Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. Cream Preparation
2.2.3. Evaluation of Sample Creams
Physical Appearance
Spreadability
Viscosity
Creaming Index
2.2.4. Optimisation of Cream Compounding
2.2.5. Confirmation Studies of Optimal Aqueous and Cetomacrogol Creams
Preparation and Evaluation of Optimal Creams
Freeze-Thaw Cycle
Optical Microscopy
Data Analysis
3. Results
3.1. DoE Cream Samples
3.1.1. Physical Appearance
3.1.2. Spreadability
3.1.3. Viscosity
3.1.4. Creaming Index
3.2. Determination of Optimal Processing Conditions
3.3. Confirmation Studies of Optimised Creams
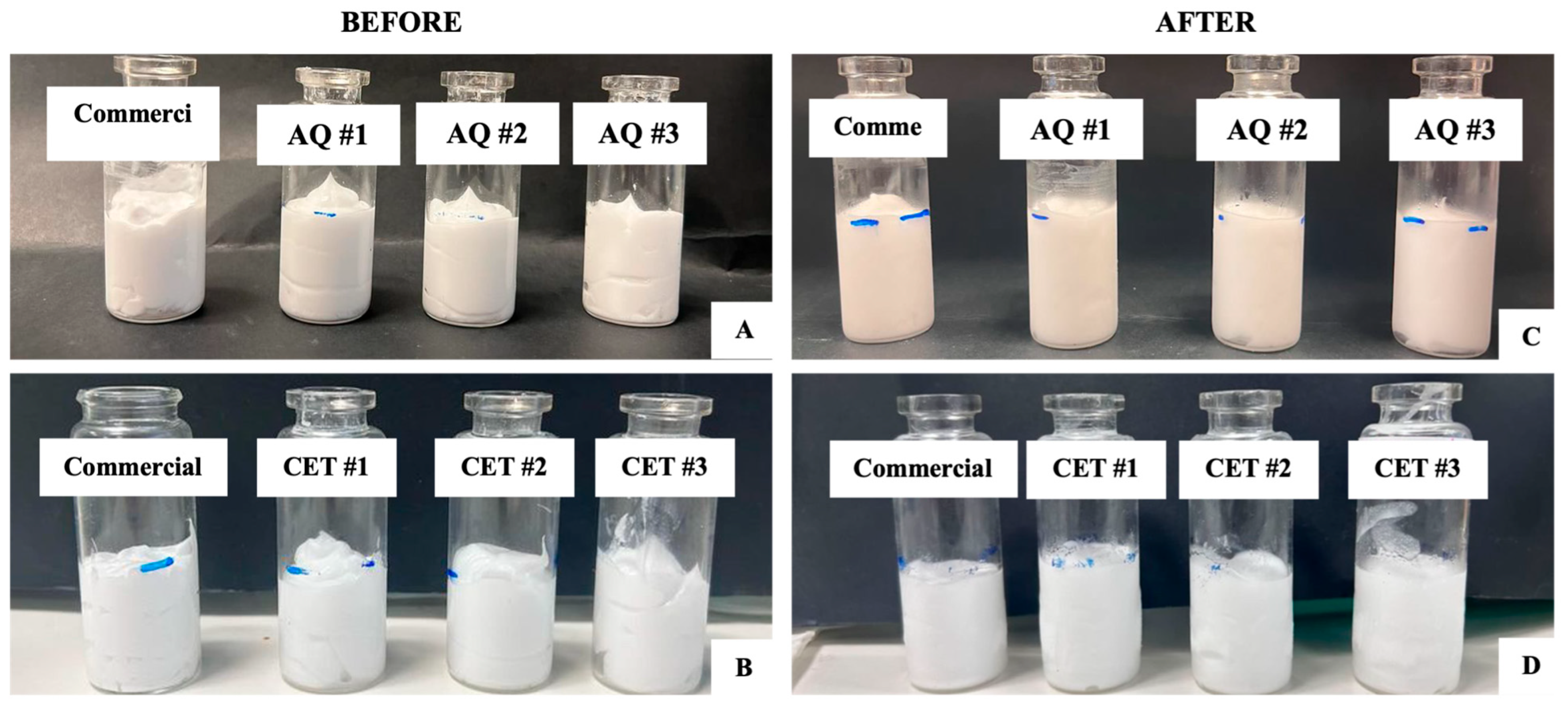
3.3.1. Evaluation of Optimised Creams
3.3.2. Freeze-Thaw Cycle
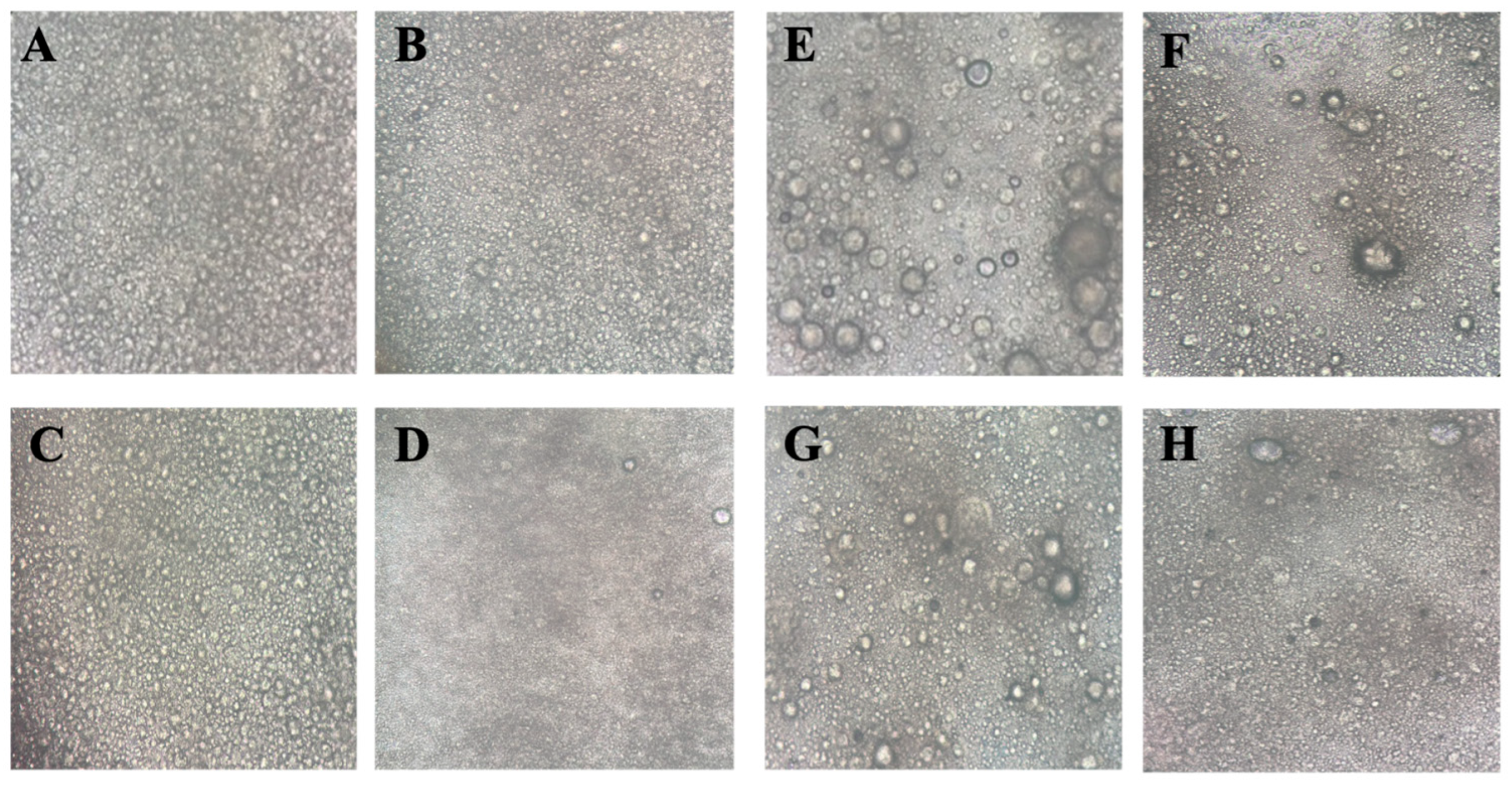
3.3.3. Optical Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guidelines on Compounding of Medicines. 2017. Available online: https://www.pharmacyboard.gov.au/documents/default.aspx?record=WD15%2f16205&dbid=AP&chksum=3QlnioMt0DhI0PsjaoB83A%3d%3d (accessed on 5 September 2024).
- Alves, T.; Arranca, D.; Martins, A.; Ribeiro, H.; Raposo, S.; Marto, J. Complying with the guideline for quality and equivalence for topical semisolid products: The case of clotrimazole cream. Pharmaceutics 2021, 13, 555. [Google Scholar] [CrossRef]
- Wibowo, C.; Ng, K.M. Product-oriented process synthesis and development: Creams and pastes. AIChE J. 2001, 47, 2746–2767. [Google Scholar] [CrossRef]
- Eccleston, G.M. Functions of mixed emulsifiers and emulsifying waxes in dermatological lotions and creams. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 169–182. [Google Scholar] [CrossRef]
- Wangpradit, N.; Macha, S.; Phooteh, N.; Yusohyo, N.; Waedoloh, A.; Manee, S. Determination of required hydrophilic-lipophilic balance of Amesiodendron chinense (Merr.) Hu oil and development of stable cream formulation. OCL 2022, 29, 29. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, H.; Pavurala, N.; Wang, J.; Sekar, V.; Martinez, M.N.; Fahmy, R.; Ashraf, M.; Cruz, C.N.; Xu, X. Formulation characteristics and In Vitro release testing of cyclosporine ophthalmic ointments. Int. J. Pharm. 2018, 544, 254–264. [Google Scholar] [CrossRef]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 26th ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2024. [Google Scholar]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef]
- Ali, A.; Skedung, L.; Burleish, S.; Lavant, E.; Ringstad, L.; Anderson, C.D.; Whalgren, M.; Engblom, J. Relationship between sensorial and physical characteristics of topical creams: A comparative study on effects of excipients. Int. J. Pharm. 2022, 613, 121370. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Lam, K.W.; Ng, K.M.; Ko, R.K.M.; Wibowo, C. An integrative approach to product development-a skin-care cream. Comput. Chem. Eng. 2009, 33, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Nguyen, N.N.T. Development and pre-clinical study of anti-allergic cream containing dexamethasone and chlorpheniramine. Turk. J. Pharm. Sci. 2018, 15, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yayé, H.S.; Faucheron, A.; Dupont, L.; El Kouari, F.; Fekkar, A.; Bellanger, A.; Tilleul, P. Management of diabetic foot ulcers: A 25% lidocaine topical cream formulation design, physicochemical and microbiological assessments. Eur. J. Hosp. Pharm. 2020, 27, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Formulation, optimization and In Vivo evaluation of fucoidan-based cream with anti-inflammatory properties. Mar. Drugs 2021, 19, 643. [Google Scholar] [CrossRef]
- Sundar, M.; Suresh, S.; Lingakumar, K. Preparation and optimization of medicated cold cream using caralluma adscendens var. Attenuata for the treatment of candida skin infection. BioTechnologia 2022, 103, 249–260. [Google Scholar] [CrossRef]
- Contreras, M.D.; Sanchez, R. Application of a factorial design to the study of the flow behavior, spreadability and transparency of a carbopol etd 2020 gel. Part ii. Int. J. Pharm. 2002, 234, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Panda, A.; Parajuli, S.; Prado, R.B.; Kundu, S.; Repka, M.; Ureña-Benavides, E.; Murthy, S.N. Effect of surfactant on quality and performance attributes of topical semisolids. Int. J. Pharm. 2021, 596, 120210. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Khattak, S.R.; Ahmad, I. Factors affecting formulation characteristics and stability of ascorbic acid in water-in-oil creams. Int. J. Cosmet. Sci. 2014, 36, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Fauzee, A.F.B.; Walker, R.B. The impact of formulation variables on the optimization of pilot scale clobetasol 17-propionate creams. Cogent Eng. 2020, 7, 1804713. [Google Scholar] [CrossRef]
- Cunningham, G.E.; Alberini, F.; Simmons, M.J.H.; O’Sullivan, J.J. Understanding the effects of processing conditions on the formation of lamellar gel networks using a rheological approach. Chem. Eng. Sci. 2021, 242, 116752. [Google Scholar] [CrossRef]
- Realdon, N.; Perin, F.; Morpurgo, M.; Ragazzi, E. Influence of processing conditions in the manufacture of o/w creams: I. Effect on dispersion grade and rheological characteristics. Il Farmaco 2001, 57, 341–347. [Google Scholar] [CrossRef]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical compounding: A history, regulatory overview, and systematic review of compounding errors. J. Med. Toxicol. 2021, 17, 197–217. [Google Scholar] [CrossRef]
- Barkat, A.; Ali Khan, B.; Naveed, A.; Muhammad, H.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A Review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- British Pharmacopoeia Commission. British Pharmacopoeia 2019; The Stationery Office: London, UK, 2018; Volume 1. [Google Scholar]
- Brayfield, A. (Ed.) Martindale: The Complete Drug Reference, 39th ed.; Pharmaceutical Press: London, UK, 2017; Volume A, pp. 1781–1782. [Google Scholar]
- Jelvehgari, M.; Montazam, H. Evaluation of Mechanical and Rheological Properties of Metronidazole Gel as Local Delivery System. Arch. Pharm. Res. 2011, 34, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, M.; Wang, X.; Zhong, J. Droplet and creaming stability of fish oil-loaded gelatin/surfactant-stabilized emulsions depends on both the adsorption ways of emulsifiers and the adjusted pH. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124847. [Google Scholar] [CrossRef]
- World Health Organisation. Guidelines for Stability Testing of Pharmaceutical Products Containing Well Established Drug Substances in Conventional Dosage Forms. 1996. Available online: https://www3.paho.org/hq/dmdocuments/2008/6_Annex_5_report_34.pdf. (accessed on 4 September 2024).
- Solutions for Your Toughest Mixing Applications in Pharmaceuticals: Production of Pharmaceutical Creams and Ointments Silverson. 2024. Available online: https://www.silverson.com/images/uploads/documents/P_Pharmaceutical_Creams_2016_US.pdf. (accessed on 5 September 2024).
- Dhoot, A.S.; Fernandes, G.J.; Naha, A.; Rathnanand, M.; Kumar, L. Design of experiments in pharmaceutical development. Pharm. Chem. J. 2019, 53, 730–735. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Developing cream formulations: Renewed interest in an old problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 19th ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2004; ISBN 0-908185-66-9. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 20th ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2006; ISBN 0-908185-77-4. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 21st ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2009; ISBN 978-0-908185-95-5. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 22nd ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2012; ISBN 978-0-646-57019-8. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 23rd ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2015; ISBN 978-0-9874550-4-8. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 24th ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2018; ISBN 978-0-9874550-6-2. [Google Scholar]
- Samsom, L. (Ed.) Australian Pharmaceutical Formulary and Handbook, 25th ed.; Pharmaceutical Society of Australia: Parkville, Australia, 2021; ISBN 978-0-908185-34-4. [Google Scholar]


| APF Aqueous Cream | APF Cetomacrogol Cream Aqueous | |
|---|---|---|
| Formulation | Emulsifying ointment 30 g Glycerol 5 mL Phenoxyethanol 1 g Purified water, freshly boiled and cooled, to 100 g | Cetomacrogol emulsifying wax 15 g Liquid paraffin 10 g White soft paraffin 10 g Chlorocresol 100 mg Propylene glycol 5 mL Purified water, freshly boiled and cooled, to 100 g |
| Compounding Instructions | Add glycerol and phenoxyethanol to 60 mL of purified water and heat to approximately 60 °C. Separately melt the emulsifying ointment. Combine the two phases and stir until a semi-solid cream forms. Make up to 100 g with warm purified water and mix thoroughly. Stir until cool. | Melt the cetomacrogol emulsifying wax in the paraffins at about 70 °C. Add the chlorocresol to a warmed 200 mL container, then add 60 mL of just-boiled purified water (>80 °C), close the container and shake to dissolve. Immediately add the propylene glycol to the aqueous phase, then mix both phases and stir until a semi-solid cream forms. Make up to 100 g with warm purified water and mix thoroughly. Stir until cool. |
| Processing Condition | Unit of Measure | Aqueous Cream Levels | Cetomacrogol Cream Levels | ||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | −1 | 0 | +1 | ||
| A 1 | °C | 60 | 70 | 80 | 60 | 70 | 80 |
| B 2 | °C | 60 | 75 | 90 | 75 | 85 | 95 |
| C 3 | °C | 30 | 20 | 10 | 30 | 20 | 10 |
| D 4 | rpm | 100 | 200 | 300 | 100 | 200 | 300 |
| E 5 | °C | 30 | 40 | 50 | 30 | 40 | 50 |
| Formulation | Phases | Ingredients | Quantities (g) |
|---|---|---|---|
| APF Aqueous Cream | Water | Glycerol | 6.3 |
| Phenoxyethanol | 1 | ||
| Water | 62.7 | ||
| Oil | Emulsifying Ointment BP | 30 | |
| TOTAL | 100 | ||
| APF Cetomacrogol Cream | Water | Chlorocresol | 0.1 |
| Propylene Glycol | 5.2 | ||
| Water | 34.8 | ||
| Oil | Cetomacrogol Emulsifying Wax | 15 | |
| Liquid Paraffin | 10 | ||
| White Soft Paraffin | 10 | ||
| TOTAL | 100 | ||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 2.22 | 1 | 2.22 | 32.63 | <0.0001 | |
| D | 2.22 | 1 | 2.22 | 32.63 | <0.0001 | 0.351 |
| Residual | 2.04 | 30 | 0.068 | |||
| Lack of Fit | 1.71 | 25 | 0.0684 | 1.04 | 0.5397 | |
| Pure Error | 0.3286 | 5 | 0.0657 | |||
| Cor Total | 4.26 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 3.29 | 7 | 0.4702 | 3.27 | 0.0138 | |
| B | 0.2689 | 1 | 0.2689 | 1.87 | 0.184 | 0.1222 |
| C | 0.245 | 1 | 0.245 | 1.71 | 0.204 | 0.1167 |
| D | 0.0022 | 1 | 0.0022 | 0.0155 | 0.9021 | 0.0111 |
| E | 0.08 | 1 | 0.08 | 0.5568 | 0.4628 | 0.0667 |
| BD | 0.81 | 1 | 0.81 | 5.64 | 0.0259 | −0.225 |
| CE | 1.32 | 1 | 1.32 | 9.2 | 0.0057 | 0.2875 |
| DE | 0.5625 | 1 | 0.5625 | 3.91 | 0.0595 | 0.1875 |
| Residual | 3.45 | 24 | 0.1437 | |||
| Lack of Fit | 2.59 | 19 | 0.1362 | 0.7921 | 0.6797 | |
| Pure Error | 0.86 | 5 | 0.172 | |||
| Cor Total | 6.74 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 2.82 × 109 | 11 | 2.56 × 108 | 39.94 | <0.0001 | |
| A | 6.58 × 106 | 1 | 6.58 × 106 | 1.03 | 0.3231 | 604.5 |
| B | 8.05 × 107 | 1 | 8.05 × 107 | 12.56 | 0.002 | 2115.06 |
| C | 4.21 × 107 | 1 | 4.21 × 107 | 6.56 | 0.0186 | 1528.61 |
| D | 1.77 × 109 | 1 | 1.77 × 109 | 276.63 | <0.0001 | 9924.5 |
| E | 12,534.72 | 1 | 12,534.72 | 0.002 | 0.9652 | 26.39 |
| AD | 3.44 × 107 | 1 | 3.44 × 107 | 5.37 | 0.0312 | 1466.25 |
| AE | 3.03 × 107 | 1 | 3.03 × 107 | 4.72 | 0.0419 | 1375.5 |
| BD | 2.57 × 107 | 1 | 2.57 × 107 | 4.01 | 0.0589 | 1267.62 |
| BE | 2.76 × 107 | 1 | 2.76 × 107 | 4.3 | 0.0512 | −1312.37 |
| CE | 4.67 × 107 | 1 | 4.67 × 107 | 7.28 | 0.0138 | 1708 |
| D2 | 7.49 × 108 | 1 | 7.49 × 108 | 116.85 | <0.0001 | −9751.73 |
| Residual | 1.28 × 108 | 20 | 6.41 × 106 | |||
| Lack of Fit | 1.22 × 108 | 15 | 8.13 × 106 | 6.53 | 0.0242 | |
| Pure Error | 6.23 × 106 | 5 | 1.25 × 106 | |||
| Cor Total | 2.94 × 109 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 5.35 × 109 | 1 | 5.35 × 109 | 33.06 | <0.0001 | |
| B | 5.35 × 109 | 1 | 5.35 × 109 | 33.06 | <0.0001 | 17,239.67 |
| Residual | 4.86 × 109 | 30 | 1.62 × 108 | |||
| Lack of Fit | 4.48 × 109 | 25 | 1.79 × 108 | 2.39 | 0.1687 | |
| Pure Error | 3.75 × 108 | 5 | 7.50 × 108 | |||
| Cor Total | 1.02 × 1010 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 196.75 | 2 | 98.38 | 17.03 | <0.0001 | |
| B | 35.14 | 1 | 35.14 | 6.08 | 0.0198 | −1.4 |
| D | 161.61 | 1 | 161.61 | 27.98 | <0.0001 | −3 |
| Residual | 167.51 | 29 | 5.78 | |||
| Lack of Fit | 140.42 | 24 | 5.85 | 1.08 | 0.5187 | |
| Pure Error | 27.1 | 5 | 5.42 | |||
| Cor Total | 364.27 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient of Final Equation in Terms of Coded Factors |
|---|---|---|---|---|---|---|
| Model | 27.36 | 5 | 5.47 | 6.6 | 0.0004 | |
| B | 10.71 | 1 | 10.71 | 12.91 | 0.0013 | 0.7713 |
| D | 3.38 | 1 | 3.38 | 4.08 | 0.0539 | 0.4334 |
| BD | 4.21 | 1 | 4.21 | 5.08 | 0.0329 | −0.5129 |
| B2 | 5.06 | 1 | 5.06 | 6.1 | 0.0204 | 1.2 |
| D2 | 9.07 | 1 | 9.07 | 10.93 | 0.0028 | −1.61 |
| Residual | 21.56 | 26 | 0.8292 | |||
| Lack of Fit | 16.34 | 21 | 0.778 | 0.7452 | 0.7144 | |
| Pure Error | 5.22 | 5 | 1.04 | |||
| Cor Total | 48.92 | 31 |
| A (°C) | B (°C) | C (°C) | D (rpm) | E (°C) | |
|---|---|---|---|---|---|
| Aqueous Cream | |||||
| Optimal Values | 60–67 | 76–90 | 10–15 | 229–270 | 48–50 |
| Confirmation study | 60 | 80 | 10 | 250 | 50 |
| Cetomacrogol Cream | |||||
| Optimal Values | 60–80 | 75 | 10–30 | 216–243 | 30–50 |
| Confirmation study | 70 | 75 | 25 | 220 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, O.; Li, W.; Ruan, S.; Syme, E.; Rodrigo, A.; Locher, C.; Sultana, S.; Lim, L.Y. Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations. Pharmaceutics 2025, 17, 1232. https://doi.org/10.3390/pharmaceutics17091232
Yoo O, Li W, Ruan S, Syme E, Rodrigo A, Locher C, Sultana S, Lim LY. Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations. Pharmaceutics. 2025; 17(9):1232. https://doi.org/10.3390/pharmaceutics17091232
Chicago/Turabian StyleYoo, Okhee, Wenting Li, Siyu Ruan, Elizabeth Syme, Alisha Rodrigo, Connelia Locher, Sharmin Sultana, and Lee Yong Lim. 2025. "Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations" Pharmaceutics 17, no. 9: 1232. https://doi.org/10.3390/pharmaceutics17091232
APA StyleYoo, O., Li, W., Ruan, S., Syme, E., Rodrigo, A., Locher, C., Sultana, S., & Lim, L. Y. (2025). Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations. Pharmaceutics, 17(9), 1232. https://doi.org/10.3390/pharmaceutics17091232







