The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Incorporation of CBD into PURASORB 5010 and PURASORB 7510
2.3. Bacterial Strains and Growth Conditions
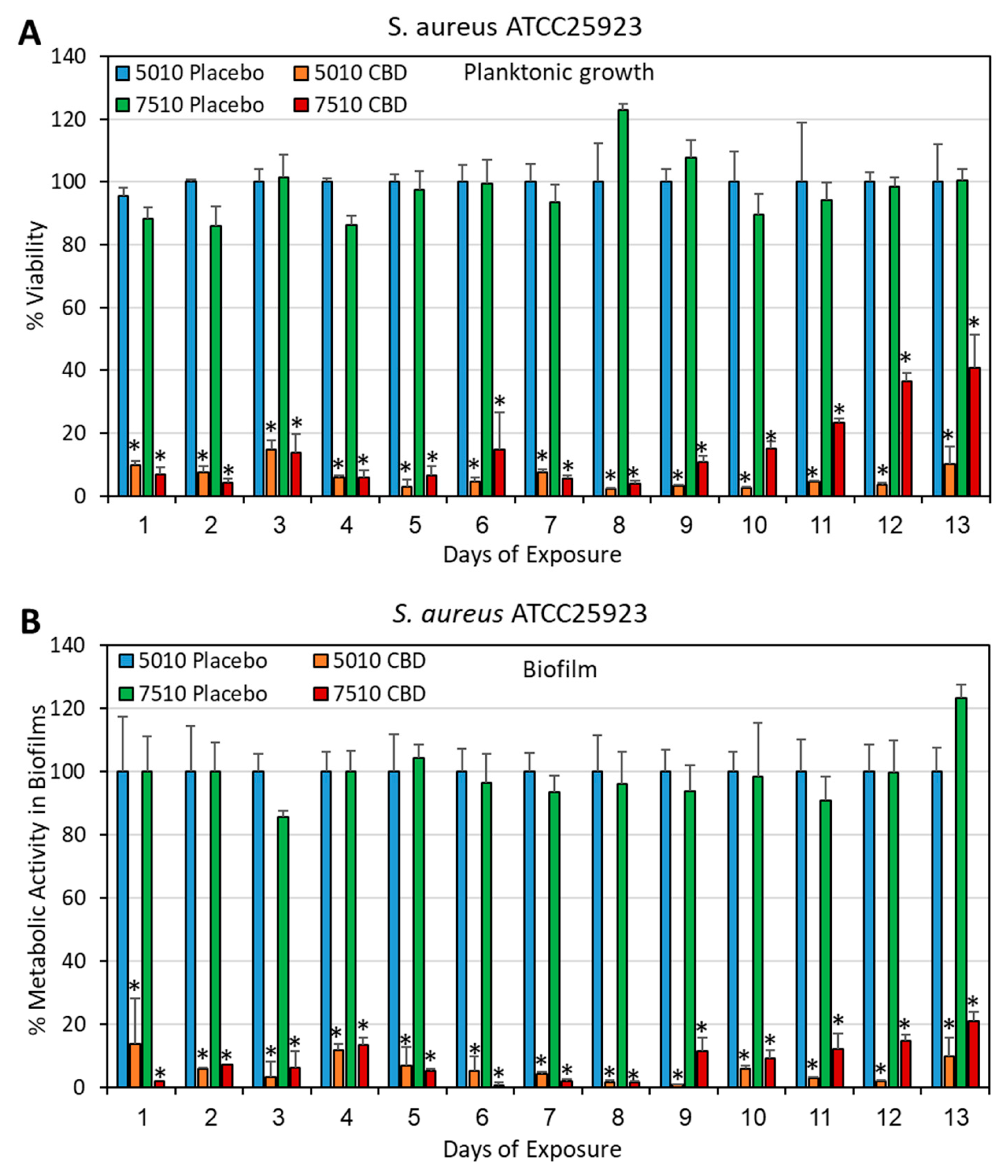
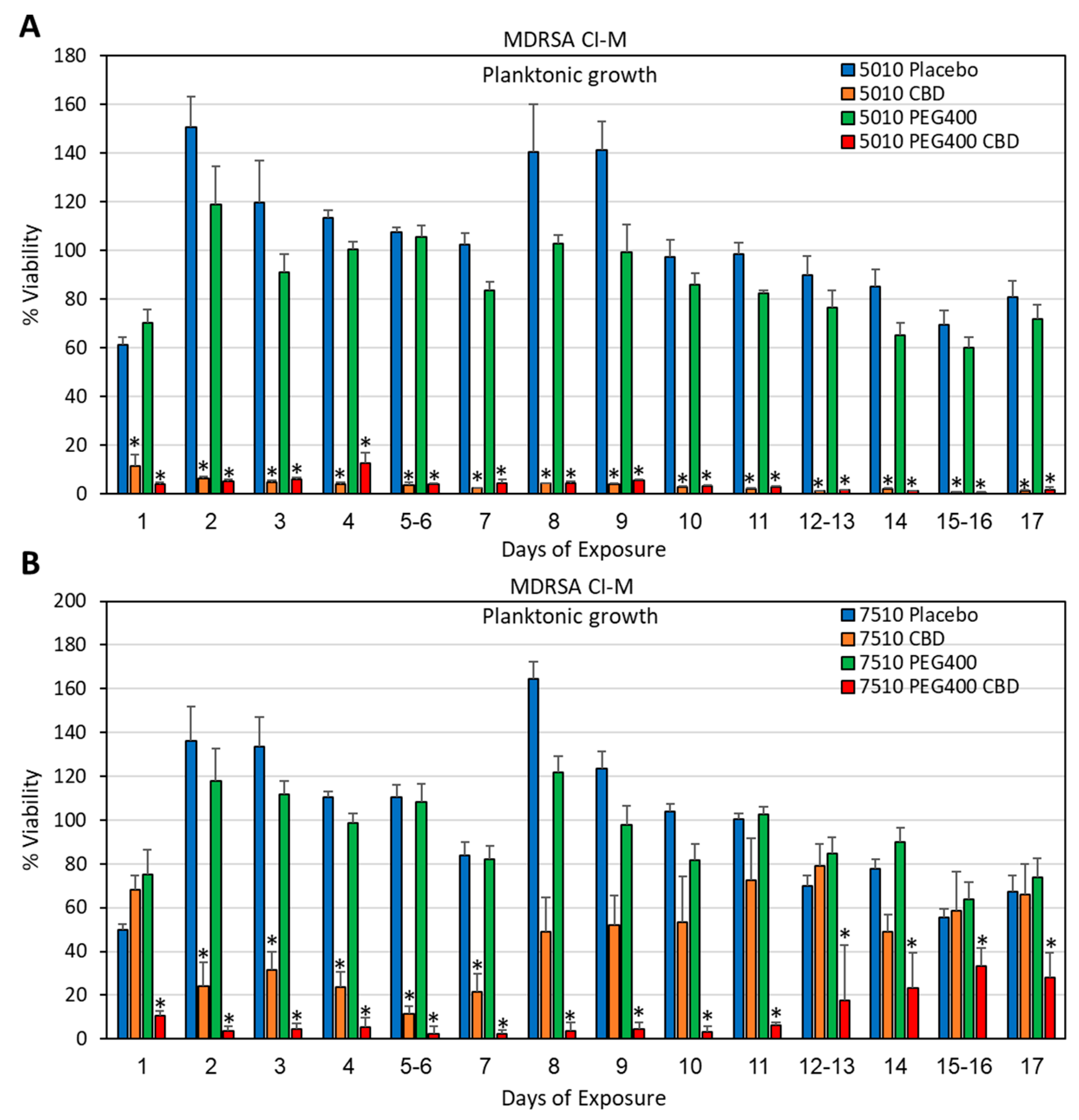
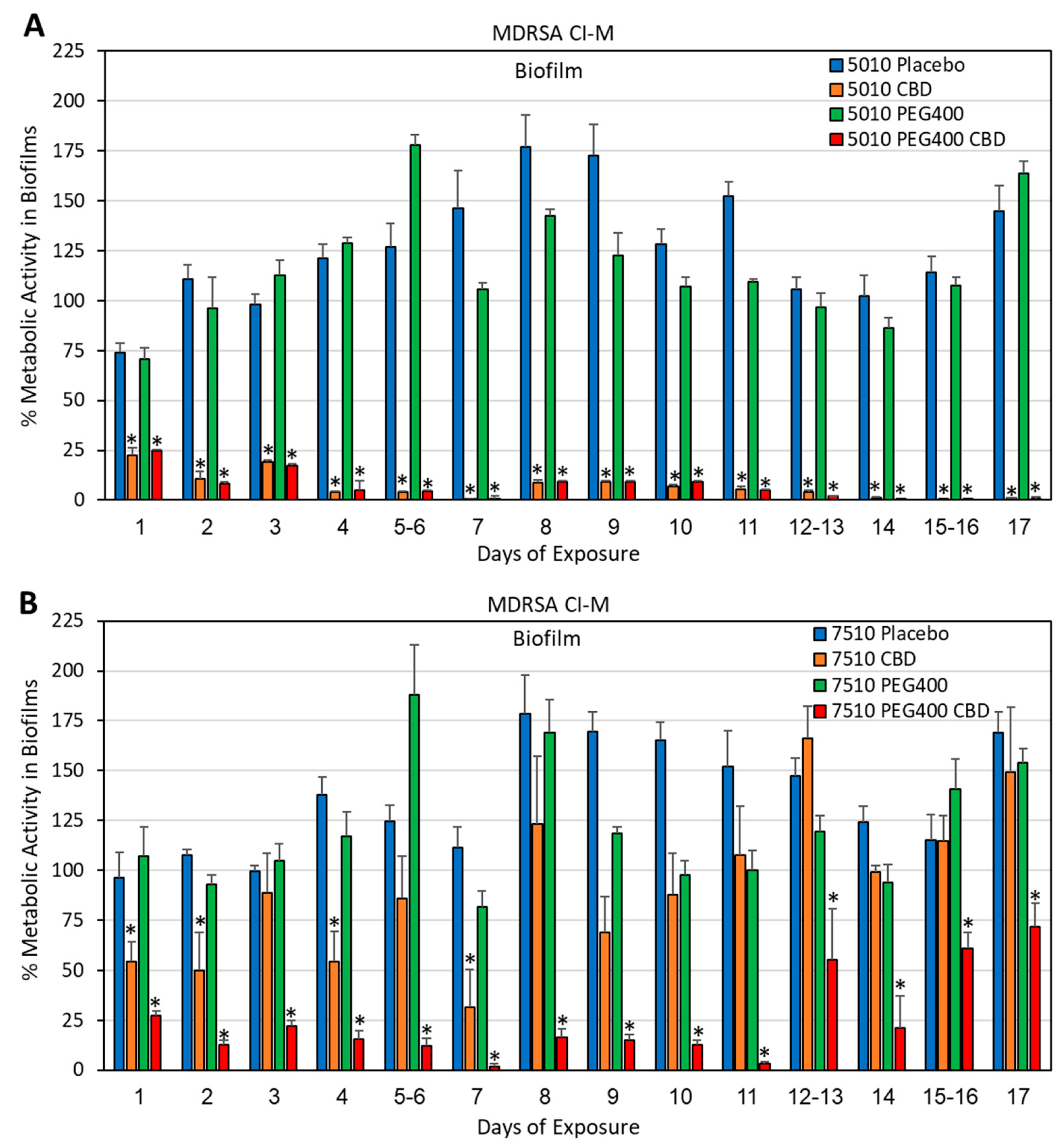
2.4. Determination of Bacterial Viability
2.5. Determination of Metabolically Active Biofilms
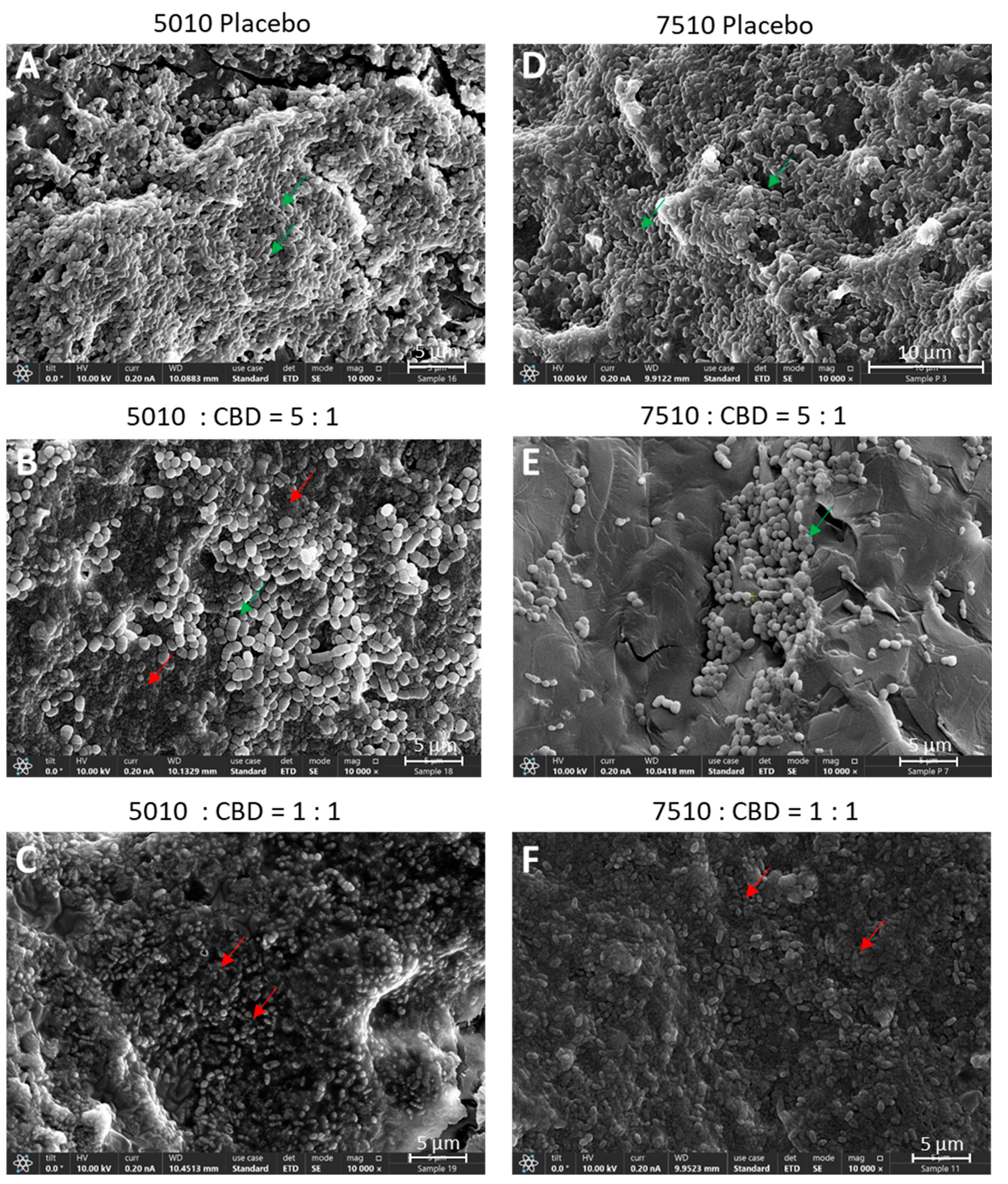
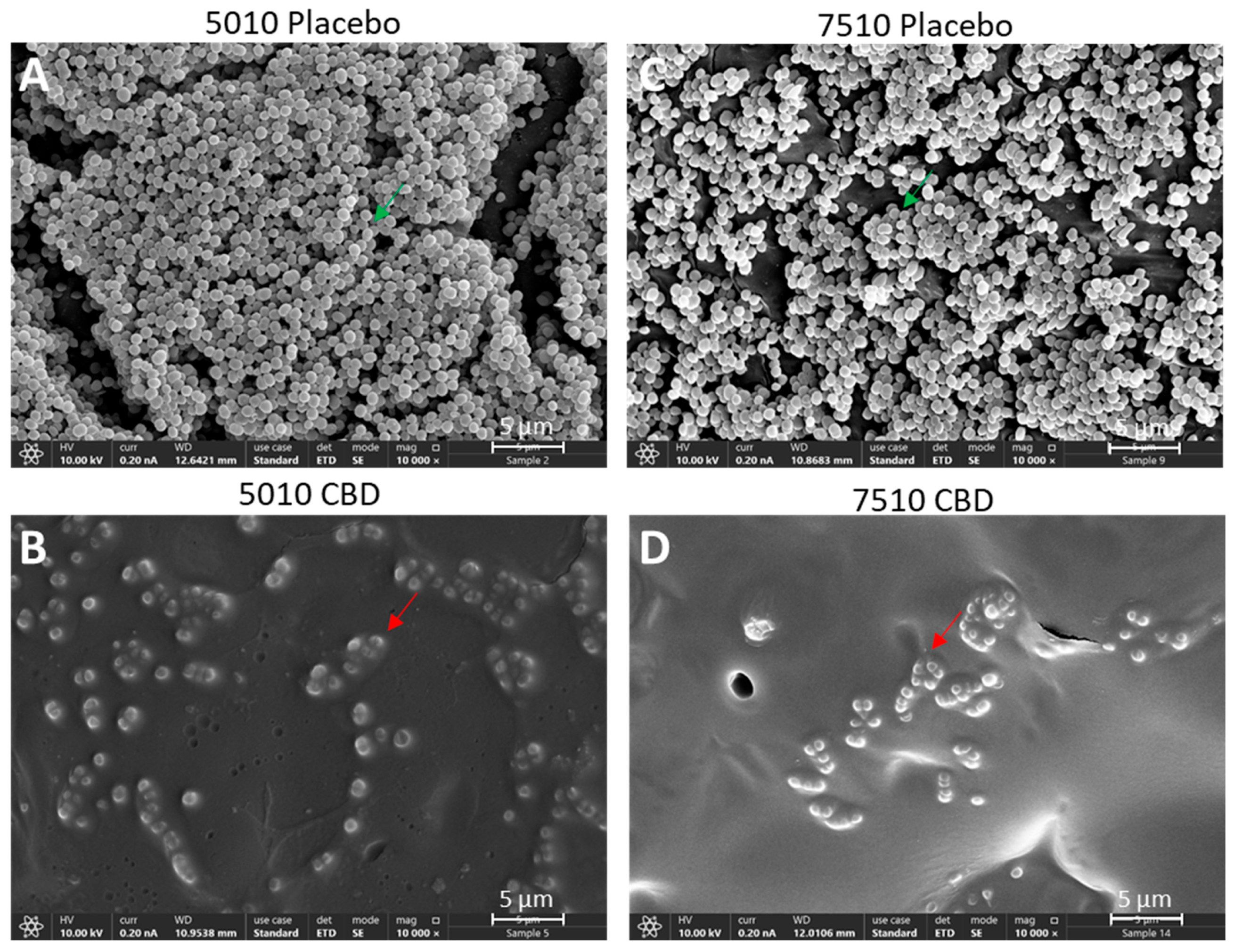
2.6. HR-SEM Imaging
2.7. Determination of IL-6 Secretion from RAW 264.7 Macrophages
2.8. Cytotoxicity Assay Using Vero Epithelial Cells
2.9. Quantification of CBD in Supernatants by Gas Chromatography–Mass Spectrometry (GC-MS)
2.10. Statistical Analysis
3. Results
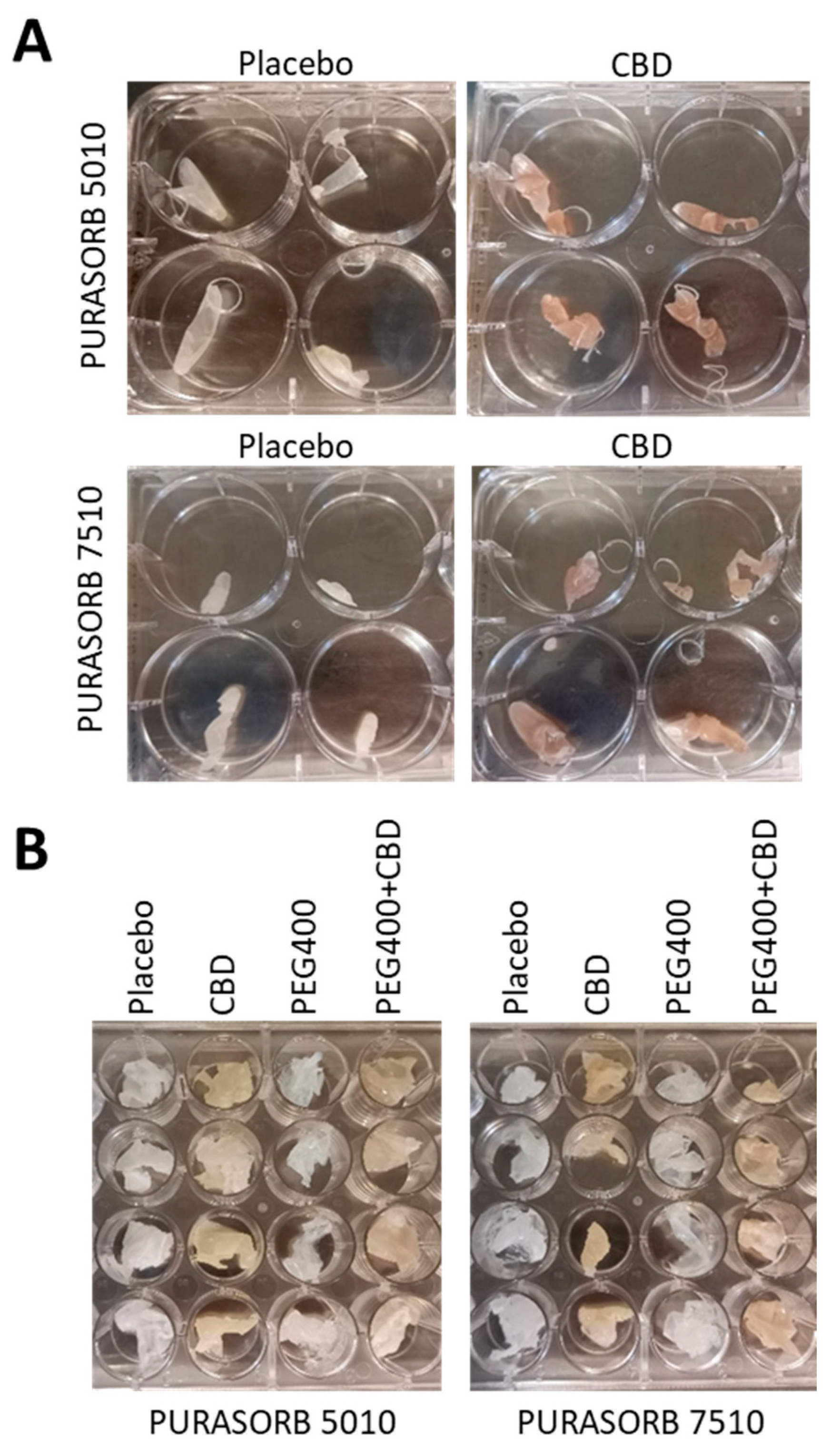
3.1. Choice of Solvent for Preparation of PURASORB/CBD Scaffolds for Sustained Release
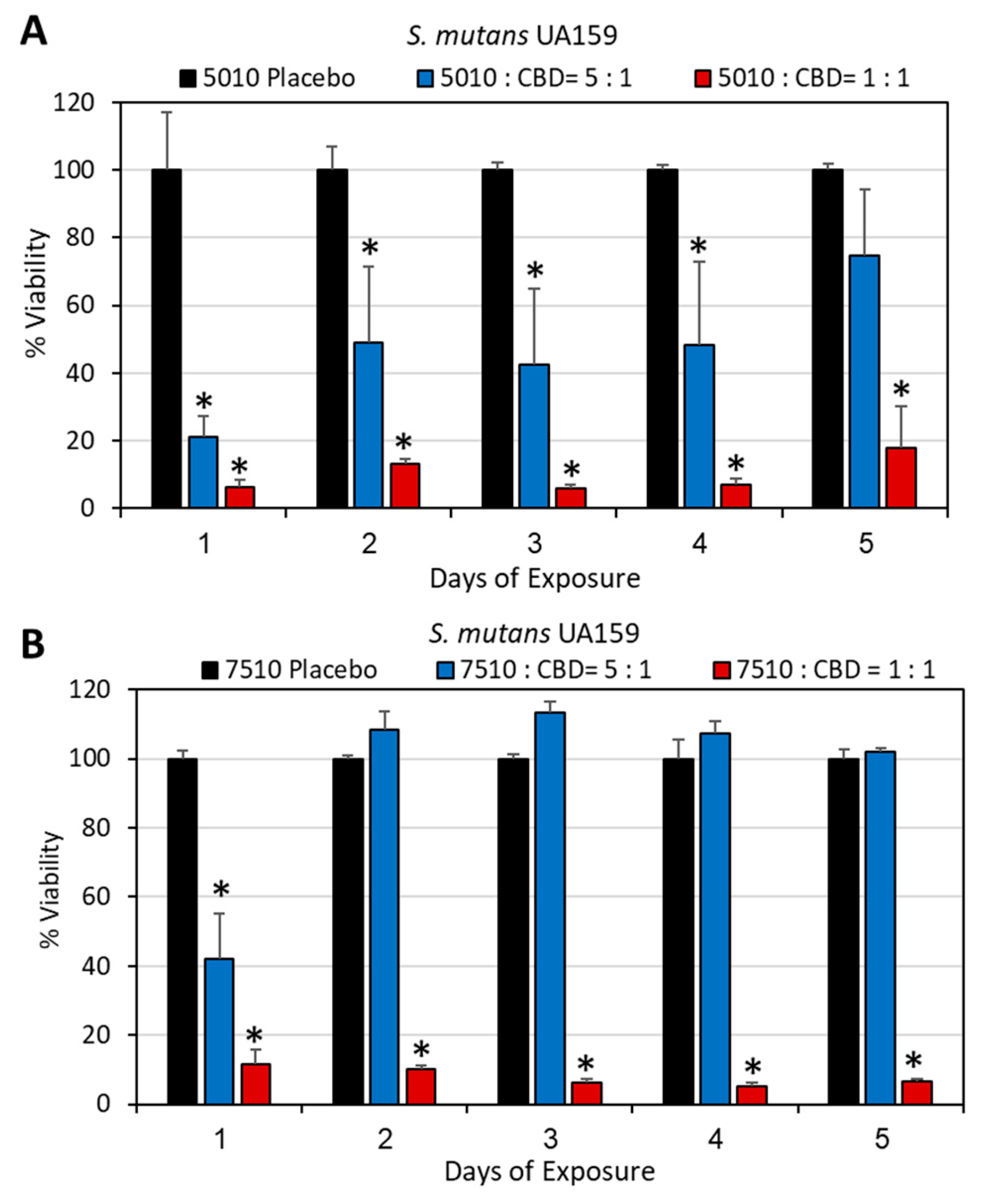
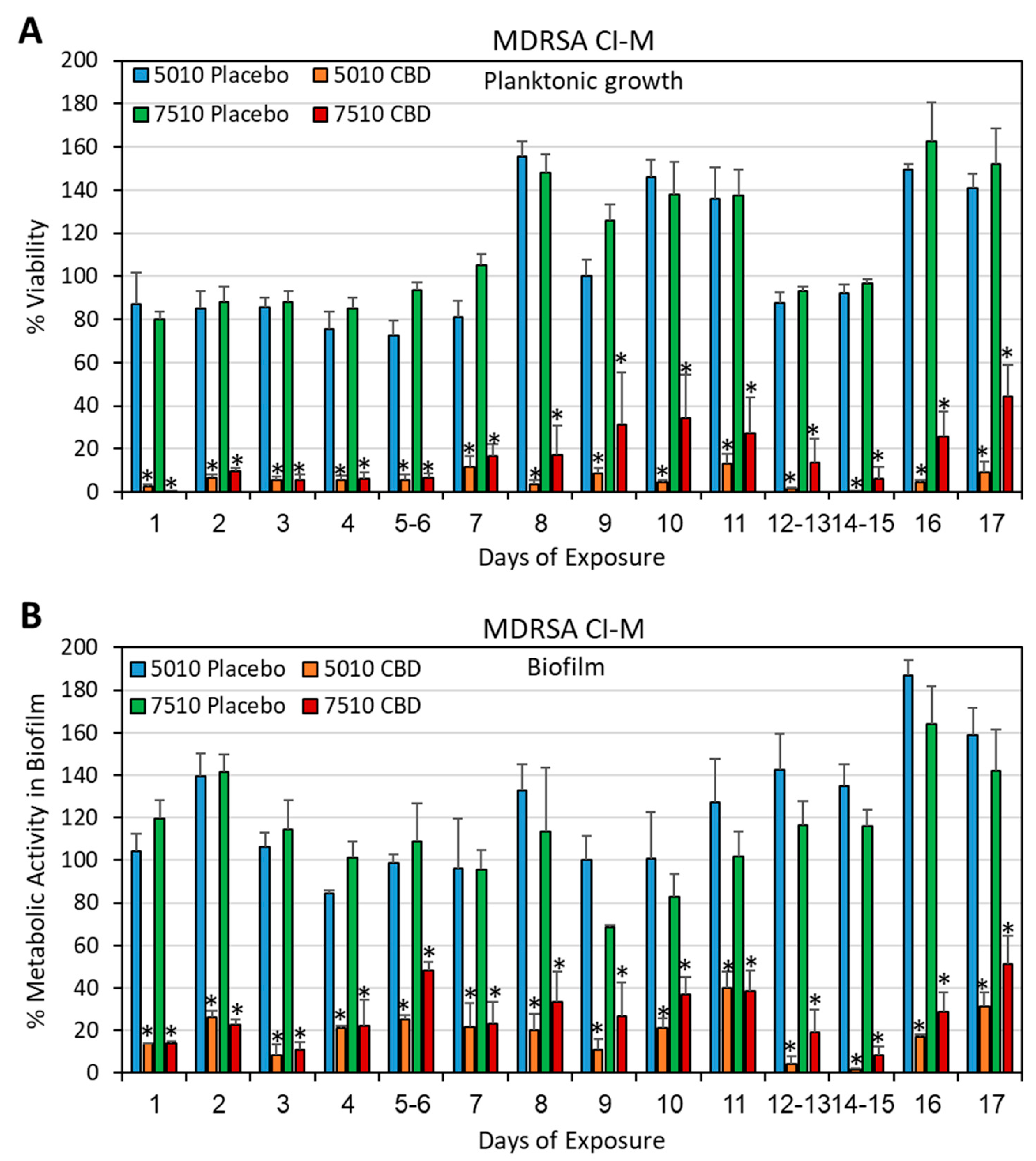
3.2. Long-Term Antibacterial Activity of PURASORB/CBD Scaffolds Prepared by Using DMSO as the Solvent
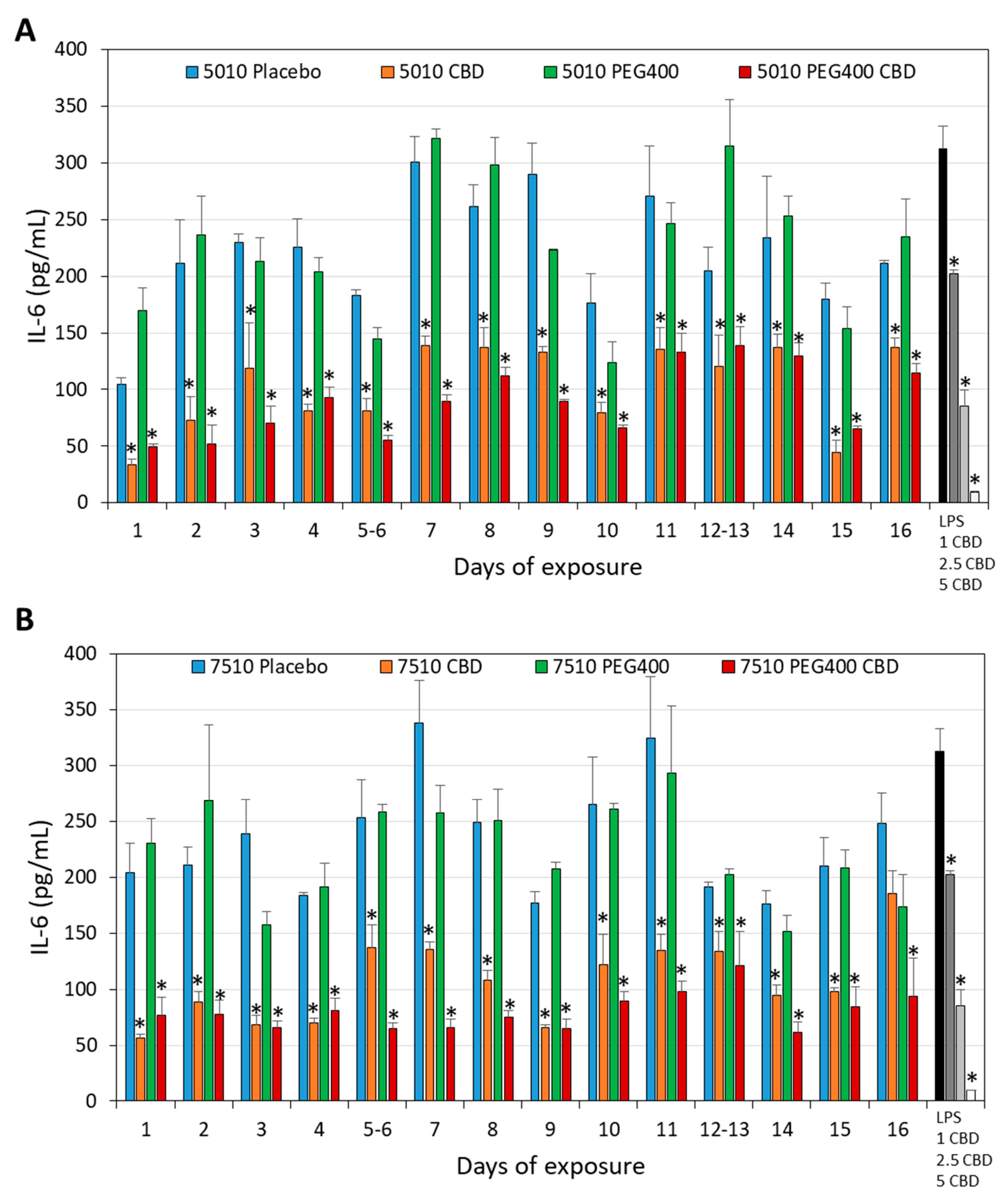
3.3. Long-Term Antibacterial Activity of the PURASORB/CBD Scaffolds Prepared by Using Acetone as the Solvent
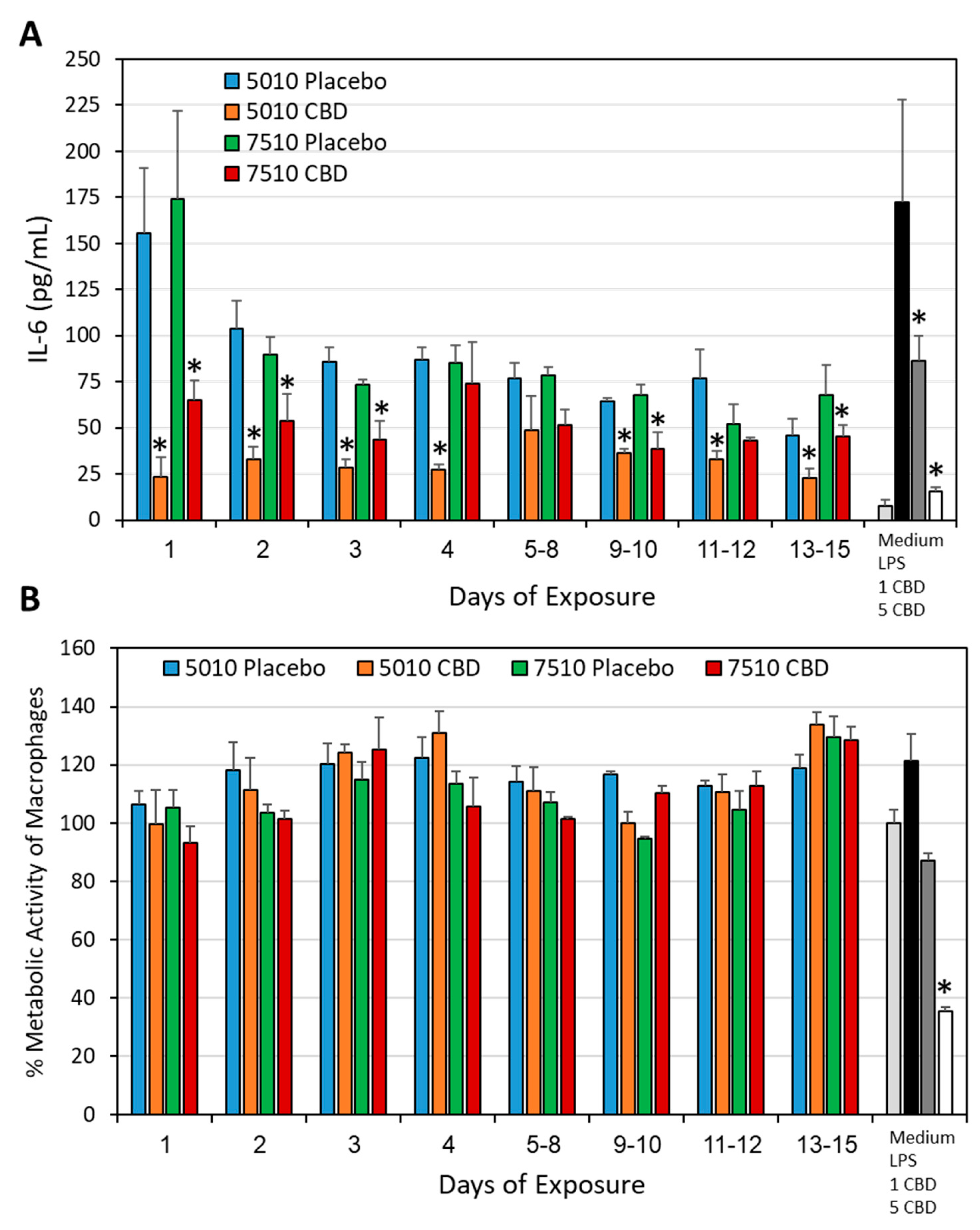
3.4. PURASORB/CBD Scaffolds Reduced IL-6 Secretion from LPS-Stimulated Macrophages
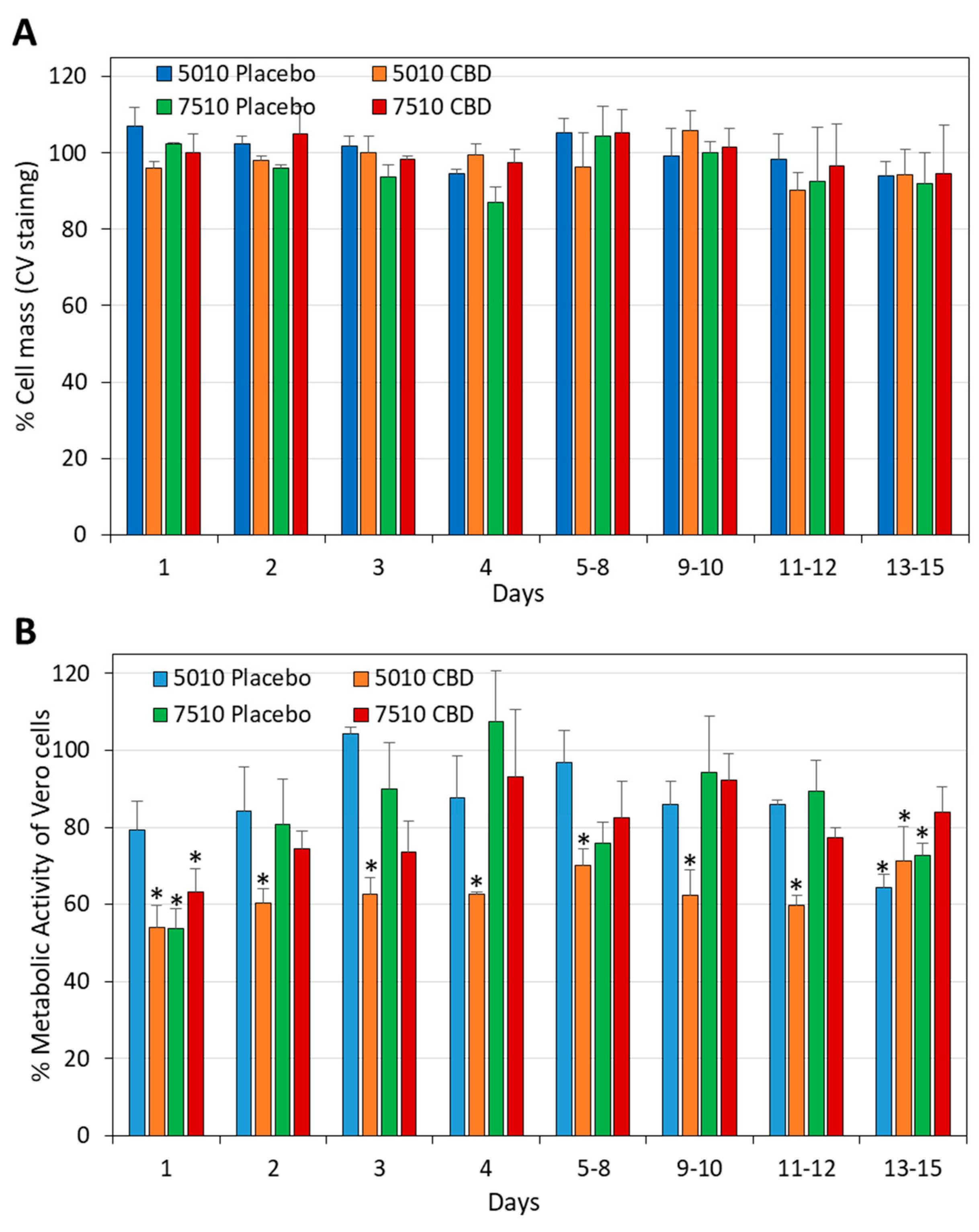
3.5. The Daily Release of CBD from PURASORB/CBD Scaffolds Was Not Cytotoxic to Vero Epithelial Cells
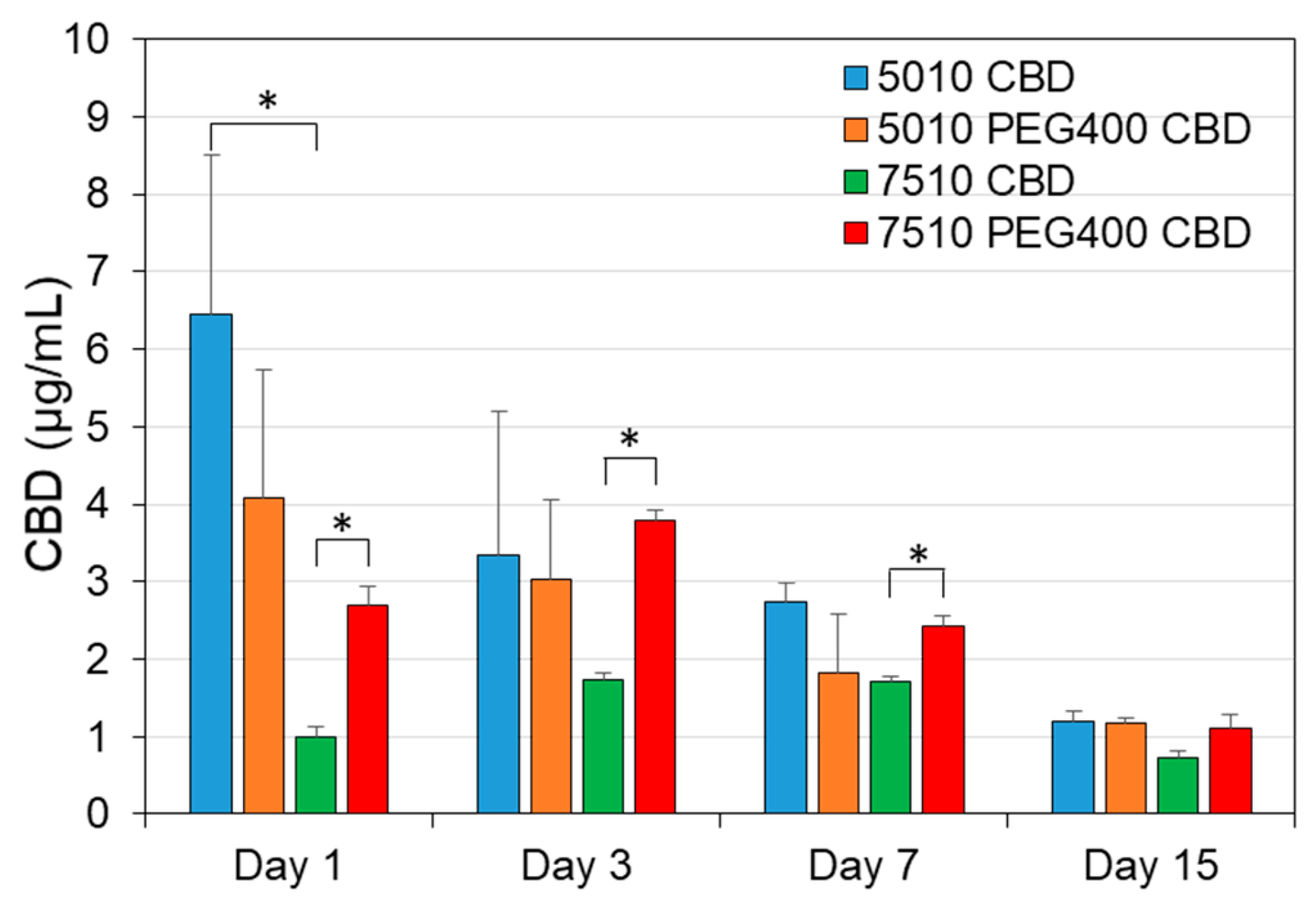
3.6. The Determination of CBD Concentration in the Scaffold-Conditioned Media by GC-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local delivery and controlled release drugs systems: A new approach for the clinical treatment of periodontitis therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Friedman, M. Sustained-release delivery of antimicrobial drugs for the treatment of periodontal diseases: Fantasy or already reality? Periodontol. 2000 2020, 84, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and transdermal drug delivery using microneedles—Fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-release drug delivery of antimicrobials in controlling of supragingival oral biofilms. Expert. Opin. Drug Deliv. 2017, 14, 571–581. [Google Scholar] [CrossRef]
- Kállai-Szabó, N.; Farkas, D.; Lengyel, M.; Basa, B.; Fleck, C.; Antal, I. Microparticles and multi-unit systems for advanced drug delivery. Eur. J. Pharm. Sci. 2024, 194, 106704. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, D.G.; Bligh, S.A. Alginate-based electrospun nanofibers and the enabled drug controlled release profiles: A review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef]
- Almoshari, Y. Osmotic pump drug delivery systems-A comprehensive review. Pharmaceuticals 2022, 15, 1430. [Google Scholar] [CrossRef]
- Johnson, A.R.; Forster, S.P.; White, D.; Terife, G.; Lowinger, M.; Teller, R.S.; Barrett, S.E. Drug eluting implants in pharmaceutical development and clinical practice. Expert. Opin. Drug Deliv. 2021, 18, 577–593. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for drug delivery and human applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Cataldo Russomando, A.; Steinberg, D.; Gati, I.; Vogt Sionov, R.; Eliashar, R.; Friedman, M.; Gross, M. Sinonasal stent coated with sustained-release varnish of mometasone furoate inhibits pro-inflammatory cytokine release from macrophages: An in vitro study. Pharmaceutics 2023, 15, 1015. [Google Scholar] [CrossRef] [PubMed]
- Cataldo Russomando, A.; Vogt Sionov, R.; Friedman, M.; Gati, I.; Eliashar, R.; Steinberg, D.; Gross, M. Sinonasal stent coated with slow-release varnish of chlorhexidine has sustained protection against bacterial biofilm growth in the sinonasal cavity: An in vitro study. Pharmaceutics 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Moustafa Elsayed, W.S.; Friedman, M.; Gati, I.; Steinberg, D.; Marei, H. Prolonged inhibition of Streptococcus mutans growth and biofilm formation by sustained release of chlorhexidine from varnish coated dental abutments: An in vitro study. Int. J. Dent. 2022, 2022, 7246155. [Google Scholar] [CrossRef]
- Gross, M.; Ashqar, F.; Sionov, R.V.; Friedman, M.; Eliashar, R.; Zaks, B.; Gati, I.; Duanis-Assaf, D.; Feldman, M.; Steinberg, D. Sustained release varnish containing chlorhexidine for prevention of Streptococcus mutans biofilm formation on voice prosthesis surface: An in vitro study. Int. Microbiol. 2022, 25, 177–187. [Google Scholar] [CrossRef]
- Sionov, R.V.; Gati, I.; Kirmayer, D.; Friedman, M.; Steinberg, D.; Gross, M. Voice Prosthesis Coated with Sustained Release Varnish Containing Clotrimazole Shows Long-Term Protection against Candida albicans: An in vitro Study. Molecules 2021, 26, 5395. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-based nanomedicine: History of advancement and development in clinical applications of multiple diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Calvo-Henriquez, C.; García-Lliberós, A.; Sánchez-Gómez, S.; Alobid, I. Assessing the effect of absorbable steroid sinus implant: A state-of-the-art systematic review. Eur. Arch. Otorhinolaryngol. 2024, 281, 3915–3928. [Google Scholar] [CrossRef]
- Hoshino, S.; Yoshida, Y.; Tanimura, S.; Yamauchi, Y.; Noritomi, T.; Yamashita, Y. A study of the efficacy of antibacterial sutures for surgical site infection: A retrospective controlled trial. Int. Surg. 2013, 98, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Niculescu, A.G.; Gaspar, B.S.; Grumezescu, A.M.; Beuran, M. A review of abdominal meshes for hernia repair-Current status and emerging solutions. Materials 2023, 16, 7124. [Google Scholar] [CrossRef]
- Abudalu, M.; Aqawi, M.; Sionov, R.V.; Friedman, M.; Gati, I.; Munz, Y.; Ohana, G.; Steinberg, D. Polyglactin 910 meshes coated with sustained-release cannabigerol varnish inhibit Staphylococcus aureus biofilm formation and macrophage cytokine secretion: An in vitro study. Pharmaceuticals 2023, 16, 745. [Google Scholar] [CrossRef]
- Kalidoss, M.; Kumar, S.; Doble, M. Combinatorial delivery of antibiotic and anti-inflammatory drugs using calcium deficient hydroxyapatite nanocarriers for the management of bone infections. Biomater. Tissue Technol. 2017, 1, 1–5. [Google Scholar]
- Ye, Y.; He, J.; Qiao, Y.; Qi, Y.; Zhang, H.; Santos, H.A.; Zhong, D.; Li, W.; Hua, S.; Wang, W.; et al. Mild temperature photothermal assisted anti-bacterial and anti-inflammatory nanosystem for synergistic treatment of post-cataract surgery endophthalmitis. Theranostics 2020, 10, 8541–8557. [Google Scholar] [CrossRef]
- Kim, D.; Kang, K.H. Anti-inflammatory and anti-bacterial potential of mulberry leaf extract on oral microorganisms. Int. J. Environ. Res. Public. Health 2022, 19, 4984. [Google Scholar] [CrossRef]
- Mai, Z.; Mai, Y.; Huang, X.; Ning, S.; Liao, H. Evaluation of anti-inflammatory and antibacterial properties of photo-thermal hydrogel as dual functional platform for management of periodontitis. Int. J. Nanomed. 2025, 20, 2923–2934. [Google Scholar] [CrossRef]
- Pattnaik, F.; Nanda, S.; Mohanty, S.; Dalai, A.K.; Kumar, V.; Ponnusamy, S.K.; Naik, S. Cannabis: Chemistry, extraction and therapeutic applications. Chemosphere 2022, 289, 133012. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef]
- Mulla, S.A.; Patil, A.; Mali, S.; Jain, A.K.; Jaiswal, H.; Sawant, H.R.; Arvind, R.; Singh, S. Unleashing the therapeutic role of cannabidiol in dentistry. J. Oral. Biol. Craniofac Res. 2024, 14, 649–654. [Google Scholar] [CrossRef]
- Boehm, E.; Droessler, L.; Amasheh, S. Cannabidiol attenuates inflammatory impairment of intestinal cells expanding biomaterial-based therapeutic approaches. Mater. Today Bio 2023, 23, 100808. [Google Scholar] [CrossRef]
- Kogan, N.M.; Melamed, E.; Wasserman, E.; Raphael, B.; Breuer, A.; Stok, K.S.; Sondergaard, R.; Escudero, A.V.; Baraghithy, S.; Attar-Namdar, M.; et al. Cannabidiol, a major non-psychotropic cannabis constituent enhances fracture healing and stimulates lysyl hydroxylase activity in osteoblasts. J. Bone Miner. Res. 2015, 30, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Lakes, J.E.; Ferrell, J.L.; Berhow, M.A.; Flythe, M.D. Antimicrobial effects of cannabidiol on select agriculturally important Clostridia. Anaerobe 2024, 87, 102843. [Google Scholar] [CrossRef]
- Avraham, M.; Steinberg, D.; Barak, T.; Shalish, M.; Feldman, M.; Sionov, R.V. Improved anti-biofilm effect against the oral cariogenic Streptococcus mutans by combined triclosan/CBD treatment. Biomedicines 2023, 11, 521. [Google Scholar] [CrossRef]
- Barak, T.; Sharon, E.; Steinberg, D.; Feldman, M.; Sionov, R.V.; Shalish, M. Anti-Bacterial Effect of Cannabidiol against the Cariogenic Streptococcus mutans Bacterium: An in vitro Study. Int. J. Mol. Sci. 2022, 23, 15878. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissimo-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.L.; Ogasawara, T.M.C.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R.; et al. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant Gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef]
- Hussein, M.; Allobawi, R.; Levou, I.; Blaskovich, M.A.T.; Rao, G.G.; Li, J.; Velkov, T. Mechanisms underlying synergistic killing of polymyxin B in combination with cannabidiol against Acinetobacter baumannii: A metabolomic study. Pharmaceutics 2022, 14, 786. [Google Scholar] [CrossRef]
- Sionov, R.V.; Korem, M.; Polacheck, I.; Steinberg, D. Cannabidiol (CBD) acts as an antioxidant on Gardnerella vaginalis, resulting in reduced metabolic activity, loss of survivability, and elimination of biofilms. Antibiotics 2025, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: A preliminary observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.R.; Alghalayini, A.; Valenzuela, S.M. Current challenges and opportunities for improved cannabidiol solubility. Int. J. Mol. Sci. 2023, 24, 14514. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef]
- Taha, I.E.; ElSohly, M.A.; Radwan, M.M.; Elkanayati, R.M.; Wanas, A.; Joshi, P.H.; Ashour, E.A. Enhancement of cannabidiol oral bioavailability through the development of nanostructured lipid carriers: In vitro and in vivo evaluation studies. Drug Deliv. Transl. Res. 2024, in press. [Google Scholar] [CrossRef]
- Jurgelane, I.; Egle, K.; Grava, A.; Galkina, D.; Brante, M.; Melnichuks, M.; Skrinda-Melne, M.; Salms, G.; Dubnika, A. Exploring the effects of cannabidiol encapsulation in liposomes on their physicochemical properties and biocompatibility. Drug Deliv. 2025, 32, 2460666. [Google Scholar] [CrossRef]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The transdermal delivery of therapeutic cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef]
- Liu, C.; Cai, A.; Li, H.; Deng, N.; Cho, B.P.; Seeram, N.P.; Ma, H. Characterization of molecular interactions between cannabidiol and human plasma proteins (serum albumin and γ-globulin) by surface plasmon resonance, microcalorimetry, and molecular docking. J. Pharm. Biomed. Anal. 2022, 214, 114750. [Google Scholar] [CrossRef]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Fowler, V.G., Jr. Intertwining clonality and resistance: Staphylococcus aureus in the antibiotic era. J. Clin. Invest. 2024, 134, e185824. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schäffer, C. Oral streptococci: Modulators of health and disease. Front. Cell Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef]
- Banerjee, S.; Sionov, R.V.; Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Anandamide alters the membrane properties, halts the cell division and prevents drug efflux in multidrug resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 8690. [Google Scholar] [CrossRef]
- Kogan, N.M.; Lavi, Y.; Topping, L.M.; Williams, R.O.; McCann, F.E.; Yekhtin, Z.; Feldmann, M.; Gallily, R.; Mechoulam, R. Novel CBG derivatives can reduce inflammation, pain and obesity. Molecules 2021, 26, 5601. [Google Scholar] [CrossRef]
- Lavi, Y.; Kogan, N.M.; Topping, L.M.; Liu, C.; McCann, F.E.; Williams, R.O.; Breuer, A.; Yekhtin, Z.; Ezra, A.F.; Gallily, R.; et al. Novel synthesis of C-methylated phytocannabinoids bearing anti-inflammatory properties. J. Med. Chem. 2023, 66, 5536–5549. [Google Scholar] [CrossRef]
- Kesavan Pillai, S.; Hassan Kera, N.; Kleyi, P.; de Beer, M.; Magwaza, M.; Ray, S.S. Stability, biofunctional, and antimicrobial characteristics of cannabidiol isolate for the design of topical formulations. Soft Matter 2024, 20, 2348–2360. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, J.; Wang, Y.; Li, S. Effect of pore former on the properties of casted film prepared from blends of Eudragit NE 30 D and Eudragit L 30 D-55. Chem. Pharm. Bull. 2007, 55, 1261–1263. [Google Scholar] [CrossRef][Green Version]
- Sacerdote, P.; Martucci, C.; Vaccani, A.; Bariselli, F.; Panerai, A.E.; Colombo, A.; Parolaro, D.; Massi, P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J. Neuroimmunol. 2005, 159, 97–105. [Google Scholar] [CrossRef]
- Sermet, S.; Li, J.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes. Toxicology 2021, 464, 153016. [Google Scholar] [CrossRef]
- Zaiachuk, M.; Suryavanshi, S.V.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. The Anti-inflammatory effects of Cannabis sativa extracts on LPS-induced cytokines release in human macrophages. Molecules 2023, 28, 4991. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Valenti, C.; Billi, M.; Pancrazi, G.L.; Calabria, E.; Armogida, N.G.; Tortora, G.; Pagano, S.; Barnaba, P.; Marinucci, L. Biological effects of cannabidiol on human cancer cells: Systematic review of the literature. Pharmacol. Res. 2022, 181, 106267. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Yu, S.; Li, C.; Gao, B.; Zhou, X. Cannabidiol attenuates periodontal inflammation through inhibiting TLR4/NF-κB pathway. J. Periodontal Res. 2023, 58, 697–707. [Google Scholar] [CrossRef]
- Jirasek, P.; Jusku, A.; Frankova, J.; Urbankova, M.; Diabelko, D.; Ruzicka, F.; Papouskova, B.; Chytilova, K.; Vrba, J.; Havlasek, J.; et al. Phytocannabinoids and gingival inflammation: Preclinical findings and a placebo-controlled double-blind randomized clinical trial with cannabidiol. J. Periodontal Res. 2024, 59, 468–479. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar]
- David, C.; de Souza, J.F.; Silva, A.F.; Grazioli, G.; Barboza, A.S.; Lund, R.G.; Fajardo, A.R.; Moraes, R.R. Cannabidiol-loaded microparticles embedded in a porous hydrogel matrix for biomedical applications. J. Mater. Sci. Mater. Med. 2024, 35, 14. [Google Scholar] [CrossRef]
- Bracaglia, L.G.; Piotrowski-Daspit, A.S.; Lin, C.Y.; Moscato, Z.M.; Wang, Y.; Tietjen, G.T.; Saltzman, W.M. High-throughput quantitative microscopy-based half-life measurements of intravenously injected agents. Proc. Natl. Acad. Sci. USA 2020, 117, 3502–3508. [Google Scholar] [CrossRef]
- Kattel, P.; Sulthana, S.; Trousil, J.; Shrestha, D.; Pearson, D.; Aryal, S. Effect of nanoparticle weight on the cellular uptake and drug delivery potential of PLGA nanoparticles. ACS Omega 2023, 8, 27146–27155. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control Release 2016, 240, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The current knowledge on the pathogenesis of tissue and medical device-related biofilm infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, T.; Lawson, L.B.; Freytag, L.C.; Blake, D.A.; Ayyala, R.S.; John, V.T. In vitro degradation and release characteristics of spin coated thin films of PLGA with a “breath figure” morphology. Biomatter 2012, 2, 77–86. [Google Scholar] [CrossRef]
- Dayyoub, E.; Hobler, C.; Nonnweiler, P.; Keusgen, M.; Bakowsky, U. Nanostructured medical device coatings based on self-assembled poly(lactic-co-glycolic acid) nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3018–3024. [Google Scholar] [CrossRef]
- Jia, Z.; Ma, C.; Zhang, H. PLGA coatings and PLGA drug-loading coatings for cardiac stent samples: Degradation characteristics and blood compatibility. Coatings 2021, 11, 1427. [Google Scholar] [CrossRef]
- Jin, Z.; Zhan, Y.; Zheng, L.; Wei, Q.; Xu, S.; Qin, Z. Cannabidiol-loaded Poly Lactic-Co-Glycolic Acid nanoparticles with improved bioavailability as a potential for osteoarthritis therapeutic. Chembiochem 2023, 24, e202200698. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Chaulagain, B.; Trivedi, R.; Singh, J. Mannose-functionalized chitosan-coated PLGA nanoparticles for brain-targeted codelivery of CBD and BDNF for the treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2024, 15, 4021–4032. [Google Scholar] [CrossRef]
- Chen, S.; Deng, C.; Zheng, W.; Li, S.; Liu, Y.; Zhang, T.; Zhang, C.; Fu, Y.; Miao, H.; Ren, F.; et al. Cannabidiol effectively promoted cell death in bladder cancer and the improved intravesical adhesion drugs delivery strategy could be better used for treatment. Pharmaceutics 2021, 13, 1415. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: In vitro and in ovo assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef]
- Lozza, I.; Martín-Sabroso, C.; Torres-Suárez, A.I.; Fraguas-Sánchez, A.I. In situ forming PLA and PLGA implants for the parenteral administration of Cannabidiol. Int. J. Pharm. 2024, 661, 124468. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zheng, Z.; Hu, L.; Wang, H.; Tang, B.; Lin, L. Development and characterization of cannabidiol-loaded alginate copper hydrogel for repairing open bone defects in vitro. Colloids Surf. B Biointerfaces 2022, 212, 112339. [Google Scholar] [CrossRef]
- Qi, X.; Liu, C.; Li, G.; Luan, H.; Li, S.; Yang, D.; Zhou, Z. Investigation of in vitro odonto/osteogenic capacity of cannabidiol on human dental pulp cell. J. Dent. 2021, 109, 103673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki-Ogawa, A.; Fukasawa, T.; Sato, S.; Yoshizaki, A. The Potential Role of Cannabidiol in Cosmetic Dermatology: A Literature Review. Am. J. Clin. Dermatol. 2024, 25, 951–966. [Google Scholar] [CrossRef]
- Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and preliminary in vitro evaluation of 3D-printed alginate films with Cannabidiol (CBD) and Cannabigerol (CBG) nanoparticles for potential wound-healing applications. Pharmaceutics 2022, 14, 1637. [Google Scholar] [CrossRef]
- Kongkadee, K.; Wisuitiprot, W.; Ingkaninan, K.; Waranuch, N. Anti-inflammation and gingival wound healing activities of Cannabis sativa L. subsp. sativa (hemp) extract and cannabidiol: An in vitro study. Arch. Oral. Biol. 2022, 140, 105464. [Google Scholar] [CrossRef]
- Bellocchio, L.; Patano, A.; Inchingolo, A.D.; Inchingolo, F.; Dipalma, G.; Isacco, C.G.; de Ruvo, E.; Rapone, B.; Mancini, A.; Lorusso, F.; et al. Cannabidiol for oral health: A new promising therapeutical tool in dentistry. Int. J. Mol. Sci. 2023, 24, 9693. [Google Scholar] [CrossRef]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Baghaban Eslaminejad, M.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 64–75. [Google Scholar] [CrossRef]
- Kamenova, K.; Momekova, D.; Grancharov, G.; Prancheva, A.; Toncheva-Moncheva, N.; Ivanov, E.; Konstantinov, S.; Petrov, P.D. In situ gelling hydroxypropyl cellulose formulation comprising cannabidiol-loaded block copolymer micelles for sustained drug delivery. Int. J. Mol. Sci. 2023, 24, 16534. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionov, R.V.; Siag, A.; Mersini, E.T.; Kogan, N.M.; Alkhazov, T.; Koman, I.; Rowlo, P.; Gutkin, V.; Gross, M.; Steinberg, D. The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus. Pharmaceutics 2025, 17, 463. https://doi.org/10.3390/pharmaceutics17040463
Sionov RV, Siag A, Mersini ET, Kogan NM, Alkhazov T, Koman I, Rowlo P, Gutkin V, Gross M, Steinberg D. The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus. Pharmaceutics. 2025; 17(4):463. https://doi.org/10.3390/pharmaceutics17040463
Chicago/Turabian StyleSionov, Ronit Vogt, Ahmad Siag, Emma Theresa Mersini, Natalya M. Kogan, Tatiana Alkhazov, Igor Koman, Praveen Rowlo, Vitaly Gutkin, Menachem Gross, and Doron Steinberg. 2025. "The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus" Pharmaceutics 17, no. 4: 463. https://doi.org/10.3390/pharmaceutics17040463
APA StyleSionov, R. V., Siag, A., Mersini, E. T., Kogan, N. M., Alkhazov, T., Koman, I., Rowlo, P., Gutkin, V., Gross, M., & Steinberg, D. (2025). The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus. Pharmaceutics, 17(4), 463. https://doi.org/10.3390/pharmaceutics17040463





