N.G.; Iorizzo, M.; Rigopoulos, D.; A working group of the European Nail Society. Onychomycosis: Recommendations for diagnosis, assessment of treatment efficacy, and specialist referral. The CONSONANCE Consensus Project. Dermatol. Ther. 2022, 12, 885–898. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Nagashima, M.; Shibanushi, T.; Iwata, A.; Kangawa, Y.; Inui, F.; Siu, W.J.J.; Pillai, R.; Nishiyama, Y. Mechanism of action of efinaconazole, a novel triazole antifungal agent. Antimicrob. Agents Chemother. 2013, 57, 2405–2409. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M. Efinaconazole in onychomycosis. Am. J. Clin. Dermatol. 2022, 23, 207–218. [Google Scholar] [CrossRef]
- Valeant Canada LP. JUBLIA™ (Efinaconazole) Topical Solution, 10% w/w [Product Monograph]; Submission Control No. 204442, 7 July 2017; Valeant Canada LP: Laval, QC, Canada, 2017. [Google Scholar]
- Raphael, A.P.; Garrastazu, G.; Sonvico, F.; Prow, T.W. Formulation design for topical drug and nanoparticle treatment of skin disease. Ther. Deliv. 2015, 6, 197–216. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; Abouelatta, S.M. Contemporary techniques and potential transungual drug delivery nanosystems for the treatment of onychomycosis. AAPS PharmSciTech 2023, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Aldeeb, M.M.E.; Wilar, G.; Suhandi, C.; Elamin, K.M.; Wathoni, N. Nanosuspension-based drug delivery systems for topical applications. Int. J. Nanomed. 2024, 19, 825–844. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Joshi, G.; Sawant, K.K. Nanotechnology in oral drug delivery: Salient aspects, state of art, and applications. In Functional Bionanomaterials. Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 165–184. [Google Scholar]
- Lunter, D.; Klang, V.; Eichner, A.; Savic, S.M.; Savic, S.; Lian, G.; Erdő, F. Progress in Topical and Transdermal Drug Delivery Research—Focus on Nanoformulations. Pharmaceutics 2024, 16, 817. [Google Scholar] [CrossRef]
- Gupta, A.K.; Polla Ravi, S.; Choi, S.Y.; Konda, A.; Cooper, E.A. Strategies for the enhancement of nail plate permeation of drugs to treat onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 243–255. [Google Scholar] [CrossRef]
- Morakul, B. Self-nanoemulsifying drug delivery systems (SNEDDS): An advancement technology for oral drug delivery. Pharm. Sci. Asia 2020, 47, 205–220. [Google Scholar] [CrossRef]
- Choi, S.A.; Park, E.J.; Lee, J.H.; Min, K.A.; Kim, S.T.; Jang, D.-J.; Maeng, H.-J.; Jin, S.G.; Cho, K.H. Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Mathew, R. Formulation and development of antifungal loaded nanoemulgel for the treatment of onychomycosis. IAJPS 2021, 08, 191–201. [Google Scholar]
- Lee, B.C.; Pangeni, R.; Na, J.; Koo, K.-T.; Park, J.W. Preparation and in vivo evaluation of a highly skin-and nail-permeable efinaconazole topical formulation for enhanced treatment of onychomycosis. Drug Deliv. 2019, 26, 1167–1177. [Google Scholar]
- Vikas, A.; Rashmin, P.; Mrunali, P.; Sandip, M.; Kaushik, T. RP-HPLC method for quantitative estimation of Efinaconazole in topical microemulsion and microemulsion-based-gel formulations and in presence of its degradation products. Microchem. J. 2020, 155, 104753. [Google Scholar]
- Lee, S.-M.; Lee, J.-G.; Yun, T.-H.; Cho, J.-H.; Kim, K.-S. Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System. Int. J. Mol. Sci. 2024, 25, 1197. [Google Scholar] [CrossRef]
- Kim, D.S.; Cho, J.H.; Park, J.H.; Kim, J.S.; Song, E.S.; Kwon, J.; Giri, B.R.; Jin, S.G.; Kim, K.S.; Choi, H.-G. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int. J. Nanomed. 2019, 15, 4949–4960. [Google Scholar]
- Emad, N.A.; Sultana, Y.; Aqil, M.; Saleh, A.; Nasr, F.A. Omega-3 fatty acid-based self-microemulsifying drug delivery system (SMEDDS) of pioglitazone: Optimization, in vitro and in vivo studies. Saudi J. Biol. Sci. 2023, 30, 103778. [Google Scholar]
- Kang, J.H.; Oh, D.H.; Oh, Y.-K.; Yong, C.S.; Choi, H.-G. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur. J. Pharm. Biopharm. 2012, 80, 289–297. [Google Scholar] [CrossRef]
- Truong, D.H.; Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Lee, H.H.; Moon, C.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Development of solid self-emulsifying formulation for improving the oral bioavailability of erlotinib. AAPS PharmSciTech 2016, 17, 466–473. [Google Scholar]
- Choi, H.J.; Kim, K.S. Development of Solid Self-nanoemulsifying Drug Delivery Systems of Ticagrelor Using Porous Carriers. J. Life Sci. 2021, 31, 502–510. [Google Scholar]
- Ramaye, Y.; Dabrio, M.; Roebben, G.; Kestens, V. Development and validation of optical methods for zeta potential determination of silica and polystyrene particles in aqueous suspensions. Materials 2021, 14, 290. [Google Scholar] [CrossRef]
- Varenne, F.; Botton, J.; Merlet, C.; Vachon, J.-J.; Geiger, S.; Infante, I.C.; Chehimi, M.M.; Vauthier, C. Standardization and validation of a protocol of zeta potential measurements by electrophoretic light scattering for nanomaterial characterization. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 486, 218–231. [Google Scholar] [CrossRef]
- Mantri, S.K.; Pashikanti, S.; Murthy, K.V.R. Development and characterization of self-nanoemulsifying drug delivery systems (SNEDDS) of atorvastatin calcium. Curr. Drug Deliv. 2012, 9, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chaurasiya, A.; Awasthi, A.; Mishra, G.; Asati, D.; Khar, R.K.; Mukherjee, R. Oral bioavailability enhancement of exemestane from self-microemulsifying drug delivery system (SMEDDS). AAPS PharmSciTech 2009, 10, 906–916. [Google Scholar] [CrossRef]
- Yun, T.; Lee, S.; Yun, S.; Cho, D.; Bang, K.; Kim, K. Investigation of stabilized amorphous solid dispersions to improve oral olaparib absorption. Pharmaceutics 2024, 16, 958. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Alsaidan, O.A.; Mohanty, D.; Zafar, A.; Das, S.; Gupta, J.K.; Khalid, M. Development of Soft Luliconazole Invasomes Gel for Effective Transdermal Delivery: Optimization to In-Vivo Antifungal Activity. Gels 2023, 9, 626. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, D.W. Development and evaluation of hot-melt–extruded diquafosol tetrasodium formulations for ophthalmic inserts: A design of experiments approach. Int. J. Pharm. 2024, 659, 124249. [Google Scholar] [CrossRef]
- Vikash, B.; Pandey, N.K.; Kumar, B.; Wadhwa, S.; Goutam, U.; Alam, A.; Al-Otaibi, F.; Chaubey, P.; Mustafa, G.; Gupta, G. Formulation and evaluation of ocular self-nanoemulsifying drug delivery system of brimonidine tartrate. J. Drug Deliv. Sci. Technol. 2023, 81, 104226. [Google Scholar] [CrossRef]
- Marzulli, F.N.; Brown, D.W.; Maibach, H.I. Techniques for studying skin penetration. Toxicol. Appl. Pharmacol. 1969, 14, 76–83. [Google Scholar] [CrossRef]
- Champmartin, C.; Seiwert, C.; Aubertin, M.; Joubert, E.; Marquet, F.; Chedik, L.; Cosnier, F. Percutaneous absorption of two bisphenol a analogues, BPAF and TGSA: Novel In vitro data from human skin. Chemosphere 2024, 367, 143564. [Google Scholar] [CrossRef]
- Bang, K.-H.; Kim, K.S. Development of trans-cinnamaldehyde self-microemulsifying drug delivery system (SMEDDS) with superior stability. J. Korea Acad.-Ind. Coop. Soc. 2019, 20, 555–562. [Google Scholar]
- Zheng, Y.; Shang, Y.; Li, M.; Li, Y.; Ouyang, W. Antifungal Activities of cis-trans Citral Isomers against Trichophyton rubrum with ERG6 as a Potential Target. Molecules 2021, 26, 4263. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Yanyun, C.; Ying, T.; Wei, K.; Hua, F.; Haijun, Z.; Ping, Z.; Shunming, X.; Jian, W. Preliminary study on antifungal mechanism of aqueous extract of cnidium monnieri against trichophyton rubrum. Front. Microbiol. 2021, 12, 707174. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Maeda, M.; Yamada, T. Effect of Topical Antifungal Luliconazole on Hyphal Morphology of Trichophyton mentagrophytes Grown on in vitro Onychomycosis Model. Mycopathologia 2022, 187, 491–496. [Google Scholar] [CrossRef]
- Limbani, M.D.; Patel, L. Studies on drug solubilization and role of lipid vehicle in pseudo ternary phase diagram in formulation development of SNEDDS containing poorly water soluble drug. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 228–237. [Google Scholar]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic–lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech 2014, 15, 456–464. [Google Scholar] [CrossRef]
- Schmied, F.-P.; Bernhardt, A.; Engel, A.; Klein, S. A customized screening tool approach for the development of a self-nanoemulsifying drug delivery system (SNEDDS). AAPS PharmSciTech 2021, 23, 39. [Google Scholar] [CrossRef]
- Ahmad, N.; Khalid, M.S.; Khan, M.F.; Ullah, Z. Beneficial effects of topical 6-gingerol loaded nanoemulsion gel for wound and inflammation management with their comparative dermatokinetic. J. Drug Deliv. Sci. Technol. 2023, 80, 104094. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Detholia, K.; Mohandas, A.; Varia, U.; Jadeja, M.; Katariya, H. Development and optimization of Ropinirole loaded self-nanoemulsifying tablets. Future J. Pharm. Sci. 2023, 9, 66. [Google Scholar] [CrossRef]
- Nair, A.B.; Aldhubiab, B.; Shah, J.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K.N.; Joseph, A.; Morsy, M.A. Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole. Pharmaceutics 2023, 15, 1422. [Google Scholar] [CrossRef]
- Asghar, Z.; Jamshaid, T.; Sajid-ur-Rehman, M.; Jamshaid, U.; Gad, H.A. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics 2023, 15, 2537. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, S.; Zuo, J.; Sun, J.; Wang, J. Reduced the food effect and enhanced the oral bioavailability of ivacaftor by self-nanoemulsifying drug delivery system (SNEDDS) using a new oil phase. Drug Des. Dev. Ther. 2022, 16, 1531–1546. [Google Scholar] [CrossRef]
- Cao, M.; Xue, X.; Pei, X.; Qian, Y.; Liu, L.; Ren, L.; Chen, G. Formulation optimization and pharmacokinetics evaluation of oral self-microemulsifying drug delivery system for poorly water soluble drug cinacalcet and no food effect. Drug Dev. Ind. Pharm. 2018, 44, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitro and in vivo assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Frei, G.; Haimhoffer, Á.; Csapó, E.; Bodnár, K.; Vasvári, G.; Nemes, D.; Lekli, I.; Gyöngyösi, A.; Bácskay, I.; Fehér, P. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Monti, D.; Mazzantini, D.; Tampucci, S.; Vecchione, A.; Celandroni, F.; Burgalassi, S.; Ghelardi, E. Ciclopirox and efinaconazole transungual permeation, antifungal activity, and proficiency to induce resistance in Trichophyton rubrum. Antimicrob. Agents Chemother. 2019, 63, e00442-19. [Google Scholar] [CrossRef]
- Devi, A.; Chaurasia, H.; Singh, R. Development of nano formuation and its optimization evaluation of efinaconazole loaded transfersomal gel for treatment of nail infection. J. Jilin Univ. 2021, 40, 23–43. [Google Scholar]
- Kwon, S.B.; Lee, H.-Y. Permeation Characteristics of Acetaminophen Using Franz Diffusion Cells. J. Biotechnol. Bioind. 2022, 10, 32–36. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as penetration enhancers for dermal and transdermal drug delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–230. [Google Scholar]
- Cole, E.T.; Cadé, D.; Benameur, H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008, 60, 747–756. [Google Scholar] [CrossRef]
- Kanwal, T.; Saifullah, S.; ur Rehman, J.; Kawish, M.; Razzak, A.; Maharjan, R.; Imran, M.; Ali, I.; Roome, T.; Simjee, S.U. Design of absorption enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for curcumin improved anti-cancer activity and oral bioavailability. J. Mol. Liq. 2021, 324, 114774. [Google Scholar] [CrossRef]
- Sarhadi, H.; Shahdadi, F.; Salehi Sardoei, A.; Hatami, M.; Ghorbanpour, M. Investigation of physio-mechanical, antioxidant and antimicrobial properties of starch–zinc oxide nanoparticles active films reinforced with Ferula gummosa Boiss essential oil. Sci. Rep. 2024, 14, 5789. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Hosny, K.M. Nano-vesicular delivery system loaded by Bifonazole: Preparation, optimization, and assessment of pharmacokinetic and antifungal activity. J. Drug Deliv. Sci. Technol. 2019, 49, 316–322. [Google Scholar] [CrossRef]
- Kassem, A.A.; Mohsen, A.M.; Ahmed, R.S.; Essam, T.M. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J. Mol. Liq. 2016, 218, 219–232. [Google Scholar] [CrossRef]
- Gräser, Y.; Kuijpers, A.; Presber, W.; De Hoog, G.S. Molecular taxonomy of Trichophyton mentagrophytes and T. tonsurans. Med. Mycol. 1999, 37, 315–330. [Google Scholar] [CrossRef]
- Chin, B.; Knight, S. Growth of Trichophyton mentagrophytes and Trichophyton rubrum in increased carbon dioxide tensions. Microbiology 1957, 16, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Švarcová, M.; Větrovský, T.; Kolařík, M.; Hubka, V. Defining the relationship between phylogeny, clinical manifestation, and phenotype for Trichophyton mentagrophytes/interdigitale complex; a literature review and taxonomic recommendations. Med. Mycol. 2023, 61, myad042. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.; Ivashechkin, A.; Alekhin, A.; Sergeeva, Y.E. Fungal spores: Dormancy, germination, chemical composition, and role in biotechnology. Appl. Biochem. Microbiol. 2012, 48, 1–11. [Google Scholar]
- Leng, W.; Liu, T.; Li, R.; Yang, J.; Wei, C.; Zhang, W.; Jin, Q. Proteomic profile of dormant Trichophyton rubrum conidia. BMC Genom. 2008, 9, 303. [Google Scholar] [CrossRef][Green Version]
- Guttentag, A.; Krishnakumar, K.; Cokcetin, N.; Hainsworth, S.; Harry, E.; Carter, D. Inhibition of dermatophyte fungi by Australian jarrah honey. Pathogens 2021, 10, 194. [Google Scholar] [CrossRef]
- Kaufman, G.; Horwitz, B.A.; Duek, L.; Ullman, Y.; Berdicevsky, I. Infection stages of the dermatophyte pathogen Trichophyton: Microscopic characterization and proteolytic enzymes. Med. Mycol. 2007, 45, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Kimura, N.; Okada, Y.; Shimizu, K.; Shibata, K.; Miyazaki, Y.; Ishida, A.; Yonezawa, K.; Shimizu, N.; Shinoda, W. Understanding the effects of ethanol on the liposome bilayer structure using microfluidic-based time-resolved small-angle X-ray scattering and molecular dynamics simulations. Nanoscale Adv. 2024, 6, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]



| SNEDDS Composition (mg) | Droplet Size (nm) | PDI | ZP (mV) | Drug Content (%) | |||
|---|---|---|---|---|---|---|---|
| Kollisolv MCT 70 | Solutol HS 15 | Labrafil M2125CS | |||||
| EFN-unloaded | 50 | 750 | 200 | 31.0 ± 0.6 | 0.13 ± 0.02 | −13.6 ± 0.5 | - |
| EFN 100 mg loaded | 31.8 ± 0.8 | 0.21 ± 0.01 | −12.3 ± 0.9 | 100.2 ± 0.3 | |||
| Parameters | EFN Suspension | EFN SNEDDS |
|---|---|---|
| Q%48 h | 0.24 ± 0.09 | 9.69 ± 0.18 * |
| Flux (J, μg/cm2/h) | 0.72 ± 0.30 | 17.31 ± 0.53 * |
| Papp (μg/h) | 3.48 × 10−5 | 8.38 × 10−4 * |
| Lag time (min) | 0.058 ± 0.017 | 0.021 ± 0.018 |
| Permeability coefficient (Kp, cm/h) | 7.34 × 10−6 | 2.47 × 10−4 * |
| Time (month) | Droplet Size (nm) | PDI | ZP (mV) | Drug Content (%) |
|---|---|---|---|---|
| (A) 25 °C, 60% RH | ||||
| 0 | 31.8 ± 0.8 | 0.21 ± 0.01 | −12.3 ± 0.9 | 100.2 ± 0.3 |
| 3 | 32.2 ± 0.2 | 0.21 ± 0.01 | −12.3 ± 0.5 | 100.2 ± 0.1 |
| 6 | 35.0 ± 0.4 | 0.22 ± 0.02 | −12.6 ± 0.6 | 100.1 ± 0.1 |
| (B) 40 °C, 75% RH | ||||
| 0 | 31.8 ± 0.8 | 0.21 ± 0.01 | −12.3 ± 0.9 | 100.2 ± 0.3 |
| 3 | 32.5 ± 0.5 | 0.23 ± 0.02 | −13.0 ± 0.4 | 100.0 ± 0.2 |
| 6 | 36.4 ± 0.2 | 0.25 ± 0.01 | −13.5 ± 0.5 | 99.8 ± 0.2 |
| (C) 60 °C | ||||
| 0 | 31.8 ± 0.8 | 0.21 ± 0.01 | −12.3 ± 0.9 | 100.2 ± 0.3 |
| 3 | 35.9 ± 0.4 | 0.25 ± 0.03 | −13.9 ± 0.2 | 100.0 ± 0.1 |
| 6 | 39.2 ± 0.6 | 0.26 ± 0.03 | −16.4 ± 0.3 | 99.5 ± 0.1 |
| (A) Zone of Inhibition (cm2)—T. rubrum | ||
| Drug conc. (mg/mL) | EFN suspension | EFN SNEDDS |
| C0 | ND | ND |
| CV | ND | ND |
| 100 | 28.97 ± 0.23 | 63.59 ± 0.00 * |
| 10 | 28.50 ± 0.03 | 52.78 ± 0.18 * |
| 1 | 14.18 ± 0.06 | 40.69 ± 0.43 * |
| 0.5 | 14.51 ± 0.20 | 33.34 ± 0.19 * |
| 0.1 | 0.79 ± 0.33 | 12.56 ± 0.27 * |
| 0.05 | ND | 2.83 ± 0.03 * |
| 0.01 | ND | ND |
| (B) Zone of Inhibition (cm2)—T. mentagrophytes | ||
| Drug conc. (mg/mL) | EFN suspension | EFN SNEDDS |
| C0 | ND | ND |
| CV | ND | ND |
| 100 | 34.45 ± 0.04 | 63.59 ± 0.00 * |
| 10 | 35.50 ± 0.14 | 54.30 ± 0.25 * |
| 1 | 17.16 ± 0.26 | 31.16 ± 0.18 * |
| 0.5 | 5.51 ± 2.00 | 23.75 ± 0.10 * |
| 0.1 | 4.15 ± 0.07 | 6.30 ± 0.03 * |
| 0.05 | 1.65 ± 0.09 | 4.09 ± 0.03 * |
| 0.01 | ND | 2.45 ± 0.06 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.W.; Lee, J.G.; Kim, C.H.; Kim, K.S. Enhanced Efinaconazole Permeation and Activity Against Trichophyton rubrum and Trichophyton mentagrophytes with a Self-Nanoemulsifying Drug Delivery System. Pharmaceutics 2025, 17, 1230. https://doi.org/10.3390/pharmaceutics17091230
Yun SW, Lee JG, Kim CH, Kim KS. Enhanced Efinaconazole Permeation and Activity Against Trichophyton rubrum and Trichophyton mentagrophytes with a Self-Nanoemulsifying Drug Delivery System. Pharmaceutics. 2025; 17(9):1230. https://doi.org/10.3390/pharmaceutics17091230
Chicago/Turabian StyleYun, Seo Wan, Jeong Gyun Lee, Chul Ho Kim, and Kyeong Soo Kim. 2025. "Enhanced Efinaconazole Permeation and Activity Against Trichophyton rubrum and Trichophyton mentagrophytes with a Self-Nanoemulsifying Drug Delivery System" Pharmaceutics 17, no. 9: 1230. https://doi.org/10.3390/pharmaceutics17091230
APA StyleYun, S. W., Lee, J. G., Kim, C. H., & Kim, K. S. (2025). Enhanced Efinaconazole Permeation and Activity Against Trichophyton rubrum and Trichophyton mentagrophytes with a Self-Nanoemulsifying Drug Delivery System. Pharmaceutics, 17(9), 1230. https://doi.org/10.3390/pharmaceutics17091230






