Conjugation of Glycine max (L.) Merrill Oligopeptide with Monosaccharides: A Novel Approach for Stability and Efficacy in Cosmeceutical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Production Process of G. max Oligopeptide
2.3. Production Process of G. max Oligopeptide–Monosaccharide Conjugates
2.4. Determination of Biological Activities Related to Cosmetic/Cosmeceutical Applications
2.4.1. Antioxidant Activities
- 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
- Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.2. Anti-Tyrosinase Activities
2.4.3. Anti-Skin Aging Activities
- Anti-Collagenase Activity
- Anti-Elastase Activity
- Anti-Hyaluronidase Activity
2.5. Irritation Test by Hen’s Egg Chorioallantoic Membrane (HET-CAM)
2.6. Stability Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. G. max Oligopeptide–Monosaccharide Conjugates
3.2. Antioxidant Activities of G. max Oligopeptide–Monosaccharide Conjugates
3.3. Anti-Tyrosinase Activities of G. max Oligopeptide–Monosaccharide Conjugates
3.4. Anti-Skin Aging Activities of G. max Oligopeptide–Monosaccharide Conjugates
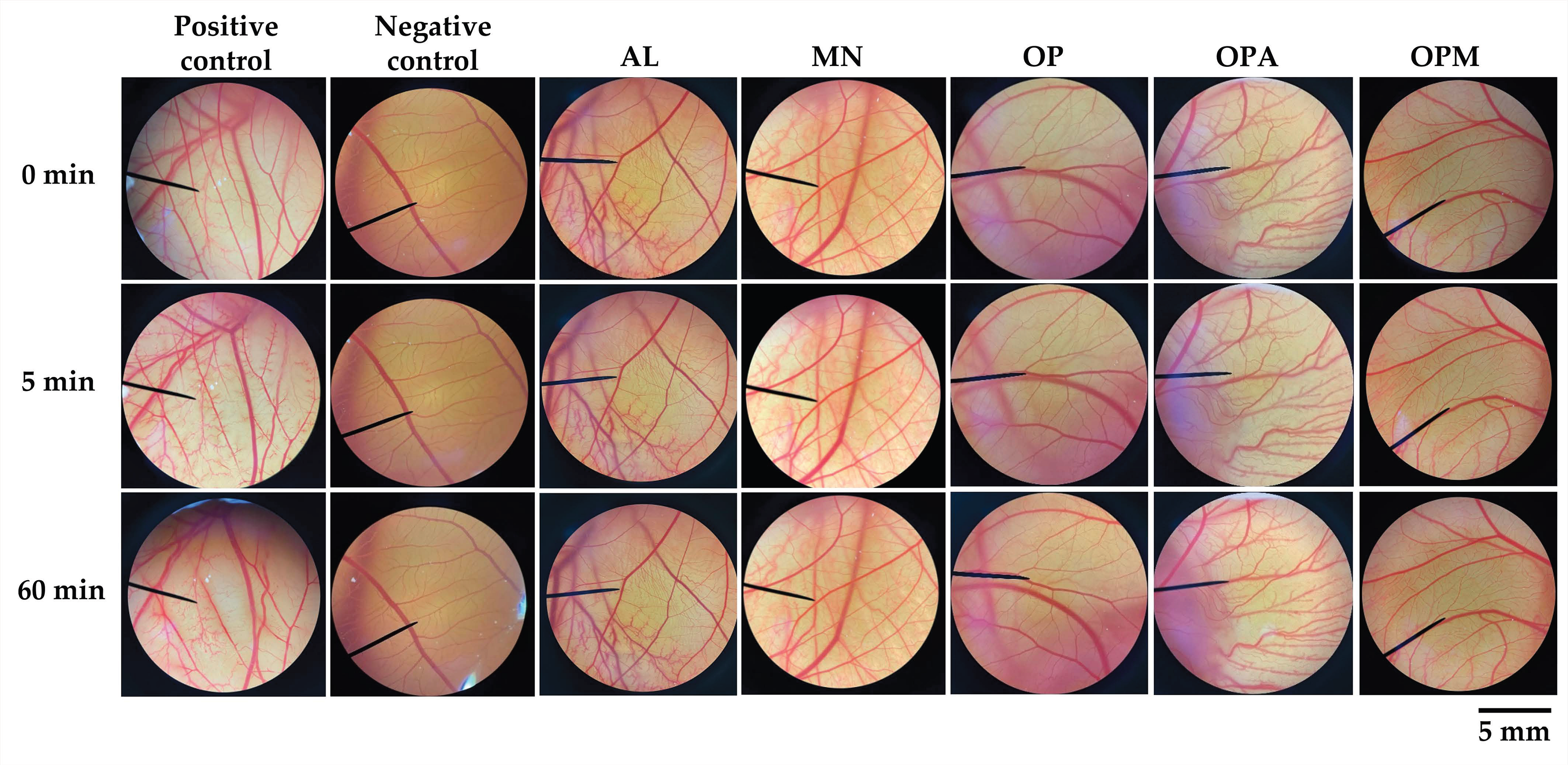
3.5. Irritation Profile of G. max Oligopeptide–Monosaccharide Conjugates
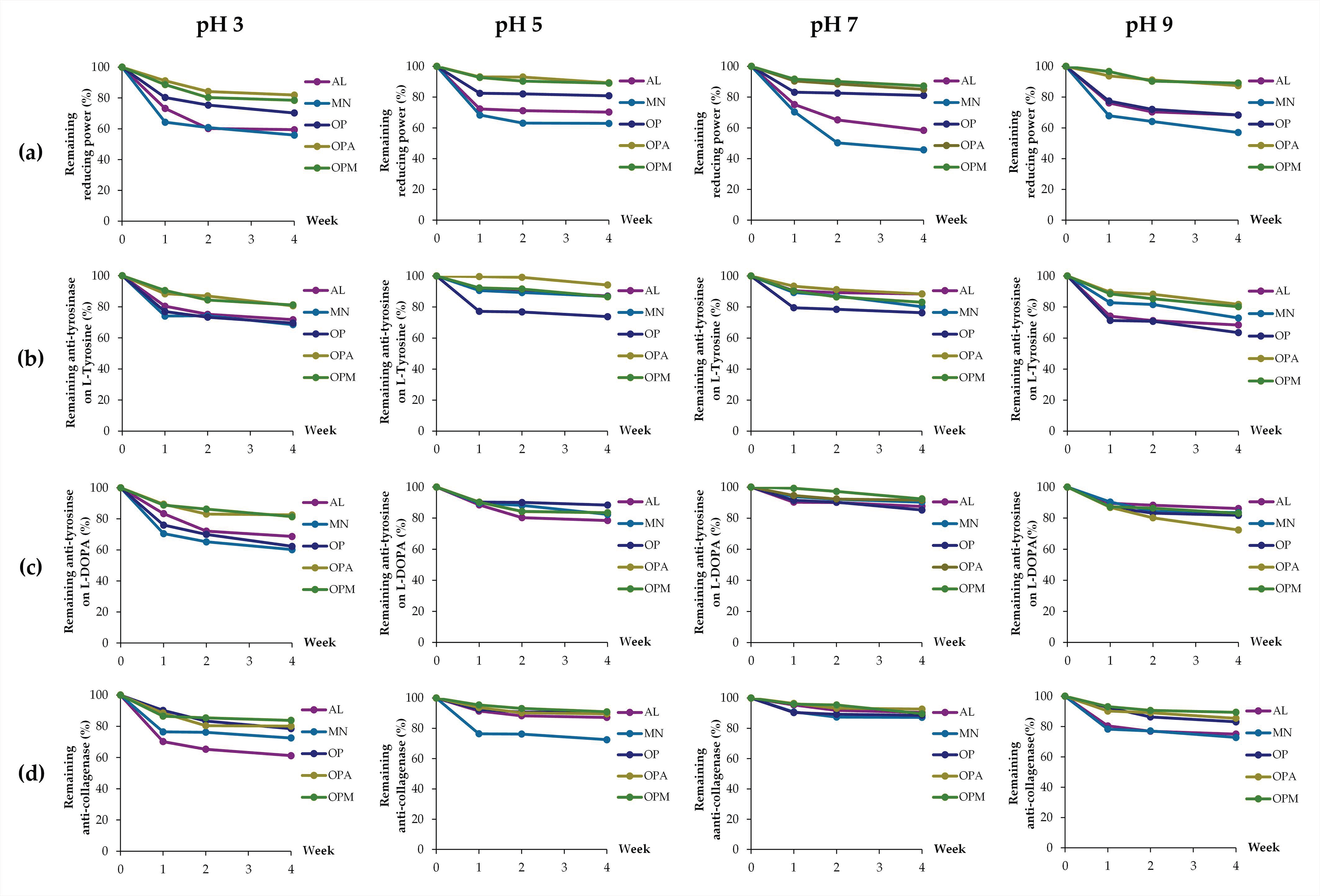
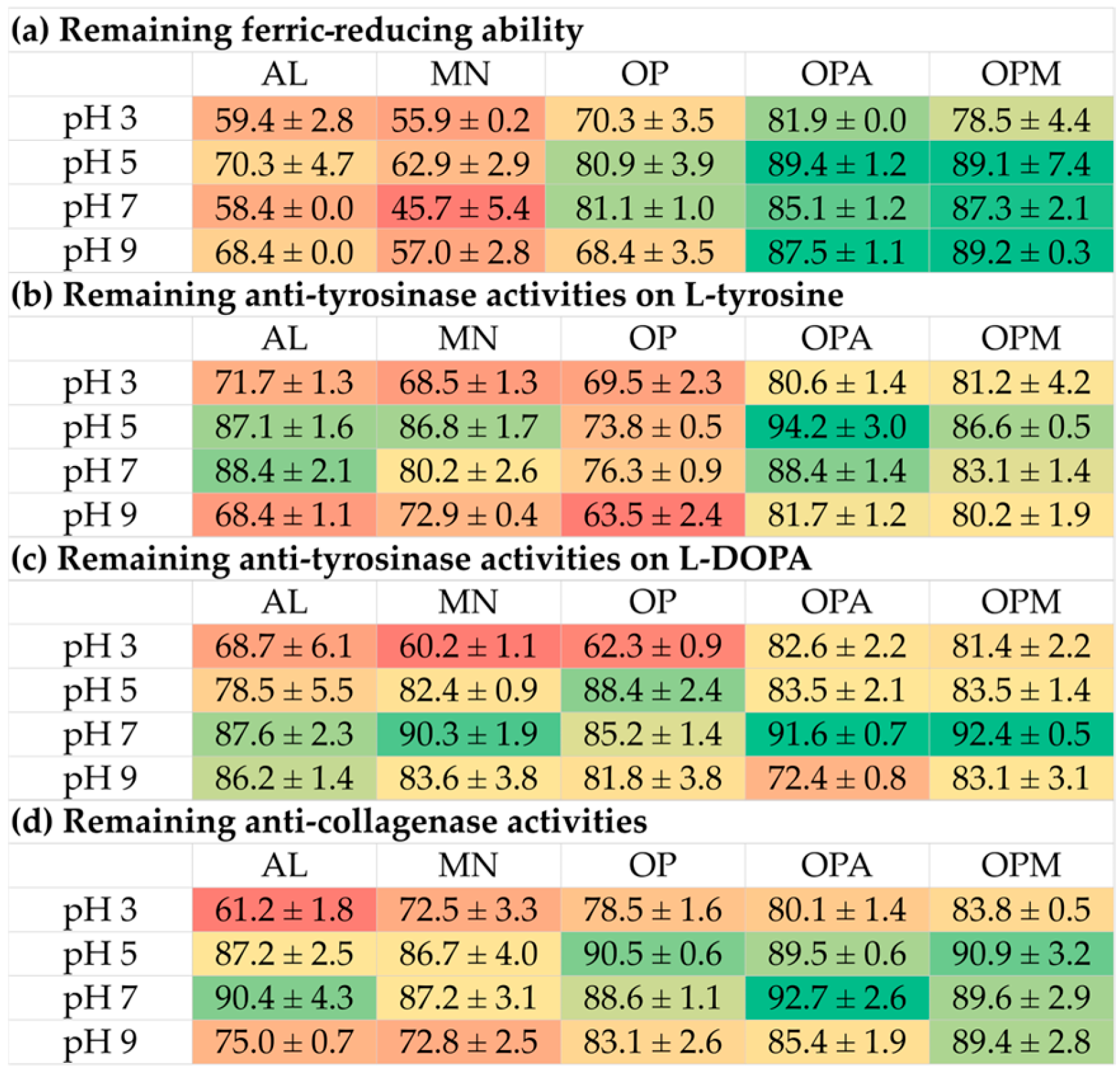
3.6. Stability of G. max Oligopeptide–Monosaccharide Conjugates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | allulose |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| HET-CAM | hen’s egg chorioallantoic membrane assay |
| IS | irritation score |
| MN | mannose |
| OP | G. max oligopeptide |
| OPA | G. max oligopeptide–allulose conjugate |
| OPM | G. max oligopeptide–mannose conjugate |
References
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Barać, M.; Stanojević, S.; Pešić, M. Biologically active components of soybeans and soy protein products: A review. Acta Period. Technol. 2005, 36, 155–168. [Google Scholar] [CrossRef]
- Ahmad, A.; Hayat, I.; Arif, S.; Masud, T.; Khalid, N.; Ahmed, A. Mechanisms involved in the therapeutic effects of soybean (Glycine max). Int. J. Food Prop. 2014, 17, 1332–1354. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of soy proteins and peptides as used in cosmetics. Int. J. Toxicol. 2023, 42 (Suppl. S2), 102S–113S. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Waqas, M.K.; Akhtar, N.; Mustafa, R.; Jamshaid, M.; Khan, H.M.; Murtaza, G. Dermatological and cosmeceutical benefits of Glycine max (soybean) and its active components. Acta Pol. Pharm. 2015, 72, 3–11. [Google Scholar]
- Zhao, R.; Bruning, E.; Rossetti, D.; Starcher, B.; Seiberg, M.; Iotsova-Stone, V. Extracts from Glycine max (soybean) induce elastin synthesis and inhibit elastase activity. Exp. Dermatol. 2009, 18, 883–886. [Google Scholar] [CrossRef]
- Paine, C.; Sharlow, E.; Liebel, F.; Eisinger, M.; Shapiro, S.; Seiberg, M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J. Investig. Dermatol. 2001, 116, 587–595. [Google Scholar] [CrossRef]
- Bomblies, K.; Priklopil, T.; Widmer, A. Adaptation of protein stability to thermally heterogeneous environments. bioRxiv 2025. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coimbra, J.S.D.R.; De Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef]
- Aplin, J.D.; Wriston, J.C.; Lee, Y.C. Preparation, properties, and applications of carbohydrate conjugates of proteins and lipid. Crit. Rev. Biotechnol. 1981, 10, 259–306. [Google Scholar] [CrossRef]
- Zhao, M.; He, H.; Ma, A.; Hou, T. Sources, chemical synthesis, functional improvement and applications of food-derived protein/peptide-saccharide covalent conjugates: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5985–6004. [Google Scholar] [CrossRef]
- Stowell, C.P.; Lee, Y.C. Neoglycoproteins the preparation and application of synthetic glycoproteins. Adv. Carbohydr. Chem. Biochem. 1980, 37, 225–281. [Google Scholar] [CrossRef]
- Wang, C.; Lin, D.; Chen, Q.; Lin, S.; Shi, S.; Chen, C. Polysaccharide peptide isolated from grass-cultured Ganoderma lucidum induces anti-proliferative and pro-apoptotic effects in the human U251 glioma cell line. Oncol. Lett. 2018, 15, 4330–4336. [Google Scholar] [CrossRef]
- Sinohara, H.; Asano, Y.; Fukui, A. Glycopeptides from silk fibroin of Bombyx mori. Biochim. Biophys. Acta-Gen. Subj. 1971, 237, 273–279. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk proteins—Their biological activities and use in cosmetics and dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef]
- Han, J.H.; Song, J.H.; Kim, Y.E.; Lee, Y.H.; Lee, J.M.; Lee, J.E. Anti-aging effects of Rosa damascena extract containing low molecular glycoprotein. J. Soc. Cosmet. Sci. Korea 2018, 44, 49–57. [Google Scholar] [CrossRef]
- Lee, M.J.; Jeong, N.H.; Jang, B.S. Antioxidative activity and antiaging effect of carrot glycoprotein. J. Ind. Eng. Chem. 2015, 25, 216–221. [Google Scholar] [CrossRef]
- Phongphisutthinant, R.; Wiriyacharee, P.; Boonyapranai, K.; Ounjaijean, S.; Taya, S.; Pitchakarn, P.; Pathomrungsiyounggul, P.; Utarat, P.; Wongwatcharayothin, W.; Somjai, C.; et al. Effect of conventional humid–dry heating through the Maillard reaction on chemical changes and enhancement of in vitro bioactivities from soy protein isolate hydrolysate–yeast cell extract conjugates. Foods 2024, 13, 380. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Buacheen, P.; Taya, S.; Karinchai, J.; Temviriyanukul, P.; Inthachat, W.; Chaipoot, S.; Wiriyacharee, P.; Phongphisutthinant, R.; Ounjaijean, S.; et al. Anti-inflammatory, cytotoxic, and genotoxic effects of soybean oligopeptides conjugated with mannose. Foods 2024, 13, 2558. [Google Scholar] [CrossRef]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal extracts as potential antioxidant, anti-aging, anti-inflammatory, and whitening cosmeceutical ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Underhill, L.A.; Dabbah, R.; Grady, L.T.; Rhodes, C.T. Alternatives to animal testing in the USP-NF: Present and future. Drug. Dev. Ind. Pharm. 1994, 20, 165–216. [Google Scholar] [CrossRef]
- Amnuaykan, P.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Enhanced antioxidant, anti-aging, anti-tyrosinase, and anti-inflammatory properties of Vanda coerulea Griff. ex Lindl. protocorm through elicitations with chitosan. Plants 2024, 13, 1770. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Wiesmann, N.; Brieger, J.; Eckrich, J. Toxicological analysis by assessment of vascularization and cell viability using the chicken’s chorioallantoic membrane (CAM Assay). In Cell Viability Assays; Methods in Molecular Biology; Friedrich, O., Gilbert, D.F., Eds.; Humana: New York, NY, USA, 2023; Volume 2644. [Google Scholar] [CrossRef]
- Jakas, A.; Ayyalasomayajula, R.; Cudic, M. Amadori and Heyns rearrangement products of bioactive peptides as potential new ligands of galectin-3. Carbohydr. Res. 2024, 542, 109195. [Google Scholar] [CrossRef]
- Barranco, S.; Cuccu, F.; Uras, M.; Frongia, A. The Heyns rearrangement: Synthetic routes beyond carbohydrate chemistry. Chem. Eur. J. 2024, 30, e202400355. [Google Scholar] [CrossRef]
- Sawettanun, S.; Inukai, R.; Yoshihara, A.; Ogawa, M. Influence of rare sugar syrup on quality attributes and acceptability of bread. Int. J. Food Sci. Technol. 2023, 58, 3693–3706. [Google Scholar] [CrossRef]
- Baek, S.H.; Kwon, S.Y.; Lee, H.G.; Baek, H.H. Maillard browning reaction of D-psicose as affected by reaction factors. Food Sci. Biotechnol. 2008, 17, 1349–1351. [Google Scholar]
- Cui, H.; Yu, J.; Zhai, Y.; Feng, L.; Chen, P.; Hayat, K.; Xu, Y.; Zhang, X.; Ho, C.T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends Food Sci. Technol. 2008, 115, 391–408. [Google Scholar] [CrossRef]
- Coca, M.; García, M.T.; González, G.; Peña, M.; García, J.A. Study of coloured components formed in sugar beet processing. Food Chem. 2004, 86, 421–433. [Google Scholar] [CrossRef]
- Mehta, A. DPPH and FRAP assays for different extracts of in vitro and in vivo grown plantlets of Bacopa monnieri L. Vegetos 2023, 36, 1570–1575. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.S. Characteristics and antioxidant activity of Maillard reaction products from fructose-glycine oligomer. Food Sci. Biotechnol. 2010, 19, 929–940. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef]
- Yadav, N.N.; Yadav, S.; Pareek, A. Structure and function of biological biomolecules: Carbohydrates, amino acids, proteins, nucleic acids, lipids and biomembranes. In Biochemistry: Fundamentals and Bioenergetics; Bentham Science Publishers: Sharjah, United Arab Emirates, 2001; pp. 118–213. [Google Scholar]
- Aversa, R.; Petrescu, R.V.V.; Apicella, A.; Petrescu, F.I.T. One can slow down the aging through antioxidants. Am. J. Eng. Appl. Sci. 2016, 9, 1112–1126. [Google Scholar] [CrossRef]
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to defend healthy and youthful skin—Current trends and future directions in cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. J. New Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Chidiebere, C.W.; Anthonia, U.A.A. Natural skin whitening compounds: Types, source and mechanism of action: A review. Afr. Sci. Rep. 2023, 2023, 95. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the aging skin. Adv. Wound Care. 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Montagna, W.; Carlisle, K. Structural changes in ageing skin. Br. J. Dermatol. 1990, 122, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chavoshnejad, P.; Foroughi, A.H.; Dhandapani, N.; German, G.K.; Razavi, M.J. Effect of collagen degradation on the mechanical behavior and wrinkling of skin. Phys. Rev. E 2021, 104, 034406. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Fisher, G.J.; Sachs, D.L.; Voorhees, J.J. Ageing: Collagenase-mediated collagen fragmentation as a rejuvenation target. Br. J. Dermatol. 2014, 171, 446–449. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; De Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. Int. J. Clin. Dermatol. Res. 2019, 7, 209–240. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Machado, R.M.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Testing vaginal irritation with the hen’s egg test-chorioallantoic membrane assay. ALTEX-Altern. Anim. Ex. 2018, 35, 495–503. [Google Scholar] [CrossRef]
- Wilson, T.D.; Steck, W.F. A modified HET–CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef]
- Smail, S.S. Ex vivo irritation evaluation of a novel brimonidine nanoemulsion using the hen’s egg test on chorioallantoic membrane (HET-CAM). Cureus 2024, 16, e68280. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Kovács, B. Protein-polysaccharide complexes and conjugates: Structural modifications and interactions under diverse treatments. J. Agric. Food Res. 2024, 18, 101510. [Google Scholar] [CrossRef]
- Rao, V.S.R.; Foster, J.F. The conformation of the pyranose rings in mono-, di-, and polysaccharides at high pH by proton magnetic resonance studies. J. Phys. Chem. 1965, 69, 636–640. [Google Scholar] [CrossRef]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Siddiquy, M.; JiaoJiao, Y.; Rahman, M.H.; Iqbal, M.W.; Al-Maqtari, Q.A.; Easdani, M.D.; Yiasmin, M.N.; Ashraf, W.; Hussain, A.; Zhang, L. Advances of protein functionalities through conjugation of protein and polysaccharide. Food Bioproc. Technol. 2024, 17, 2077–2097. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, S.; Wan, H.; Zhang, X.; Sun, M. Characterization and functional properties of conjugates of rice protein with exopolysaccharides from Arthrobacter ps-5 by Maillard reaction. Food Sci. Nutr. 2021, 9, 4745–4757. [Google Scholar] [CrossRef]
- Smith, W.J.; Ahl, P.L.; Wang, B.; Hamm, M.; Rustandi, R.R.; Winters, M.A.; Blue, J.T. Analytical technology development to monitor the stability of polysaccharide-protein conjugate vaccines. Vaccine 2022, 40, 4182–4189. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Borda, D.; Aprodu, I. Alternative processing options for improving the proteins functionality by Maillard conjugation. Foods 2023, 12, 3588. [Google Scholar] [CrossRef]


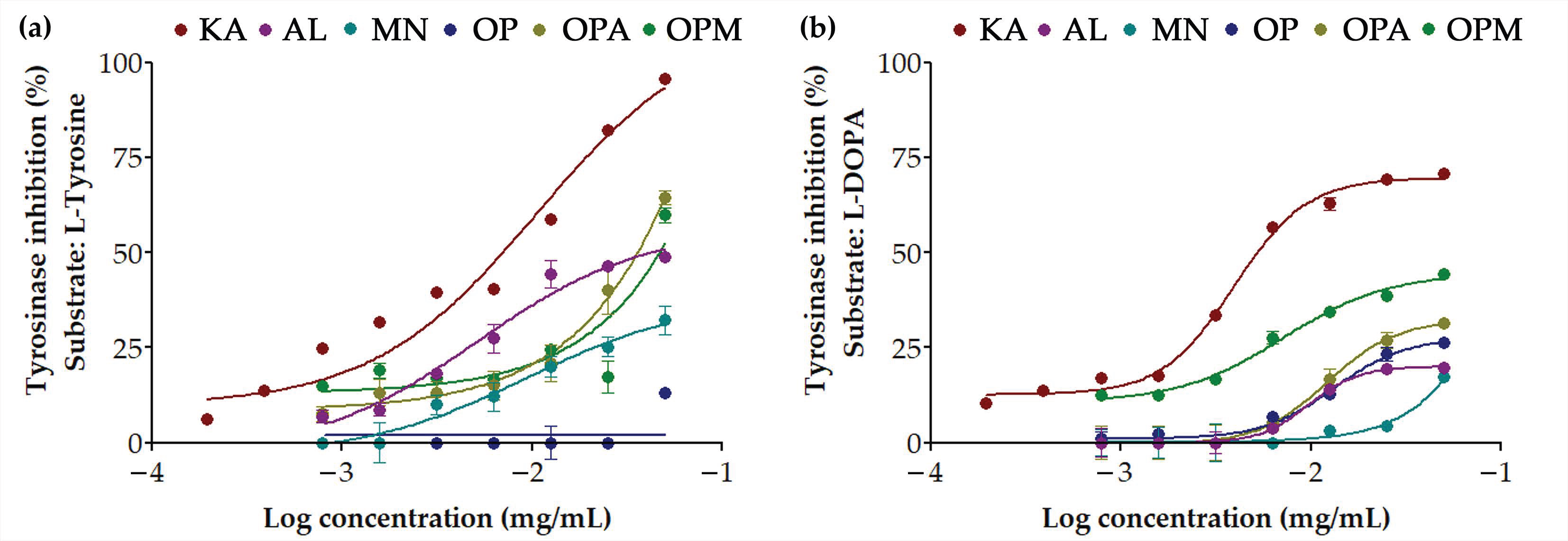





| Samples | Anti-Tyrosinase Activities (%) | |
|---|---|---|
| L-Tyrosine | L-DOPA | |
| KA | 98.0 ± 0.4 a | 72.6 ± 0.5 a |
| AL | 48.7 ± 2.3 b | 19.8 ± 1.4 e |
| MN | 32.2 ± 6.4 c | 17.2 ± 0.5 e |
| OP | 7.6 ± 3.2 d | 26.2 ± 1.4 d |
| OPA | 44.4 ± 3.2 b | 31.5 ± 1.9 c |
| OPM | 39.9 ± 3.3 b | 44.4 ± 1.7 b |
| Samples | Irritation Score (IS) | Irritation Severity |
|---|---|---|
| 1% w/v SLS | 12.5 ± 0.3 a | Severe irritation |
| NSS | 0.0 ± 0.0 b | No irritation |
| AL | 0.0 ± 0.0 b | No irritation |
| MN | 0.0 ± 0.0 b | No irritation |
| OP | 0.0 ± 0.0 b | No irritation |
| OPA | 0.0 ± 0.0 b | No irritation |
| OPM | 0.0 ± 0.0 b | No irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyana, W.; Jiamphun, S.; Phongphisutthinant, R.; Chaipoot, S.; Wiriyacharee, P. Conjugation of Glycine max (L.) Merrill Oligopeptide with Monosaccharides: A Novel Approach for Stability and Efficacy in Cosmeceutical Applications. Pharmaceutics 2025, 17, 530. https://doi.org/10.3390/pharmaceutics17040530
Chaiyana W, Jiamphun S, Phongphisutthinant R, Chaipoot S, Wiriyacharee P. Conjugation of Glycine max (L.) Merrill Oligopeptide with Monosaccharides: A Novel Approach for Stability and Efficacy in Cosmeceutical Applications. Pharmaceutics. 2025; 17(4):530. https://doi.org/10.3390/pharmaceutics17040530
Chicago/Turabian StyleChaiyana, Wantida, Sudarat Jiamphun, Rewat Phongphisutthinant, Supakit Chaipoot, and Pairote Wiriyacharee. 2025. "Conjugation of Glycine max (L.) Merrill Oligopeptide with Monosaccharides: A Novel Approach for Stability and Efficacy in Cosmeceutical Applications" Pharmaceutics 17, no. 4: 530. https://doi.org/10.3390/pharmaceutics17040530
APA StyleChaiyana, W., Jiamphun, S., Phongphisutthinant, R., Chaipoot, S., & Wiriyacharee, P. (2025). Conjugation of Glycine max (L.) Merrill Oligopeptide with Monosaccharides: A Novel Approach for Stability and Efficacy in Cosmeceutical Applications. Pharmaceutics, 17(4), 530. https://doi.org/10.3390/pharmaceutics17040530








