Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications
Abstract
1. Introduction
2. Bioavailability and Stability Challenges
2.1. Bioavailability
2.2. Stability in Solid State
2.3. Stability in Aqueous Solution
3. Functional Role of Nanoparticles in Phytochemical Delivery Systems
3.1. Phytosomes
3.2. Liposomes
3.3. Invasomes
3.4. Niosomes
3.5. Bilosomes
3.6. Transferosomes
3.7. Ethosomes
3.8. Transethosomes
3.9. Cubosomes
4. Preparation Methods
4.1. Thin Film Hydration
4.2. Anti-Solvent Precipitation Technique
4.3. Cosolvency Method
4.4. Salting-Out Method
4.5. Freeze-Drying Technique
4.6. Supercritical Fluid Technique
5. In Vitro and In Vivo Tests for Vesicular Carriers
6. Clinical Trials and Patents
7. Future Perspectives, Challenges and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, P.Q.; Ling, L.S.C.; Chellian, J.; Madheswaran, T.; Panneerselvam, J.; Kunnath, A.P.; Gupta, G.; Satija, S.; Mehta, M.; Hansbro, P.M.; et al. Applications of Nanocarriers as Drug Delivery Vehicles for Active Phytoconstituents. Curr. Pharm. Des. 2020, 26, 4580–4590. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers 2021, 13, 2262. [Google Scholar] [CrossRef] [PubMed]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Akhtar, M.J.; Khan, S.A. Role of Natural Phytoconstituents as a Potential Bioenhancer of Anti-Cancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents. Processes 2024, 12, 2060. [Google Scholar] [CrossRef]
- Thorat, S.S.; Gujar, K.N.; Karale, C.K. Bioenhancers from mother nature: An overview. Future J. Pharm. Sci. 2023, 9, 20. [Google Scholar] [CrossRef]
- Ara, N.; Sultana, T.; Bolleddu, R.; Venkatesh, S.; Kiran, A.R. A strategy to enhance bioavailability of drug candidates: Natural bioenhancers. Int. J. Pharm. Bio Med. Sci. 2021, 1, 10–14. [Google Scholar]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Breedveld, P.; Beijnen, J.H.; Schellens, J.H. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol. Sci. 2006, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Cho, J.Y.; Shim, B.H.; Kim, D.K.; Lee, J. Bioavailability enhancing activities of natural compounds from medicinal plants. J. Med. Plants Res. 2009, 3, 1204–1211. [Google Scholar]
- Makhov, P.; Golovine, K.; Canter, D.; Kutikov, A.; Simhan, J.; Corlew, M.M.; Uzzo, R.G.; Kolenko, V.M. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate 2012, 72, 661–667. [Google Scholar] [CrossRef]
- Kumar, V.; Mittal, V.; Jalwal, P. Role of herbal bioenhancers (Piperine and Curcumin) on the oral bioavailability of Tamoxifen using experimental rats. J. Pharmacogn. Phytochem. 2015, 4, 133–136. [Google Scholar]
- Chakraborty, T.; Gupta, S.; Nair, A.; Chauhan, S.; Saini, V. Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102601. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals based chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phytother. Res. 2019, 33, 3064–3089. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Seenivasan, R.; Halagali, P.; Nayak, D.; Tippavajhala, V.K. Transethosomes: A Comprehensive Review of Ultra-Deformable Vesicular Systems for Enhanced Transdermal Drug Delivery. AAPS PharmSciTech 2025, 26, 41. [Google Scholar] [CrossRef]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simões, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological breakthroughs in the development of topical phytocompounds-based formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P. Development of asialoglycoprotein receptor-targeted nanoparticles for selective delivery of gemcitabine to hepatocellular carcinoma. Molecules 2019, 24, 4566. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Morsy, M.A.; Nair, A.B. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int. J. Pharm. 2018, 552, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.A.; Farahani, M.H.; Daryab, M.; Motahari, A.; Gholami, S.; Salmani, F.; Karimi, F.; Samaei, S.S.; Rezaee, A.; Rahmanian, P.; et al. Stimuli-responsive (nano)architectures for phytochemical delivery in cancer therapy. Biomed. Pharmacother. 2023, 166, 115283. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Z.; Li, C.; Zhou, W.; Shaw, J.P.; Baguley, B.C.; Liu, J.; Zhang, W. Optimization of Weight Ratio for DSPE-PEG/TPGS Hybrid Micelles to Improve Drug Retention and Tumor Penetration. Pharm. Res. 2018, 35, 13. [Google Scholar] [CrossRef]
- Wang, J. Combination Treatment of Cervical Cancer Using Folate-Decorated, pH-Sensitive, Carboplatin and Paclitaxel Co-Loaded Lipid-Polymer Hybrid Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 823–832. [Google Scholar] [CrossRef]
- Seder, I.; Zheng, T.; Zhang, J.; Rojas, C.C.; Helalat, S.H.; Téllez, R.C.; Sun, Y. A Scalable Microfluidic Platform for Nanoparticle Formulation: For Exploratory- and Industrial-Level Scales. Nano Lett. 2024, 24, 5132–5138. [Google Scholar] [CrossRef]
- Lin, H.; Leng, J.; Fan, P.; Xu, Z.; Ruan, G. Scalable production of microscopic particles for biological delivery. Mater. Adv. 2023, 4, 2885–2908. [Google Scholar] [CrossRef]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 5764. [Google Scholar] [CrossRef]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Afzal, O.; Rizwanullah, M.; Altamimi, A.S.A.; Alossaimi, M.A.; Kamal, M.; Ahmad, J. Harnessing natural polysaccharides-based nanoparticles for oral delivery of phytochemicals: Knocking down the barriers. J. Drug Deliv. Sci. Technol. 2023, 82, 104368. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crișan, G. Bioaccessibility and bioactive potential of different phytochemical classes from nutraceuticals and functional foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Negi, P.S. Stability of phytochemicals at the point of sale. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 375–395. [Google Scholar]
- Mrázková, M.; Sumczynski, D.; Orsavová, J. Influence of Storage Conditions on Stability of Phenolic Compounds and Antioxidant Activity Values in Nutraceutical Mixtures with Edible Flowers as New Dietary Supplements. Antioxidants 2023, 12, 962. [Google Scholar] [CrossRef]
- Li, D.; Xiao, Y.; Zhang, Z.; Liu, C. Light-induced oxidation and isomerization of all-trans-β-cryptoxanthin in a model system. J. Photochem. Photobiol. B Biol. 2015, 142, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhao, Y. Photooxidation of phytochemicals in food and control: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, X.; Zhao, D. Factors affecting phytochemical stability. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 332–374. [Google Scholar]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Darji, M.A.; Lalge, R.M.; Marathe, S.P.; Mulay, T.D.; Fatima, T.; Alshammari, A.; Lee, H.K.; Repka, M.A.; Narasimha Murthy, S. Excipient Stability in Oral Solid Dosage Forms: A Review. AAPS PharmSciTech 2018, 19, 12–26. [Google Scholar] [CrossRef]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Salmani, J.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Xia, S.; Muhoza, B.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Sodium carboxymethyl cellulose modulates the stability of cinnamaldehyde-loaded liposomes at high ionic strength. Food Hydrocoll. 2019, 93, 10–18. [Google Scholar] [CrossRef]
- Gabrič, A.; Hodnik, Ž.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. [Google Scholar] [CrossRef]
- Zhou, F.; Peterson, T.; Fan, Z.; Wang, S. The Commonly Used Stabilizers for Phytochemical-Based Nanoparticles: Stabilization Effects, Mechanisms, and Applications. Nutrients 2023, 15, 3881. [Google Scholar] [CrossRef]
- Bakrim, S.; Aboulaghras, S.; El Menyiy, N.; El Omari, N.; Assaggaf, H.; Lee, L.H.; Montesano, D.; Gallo, M.; Zengin, G.; AlDhaheri, Y.; et al. Phytochemical Compounds and Nanoparticles as Phytochemical Delivery Systems for Alzheimer’s Disease Management. Molecules 2022, 27, 9043. [Google Scholar] [CrossRef] [PubMed]
- Melim, C.; Magalhães, M.; Santos, A.C.; Campos, E.J.; Cabral, C. Nanoparticles as phytochemical carriers for cancer treatment: News of the last decade. Expert Opin. Drug Deliv. 2022, 19, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Bhairam, M.; Shukla, S.S.; Gidwani, B. Colloidal and vesicular delivery system for herbal bioactive constituents. DARU J. Pharm. Sci. 2021, 29, 415–438. [Google Scholar] [CrossRef]
- Nair, A.B.; Dalal, P.; Kadian, V.; Kumar, S.; Garg, M.; Rao, R.; Almuqbil, R.M.; Alnaim, A.S.; Aldhubiab, B.; Alqattan, F. Formulation Strategies for Enhancing Pharmaceutical and Nutraceutical Potential of Sesamol: A Natural Phenolic Bioactive. Plants 2023, 12, 1168. [Google Scholar] [CrossRef]
- Kotta, S.; Eshmawi, B.A.; Alahmadi, A.A.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B. Bioactivities, Biopharmaceutics, and Advanced Drug Delivery Systems of Magnolol. Indian J. Pharm. Educ. Res. 2024, 58, s1102–s1121. [Google Scholar]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, X.; Zhang, Q.; Wang, B.; Chen, Y.; Zang, C.; Wang, Y.; Dong, T.T.; Zhang, T. 20(S)-Protopanaxadiol Phospholipid Complex: Process Optimization, Characterization, In Vitro Dissolution and Molecular Docking Studies. Molecules 2016, 21, 1396. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Babazadeh, A.; Zeinali, M.; Hamishehkar, H. Nano-Phytosome: A Developing Platform for Herbal Anti-Cancer Agents in Cancer Therapy. Curr. Drug Targets 2018, 19, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. 2009, 14, 226–246. [Google Scholar] [PubMed]
- Talebi, M.; Shahbazi, K.; Dakkali, M.S.; Akbari, M.; Almasi Ghale, R.; Hashemi, S.; Sashourpour, M.; Mojab, F.; Aminzadeh, S. Phytosomes: A promising nanocarrier system for enhanced bioavailability and therapeutic efficacy of herbal products. Phytomed. Plus 2025, 5, 100779. [Google Scholar] [CrossRef]
- Giori, A.; Franceschi, F. Phospholipid Complexes of Curcumin Having Improved Bioavailability. U.S. Patent 20090131373A1, 31 March 2020. [Google Scholar]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar]
- Li, F.; Yang, X.; Yang, Y.; Li, P.; Yang, Z.; Zhang, C. Phospholipid complex as an approach for bioavailability enhancement of echinacoside. Drug Dev. Ind. Pharm. 2015, 41, 1777–1784. [Google Scholar] [CrossRef]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Petrangolini, G.; Ronchi, M.; Frattini, E.; De Combarieu, E.; Allegrini, P.; Riva, A. A New Food-grade Coenzyme Q10 Formulation Improves Bioavailability: Single and Repeated Pharmacokinetic Studies in Healthy Volunteers. Curr. Drug Deliv. 2019, 16, 759–767. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Medica 2019, 110, 524–529. [Google Scholar] [CrossRef]
- Di Pierro, F.; Menghi, A.B.; Barreca, A.; Lucarelli, M.; Calandrelli, A. Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: A clinical trial. Altern. Med. Rev. 2009, 14, 154–160. [Google Scholar]
- Riva, A.; Allegrini, P.; Franceschi, F.; Togni, S.; Giacomelli, L.; Eggenhoffner, R. A novel boswellic acids delivery form (Casperome®) in the management of musculoskeletal disorders: A review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5258–5263. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Pugliese, V.; Fontana, S.; Jirillo, E. Human use of Leucoselect® Phytosome® with special reference to inflammatory-allergic pathologies in frail elderly patients. Curr. Pharm. Des. 2014, 20, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Togni, S.; Franceschi, F.; Eghenhofner, R.; Giacomelli, L. Effects of standardized Ginkgo biloba extract complexed with phosphatidylserine (Virtiva®) on physiological response to prolonged, intense physical activity. Minerva Ortop. Traumatol. 2016, 67, 119–123. [Google Scholar]
- Sbrini, G.; Brivio, P.; Fumagalli, M.; Giavarini, F.; Caruso, D.; Racagni, G.; Dell’Agli, M.; Sangiovanni, E.; Calabrese, F. Centella asiatica L. Phytosome Improves Cognitive Performance by Promoting Bdnf Expression in Rat Prefrontal Cortex. Nutrients 2020, 12, 355. [Google Scholar] [CrossRef]
- El-Menshawe, S.F.; Ali, A.A.; Rabeh, M.A.; Khalil, N.M. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: An approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018, 13, 307–318. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Commercially bioavailable proprietary technologies and their marketed products. Drug Discov. Today 2013, 18, 936–949. [Google Scholar] [CrossRef]
- Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. [Google Scholar] [CrossRef]
- Pottathil, S.; Nain, P.; Morsy, M.A.; Kaur, J.; Al-Dhubiab, B.E.; Jaiswal, S.; Nair, A.B. Mechanisms of antidiabetic activity of methanolic extract of Punica granatum leaves in nicotinamide/streptozotocin-induced type 2 diabetes in rats. Plants 2020, 9, 1609. [Google Scholar] [CrossRef]
- Gorain, B.; Al-Dhubiab, B.E.; Nair, A.; Kesharwani, P.; Pandey, M.; Choudhury, H. Multivesicular Liposome: A Lipid-based Drug Delivery System for Efficient Drug Delivery. Curr. Pharm. Des. 2021, 27, 4404–4415. [Google Scholar]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Tahover, E.; Golan, T.; Geva, R.; Perets, R.; Amitay, Y.; Shmeeda, H.; Ohana, P. Pharmacokinetics of mitomycin-c lipidic prodrug entrapped in liposomes and clinical correlations in metastatic colorectal cancer patients. Investig. New Drugs 2020, 38, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Kolter, M.; Wittmann, M.; Köll-Weber, M.; Süss, R. The suitability of liposomes for the delivery of hydrophobic drugs—A case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 124–132. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, J.; Chen, Q. Synthesis, Characterization of Liposomes Modified with Biosurfactant MEL—A Loading Betulinic Acid and Its Anticancer Effect in HepG2 Cell. Molecules 2019, 24, 3939. [Google Scholar] [CrossRef]
- Righeschi, C.; Coronnello, M.; Mastrantoni, A.; Isacchi, B.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Strategy to provide a useful solution to effective delivery of dihydroartemisinin: Development, characterization and in vitro studies of liposomal formulations. Colloids Surf. B Biointerfaces 2014, 116, 121–127. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskac, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New delivery systems to improve the bioavailability of resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of phytochemicals by liposome cargos: Recent progress, challenges and opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-loaded liposomes for anticancer therapy: An updated review. Nanomedicine 2022, 17, 547–568. [Google Scholar] [CrossRef]
- Dutt, Y.; Pandey, R.P.; Dutt, M.; Gupta, A.; Vibhuti, A.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. Liposomes and phytosomes: Nanocarrier systems and their applications for the delivery of phytoconstituents. Coord. Chem. Rev. 2023, 491, 215251. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Sahu, R.K.; Aboulthana, W.M.; Mehta, D.K. Phyto-Phospholipid Complexation as a Novel Drug Delivery System for Management of Cancer with Better Bioavailability: Current Perspectives and Future Prospects. Anticancer Agents Med. Chem. 2021, 21, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Allaw, M.; Manca, M.L.; Castangia, I.; Manconi, M. From plants to phospholipid vesicles: A comprehensive review on the incorporation of phytochemicals into phospholipid vesicles designed for skin applications with special focus on scalability and in vitro and in vivo efficacy. J. Drug Deliv. Sci. Technol. 2022, 67, 103049. [Google Scholar] [CrossRef]
- Sharma, D.; Rani, A.; Singh, V.D.; Shah, P.; Sharma, S.; Kumar, S. Glycerosomes: Novel Nano-Vesicles for Efficient Delivery of Therapeutics. Recent Adv. Drug Deliv. Formul. 2023, 17, 173–182. [Google Scholar] [CrossRef]
- Allaw, M.; Manconi, M.; Aroffu, M.; Marongiu, F.; Porceddu, M.; Bacchetta, G.; Usach, I.; Rached, R.A.; Rajha, H.N.; Maroun, R.G.; et al. Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress. Pharmaceutics 2020, 12, 1010. [Google Scholar] [CrossRef]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’Hallewin, G.; Zaru, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Biophysical characterization of lutein or beta carotene-loaded cationic liposomes. RSC Adv. 2020, 10, 32409–32422. [Google Scholar] [CrossRef]
- Franca, L.; Ferraz, M.; Barros, M.C.; Gibson, V.; Xavier-Júnior, F.H.; Magalhães, N.S.S.; Lira-Nogueira, M. ConA-Coated Liposomes as a System to Delivery β-Lapachone to Breast Cancer Cells. Anticancer Agents Med. Chem. 2022, 22, 968–977. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci. Technol. 2014, 57, 686–695. [Google Scholar] [CrossRef]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid Interface Sci. 2021, 586, 391–403. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Mikheeva, I.B.; Yashin, V.A.; Penkov, N.V.; Vydrina, V.A.; Ishmuratov, G.Y.; Sharapov, V.A.; Khoroshavina, E.I.; et al. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183383. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Razavi, S.H.; Nácher, A.; Díez-Sales, O.; Peris, J.E.; Allaw, M.; Terencio, M.C.; Usach, I.; Manconi, M. Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration. Biomedicines 2022, 10, 157. [Google Scholar] [CrossRef]
- Hong, S.-C.; Park, K.-M.; Hong, C.R.; Kim, J.-C.; Yang, S.-H.; Yu, H.-S.; Paik, H.-D.; Pan, C.-H.; Chang, P.-S. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124670. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, F.; Swing, C.J.; Xia, S.; Zhang, X. Modulation effect of core-wall ratio on the stability and antibacterial activity of cinnamaldehyde liposomes. Chem. Phys. Lipids 2019, 223, 104790. [Google Scholar] [CrossRef]
- Ou, Z.; You, Y.; Yi, H.; Liu, X.; Tong, Y.; Liu, D.; Wang, J. Key Lipoprotein Receptor Targeted Echinacoside-Liposomes Effective Against Parkinson’s Disease in Mice Model. Int. J. Nanomed. 2024, 19, 8463–8483. [Google Scholar] [CrossRef]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef]
- Renault-Mahieux, M.; Vieillard, V.; Seguin, J.; Espeau, P.; Le, D.T.; Lai-Kuen, R.; Mignet, N.; Paul, M.; Andrieux, K. Co-Encapsulation of Fisetin and Cisplatin into Liposomes for Glioma Therapy: From Formulation to Cell Evaluation. Pharmaceutics 2021, 13, 970. [Google Scholar] [CrossRef]
- Song, Y.Y.; Yuan, Y.; Shi, X.; Che, Y.Y. Improved drug delivery and anti-tumor efficacy of combinatorial liposomal formulation of genistein and plumbagin by targeting Glut1 and Akt3 proteins in mice bearing prostate tumor. Colloids Surf. B Biointerfaces 2020, 190, 110966. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-Modified Honokiol-Loaded Liposomes Targeted Therapy for Osteosarcoma. Int. J. Nanomed. 2022, 17, 5137–5151. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Q.; Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, Y.; Guo, S.; Zhang, H.; Chen, G.; Xu, X. The efficacy of anti-VEGF antibody-modified liposomes loaded with paeonol in the prevention and treatment of hypertrophic scars. Drug Dev. Ind. Pharm. 2019, 45, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Qamar, W.; Gilani, S.J.; Zafar, A.; Alruwaili, N.K.; Alanazi, S.; Almutairy, B.K. Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation. Molecules 2021, 26, 3281. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, T.; Song, Y.; Lu, Y.; Chen, T.; Chen, P.; Hui, A.; Chen, Y.; Wang, H.; Zhang, W. Optimization on conditions of podophyllotoxin-loaded liposomes using response surface methodology and its activity on PC3 cells. J. Liposome Res. 2019, 29, 133–141. [Google Scholar] [CrossRef]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Sun, R.; Zhang, K.; Chen, Y.; Gong, Z. Phospholipids of inhaled liposomes determine the in vivo fate and therapeutic effects of salvianolic acid B on idiopathic pulmonary fibrosis. J. Control. Release 2024, 371, 1–15. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Calvani, M.; Pellegrini-Giampietro, D.E.; Bergonzi, M.C. Evaluation of Conventional and Hyaluronic Acid-Coated Thymoquinone Liposomes in an In Vitro Model of Dry Eye. Pharmaceutics 2023, 15, 578. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Q.; Zhou, C.; Lin, Y. Liposomes co-loaded with ursolic acid and ginsenoside Rg3 in the treatment of hepatocellular carcinoma. Acta Biochim. Pol. 2021, 68, 711–715. [Google Scholar] [CrossRef]
- Isacchi, B.; Bergonzi, M.C.; Iacopi, R.; Ghelardini, C.; Galeotti, N.; Bilia, A.R. Liposomal Formulation to Increase Stability and Prolong Antineuropathic Activity of Verbascoside. Planta Medica 2017, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wu, F.; Lee, R.J.; Lu, W.; Wang, J. Development of a stable single-vial liposomal formulation for vincristine. Int. J. Nanomed. 2019, 14, 4461–4474. [Google Scholar] [CrossRef]
- Tian, J.; Wang, L.; Wang, L.; Ke, X. A wogonin-loaded glycyrrhetinic acid-modified liposome for hepatic targeting with anti-tumor effects. Drug Deliv. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Kumar, B.; Pandey, M.; Aggarwal, R.; Sahoo, P.K. A comprehensive review on invasomal carriers incorporating natural terpenes for augmented transdermal delivery. Future J. Pharm. Sci. 2022, 8, 50. [Google Scholar] [CrossRef]
- Nangare, S.; Dugam, S. Smart invasome synthesis, characterizations, pharmaceutical applications, and pharmacokinetic perspective: A review. Future J. Pharm. Sci. 2020, 6, 123. [Google Scholar] [CrossRef]
- Duangjit, S.; Nimcharoenwan, T.; Chomya, N.; Locharoenrat, N.; Ngawhirunpat, T. Design and development of optimal invasomes for transdermal drug delivery using computer program. Asian J. Pharm. Sci. 2016, 11, 52–53. [Google Scholar] [CrossRef][Green Version]
- Kumar, B.; Sahoo, P.K.; Manchanda, S. Development of eucalyptol enriched nano vesicles for better transdermal delivery of curcumin: Preparation, characterisation and ex vivo skin analysis. Nanomed. J. 2022, 9, 223–230. [Google Scholar] [CrossRef]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Antiarthritic potential of berberine loaded invasomal gel. Phytomed. Plus 2022, 2, 100373. [Google Scholar] [CrossRef]
- Kumar, B.; Sahoo, P.K. Augmented Transdermal Delivery of Curcumin for the Effective Management of Plaque Psoriasis—Design, Formulation, Characterisation, and In Vivo Studies. AAPS PharmSciTech 2023, 24, 134. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and In Vivo Skin Deposition Assay. AAPS PharmSciTech 2018, 19, 2174–2184. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Bacchetta, G.; Pons, R.; Demurtas, D.; Diez-Sales, O.; Fadda, A.M.; Manconi, M. Santosomes as natural and efficient carriers for the improvement of phycocyanin reepithelising ability in vitro and in vivo. Eur. J. Pharm. Biopharm. 2016, 103, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shinu, P.; Nair, A.B.; Kumari, B.; Jacob, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Venugopala, K.N.; Attimarad, M.; Nagaraja, S. Recent Advances and Appropriate use of Niosomes for the Treatment of Skin Cancer. Indian J. Pharm. Educ. Res. 2022, 56, 1–14. [Google Scholar]
- Shehata, T.M.; Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Alhaider, I.A.; Attimarad, M.; Elsewedy, H.S.; Ibrahim, M.M. Vesicular emulgel based system for transdermal delivery of insulin: Factorial design and in vivo evaluation. Appl. Sci. 2020, 10, 5341. [Google Scholar] [CrossRef]
- Shah, J.; Nair, A.B.; Shah, H.; Jacob, S.; Shehata, T.M.; Morsy, M.A. Enhancement in antinociceptive and anti-inflammatory effects of tramadol by transdermal proniosome gel. Asian J. Pharm. Sci. 2020, 15, 786–796. [Google Scholar] [CrossRef]
- Momekova, D.B.; Gugleva, V.E.; Petrov, P.D. Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery. ACS Omega 2021, 6, 33265–33273. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef]
- Raafat, K.M.; El-Zahaby, S.A. Niosomes of active Fumaria officinalis phytochemicals: Antidiabetic, antineuropathic, anti-inflammatory, and possible mechanisms of action. Chin. Med. 2020, 15, 40. [Google Scholar] [CrossRef]
- Agarwal, S.; Mohamed, M.S.; Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv. 2018, 8, 32621–32636. [Google Scholar] [CrossRef]
- Parnian, F.; Hekmati-Moghaddam, S.H.; Majdizadeh, M.; Jebali, A.; Haghiralsadat, B.F. Fabrication of niosomal nano-carriers containing aqueous extract of hedera helix and comparison of toxicity of free extract and niosome extract on HT29 colorectal cancer cell line. J. Knowl. Health Basic Med. Sci. 2020, 15, 31–45. [Google Scholar] [CrossRef]
- Shahbazi, R.; Mirjafary, Z.; Zarghami, N.; Saeidian, H. Efficient PEGylated magnetic nanoniosomes for co-delivery of artemisinin and metformin: A new frontier in chemotherapeutic efficacy and cancer therapy. Sci. Rep. 2024, 14, 27380. [Google Scholar] [CrossRef]
- El-Banna, A.H.; Abo El-Ela, F.I.; Abdel-Wahab, A.; Gamal, A.; Abdel-Razik, A.-R.H.; El-Banna, H.A.; Elsamannoudy, S.I.; Ibrahim, M.A.; Abdelghany, A.K. Therapeutic efficacy of amygdaline and amygdaline-loaded niosomes in a rat model of Alzheimer’s disease via oxidative stress, brain neurotransmitters, and apoptotic pathway. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 117. [Google Scholar] [CrossRef]
- Palozza, P.; Muzzalupo, R.; Trombino, S.; Valdannini, A.; Picci, N. Solubilization and stabilization of beta-carotene in niosomes: Delivery to cultured cells. Chem. Phys. Lipids 2006, 139, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ravaghi, M.; Razavi, S.H.; Mousavi, S.M.; Sinico, C.; Fadda, A.M. Stabilization of natural canthaxanthin produced by Dietzia natronolimnaea HS-1 by encapsulation in niosomes. LWT 2016, 73, 498–504. [Google Scholar] [CrossRef]
- Gadapa, S.; Battula, S.N.; Mor, S.; Pushpadass, H.A.; Naik, L.N.; Emerald, M.E. Green tea catechin loaded niosomes: Formulation and their characterization for food fortification. J. Food Sci. Technol. 2022, 59, 3669–3682. [Google Scholar] [CrossRef]
- Esmaeili Rad, M.; Egil, A.C.; Ozaydin Ince, G.; Yuce, M.; Zarrabi, A. Optimization of curcumin loaded niosomes for drug delivery applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129921. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Parvaz, N.; Barani, M.; Khoshdel, A.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. Diosgenin-loaded niosome as an effective phytochemical nanocarrier: Physicochemical characterization, loading efficiency, and cytotoxicity assay. DARU J. Pharm. Sci. 2019, 27, 329–339. [Google Scholar] [CrossRef]
- Karaman Evren, D.; Kozgus Guldu, O.; Tut, E.; Medine, E.I. Nanoencapsulation of green tea catechin (−)-Epigallocatechin-3-Gallate (EGCG) in niosomes and assessment of its anticancer activity against lung cancer. J. Drug Deliv. Sci. Technol. 2024, 93, 105412. [Google Scholar] [CrossRef]
- Kanpipit, N.; Mattariganont, S.; Janphuang, P.; Rongsak, J.; Daduang, S.; Chulikhit, Y.; Thapphasaraphong, S. Comparative Study of Lycopene-Loaded Niosomes Prepared by Microfluidic and Thin-Film Hydration Techniques for UVB Protection and Anti-Hyperpigmentation Activity. Int. J. Mol. Sci. 2024, 25, 1717. [Google Scholar] [CrossRef]
- He, R.X.; Ye, X.; Li, R.; Chen, W.; Ge, T.; Huang, T.Q.; Nie, X.J.; Chen, H.J.; Peng, D.Y.; Chen, W.D. PEGylated niosomes-mediated drug delivery systems for Paeonol: Preparation, pharmacokinetics studies and synergistic anti-tumor effects with 5-FU. J. Liposome Res. 2017, 27, 161–170. [Google Scholar] [CrossRef]
- Khederzadeh, A.; Ebrahimnejad, P.; Seyedabadi, M.; Babaei, A.; Amiri, F.T.; Aslani, N.; Mojarad-Jabali, S.; Mohammadi, H. Synergistic effect of curcumin and Piperine loaded Niosomal nanoparticles on acute pulmonary toxicity induced by Paraquat in mice. Toxicol. Res. 2024, 13, tfae181. [Google Scholar] [CrossRef]
- Tyagi, R.; Waheed, A.; Kumar, N.; Ahad, A.; Bin Jardan, Y.A.; Mujeeb, M.; Kumar, A.; Naved, T.; Madan, S. Formulation and Evaluation of Plumbagin-Loaded Niosomes for an Antidiabetic Study: Optimization and In Vitro Evaluation. Pharmaceuticals 2023, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-Sardou, M. Evaluation of Carum-loaded Niosomes on Breast Cancer Cells:Physicochemical Properties, In Vitro Cytotoxicity, Flow Cytometric, DNA Fragmentation and Cell Migration Assay. Sci. Rep. 2019, 9, 7139. [Google Scholar] [CrossRef]
- Amiri, B.; Ahmadvand, H.; Farhadi, A.; Najmafshar, A.; Chiani, M.; Norouzian, D. Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells. Drug Dev. Ind. Pharm. 2018, 44, 1371–1376. [Google Scholar] [CrossRef]
- Chauhan, M.K.; Sahoo, P.K.; Rawat, A.S.; Singh, A.; Bamrara, A.; Sharma, D. Bilosomes: A Novel Approach to Meet the Challenges in Oral Immunization. Recent Pat. Drug Deliv. Formul. 2015, 9, 201–212. [Google Scholar] [CrossRef]
- Can, A.; Tyler, A.I.; Mackie, A.R. Potential use of bile salts in lipid self-assembled systems for the delivery of phytochemicals. Curr. Opin. Colloid Interface Sci. 2021, 56, 101502. [Google Scholar]
- Mitrović, D.; Zaklan, D.; Đanić, M.; Stanimirov, B.; Stankov, K.; Al-Salami, H.; Pavlović, N. The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery. Molecules 2025, 30, 1181. [Google Scholar] [CrossRef]
- Ahmad, J.; Singhal, M.; Amin, S.; Rizwanullah, M.; Akhter, S.; Kamal, M.A.; Haider, N.; Midoux, P.; Pichon, C. Bile Salt Stabilized Vesicles (Bilosomes): A Novel Nano-Pharmaceutical Design for Oral Delivery of Proteins and Peptides. Curr. Pharm. Des. 2017, 23, 1575–1588. [Google Scholar] [CrossRef]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Song, Y.; Hu, C. Hyaluronic acid-functionalized bilosomes for targeted delivery of tripterine to inflamed area with enhancive therapy on arthritis. Drug Deliv. 2019, 26, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef] [PubMed]
- Kaurav, H.; Tripathi, M.; Kaur, S.D.; Bansal, A.; Kapoor, D.N.; Sheth, S. Emerging Trends in Bilosomes as Therapeutic Drug Delivery Systems. Pharmaceutics 2024, 16, 697. [Google Scholar] [CrossRef]
- Eid, H.M.; Ali, A.A.; Ali, A.M.A.; Eissa, E.M.; Hassan, R.M.; Abo El-Ela, F.I.; Hassan, A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022, 17, 555–575. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Almalki, R.K.H.; Hussain, A.; Bukhari, S.I.; Mahdi, W.A.; Qamar, W. Formulation of Chitosan-Coated Apigenin Bilosomes: In Vitro Characterization, Antimicrobial and Cytotoxicity Assessment. Polymers 2022, 14, 921. [Google Scholar] [CrossRef]
- Shahbazi, R.; Jafari-Gharabaghlou, D.; Mirjafary, Z.; Saeidian, H.; Zarghami, N. Design and optimization various formulations of PEGylated niosomal nanoparticles loaded with phytochemical agents: Potential anti-cancer effects against human lung cancer cells. Pharmacol. Rep. 2023, 75, 442–455. [Google Scholar] [CrossRef]
- Deng, F.; Bae, Y.H. Bile acid transporter-mediated oral drug delivery. J. Control. Release 2020, 327, 100–116. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Khalid, M.; Amir, M.; Singh, L. Pegylated bilosomes for improvement of oral delivery of Biochanin A: Development to preclinical evaluation. S. Afr. J. Bot. 2023, 162, 633–643. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, Y.; Zhao, Z.; Liu, C.; Li, M.; Liu, J.; Wang, S.; Yang, D.; Luo, F.; et al. LS-HB-Mediated Photodynamic Therapy Inhibits Proliferation and Induces Cell Apoptosis in Melanoma. Mol. Pharm. 2022, 19, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Waglewska, E.; Kulbacka, J.; Bazylinska, U. Superior Drug Delivery Performance of Multifunctional Bilosomes: Innovative Strategy to Kill Skin Cancer Cells for Nanomedicine Application. Int. J. Nanomed. 2024, 19, 4701–4717. [Google Scholar] [CrossRef]
- Alamoudi, A.J.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Fahmy, U.A.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Asfour, H.Z.; Mohamed, G.A.; Ibrahim, S.R.M.; Abdel-Naim, A.B.; et al. Optimized bilosome-based nanoparticles enhance cytotoxic and pro-apoptotic activity of costunolide in LS174T colon cancer cells. Biomed. Pharmacother. 2023, 168, 115757. [Google Scholar] [CrossRef]
- Hashem, F.M.; Elkhateeb, D.; Ali, M.M.; Abdel-Rashid, R.S. In-vivo and in-vitro assessment of curcumin loaded bile salt stabilized nanovesicles for oral delivery. DARU J. Pharm. Sci. 2025, 33, 9. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Yasir, M.; Ghoneim, M.M.; Alshehri, S.; Anwer, M.K.; Almurshedi, A.S.; Alanazi, A.S. Development and evaluation of luteolin loaded pegylated bilosome: Optimization, in vitro characterization, and cytotoxicity study. Drug Deliv. 2021, 28, 2562–2573. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; Sultan, A.A.; Negm, W.A.; Attallah, N.G.M.; Alqahtani, M.J.; Hussein, I.A.; Shaldam, M.A.; El-Sherbeni, S.A.; Elekhnawy, E. Bilosomes as Nanoplatform for Oral Delivery and Modulated In Vivo Antimicrobial Activity of Lycopene. Pharmaceuticals 2022, 15, 1043. [Google Scholar] [CrossRef]
- Youness, R.A.; Al-Mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef]
- Waglewska, E.; Misiaszek, T.; Bazylińska, U. Nanoencapsulation of poorly soluble sea-buckthorn pulp oil in bile salt-origin vesicles: Physicochemical characterization and colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129113. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Youssef, N.; Ali, M.E.; Ragab, M.F.; Kamal, M.F.; Noureldin, M.H.; Abbas, H. Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals 2022, 16, 36. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A. Transfersomes: The Ultra-Deformable Carrier System for Non-Invasive Delivery of Drug. Curr. Drug Deliv. 2021, 18, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a transdermal drug delivery system: Dermal kinetics and recent developments. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1918. [Google Scholar] [CrossRef]
- Chen, R.P.; Chavda, V.P.; Patel, A.B.; Chen, Z.S. Phytochemical Delivery Through Transferosome (Phytosome): An Advanced Transdermal Drug Delivery for Complementary Medicines. Front. Pharmacol. 2022, 13, 850862. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Ontong, J.C.; Singh, S.; Siriyong, T.; Voravuthikunchai, S.P. Transferosomes stabilized hydrogel incorporated rhodomyrtone-rich extract from Rhodomyrtus tomentosa leaf fortified with phosphatidylcholine for the management of skin and soft-tissue infections. Biotechnol. Lett. 2024, 46, 127–142. [Google Scholar] [CrossRef]
- Wang, W.H.; Tyan, Y.C.; Chen, Z.S.; Lin, C.G.; Yang, M.H.; Yuan, S.S.; Tsai, W.C. Evaluation of the antioxidant activity and antiproliferative effect of the jaboticaba (Myrciaria cauliflora) seed extracts in oral carcinoma cells. BioMed Res. Int. 2014, 2014, 185946. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Chutoprapat, R.; Khankaew, P.; Titapiwatanakun, V.; Ruksiriwanich, W.; Boonpisuttinant, K. Asiatic acid-entrapped transfersomes for the treatment of hypertrophic scars: In vitro appraisal, bioactivity evaluation, and clinical study. Int. J. Pharm. 2024, 651, 123738. [Google Scholar] [CrossRef]
- Retnaningtyas, E.; Susatia, B.; Khotimah, H.; Rudijanto, A.; Ali Ahmed Abousouh, A.; Setiawan, A. Centella asiatica transfersomes and Bergamot essential oil nanoemulsion combined in gel exhibited anti-photoaging effects on UVB-radiated BALB/c mice. J. King Saud Univ. Sci. 2024, 36, 103207. [Google Scholar] [CrossRef]
- Tuntiyasawasdikul, S.; Sripanidkulchai, B. Curcuma comosa loaded transfersomal gel for transdermal application: Formulation, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2021, 47, 1824–1834. [Google Scholar] [CrossRef]
- Nangare, S.; Bhatane, D.; Mali, R.; Shitole, M. Development of a Novel Freeze-dried Mulberry Leaf Extract-based Transfersome Gel. Turk. J. Pharm. Sci. 2021, 18, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Sklenarova, R.; Allaw, M.; Perra, M.; Castangia, I.; Frankova, J.; Luis Pedraz, J.; Letizia Manca, M.; Manconi, M. Co-delivering of oleuropein and lentisk oil in phospholipid vesicles as an effective approach to modulate oxidative stress, cytokine secretion and promote skin regeneration. Eur. J. Pharm. Biopharm. 2023, 185, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Chamsai, B.; Rangsimawong, W.; Suriyaamporn, P.; Opanasopit, P.; Samprasit, W. Development of radish extract-loaded transfersomes blended sunscreen formulation for tyrosinase melanin and photoprotective sunscreening effect. J. Drug Deliv. Sci. Technol. 2024, 101, 106230. [Google Scholar] [CrossRef]
- Sahu, N.; Alam, P.; Ali, A.; Kumar, N.; Tyagi, R.; Madan, S.; Walia, R.; Saxena, S. Optimization, In Vitro and Ex Vivo Assessment of Nanotransferosome Gels Infused with a Methanolic Extract of Solanum xanthocarpum for the Topical Treatment of Psoriasis. Gels 2024, 10, 119. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Ragaie, M.H.; Elmowafy, E. Unraveling the pharmaceutical and clinical relevance of the influence of syringic acid loaded linoleic acid transferosomes on acne. Int. J. Pharm. 2023, 639, 122940. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded With Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef]
- Javed, N.; Ijaz, S.; Akhtar, N.; Khan, H.M.S. Nanostructured Ethosomal Gel Loaded with Arctostaphylosuva-Ursi Extract; In-Vitro/In-Vivo Evaluation as a Cosmeceutical Product for Skin Rejuvenation. Curr. Drug Deliv. 2022, 19, 706–720. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef]
- Khan, P.; Akhtar, N. Phytochemical investigations and development of ethosomal gel with Brassica oleraceae L. (Brassicaceae) extract: An innovative nano approach towards cosmetic and pharmaceutical industry. Ind. Crop. Prod. 2022, 183, 114905. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Sasindran, S.; Easwaran, M.; Shyamala, G.; Karuppaiah, A.; Siram, K.; Veintramuthu, S. Phytochemical screening and cytotoxicity evaluation of crude extracts: Toxicity comparison of crude extracts and its ethosomal formulations. J. Cosmet. Dermatol. 2020, 19, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Prazeres, I.; Mestre, H.; Bento-Silva, A.; Rodrigues, M.J.; Duarte, N.; Serra, A.T.; Bronze, M.R.; Rijo, P.; Gaspar, M.M.; et al. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals 2021, 14, 467. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Cui, Y.; Zheng, M.; Yang, Z.; Wang, X. Preparation of Ethosome Gel with Total Flavonoids from Vernonia anthelmintica (L.) Willd. for the Treatment of Vitiligo. Gels 2025, 11, 73. [Google Scholar] [CrossRef]
- Adnan, M.; Afzal, O.; Altamimi, A.S.A.; Alamri, M.A.; Haider, T.; Faheem Haider, M. Development and optimization of transethosomal gel of apigenin for topical delivery: In-vitro, ex-vivo and cell line assessment. Int. J. Pharm. 2023, 631, 122506. [Google Scholar] [CrossRef]
- Vera Pérez, J.; Martínez Cortés, D.M.; Gómez y Gómez, Y. Potential use of transethosomes as a transdermal delivery system for metabolites from Chenopodium murale. Mater. Today Commun. 2022, 30, 103165. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 795–813. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box-Behnken design, optimization, in vitro skin penetration, vesicles-skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hofni, A.; Abourehab, M.A.S.; Abdel-Rahman, I.A.M. Ginger Extract-Loaded Transethosomes for Effective Transdermal Permeation and Anti-Inflammation in Rat Model. Int. J. Nanomed. 2023, 18, 1259–1280. [Google Scholar] [CrossRef]
- Yasmeen; Khan, M.A.; Iqbal, Z.; Aqil, M. Carbopol 934-based transethosomal gel of Glycyrrhizic acid for the management of skin cancer. J. Drug Deliv. Sci. Technol. 2024, 97, 105825. [Google Scholar] [CrossRef]
- Aodah, A.H.; Hashmi, S.; Akhtar, N.; Ullah, Z.; Zafar, A.; Zaki, R.M.; Khan, S.; Ansari, M.J.; Jawaid, T.; Alam, A.; et al. Formulation Development, Optimization by Box-Behnken Design, and In Vitro and Ex Vivo Characterization of Hexatriacontane-Loaded Transethosomal Gel for Antimicrobial Treatment for Skin Infections. Gels 2023, 9, 322. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Al-Jenoobi, F.I. Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid. Pharmaceutics 2023, 15, 2391. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Alfehaid, F.S.; Nair, A.B.; Shah, H.; Aldhubiab, B.; Shah, J.; Mewada, V.; Jacob, S.; Attimarad, M. Enhanced transdermal delivery of apremilast loaded ethosomes: Optimization, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2024, 91, 105211. [Google Scholar]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as Vesicular System for Cutaneous Administration of Mangiferin: Formulative Study and 3D Skin Tissue Evaluation. Pharmaceutics 2021, 13, 1124. [Google Scholar] [CrossRef]
- Touitou, E.; Natsheh, H. The Evolution of Emerging Nanovesicle Technologies for Enhanced Delivery of Molecules into and across the Skin. Pharmaceutics 2024, 16, 267. [Google Scholar] [CrossRef]
- Bajaj, K.J.; Parab, B.S.; Shidhaye, S.S. Nano-transethosomes: A novel tool for drug delivery through skin. Indian J. Pharm. Educ. Res. 2021, 55, S1–S10. [Google Scholar]
- Munir, M.; Zaman, M.; Waqar, M.A.; Hameed, H.; Riaz, T. A comprehensive review on transethosomes as a novel vesicular approach for drug delivery through transdermal route. J. Liposome Res. 2024, 34, 203–218. [Google Scholar] [CrossRef]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Malang, S.D.; Shambhavi; Sahu, A.N. Transethosomal gel for enhancing transdermal delivery of natural therapeutics. Nanomedicine 2024, 19, 1801–1819. [Google Scholar] [CrossRef]
- Nayak, B.S.; Mohanty, B.; Mishra, B.; Roy, H.; Nandi, S. Transethosomes: Cutting edge approach for drug permeation enhancement in transdermal drug delivery system. Chem. Biol. Drug Des. 2023, 102, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.R.; Abdassah, M.; Supratman, U.; Shiono, Y.; Rahayu, D.; Sopyan, I.; Wilar, G. Nanoparticulate System for the Transdermal Delivery of Catechin as an Antihypercholesterol: In Vitro and In Vivo Evaluations. Pharmaceuticals 2022, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Oh, G.-H.; Jang, G.-H.; Kim, Y.-M.; Park, Y.-J. Transformer-ethosomes with palmitoyl pentapeptide for improved transdermal delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 460–467. [Google Scholar]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Jahan, S.; Ali, A.; Sultana, N.; Qizilbash, F.F.; Ali, H.; Aqil, M.; Mujeeb, M.; Ali, A. An overview of phospholipid enriched-edge activator-based vesicle nanocarriers: New paradigms to treat skin cancer. J. Drug Target. 2025, 33, 17–41. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines 2023, 11, 1114. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef]
- Gorantla, S.; Saha, R.N.; Singhvi, G. Exploring the affluent potential of glyceryl mono oleate–myristol liquid crystal nanoparticles mediated localized topical delivery of Tofacitinib: Study of systematic QbD, skin deposition and dermal pharmacokinetics assessment. J. Mol. Liq. 2022, 346, 117053. [Google Scholar] [CrossRef]
- Nath, A.G.; Dubey, P.; Kumar, A.; Vaiphei, K.K.; Rosenholm, J.M.; Bansal, K.K.; Gulbake, A. Recent Advances in the Use of Cubosomes as Drug Carriers with Special Emphasis on Topical Applications. J. Lipids 2024, 2024, 2683466. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef]
- Faisal, M.M.; Gomaa, E.; Ibrahim, A.E.; El Deeb, S.; Al-Harrasi, A.; Ibrahim, T.M. Verapamil-Loaded Cubosomes for Enhancing Intranasal Drug Delivery: Development, Characterization, Ex Vivo Permeation, and Brain Biodistribution Studies. AAPS PharmSciTech 2024, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Ther. 2015, 9, 4209–4218. [Google Scholar] [CrossRef]
- Khattab, A.; Ismail, S.; Abd-Elrazek, A.M. Mucoadhesive Chitosan Composite Sponge as a Carrier for β-Sitosterol Cubosomes for Thermal Burn Treatment. AAPS PharmSciTech 2024, 25, 148. [Google Scholar] [CrossRef]
- Alyami, M.H.; Hamdan, D.I.; Khalil, H.M.A.; Orabi, M.A.A.; Aborehab, N.M.; Osama, N.; Abdelhafez, M.M.; Al-Mahallawi, A.M.; Alyami, H.S. Preparation and in vivo evaluation of nano sized cubosomal dispersion loaded with Ruta graveolens extracts as a novel approach to reduce asthma-mediated lung inflammation. Saudi Pharm. J. 2024, 32, 101968. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Garg, M. Box-Behnken Design (BBD) Based Optimization of Beta-Carotene Loaded Cubosomes for Anti-Oxidant Activity Using DPPH Assay. BioNanoScience 2023, 13, 466–480. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir ACS J. Surf. Colloids 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Attri, S.; Kaur, P.; Rashid, F.; Bedi, N.; Haque, S.; Janahi, E.M.; Arora, S. Development and optimization of nanoparticles loaded with erucin, a dietary isothiocyanate isolated from Eruca sativa: Antioxidant and antiproliferative activities in ehrlich-ascites carcinoma cell line. Front. Pharmacol. 2022, 13, 1080977. [Google Scholar] [CrossRef]
- Zhu, C.; Duan, W.; Jing, H.; Long, J.; Huang, Y.; Huang, D.; Wu, C. Improving the stability and transdermal permeability of phycocyanin loaded cubosomes. Front. Nanotechnol. 2024, 6, 1359219. [Google Scholar]
- Raafat, K.M.; Abdelwahab, I.A.; El-Zahaby, S.A. Nano-cubosomes of the phyto-active principle in Withania somnifera: LC-MS-NMR, anti-microbial, and insights of the anti-neuropathic and anti-inflammatory mechanism. Fitoterapia 2024, 178, 106196. [Google Scholar] [CrossRef] [PubMed]
- Salaheldin Abdelhamid Ibrahim, S.; Bassam, S.M.; El-Hawary, S.; Sheta, E.; Masoud, I.M.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Hammad, G.O. The gastroprotective effect of Yucca filamentosa standardized crude leaves extract versus its nano-cubosomal formulation in ethanol-induced gastric injury. Int. Immunopharmacol. 2024, 137, 112440. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Dragićević, N. Invasomes as Drug Nanocarriers for Innovative Pharmaceutical Dosage Forms; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Roostaee, M.; Derakhshani, A.; Mirhosseini, H.; Banaee Mofakham, E.; Fathi-Karkan, S.; Mirinejad, S.; Sargazi, S.; Barani, M. Composition, preparation methods, and applications of nanoniosomes as codelivery systems: A review of emerging therapies with emphasis on cancer. Nanoscale 2024, 16, 2713–2746. [Google Scholar] [CrossRef]
- Nayak, D.; Rathnanand, M.; Tippavajhala, V.K. Unlocking the Potential of Bilosomes and Modified Bilosomes: A Comprehensive Journey into Advanced Drug Delivery Trends. AAPS PharmSciTech 2023, 24, 238. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Khan, M.A.; Hameed, A.; Ereej, N.; Ihsan, H. Ethosomes: A potential nanovesicular carrier to enhancing the drug delivery against skin barriers. J. Microencapsul. 2024, 41, 204–225. [Google Scholar] [CrossRef]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Q.; Du, P.; Zhao, J.; Zhang, T. Preparation and characterization of solidified oleanolic acid–phospholipid complex aiming to improve the dissolution of oleanolic acid. Asian J. Pharm. Sci. 2016, 11, 241–247. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M.; et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef]
- Andishmand, H.; Yousefi, M.; Jafari, N.; Azadmard-Damirchi, S.; Homayouni-Rad, A.; Torbati, M.; Hamishehkar, H. Designing and fabrication of colloidal nano-phytosomes with gamma-oryzanol and phosphatidylcholine for encapsulation and delivery of polyphenol-rich extract from pomegranate peel. Int. J. Biol. Macromol. 2024, 256, 128501. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Yooying, R. Enhancement of solubility, thermal stability and bioaccessibility of vitexin using phosphatidylcholine-based phytosome. NFS J. 2023, 31, 28–38. [Google Scholar] [CrossRef]
- Demir, B.; Barlas, F.; Guler, E.; Gumus, P.; Can, M.; Yavuz, M.; Coskunol, H.; Timur, S. Gold nanoparticle loaded phytosomal systems: Synthesis, characterization and in vitro investigations. RSC Adv. 2014, 4, 34687–34695. [Google Scholar]
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, S.; Khar, R.K. Murraya koenigii extract loaded phytosomes prepared using antisolvent precipitation technique for improved antidiabetic and hypolidemic activity. Indian J. Pharm. Educ. Res. 2022, 56, s326–s338. [Google Scholar] [CrossRef]
- Damle, M.; Mallya, R. Development and Evaluation of a Novel Delivery System Containing Phytophospholipid Complex for Skin Aging. AAPS PharmSciTech 2016, 17, 607–617. [Google Scholar] [CrossRef]
- Karole, S.; Gupta, G. Preparation and evaluation of phytosomes containing ethanolic extract of leaves of Bombax ceiba for hepatoprotective activity. Pharma Innov. J. 2019, 8, 22–26. [Google Scholar]
- Habbu, P.; Madagundi, S.; Shastry, R.; Vanakudri, R.; Kulkarni, V. Preparation and evaluation of antidiabetic activity of Allium cepa-phospholipid complex (phytosome) in streptozotocin induced diabetic rats. RGUHS J. Pharm. Sci. 2015, 5, 132–141. [Google Scholar]
- Dewi, M.K.; Muhaimin, M.; Joni, I.M.; Hermanto, F.; Chaerunisaa, A.Y. Fabrication of Phytosome with Enhanced Activity of Sonneratia alba: Formulation Modeling and in vivo Antimalarial Study. Int. J. Nanomed. 2024, 19, 9411–9435. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: Optimization and ex vivo permeation. Int. J. Nanomed. 2013, 8, 2385–2397. [Google Scholar] [CrossRef]
- Human, C.; Aucamp, M.; de Beer, D.; van der Rijst, M.; Joubert, E. Food-grade phytosome vesicles for nanoencapsulation of labile C-glucosylated xanthones and dihydrochalcones present in a plant extract matrix—Effect of process conditions and stability assessment. Food Sci. Nutr. 2023, 11, 8093–8111. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Tatode, A.A.; Anand, S.; Dave, V.S. Kaempferol-phospholipid complex: Formulation, and evaluation of improved solubility, in vivo bioavailability, and antioxidant potential of kaempferol. J. Excip. Food Chem. 2016, 7, 89–116. [Google Scholar]
- Li, Y.; Yang, D.J.; Chen, S.L.; Chen, S.B.; Chan, A.S. Process parameters and morphology in puerarin, phospholipids and their complex microparticles generation by supercritical antisolvent precipitation. Int. J. Pharm. 2008, 359, 35–45. [Google Scholar] [CrossRef]
- Goel, H.; Saini, K.; Razdan, K.; Khurana, R.K.; Elkordy, A.A.; Singh, K.K. Chapter 3—In vitro physicochemical characterization of nanocarriers: A road to optimization. In Nanoparticle Therapeutics: Production Technologies, Types of Nanoparticles, and Regulatory Aspects; Kesharwani, P., Singh, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 133–179. [Google Scholar] [CrossRef]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Chapter 2—Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 33–58. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- AlMadalli, H.J.; Abdul Rasool, B.K.; Shehab, N.G.; Sala, F.D.; Borzacchiello, A. Pomegranate extract-loaded sphingosomes for the treatment of cancer: Phytochemical investigations, formulation, and antitumor activity evaluation. PLoS ONE 2024, 19, e0293115. [Google Scholar] [CrossRef]
- Maurizi, L.; Lasalvia, A.; Fabiano, M.G.; D’Intino, E.; Del Cioppo, F.; Fraschetti, C.; Filippi, A.; Ammendolia, M.G.; Conte, A.L.; Forte, J.; et al. Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp. Pharmaceutics 2024, 16, 927. [Google Scholar] [CrossRef]
- Bennion, B.J.; Be, N.A.; McNerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C.; et al. Predicting a Drug’s Membrane Permeability: A Computational Model Validated with in Vitro Permeability Assay Data. J. Phys. Chem. B 2017, 121, 5228–5237. [Google Scholar] [CrossRef]
- Bansal, G.; Suthar, N.; Kaur, J.; Jain, A. Stability Testing of Herbal Drugs: Challenges, Regulatory Compliance and Perspectives. Phytother. Res. 2016, 30, 1046–1058. [Google Scholar] [CrossRef]
- Isleroglu, H.; Turker, I. Ultrasonic-assisted extraction and thermal stability of phytochemicals from fenugreek leaves. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100390. [Google Scholar]
- İnanç Horuz, T.; Maskan, M. Effect of the phytochemicals curcumin, cinnamaldehyde, thymol and carvacrol on the oxidative stability of corn and palm oils at frying temperatures. J. Food Sci. Technol. 2015, 52, 8041–8049. [Google Scholar] [CrossRef]
- Baba, W.N.; Abdelrahman, R.; Maqsood, S. Production and utilization of non-covalent dairy-based proteins complexed with date palm leave polyphenols for improving curcumin stability. Food Biosci. 2023, 53, 102690. [Google Scholar] [CrossRef]
- Thomas Pannakal, S.; Eilstein, J.; Prasad, A.; Ekhar, P.; Shetty, S.; Peng, Z.; Bordier, E.; Boudah, S.; Paillat, L.; Marrot, L.; et al. Comprehensive characterization of naturally occurring antioxidants from the twigs of mulberry (Morus alba) using on-line high-performance liquid chromatography coupled with chemical detection and high-resolution mass spectrometry. Phytochem. Anal. 2022, 33, 105–114. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.U.; Tabassum, F.; Khursheed, A.; Zaman, Q.U.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef]
- Rathaur, P.; Johar, K., Sr. Metabolism and Pharmacokinetics of Phytochemicals in the Human Body. Curr. Drug Metab. 2019, 20, 1085–1102. [Google Scholar] [CrossRef]
- Ghalbi Ahangari, M.; Farimani, M.M.; Erfani, M.; Goudarzi, M. Technetium-99m radiolabeling through conjugation with l,l-ethylene dicysteine chelator of a trimethoxylated flavone and its bioevaluation in rat with induced C6 glioma tumor as a new cancer diagnostic agent. Radiochim. Acta 2024, 112, 327–337. [Google Scholar] [CrossRef]
- Sousa Carvalho, G.F.; Marques, L.K.; Sousa, H.G.; Silva, L.R.; Leão Ferreira, D.C.; Pires de Moura do Amaral, F.; Martins Maia Filho, A.L.; Figueredo-Silva, J.; Alves, W.D.S.; Oliveira, M.; et al. Phytochemical study, molecular docking, genotoxicity and therapeutic efficacy of the aqueous extract of the stem bark of Ximenia americana L. in the treatment of experimental COPD in rats. J. Ethnopharmacol. 2020, 247, 112259. [Google Scholar] [CrossRef]
- Aurori, M.; Niculae, M.; Hanganu, D.; Pall, E.; Cenariu, M.; Vodnar, D.C.; Bunea, A.; Fiţ, N.; Andrei, S. Phytochemical Profile, Antioxidant, Antimicrobial and Cytoprotective Effects of Cornelian Cherry (Cornus mas L.) Fruit Extracts. Pharmaceuticals 2023, 16, 420. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. C. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar] [CrossRef]
- Gul, B.; Anwar, R.; Saleem, M.; Noor, A.; Ullah, M.I. Cassia absus-mediated upregulation of IL-4, IL-10 and downregulation of IL-1β, IL-6, TNF-α, NF-κB, IFN-γ in CFA-induced arthritis model. Inflammopharmacology 2023, 31, 1241–1256. [Google Scholar] [CrossRef]
- Jantan, I.; Arshad, L.; Septama, A.W.; Haque, M.A.; Mohamed-Hussein, Z.A.; Govender, N.T. Antiviral effects of phytochemicals against severe acute respiratory syndrome coronavirus 2 and their mechanisms of action: A review. Phytother. Res. 2023, 37, 1036–1056. [Google Scholar] [CrossRef]
- Ndezo Bisso, B.; Njikang Epie Nkwelle, R.; Tchuenguem Tchuenteu, R.; Dzoyem, J.P. Phytochemical Screening, Antioxidant, and Antimicrobial Activities of Seven Underinvestigated Medicinal Plants against Microbial Pathogens. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1998808. [Google Scholar] [CrossRef]
- Noushida, N.; Nayak, R.P.; Sultana, R.; Salian, T.R.; Alobid, S.; Almadani, M.E.; Ahmad, F.; Gilkaramenthi, R.; Asdaq, S.M.B.; Almoteer, A.I. Cardioprotective effects of Callicarpa tomentosa leaf extract in Wistar albino rats against isoproterenol-induced myocardial necrosis: Phytochemical analysis and in vitro antioxidant study. J. King Saud Univ.-Sci. 2024, 36, 103100. [Google Scholar]
- da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef]
- Yolin Angel, P.; Jeyakumar, P.; Jasmin Suriya, A.R.; Sheena, A.; Karuppiah, P.; Periyasami, G.; Stalin, A.; Murugan, K. Topical antifungal keratitis therapeutic potential of Clitoria ternatea Linn. flower extract: Phytochemical profiling, in silico modelling, and in vitro biological activity assessment. Front. Microbiol. 2024, 15, 1343988. [Google Scholar] [CrossRef]
- Rani, A.; Uzair, M.; Ali, S.; Qamar, M.; Ahmad, N.; Abbas, M.W.; Esatbeyoglu, T. Dryopteris juxtapostia Root and Shoot: Determination of Phytochemicals; Antioxidant, Anti-Inflammatory, and Hepatoprotective Effects; and Toxicity Assessment. Antioxidants 2022, 11, 1670. [Google Scholar] [CrossRef]
- El Faqer, O.; Bendiar, S.; Rais, S.; Elkoraichi, I.; Dakir, M.; Elouaddari, A.; El Amrani, A.; Oudghiri, M.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous, ethanolic extracts and essential oil of Syzygium aromaticum L. on human neutrophils. Sci. Afr. 2022, 18, e01395. [Google Scholar]
- Ullah, M.; Mehmood, S.; Khan, R.A.; Ali, M.; Fozia, F.; Waqas, M.; Ullah, R.; Alotaibi, A.; Sultan, M.A. Assessment of antidiabetic potential and phytochemical profiling of Viscum album, a traditional antidiabetic plant. J. Food Qual. 2022, 2022, 5691379. [Google Scholar]
- Zhang, J.; Jiang, M.; Zhao, H.; Han, L.; Jin, Y.; Chen, W.; Wang, J.; Zhang, Z.; Peng, C. Synthesis of Paeonol-Ozagrel Conjugate: Structure Characterization and In Vivo Anti-Ischemic Stroke potential. Front. Pharmacol. 2020, 11, 608221. [Google Scholar] [CrossRef]
- Chudasma, M.P.; Shah, S.A.; Qureshi, M.H.N.; Shah, N.; Shah, D.; Trivedi, R.; Shah, V.H. Brief insight on nanovesicular liposomes as drug-delivery carriers for medical applications. J. Explor. Res. Pharmacol. 2023, 8, 222–236. [Google Scholar]
- Su, S.; Peter, M.K. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Wi, T.I.; Won, J.E.; Lee, C.M.; Lee, J.W.; Kang, T.H.; Shin, B.C.; Han, H.D.; Park, Y.M. Efficacy of Combination Therapy with Linalool and Doxorubicin Encapsulated by Liposomes as a Two-in-One Hybrid Carrier System for Epithelial Ovarian Carcinoma. Int. J. Nanomed. 2020, 15, 8427–8436. [Google Scholar] [CrossRef]
- Castro, N.R.; Pinto, C.S.C.; Santos, E.P.; Mansur, C.R.E. Hybrid Vesicular Nanosystems Based on Lipids and Polymers Applied in Therapy, Theranostics, and Cosmetics. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 271–303. [Google Scholar] [CrossRef]

| Name | Structure | Botanical Sources | Type | Biological Role | Formulation Challenges |
|---|---|---|---|---|---|
| Allicin | 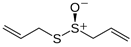 | Allium sativum | Thiosulfinate | Antimicrobial, antioxidant, anti-inflammatory, cardioprotective, anticancer | Highly unstable, short shelf life, strong odor, low oral bioavailability |
| Artemisinin |  | Artemisia annua | Sesquiterpene lactone | Antimalarial, anticancer, anti-inflammatory, antiviral, antiparasitic | Low solubility, short-half life, chemical instability, limited shelf life, drug resistance |
| β-carotene |  | Daucus carota, Ipomoea batatas, Cucurbita maxima, Spinacia oleracea | Carotenoid | Provitamin A, antioxidant | Insoluble in water, prone to oxidation |
| β-Lapachone |  | Tabebuia avellanedae | Quinone | Antioxidant, anti-inflammatory, cardioprotective, anticancer, antimicrobial | Poor solubility, low bioavailability, toxicity, short half-life |
| Betulinic acid |  | Betula pendula, Syzygium formosanum, Ziziphus mauritiana, Diospyros melanoxylon | Pentacyclic triterpenoid | Anti-cancer, anti-HIV, anti-inflammatory | Low water solubility, limited bioavailability |
| Canthaxanthin |  | Cantharellus cinnabarinus, Capsicum annuum, Daucus carota | Keto-carotenoid | Antioxidant | Insoluble in water, prone to oxidation |
| Catechins |  | Camellia sinensis, Theobroma cacao, Vitis vinifera | Polyphenolic flavonoid | Antioxidant, anti-inflammatory, cardiovascular, anticancer, metabolic health, neuroprotective, antimicrobial | Poor solubility, low stability, rapid metabolism, low oral bioavailability, |
| Cinnamaldehyde |  | Cinnamomum cassia, Cinnamomum verum | Phenylpropanoid | Antimicrobial, antiparasitic, antioxidant, antidiabetic, anticancer, cardiovascular, neuroprotective | Volatility, low stability, poor solubility, mucosal irritation, rapid metabolism |
| Curcumin |  | Curcuma longa | Diarylheptanoid | Anti-inflammatory, antioxidant, anti-cancer | Poor bioavailability, rapid metabolism |
| Echinacoside |  | Echinacea purpurea, Cistanche deserticola, Leonurus japonicus | Phenylethanoid glycoside | Antioxidant, anti-inflammatory, neuroprotective, immunomodulatory, antiaging, wound healing, hepatoprotective | Low stability, poor solubility, low bioavailability, short half-life |
| Fisetin |  | Nelumbo nucifera | Flavonoid | Antioxidant, anti-inflammatory, senolytic, neuroprotective, anti-cancer, cardioprotective | Poor solubility, sensitive to heat, oxygen and light, first-pass metabolism |
| Ginsenoside Rg3 |  | Panax ginseng, Panax notoginseng | Saponin | Anticancer, neuroprotective, anti-inflammatory, cardioprotective, antifatigue, immunomodulatory, antidiabetic | Poor solubility, low bioavailability, unstable, low permeability, first-pass metabolism |
| Honokiol |  | Magnolia officinalis, Magnolia grandiflora | Biphenol | Anticancer, neuroprotective, anti-inflammatory, antimicrobial, antiviral, cardioprotective, anxiolytic, antidepressant | Poor solubility, rapid metabolism, low stability, low intestinal permeability |
| Lutein |  | Spinacia oleracea, Brassica oleracea | Carotenoid | Antioxidant | Instability to light, poor solubility |
| Lycopene |  | Solanum lycopersicum L., Citrullus lanatus, Psidium guajava | Acyclic carotenoid | Cardiovascular, reduce oxidative stress | Poor solubility |
| Paeonol |  | Paeonia suffruticosa, Paeonia lactiflora | Phenolic compound | Anti-inflammatory, analgesic, antioxidant, antimicrobial, immunomodulatory, cardioprotective, anticancer | Poor solubility, volatility, low stability, low bioavailability |
| Piperine |  | Piper nigrum, Piper longum | Alkaloid | Antioxidant, anti-inflammatory, antimicrobial, anti-carcinogenic, neuroprotective | Poor solubility, low bioavailability, light/heat sensitive |
| Plumbagin |  | Plumbago zeylanica, Diospyros lotus, Drosera rotundifolia | Naphthoquinone | Anticancer, anti-inflammatory, antioxidant, cardioprotective, neuroprotective, antidiabetic, antimicrobial | Low stability, poor solubility, low oral bioavailability, short half-life, toxicity |
| Quercetin |  | Capparis spinosa, Malus domestica, Vaccinium corymbosum | Flavonoid | Antioxidant, anti-inflammatory, anticancer, antiviral, antimicrobial, cardioprotective, neuroprotective | Poor solubility, low bioavailability, stability, low permeability |
| Resveratrol |  | Vitis vinifera, Arachis, Punica granatum, Polygonum cuspidatum | Stilbene | Antioxidant, anti-inflammatory, anti-carcinogenic | Slightly soluble in water |
| Salvianolic acid B |  | Salvia miltiorrhiza | Polyphenol | Antioxidant, anti-inflammatory, cardioprotective, neuroprotective, anti-fibrotic, anticancer, antidiabetic | Low oral bioavailability, instability, first-pass metabolism, short half-life, poor permeability |
| Thymoquinone | 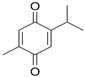 | Nigella sativa | Benzoquinone | Hepato-protective, neuroprotective, nephroprotective, cardioprotective, anti-inflammatory, antimicrobial, anti-carcinogenic, antidiabetic, immunomodulatory | Poor solubility, instability, low bioavailability, bitter taste |
| Ursolic acid |  | Rosmarinus officinalis, Prunella vulgaris, Ocimum sanctum | Triperpenoid | Antioxidant, anti-inflammatory, anti-cancer, antimicrobial, cardioprotective | Poor solubility, low bioavailability, unstable |
| Verbascoside | 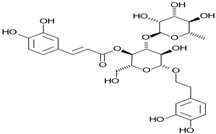 | Verbascum thapsus | Phenylethanoid glycoside | Antioxidant, anti-inflammatory, neuroprotective, hepatoprotective, antimicrobial, nephroprotective, wound healing, anticancer | Low stability, poor solubility, low bioavailability, short half-life |
| Wogonin | 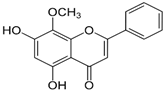 | Scutellaria baicalensis | Flavonoid | Anticancer activity, neuroprotective, anti-inflammatory, antiviral | Poor solubility, low stability, first-pass metabolism |
| Carrier | Advantages | Limitations | Skin Permeation | Entrapment Efficiency | Drug Loading | Scalability | Stability | Cost |
|---|---|---|---|---|---|---|---|---|
| Phytosomes | Enhanced bioavailability, natural origin, suitable for phytoactives | Limited to amphiphilic/lipophilic phytochemicals, cost of phospholipid | Moderate | High | Moderate to high | Good, relatively simple formulation methods | Moderate, sensitive to oxidation | High due to phospholipids |
| Liposomes | Biocompatible, versatile, enhanced drug delivery | Low stability, high cost, potential leakage | Moderate, depending on formulation | Moderate to high | Moderate | Challenging due to cost and stability issues | Low to moderate, prone to oxidation | High due to phospholipids |
| Invasomes | Enhanced active delivery through stratum corneum | Volatility of terpenes, moderate stability | High | Moderate to high | Moderate | Moderate, due to terpene handling | Moderate, sensitive to terpene loss | Moderate, terpene inclusion increases cost slightly |
| Niosomes | Stable, cost-effective, customizable | Less biocompatible compared to liposomes, may require specific surfactants | Moderate to high, dependent on surfactant type | High | Moderate to high | Good, cost-effective methods available | Good, more stable than liposomes | Low to moderate, cost-effective materials |
| Bilosomes | Enhanced oral bioavailability, protects against gastric degradation, stable under physiological conditions | Limited skin delivery application, bile salts may interact with other excipients | Low to moderate | High | Moderate to high | Good, suitable for large-scale production | High, stable in GI tract and physiological pH | Low to moderate, bile salts are cost-effective |
| Transferosomes | High deformability, deep skin penetration | Requires special handling, potential instability | High due to deformability | Moderate to high | Moderate | Moderate, requires specific techniques | Moderate, edge activators may affect stability | Moderate, depends on edge activators |
| Ethosomes | Improved skin permeation due to ethanol, effective for transdermal delivery | Ethanol may irritate, stability issues | High due to ethanol’s effect on skin lipids | High | Moderate to high | Moderate to high, depends on ethanol handling | Moderate, ethanol can evaporate over time | Moderate, ethanol adds to cost |
| Transethosomes | Combines ethosome and transferosome benefits, superior penetration | Complex composition requires precise formulation | Very high, superior to ethosomes and transferosomes | Very high | High | Moderate, due to complexity | Moderate, requires optimized storage | Moderate to high, complex formulation increases cost |
| Cubosomes | High stability, controlled and sustained drug release, large surface area, high drug-loading capacity | Complex preparation process, potential viscosity issues, limited scalability | Moderate to high, depends on formulation | High, due to large internal surface area and cubic structure | High, suitable for both hydrophilic and lipophilic drugs | Moderate, requires specialized techniques like high pressure homogenization | High, resistant to degradation, but sensitive to temperature and pH fluctuations | Moderate, influenced by surfactant and stabilizer costs |
| Phytochemical | Preparation Technique | Composition | Disease/Therapeutic Application | Highlights | Ref. |
|---|---|---|---|---|---|
| β-carotene and lutein | Thin film hydration with ultrasonication | Dipalmitoyl-phosphatidylcholine, L-α-phosphatidyl-ethanolamine, L-α-cephalin (3-snphosphatidyl-ethanolamine), stearylamine | Antioxidants, cancer, cardiovascular, skin diseases, eye health, anti-inflammatory effects | β-carotene adheres to the liposomal boundary, causing external morphological changes. Encapsulation efficiencies of lutein and β-carotene are over 98.8% and 87%, respectively. Lutein in cationic liposomes demonstrates better in vitro release stability (30%) compared to β-carotene (45%) between 3 and 6 h, with a lower leakage rate ensuring higher lutein retention. | [102] |
| β-lapachone | Thin film hydration | Soybean phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-phosphoethanol amine-[N-(Carbonyl-methoxypolyethylene glycol-2000), cholesterol | Breast cancer | Uncoated and concanavalin A (ConA) coated β-lapachone liposomes exhibit nano size (100–150 nm). Hemagglutination assays verified ConA avidity, showing that Lipo-ConA and Lipo-PEG-ConA could hemagglutinate red blood cells. Site-specific liposomes demonstrated enhanced toxicity. ConA-coated liposomes achieved greater internalization in MCF7 cells compared to uncoated liposomes. | [103] |
| Allicin | Reverse phase evaporation | Lecithin, cholesterol | Diarrhea, flatulence, edema, arthritis, worm infestation and pulmonary complaints | Optimized allicin liposomes showed higher EE, low vesicle size and good stability. The formulation demonstrated sustained release behavior, highlighting their potential for controlled release for wider applications. | [104] |
| Artemisinin | Thin film hydration | Soy lecithin, cholesterol,1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(polyethylene glycol)-2000 | Antimalarial | Artemisinin-loaded liposomes, modified with a reversibly activatable cell penetrating peptide ((HE)10-G5-R6 or HE-R6), demonstrated sustained release, enhanced internalization, and increased cytotoxicity compared to non-modified liposomes. Additionally, modified liposomes exhibited prolonged tumor retention and improved tumor suppression. | [105] |
| Betulin and betulinic acid | Lipid hydration with extrusion | Dry egg-phosphatidylcholine/dipalmitoyl-phosphatidylcholine | Cytotoxic properties | Betulin and betulinic acid influence mitochondrial bioenergetics and membrane behavior by inhibiting oxidative phosphorylation, affecting H2O2 production, and inducing mitochondrial and liposome aggregation. Laurdan fluorescence studies reveal that these compounds cause phase heterogeneity in lipid systems, linked to their interaction with lipid bilayers. | [106] |
| Canthaxanthin | Hydration with sonication | Soy lecithin | Photoprotectors, antioxidants, anti-inflammatory, immunity enhancer and reproductive health. | Canthaxanthin-loaded vesicles (63–87 nm) were stable, biocompatible, and effective in protecting skin cells. Vesicles preserved fibroblast viability, reduced oxidative and inflammatory responses, and promoted cell migration, showcasing the potential for skin protection and regeneration through their antioxidant, anti-inflammatory, and antiaging effects. | [107] |
| Catechins and curcumin | Microfluidic | Dipalmitoylphosphatidylcholine, cholesterol | Tumor | Dual-loaded liposomes with curcumin and catechin exhibited nanoscale size (<200 nm) with encapsulation efficiencies of 100% for curcumin and 16.77% for catechin. While curcumin, catechin, and liposomes individually enhanced antiproliferation activity in colon cancer cells, the dual-loaded liposomes showed significantly greater inhibitory effects (p < 0.05). The results highlight the potential of dual encapsulation. | [108] |
| Cinnamaldehyde | Ethanol injection | Egg yolk lecithin, tween 80 | Antibacterial | Higher cinnamaldehyde loading reduced liposome membrane fluidity. Antibacterial studies revealed that liposome-encapsulated cinnamaldehyde retained its ability to inhibit Staphylococcus aureus by disrupting cell membrane integrity, demonstrating greater persistence than pure cinnamaldehyde. | [109] |
| Curcumin | Film hydration with hand extrusion | 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000], cholesterol | Anticancer | Liposomes loaded with curcumin demonstrated low particle size and excellent physical stability. Dilution showed rapid partitioning to the lipid bilayer. The in vivo efficacy of curcumin is unlikely to improve with liposomes, as cytotoxicity and uptake studies revealed reduced effectiveness in the 3D cell model. | [85] |
| Echinacoside | Thin film hydration | Soybean phosphatidylcholine, cholesterol, peptide angiopep-2, DSPE-PEG2K-MAL, DSPE-PEG 2K | Parkinson’s disease | Peptide angiopep-2 modified PEGylated echinacoside-loaded liposomes showed significantly higher brain uptake than others. It also showed superior efficacy in reducing MPTP-induced behavioral impairments, oxidative stress, dopamine depletion, and dopaminergic neuron death. This formulation significantly enhanced the neuroprotective effects of drug in the Parkinson’s disease model. | [110] |
| Epigallocatechin gallate and quercetin | Thin film hydration | Lecithin, cholesterol, tween 80 | Antioxidant | Liposomes showed nanosized with encapsulation efficiencies of 64.05% for epigallocatechin gallate and 61.73% for quercetin. After 30 days, size increased slightly by 4.05%, with no significant changes in stability. The DPPH assay confirmed a synergistic antioxidant effect of the encapsulated compounds. | [111] |
| Fisetin | Film hydration | Dioleyl-phosphatidylcholine, cholesterol, 2-dioctadecylcarbamoyl-methoxyacetylamino) acetic Acid-(Ω-methoxy)-polyethylene glycol 2000 ester | Glioblastoma | The formulation exhibited a particle size of 173 ± 8 nm, with drug loadings of 1.7 ± 0.3% for fisetin and 0.8 ± 0.1% for cisplatin, and demonstrated stability over time. Encapsulation preserved the antiangiogenic activity of fisetin, and the formulation displayed an additive therapeutic effect of fisetin and cisplatin on GBM cells. | [112] |
| Genistein and plumbagin | Lipid film | L-α-phosphatidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] ammonium salt | Prostate tumor | Nanoliposomes (~100 nm) encapsulating plumbagin and genistein demonstrated ~80% inhibition of prostate tumor growth in xenograft models without significant toxicity. The formulation inhibited the PI3K/AKT3 signaling pathway and reduced Glut-1 transporter levels, slowing tumor growth. The observed antitumor effects were linked to reduced cell proliferation and decreased blood vessel formation. | [113] |
| Honokiol | Thin film hydration with sonication | Soybean phospholipids, cholesterol, DSPE-mpeg2000 | Osteosarcoma | Hyaluronic acid phospholipid conjugates were used to combine honokiol-loaded liposomes which have low particle size and high encapsulation efficiency. The developed formulation inhibited cell proliferation, induced apoptosis, blocked the cell cycle and disrupted mitochondrial activity. | [114] |
| Lycopene | Thin film hydration | Soybean phosphatidylcholine, cholesterol | Antitumor | Lycopene-loaded liposomes possess low particle size. In vitro, the combination of lycopene and doxorubicin significantly increased cytotoxicity, while in vivo it reduced tumor size in B16 melanoma bearing mice and alleviated doxorubicin induced cardiotoxicity. | [115] |
| Paeonol | Film dispersion | Lecithin, cholesterol, polyethylene glycol-p-nitrophenyl ester, dioleoyl-phosphatidyl-ethanolamine | Hypertrophic scars | The particle size of prepared formulation was 235.7 nm. They significantly lowered scar proliferation, improved collagen structure, and reduced VEGF, TGF-β1, and TNF-α levels, demonstrating strong therapeutic potential for scar treatment. | [116] |
| Piperine | Thin layer dispersion | Cholesterol, phosphatidylcholine, sodium cholate, chitosan | Breast cancer | The drug included liposomes and chitosan-coated drug-loaded liposomes had nanometric sizes (165.7 to 243.4 nm). A biphasic release, improved permeation, and better in vitro antioxidant activity were noticed with liposomes when compared to piperine. The anticancer study with selected liposomes showed significant reduction of IC50 (p < 0.001) compared to pure drug. | [117] |
| Podophyllotoxin | Thin layer dispersion | Lecithin, cholesterol | Prostate cancer | In vitro drug release showed a gradual, time-dependent release pattern, with a cumulative release of 70.3% after 24 h. Cell viability tests on PC3 cells indicated that PPT-Lips had more effective anticancer activity than free PPT. | [118] |
| Quercetin | Thin film hydration | Soybean lecithin, cholesterol, polyethylene glycol 4000 | Diabetic nephropathy | The antidiabetic effects of liposomes were demonstrated in rats with diabetic nephropathy. Liposomes and free drug improved biochemical and pathological markers of nephropathy. Liposomes showed higher drug plasma than pure drug. | [119] |
| Resveratrol and curcumin | Thin film evaporation with ultrasonication | Egg yolk phosphatidylcholine, tween 80 | Antioxidant | The liposome formulation co-loading curcumin and resveratrol in a 5:1 ratio exhibited small particle size. It demonstrated superior antioxidant properties and better stability compared to liposomes loaded with individual polyphenols. | [120] |
| Salvianolic acid B | Soybean phospholipid, hydrogenated soybean phosphatidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phospho-(1-rac-glycerol) | Idiopathic pulmonary fibrosis | Liposomes made of saturated neutral and anionic phospholipids have higher stability and permeability compared to unsaturated or cationic phospholipids. Better efficacy was observed in bleomycin induced idiopathic pulmonary fibrosis mice model when inhaled salvianolic acid B-loaded liposomes, attributed to inflammation inhibition and regulation of the coagulation fibrinolytic system. | [121] | |
| Thymoquinone | Thin layer evaporation with ultrasonication | Egg phosphatidylcholine, phospholipon 90 G, plurol oleique, sodium hyaluronate | Dry eye disease | Drug-loaded liposomes reduced proinflammatory markers (Interleukin-1β, Interleukin-6, and tumor necrosis factor) and mitochondrial reactive oxygen species. These findings demonstrated the developed liposomes can enhance the efficacy of thymoquinone in treating dry eye disease. | [122] |
| Ursolic acid and ginsenoside Rg3 | Reverse evaporation and pH gradient | Lecithin, cholesterol and (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[poly(ethylene glycol)]-2000) with glycyrrhetinic acid | Hepatocellular carcinoma | Liposomes containing ursolic acid and ginsenoside Rg3 showed good EE, drug loading, and sustained drug release. Cell experiments confirmed that codelivery of these actives significantly reduced cell viability, increased apoptosis, and elevated the proportion of cells. | [123] |
| Verbascoside | Film hydration | Soy phosphatidylcholine, cholesterol | Antineuropathic activity | Prepared liposomes have low particle size and sustained drug release (~82.28% in 24 h). In chronic constriction injury rat models, the liposomal formulation (100 mg/kg, i.p.) provided an extended antihyperalgesic effect compared to drug solution, with effects appearing within 15 min and lasting up to 60 min. | [124] |
| Vincristine | Ethanol injection with extrusion | Sphingomyelin, cholesterol, 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] | Hematological malignancies and solid tumors | Liposomes with drug lipid ratio (1:5) showed reduced degradation (2.9% in 12 months at 2–8 °C) and extended in vivo half-life (22.7 h). This formulation demonstrated lower toxicity and better antitumor efficacy in a human melanoma model. | [125] |
| Wogonin | Reverse evaporation | Soybean phospholipids, cholesterol, 3-succinyl 30-stearyl glycyrrhetinic acid | Liver cancer | Three types of formulations were tested for antitumor efficacy: drug solution, liposomes, and glycyrrhetinic acid-modified liposomes. The modified liposomes exhibit greater entrapment, cellular uptake, liver accumulation, prolonged retention, and anticancer effects compared to others. | [126] |
| Phytochemical | Preparation Technique | Composition | Disease/ Therapeutic Application | Highlights | Ref. |
|---|---|---|---|---|---|
| Amygdalin | Thin film hydration | Dihexadecyl phosphate, tween 60, cholesterol | Alzheimer’s disease | Nanocarrier enhanced working memory and recognition, reduced oxidative stress, and restored brain monoamine and neurotransmitter levels. Gene expression analysis revealed significant downregulation of BAX, with upregulation of BCL2, acetylcholinesterase, and monoamine oxidase. | [144] |
| Artemisinin and metformin | Thin film hydration | Cholesterol, span 60 | Lung cancer | PEGylated magnetic niosomes developed facilitated rapid tumor-targeted drug delivery. In vitro studies on A549 lung cancer cells showed significant toxicity under a magnetic field, increased pro-apoptotic Bax expression, decreased antiapoptotic Bcl2 expression, and effective cellular uptake via endocytosis. | [143] |
| β-carotene | Thin film hydration | Spans 40, 60, 80, tween 20, 40, 60, cholesterol | Cancer, cardiovascular diseases, arteriosclerosis, and macular degeneration | The developed formulation has good stability against sunlight, temperatures, oxidative stress, and was stable in culture medium. It was effectively taken up by immortalized and transformed cells at concentrations of 0.1–2 μM, suggesting the formulation as an efficient method for β carotene delivery. | [145] |
| Canthaxanthin | Thin film hydration with sonication | Span 60, cholesterol | Antioxidant and free radical scavenging | Niosomes prepared with span 60 and cholesterol at a 1:1 molar ratio showed smaller, uniform sizes with higher EE. Stability tests indicated higher degradation under light and higher temperatures. The addition of cholesterol significantly improved drug stability. | [146] |
| Catechin | Thin film hydration | Tween 60, lauryl alcohol, cetyl alcohol, cholesterol | Antioxidant | Developed carrier has nanosize, narrow size distribution, good EE (85.82%), and sustained catechin release. Milk fortified with niosomes maintained its sensory and physicochemical properties while exhibiting enhanced antioxidant activity. | [147] |
| Curcumin | Thin film hydration with ultrasonication | Span 60, cholesterol | Anticancer | Developed niosomes showed high entrapment (~99.8%), loading (68.33%), and sustained release (~25.49 in 336 h). The formulation was biocompatible and exhibited dose-dependent toxicity against cancer cells (IC50 at 200 µg/mL, containing 66.75 µg drug). | [148] |
| Diosgenin | Thin film hydration | Span 40, tween 40, cholesterol | Anticancer | Diosgenin niosomes exhibited nanosize, higher loading efficiency (~89%), controlled and sustained drug release. The viability of HepG2 cell line treated with free diosgenin was 61.25%, which decreased to 28.32% upon niosomes, significantly enhancing its anticancer efficacy. | [149] |
| Epigallocatechin-3-gallate | Thin film hydration | Span-60, tween-60, cholesterol, DSPE-PEG (2000)-carboxylic acid | Lung cancer | Prepared niosomes showed ideal pharmaceutical characteristics. In vitro studies of cetuximab conjugated to niosomes on A549 lung carcinoma and BEAS-2B normal bronchial cells assessed cytotoxicity, apoptosis, fluorescence imaging, and theranostic potential. | [150] |
| Fumaria officinalis active rich fraction | Ether injection | Span 60, brij 52, cholesterol | Antidiabetic, anti-neuropathic, anti-inflammatory | Optimized formulation (Nio-2) with stylopine (48.3%) and sanguinarine (51.6%) demonstrated high entrapment and stability. Fumaria officinalis, active rich fraction, and Nio-2 showed significant antidiabetic and anti-inflammatory activity, hence suggested as an alternative therapy in inflammations and neuropathic pain. | [140] |
| Lycopene | Microfluidic and thin film hydration with probe sonication | Span 60, cholesterol | UVB protection and anti-hyperpigmentation | Niosomes displayed good uniformity, nano size, and low PDI when processed through a hydrodynamic flow-focusing platform. The drug release showed Korsmeyer–Peppas kinetics, and the formulation showed enhanced stability, strong UVB protection, and anti-melanogenesis effects. | [151] |
| Paeonol | Modified ethanol injection | Span 60, cholesterol, polyethylene glycol monostearate | Anticancer | Optimized PEGylated niosomes exhibited nano size, good entrapment, and sustained release. Pharmacokinetic studies in rats showed a higher elimination half-life (87.5 vs. 17.0 min) and a higher AUC (38.0 vs. 19.48 μg/mL·min) compared to the free drug. Enhanced cytotoxicity was observed against HepG2 cells, with IC50 values of 22.47 μg/mL. | [152] |
| Piperine and curcumin | Thin film hydration | Span 60, cholesterol | Antioxidant, Protection against Paraquat-induced pulmonary toxicity | Optimized niosomes demonstrated high encapsulation efficiency (>85%), nanosize (264–286 nm), a narrow PDI (0.19–0.23), and stability for 90 days. Co-treatment with curcumin and piperine-loaded nanoparticles effectively restore mitochondrial function, hence reduce acute pulmonary toxicity. | [153] |
| Plumbagin | Solvent evaporation | Span 20, cholesterol | Antidiabetic | The optimized niosomes demonstrated antioxidant activity and enzyme inhibition of α-amylase (90.69%) and α-glucosidase (88.43%). The data in this study show significant potential for diabetes management by inhibiting oxygen radicals and key enzymes involved in glucose metabolism by the developed carrier. | [154] |
| Quercetin | Hydration with high pressure homogenization | Span 60, creemophor RH40 | Skin whitening and antioxidant | The best formulation was prepared with span 60 Cremophor RH40 ratio of 9:11. Niosomes enhanced quercetin’s solubility and photostability while offering sustained release and superior transdermal penetration. Skin retention of quercetin niosomes was 2.95 times higher than that of quercetin solution. | [155] |
| Thymoquinone | Solvent evaporation and hydration with sonication | Span 20, 40, 60, tween 20, 40, 80, cholesterol, soya lecithin | Anticancer, antioxidant, anti-inflammatory, and antimicrobial | Optimized niosomes (TMQNIOS) demonstrated low vesicle size (~157.32 to 211.44 nm) and encapsulation (~60–80%). Drug release followed Higuchi’s release kinetics and greater permeation across intestinal mucosa from developed carriers. | [156] |
| Thymoquinone and carum extract | Thin film hydration | Span 60, tween 60, ergosterol | Breast cancer | Two formulations, namely Nio/TQ (thymoquinone) and Nio/Carum (carum extract), were developed. The MTT assay showed these carriers have greater anticancer activity than their pure form against MCF-7 cancer cell line. Cell cycle analysis revealed G2/M arrest in the TQ, Nio/TQ, and Nio/Carum formulations. | [157] |
| Vincristine | Thin film hydration | Span 60, tween 60, cholesterol-PEG 600: diacetyl phosphate | Lung cancer | PEGylated drug-containing niosomes demonstrated significantly higher cytotoxicity against TC-1 lung cancer cells and stronger tumor inhibitory effects in lung tumor bearing C57BL/6 mice compared to free drug, suggesting its potential as a promising delivery system for vinblastine in cancer treatment. | [158] |
| Phytochemical | Preparation Technique | Composition | Disease/ Therapeutic Potential | Highlights | Ref. |
|---|---|---|---|---|---|
| Enteromorpha intestinalis-sulfated polysaccharide-protein complexes | Thin film hydration | Sodium cholate, span 65 | Hepatocellular carcinoma | Optimized formulation consisted of spherical vesicles (181.1 ± 16.80 nm) with moderate drug entrapment efficiency (71.60 ± 0.25%) and exhibited controlled release behavior. In disease-induced rats, treatment with bilosomes significantly reduced serum levels of α-fetoprotein, endoglin, lipocalin-2, and heat shock protein 70 compared to untreated rats. Liver histology revealed focal degeneration of pleomorphic hepatocytes with mild fibrosis extending from the portal area. | [163] |
| Tripterine | Thin film hydration | Soybean phosphatidylcholine,1, 2-stearoyl-3-trimethylammonium-propane, Sodium deoxycholate, hyaluronic acid | Arthritis | Hyaluronic acid-coated tri-loaded bilosomes had a particle size of 118.5 nm and high entrapment efficiency (99.56%). Demonstrated excellent cellular uptake and significantly enhanced intra-articular bioavailability—799.9% > Tri solution. In arthritic animal models, coated tri-loaded bilosomes showed markedly improved pharmacokinetics and superior antiarthritic efficacy compared to uncoated Tri-BLs, leading to notable inflammation reduction. | [164] |
| Berberine | Thin film hydration | Soybean lecithin, sodium deoxycholate, cholesterol, chitosan, carbopol 974 NF | Rheumatoid arthritis | Optimized chitosan-coated, berberine-loaded bilosomes had a mean particle size of 202.3 nm, entrapment efficiency of 83.8%, and a surface charge of +30.8 mV. Demonstrated a sustained in vitro release profile, enhanced ex vivo skin permeability and confirmed non-irritant nature via histological studies. Topical application of the berberine-loaded bilosomal gel significantly reduced inflammation in a rat model of carrageenan-induced paw edema. | [169] |
| Apigenin | Solvent evaporation method | Cholesterol, sodium deoxy cholate, tween 80, phosphatidylcholine, chitosan | Antimicrobial, anticarcinogenic | Optimized chitosan-coated bilosome formulation showed an increased vesicle size (298 ± 3.56 nm), positive surface charge (+17 mV), high encapsulation efficiency (88.1 ± 1.48%), and enhanced drug release (69.37 ± 1.34%). TEM analysis revealed smooth, non-aggregated vesicles. Antimicrobial and cytotoxicity studies confirmed its superior efficacy, displaying larger inhibition zones and greater activity against two cancer cell lines. | [170] |
| Biochanin A | Thin film hydration | Sodium deoxy cholate, span 60, polyethylene glycol-2000SA | Anti-inflammatory | Selected PEGylated bilosome formulation displayed a vesicle size of 216 ± 6.62 nm, entrapment efficiency of 80.54 ± 1.02%, polydispersity index of 0.231, and zeta potential of –15.4 mV. X-ray diffraction confirmed successful drug encapsulation within the bilosomal matrix. It showed a sustained release profile (88.23 ± 3.54% over 24 h) and high ex vivo intestinal permeation (56.97 ± 2.76% in 6 h). Compared to pure Biochanin A dispersion, the optimized Pegylated formulation demonstrated 4.7-fold greater bioavailability and enhanced anti-inflammatory activity. | [173] |
| Curcumin | Thin film hydration method | L-α-phosphatidylcholine, sodium cholate, pluronic® P123, and cholesterol | Melanoma | PEGylated bilosomes demonstrated protective effects and enhanced cellular uptake in A375, Me45, and HaCaT cell lines. They showed selective targeting of tumor cells, with significantly greater cytotoxicity against Me45 melanoma cells, reducing their viability to below 20% after 24 h of phyto-photodynamic treatment. | [175] |
| Costunolide | Thin film hydration | Span 85, cholesterol, bile salt | Colon cancer | Formulation exhibited superior cytotoxicity against LS174T colon cancer cells and demonstrated safety and selectivity in normal colonic epithelial cells, as compared to raw drug. Bilosomes were found to be more effective in up-regulating proapoptotic genes, down-regulating antiapoptotic BCL2 mRNA, enhancing cytochrome C release, increasing reactive oxygen species generation, and disrupting mitochondrial membrane integrity. | [176] |
| Curcumin | Thin film hydration | Span 60, sodium deoxycholate, cholesterol | Hepatoprotective and renal protective effect | The formulation showed ideal pharmaceutical characteristics, drug release, good stability, and reduced cytotoxicity compared to free drug. The formulation demonstrated effective hepatic and renal protection in liver cirrhosis-induced rats, preserving organ function and histological integrity. | [177] |
| Epigallocatechin gallate | Ethanol injection | Tween 40, cholesterol | Antioxidant, anti-cancer, regulation of fatty acid metabolism, improvement of intestinal immunity | Bilosomes demonstrated superior gastrointestinal stability, improved drug bioavailability by 1.98 times. These results highlight the potential of bilosomes as an effective delivery system to enhance the stability and bioavailability of epigallocatechin gallate. | [178] |
| Luteolin | Thin film hydration | Cholesterol, span 60, bile salt, PEG 2000 | Antioxidant, antimicrobial, and breast cancer | The selected PEGylated bilosomes have nanosize, higher encapsulation (~75.05), showed biphasic release, and greater permeation. Additionally, the nanocarriers showed higher antioxidant activity, greater cell viability on cancer cell lines, and superior antibacterial activity against Staphylococcus aureus and Escherichia coli compared to pure drug. | [179] |
| Lycopene | Lipid hydration | Span 60, cholesterol, sodium cholate | Antibacterial effect | The formulation showed strong antibacterial activity against multidrug-resistant K. pneumoniae. In vivo studies in a mouse lung infection model demonstrated significant therapeutic effects, including reduced lung inflammation and congestion, restored normal lung architecture, and decreased pulmonary fibrosis. | [180] |
| Psoralidin | Thin film hydration | Phosphatidylcholine, cholesterol, span 60, sodium deoxycholate | Breast and lung cancer | Developed formulation has shown better mucoadhesivity. In vitro studies revealed significantly improved apoptotic and necrotic effects on breast (MCF-7) and lung (A549) cancer cell lines, highlighting the potential of bilosomes as an effective oral treatment. | [181] |
| Sea-buckthorn pulp oil | Thin film hydration with ultrasonication | L-α-phosphatidylcholine, cholesterol, pluronic P123 | Antioxidant, anti-inflammatory, wound healing, gastroprotective | Attenuated total reflection Fourier-transform infrared spectroscopy provided insights into bilosomes oil interactions. This study presents a reliable method to develop highly stable bilosome delivery systems with nanosize suitable for application in food and cosmetics. | [182] |
| Spirulina | Thin film hydration | Phosphatidylcholine, cholesterol, sodium deoxycholate | Photoaging effect | In vivo studies evaluated the photoprotective and antiaging effects of formulation through biochemical analysis of antioxidant, anti-inflammatory, and anti-wrinkling markers. Biochemical and histopathological findings confirmed that formulation offered superior antiaging benefits compared to pure drug. | [183] |
| Phytochemical | Preparation Technique | Composition | Highlights | Disease/ Therapeutic Application | Ref. |
|---|---|---|---|---|---|
| Transferosomes | |||||
| Asiatic acid | High pressure homogenization | Sodium deoxycholate, tween 80 | Small particle size (27.15–63.54 nm), high encapsulation (90.84%), and 71.65% anti-inflammatory activity were observed with the formulation. In clinical trials, the transfersomal gel showed no adverse effects, along with significant improvements in melanin index and skin elasticity at 2, 4, and 8 weeks, demonstrating its potential as an effective treatment for hypertrophic scars. | Hypertrophic scars | [191] |
| Centella asiatica | Thin film hydration | Soyaphosphatidyl choline, tween 80, propylene glycol | The Centella asiatica transfersomes and Bergamot essential oil nanoemulsions combination showed effectiveness in protecting against UVB-induced skin damage in BALB/c mice. It reduced oxidative stress by enhancing SOD activity, lowering MDA levels, and suppressing proinflammatory cytokines. It also promoted collagen production, highlighting its potential as a therapeutic agent for UVB-induced skin protection. | Antiphotoaging effects | [192] |
| Curcuma comosa extract | Thin film hydration | Phospholipon 90G, transcutol P, cremophor RH 40 | Optimal transfersomes, containing diarylheptanoids dispersed in Carbopol gel followed zero order release kinetics and exhibited greater permeation. In vivo pharmacokinetics in rats showed a peak concentration of ~220 ng/mL and sustained plasma levels for over 12 h. | Estrogenic activity | [193] |
| Jabuticaba peel | Dispersion technique with ultrasonication | Lipoid S75, tween 20, hydroxyethyl cellulose, sodium hyaluronate | The extract was encapsulated in transfersomes, with or without polymer modifications (hydroxyethyl cellulose and sodium hyaluronate). Polymer-enriched transfersomes had larger sizes but were more stable and showed superior bioactivity compared to the extract solution, effectively reducing hydrogen peroxide toxicity and accelerating wound healing in cell models. | Antioxidant, wound healing | [190] |
| Mulberry leaf extract | Thin film hydration | Phospholipon 90G, tween 80 | Optimized transferosome gel showed higher antioxidant activity, drug content, EE, ex vivo drug release, spreadability, homogeneity, and stability. | Antioxidant | [194] |
| Oleuropein and lentisk oil | Lipid hydration | Soy phospholipid, tween 80 | Developed transferosomes formulation effectively reduced the overexpression of inflammatory markers, particularly MMP-1 and IL-6, mitigated oxidative stress induced by hydrogen peroxide, and accelerated wound healing in fibroblast monolayers in vitro. | Skin regeneration | [195] |
| Radish sprouts extract | Thin film hydration with sonication | Phospholipon 90G, polysorbate 20, 40, 60, and 80 | The extract-loaded transferosomes reduce tyrosinase activity and melanin content while being safe for skin cells. Combined with sunscreen emulgels, the formulation enhanced cytotoxic safety, demonstrated stability, provided effective UVA and UVB protection and prolonged efficacy. | UV protection and antiaging effect | [196] |
| Solanum xanthocarpum methanolic extract | Thin film hydration | Phospholipon 90G, cholesterol, sodium cholate | The developed formulation showed nanosize, high entrapment, loading and antioxidant activity. Ex vivo studies confirmed significantly better skin permeation (82.86%) and retention compared to the conventional gel (35.28%), highlighting its potential for effective topical delivery. | Psoriasis | [197] |
| Rhodomyrtus tomentosa leaf extract | Thin film hydration | L-α-phosphatidylcholine, cholesterol, tween 80, tween 20, span 80, span 20, sodium deoxycholate | Spherical nanovesicles (405.3 ± 2.0 nm), high encapsulation efficiency (81.90 ± 0.31%), low polydispersity index (0.16 ± 0.08), and stable zeta potential (−61.62 ± 0.86 mV). The MIC and MBC values ranged from 8 to 256 and 64 to 1024 μg/mL, respectively. Exhibited strong antioxidant activity against DPPH and ABTS radicals and moderate tyrosinase inhibition. A significant reduction in nitric oxide production (6.78–88.25%) was observed. | Soft tissue infections | [188] |
| Syringic acid | Ethanol injection | Phosphatidylcholine, tween 80, d-α-tocopheryl polyethylene glycol 1000 succinate, diphenyl-1-picrylhydrazyl | Optimized transferosomes demonstrated significant antioxidant activity (IC50 = 11.1 µg/mL), and excellent skin deposition (78.72%). Additionally, it reduced acne lesions by 79.5%, outperforming Adapalene® gel (18.7% reduction) without causing irritation or erythema, highlighting its potential as an effective and skin friendly acne treatment. | Acne | [198] |
| Ethosomes | |||||
| Achillea millefolium L. extract | Cold | Lipoid, propylene glycol, ethanol | Optimized ethosomal carrier demonstrated 88% free radical scavenging activity, along with high phenolic and flavonoid contents. The topical gel was stable and showed release of 79.8%. | Antioxidant | [199] |
| Arctostaphylos uva-ursi extract | Lipid dispersion | Phospholipid, ethanol, propylene glycol | Optimized formulations possess all pharmaceutical characteristics. The gel formulation demonstrated significant effects in reducing skin erythema, melanin, and sebum levels while enhancing skin hydration and elasticity. | Skin rejuvenation and depigmentation | [200] |
| Berberine chlride and evodiamine | Modified single step injection technique | Soybean lecithin, cholesterol, ethanol, propylene glycol | The developed formulation showed highest drug deposition in the epidermis cell viability tests confirmed that the optimized ethosomes enhanced the inhibitory effect on B16 melanoma cells. | Melanoma | [201] |
| Brassicaceae extract | Cold | Phospholipon 90 G, cholesterol, ethanol | Optimized ethosomal gel showed characteristics suitable for skin application. The release of bioactives follows the Korsmeyer–Peppas model and shows higher flux values. | Photoprotectiion | [202] |
| Caffeic acid | Lipid dispersion | Soybean lecithin, pluronic F127 | Permeability of caffeic acid increased drastically when formulated as ethosomes. Skin-covered oxygen electrode experiments confirmed improved delivery and antioxidant activity of caffeic acid to porcine skin compared to a simple solution. | Antioxidant | [203] |
| Phyllanthus nruri, croton tiglium, zingiber officinale extract | Solvent dispersion with probe sonication | Soya lecithin, ethanol | Studies in cell line (HaCaT) revealed crude extracts-loaded ethosomal formulation inhibits testosterone and improves the cell viability similar to minoxidil. Preclinical safety was confirmed through in vitro cytotoxicity and histopathological studies. | Antiandrogenic and cytoprotectant | [204] |
| Sambucus nigra L. extract | Lipid hydration | Soybean phosphatidylcholine, ethanol | Prepared ethosomes showed slow release profile over 24 h and exhibited collagenase inhibition activity and excellent skin compatibility in human applications. These results indicate the potential of extract-loaded ethosomes as a promising cosmeceutical ingredient for skin care. | Skin care | [205] |
| Vernonia anthelmintica (L.) Willd. | Injection with ultrasonication | Soybean lecithin, cholesterol, ethanol | Ethosome formulation exhibited good physicochemical properties. In vitro studies showed significant therapeutic efficacy against vitiligo caused by hydroquinone exposure. Histological analysis of mouse skin showed increased melanin, elevated enzyme activities, and reduced antioxidant levels. | Vitiligo | [206] |
| Transethosomes | |||||
| Apigenin | Thin film hydration | Soya phospholipid, span 80 | Optimized formulation demonstrated good EE, low vesicle size, and greater drug release. Ex vivo permeation showed superior apigenin delivery while cytotoxicity studies confirmed that formulation significantly reduced cell viability more effectively than the conventional gel. | Skin cancer | [207] |
| Chenopodium murale extract | Injection | Soybean phosphatidylcholine, cholesterol, tween 80 | Drug-loaded transethosomes exhibited good physicochemical properties. The formulation maintained the extract’s macrostructural integrity, enabling enhanced skin permeation and achieving higher drug release. | Anti-inflammatory, antimicrobial, antioxidant and wound healing | [208] |
| Colchicine | Cold | Phospholipon 90G, tween 20, sodium taurocholate, or labrafil | Formulation was optimized by 22 full factorial design and has exhibited desirable properties for transdermal drug delivery. Ex vivo studies confirmed higher flux compared to normal gel and demonstrated satisfactory stability. | Gout, and familial Mediterranean fever | [209] |
| Fisetin | Lipid film hydration with ultrasonication | Lipoid S100, sodium cholate | Transethosomes vesicles showed suitable physicochemical properties for transdermal delivery. In vivo data indicated that the formulation fluidized the rigid membranes of rat skin and deeper skin penetration. | Skin cancer | [210] |
| Ginger extract | Cold injection | Phospholipon 90G, cholesterol | Transethosomal formulation demonstrated enhanced flux and skin deposition compared to free drug hydrogel. In vivo studies confirmed significant edema reduction by the transethosomal hydrogel compared to free drug and ketoprofen gels. Treated animals exhibited marked decrease in reactive oxygen species and prostaglandin E2 levels. | Anti-inflammatory effect | [211] |
| Glycyrrhizic acid | Lipid film hydration | Phospholipid 90G, sodium cholate | Transethosome was optimized using the Box–Behnken design, which exhibited ideal characteristics for skin application. Ex vivo data demonstrated enhanced permeation and distribution of the drug, which highlighted superior skin deposition and retention of the formulation compared to conventional gels. | Skin cancer | [212] |
| Hexatriacontane | Lipid film hydration | Lipoid S 100, sodium cholate | Developed formulation showed optimized characteristics and Higuchi release kinetics. Dermatokinetic studies confirmed higher drug deposits in epidermal layers. Formulation exhibited effective antibacterial activity against S. aureus and E. coli. | Skin infections | [213] |
| Sinapic acid | Thin film hydration | Phospholipon 90 G, sodium deoxycholate | Optimized transethosome formulation demonstrated characteristics ideal for skin delivery. Higher antioxidant activity and greater penetration across the Strat-M® membrane were observed. | Anti-inflammatory, antioxidant, anticancer, and antibacterial activities | [214] |
| Phytochemical | Preparation Technique | Composition | Disease/Therapeutic Application | Highlights | Ref. |
|---|---|---|---|---|---|
| β-carotene | High pressure homogenization | Glyceryl monooleate, poloxamer 407 | Antioxidant | Design of experiment-based optimized formulation has 1800 mg glyceryl monooleate and 200 mg poloxamer 407. The optimized formulation demonstrated higher drug release. Incorporating β carotene into cubosomal formulations enhanced ~2-fold in its antioxidant potential. | [239] |
| Brucea javanica oil, doxorubicin hydrochloride | High pressure homogenization | Glycerol monooleate, pluronic F127 | Antitumor | pH-responsive cubosomes exhibited phase transitions under different pH conditions and enhanced interaction in acidic tumor environments. Demonstrated antitumor activity against MCF-7 and doxorubicin-resistant MCF-7 cells, with the potential to overcome doxorubicin resistance. | [240] |
| Curcumin, fish oil | Thin film hydration | Monoolein, PEG 1000 | Neuroprotection | Optimized cubosome showed negligible cytotoxicity at concentrations of 300 and 500 nM. In neuronally derived SH-SY5Y cells, this formulation effectively mitigated hydrogen peroxide-induced oxidative stress by reducing intracellular ROS levels and apoptosis. Higher neuroprotective potential noticed here suggests combination treatments in neurodegenerative disorders. | [241] |
| Erucin | Solvent evaporation | Monoolein, pluronic 84 | Antioxidant and antiproliferative activities | The antioxidant potential of the developed vesicle was evaluated using DNA nicking, DPPH, and phosphomolybdate assays, and demonstrated significantly improved results compared to erucin alone. Additionally, it exhibited enhanced anticancer activity with a lower IC50 value in MTT assays. | [242] |
| Phycocyanin | Emulsification with homogenization | Glyceryl monooleate, poloxamer 407 | Antioxidant | Optimized formulation is ideal for skin therapy, better stability and drug release followed the Higuchi model. Drug formulation showed superior skin permeability compared to the phycocyanin solution and was readily taken up by keratinocytes, providing prolonged antioxidative effects. | [243] |
| Ruta graveolens extract | Hot emulsification | Glyceryl monooleate, pluronic F108 | Anti-asthmatic | Cubosomal formulation reduced asthma scores and improved lung function by restoring the FEV1/FVC ratio to normal levels. The nanocarrier demonstrated strong antioxidant and anti-inflammatory effects by reducing malondialdehyde, IL-4, IL-7, TGF-β, and Ig-E levels while increasing superoxide dismutase and INF-γ levels in bronchoalveolar lavage fluid. | [238] |
| Withanolide A | Hot emulsification | Glycerol monooleate, poloxamer 403, pluronic F127 | Anti-diabetic, anti-neuropathic, anti-inflammatory, and anti-bacterial activities | Developed formulation showed superior antidiabetic, antineuropathic, anti-inflammatory, and antibacterial activities than the drug alone. Effects like HbA1c normalization, insulin secretagogue potential, oxidative stress reduction, and modulation of pro-inflammatory cytokines indicate the potential of this carrier in oral therapy. | [244] |
| Yucca filamentosa | Hot emulsification | Glyceryl monooleate, pluronic F108 | Gastroprotective effect | The formulation showed superior gastroprotective, antioxidant, and anti-inflammatory effects in rat models of ethanol induced gastric injury and was comparable to famotidine. Developed formulations demonstrated potential in preventing peptic ulcer recurrence through modulation of the HMGB-1/RAGE/TLR4/NF-κB pathway. | [245] |
| Vesicular Nanocarrier | Preparation Techniques | Commonly Used Excipients | Key Features and Stability | Ref. |
|---|---|---|---|---|
| Liposomes | Thin film hydration, electroformation, vesicle fusion, solvent injection, detergent dialysis, reverse phase evaporation, solvent spherule, size reduction, microfluidic, supercritical fluid, freeze-drying of double emulsions, membrane contactor method. | Phospholipids (e.g., phosphatidylcholine), cholesterol, cryoprotectants (e.g., trehalose) | Encapsulate hydrophilic and lipophilic drugs. Stability: Sensitive to oxidation, requires antioxidants or lyophilization for improved stability. | [246] |
| Invasomes | Thin film hydration, sonication, extrusion | Phospholipids, cholesterol, terpenes (e.g., cineole, limonene) | Enhanced dermal and transdermal drug delivery via terpene-mediated membrane fluidization. Stability: Moderate; terpene volatility may impact long-term stability. | [248] |
| Niosomes | Thin film hydration, ether injection, micro fluidization, reverse phase evaporation | Non-ionic surfactants (e.g., Span 60, Tween 80), cholesterol | Cost-effective alternative to liposomes. Stability: More stable than liposomes; however, sensitive to temperature and pH changes. | [249] |
| Bilosomes | Thin film hydration, sonication, extrusion | Bile salts (e.g., sodium deoxycholate), phospholipids, cholesterol | Improved oral bioavailability via bile salt stabilization. Stability: Enhanced resistance to gastrointestinal degradation; stable under physiological pH. | [165,250] |
| Transferosomes | Thin film hydration with edge activators, ultrasonic dispersion, ethanol injection | Phospholipids, edge activators (e.g., sodium cholate, span 80), ethanol | Highly deformable vesicles for transdermal delivery. Stability: Sensitive to environmental conditions like ethanol evaporation or oxidation. | [251] |
| Ethosomes | Hot or cold method, sonication, extrusion | Ethanol, phospholipids (e.g., phosphatidylcholine), propylene glycol | Enhanced skin penetration. Stability: Sensitive to ethanol evaporation; proper sealing is needed to maintain integrity. | [252] |
| Transethosomes | Combination of ethosomes and transferosomes techniques, sonication, extrusion | Phospholipids, ethanol, edge activators (e.g., Tween 80) | Combines properties of ethosomes and transferosomes. Stability: Sensitive to ethanol loss and oxidation; requires proper storage. | [221,253] |
| Cubosomes | Top-down approach, bottom-up approach | Lipids (e.g., glyceryl monooleate), polymers (e.g., pluronic F127) | High internal surface area for drug encapsulation. Stability: More stable than vesicular systems; sensitive to dilution and surfactant imbalance. | [229] |
| Test Type | Principle | Equipment Used | Evaluation Parameters | Ref. |
|---|---|---|---|---|
| Particle size analysis | Determines vesicle size distribution | Dynamic light scattering (DLS) | Particle size, polydispersity index | [271] |
| Zeta potential | Measures surface charge of vesicles | Zeta potential analyzer | Surface charge, colloidal stability | [272] |
| In vitro drug release | Measures drug diffusion through a membrane | Franz diffusion cell, dialysis membrane | Drug release rate, cumulative release profile | [273] |
| Permeability | Simulates passive drug permeation | Permeability apparatus, membrane models | Permeability coefficient, flux rate | [274] |
| Stability testing | Assesses long-term stability | Stability chambers, DLS, HPLC | Particle size, drug retention, degradation products | [275] |
| Thermal stability | Evaluates stability under varying temperature conditions | Thermal analyzer, stability chamber | Degradation rate, active ingredient stability | [276] |
| Oxidative stability | Assesses stability in the presence of oxygen or reactive species | Oxidative chamber, spectrophotometer | Oxidation products, active ingredient retention | [277] |
| Photostability test | Assesses stability under ultraviolet (UV) or light exposure | UV chamber, spectrophotometer | Degradation products, remaining active phytochemical | [278] |
| Antioxidant assay | Evaluates antioxidant activity (e.g., DPPH, ABTS, FRAP, ORAC, FIC, CUPRAC) | UV–vis spectrophotometer | Percentage inhibition, antioxidant capacity | [279] |
| Anti-inflammatory assay | Measures inhibition of inflammatory mediators | Enzyme-linked immunosorbent assay (ELISA), cell culture models | Cytokine levels (e.g., TNF-α, IL-6) | [280] |
| Pharmacokinetic study | Measures drug absorption, distribution, metabolism, and excretion | HPLC, mass spectrometry | Cmax, Tmax, AUC, half-life | [281] |
| Biodistribution study | Analyzes drug accumulation in tissues | Fluorescent imaging, gamma counters | Organ-specific drug concentration, clearance | [282] |
| Therapeutic efficacy | Evaluate pharmacological effects (e.g., anticancer, neuroprotection) | Animal models, histopathology | Disease regression, biomarker levels | [283] |
| Cell viability | Assesses cell metabolic activity post-exposure (e.g., MTT) | Microplate reader, cultured cells | Cell viability, IC50 | [284] |
| Oxidative stress markers | Analyzes ROS, lipid peroxidation, or protein oxidation | ELISA, biochemical assays | ROS levels, malondialdehyde, protein oxidation | [285] |
| Cytokine profiling | Measures inflammatory and immunomodulatory effects | ELISA, multiplex assays | Cytokine expression (e.g., IL-10, IFN-γ) | [286] |
| Antiviral activity assay | Evaluates inhibition of viral replication | Cell culture models, PCR | Viral load, cytopathic effect | [287] |
| Antimicrobial assay | Determines antimicrobial efficacy | Microdilution plates, colony counter | Minimum inhibitory concentration, zone of inhibition | [288] |
| Cardioprotective assay | Assesses protection against cardiac injury or dysfunction | Animal models, echocardiography | Cardiac biomarkers, ECG, histology | [289] |
| Neuroprotective assay | Evaluate protective effects against neuronal damage | Neuronal cell lines, animal models | Neuronal viability, ROS levels, neuroinflammation markers | [290] |
| Anticancer assay | Measures inhibition of cancer cell growth | MTT assay, flow cytometry | Cell viability, apoptosis rate, tumor volume | [291] |
| Hepatoprotective assay | Assesses protection against liver damage | Animal models, liver function tests | Liver enzymes (ALT, AST), histopathology | [292] |
| Immunomodulatory assay | Evaluates modulation of immune response | ELISA, multiplex cytokine assays | Cytokine profile, immune cell activation | [293] |
| Antidiabetic assay | Measures blood glucose regulation and insulin sensitivity | Animal models, glucometer | Blood glucose, insulin levels, HbA1c | [294] |
| Application ID | Publication Date | Title | Summary of Invention | Potential Benefits |
|---|---|---|---|---|
| 11868251 | 5 October 2007 | Liposomal curcumin for treatment of neurofibromatosis | The invention presents a method to treat neurofibromatosis types 1 and 2 using curcumin or its analogues encapsulated in colloidal drug delivery systems, such as liposomes, nanoparticles, or micelles. The system targets Merlin and related pathway proteins and is administered parenterally with a pharmaceutically acceptable carrier. | Neurofibromatosis |
| WO/2009/061787 | 14 May 2009 | Coated devices and method of making coated devices that reduce smooth muscle cell proliferation and platelet activity | The invention features drug eluting stents and coated devices that release resveratrol and quercetin to maintain blood flow, reduce smooth muscle cell proliferation, and limit restenosis. | Atherosclerosis, stenosis, and clotting disorders. |
| 201010158483.X | 22 March 2010 | Allicin liposome for resisting microbe, protozoan and tumor and preparation method thereof | The invention introduces an allicin liposome formulation to treat microbes, protozoa, tumors, and viruses. Its enhanced properties expand allicin’s applications beyond bacterial and fungal infections, offering new therapeutic possibilities. | Antimicrobial, protozoal and viral infection |
| 201210041878.0 | 23 February 2012 | Wogonin liposome preparation modified with glycyrrhetinic acid and preparation method thereof | The invention presents a glycyrrhetinic acid-modified wogonin liposome, prepared through the reverse evaporation method, which enhances efficacy against liver tumor cells and improves liver cancer treatment. | Hepatic carcinoma |
| 14777160 | 15 March 2013 | Compositions and methods including celecoxib and plumbagin relating to treatment of cancer | The invention presents a method for treating proliferative diseases, especially skin cancer, using a combination of celecoxib and plumbagin for improved therapeutic outcomes. | Skin cancer |
| WO/2016/005786 | 14 January 2016 | Liposomal formulations comprising thymoquinone and taxane, and methods of treating cancer using same | The invention describes liposomal compositions with taxane and thymoquinone, offering synergistic anticancer effects, improved drug encapsulation, enhanced stability, and consistent taxane release for more effective cancer treatment. | Synergistic Anticancer effects |
| 201711105675.2 | 10 November 2017 | Brain-targeting liposome with paeonol-ozagrel conjugate and preparation method thereof | The invention describes a brain-targeting liposome containing a paeonol-ozagrel conjugate, designed to enhance drug delivery across the BBB. It prolongs drug circulation time and enables targeted treatment of various thrombotic cardiovascular and cerebrovascular diseases. | cardiac and cerebral ischemia or infarction |
| 202311320134.7 | 12 October 2023 | Echinacoside liposome, LRP1 targeted echinacoside-loaded liposome, and preparation method and application of echinacoside-loaded liposome and LRP1 targeted echinacoside-loaded liposome | The invention features an LRP1-targeted echinacoside liposome that enhances brain delivery, improves dopamine levels, reduces oxidative stress, and restores motor function in neurodegenerative disease models. | Neurodegenerative diseases |
| 202311327683.7 | 13 October 2023 | Aptamer-macrophage membrane composite nano delivery system for targeting breast cancer and method for analyzing anti-breast cancer action mechanism | The invention describes a nano-delivery system utilizing betulinic acid encapsulated in macrophage membranes modified with nucleic acid aptamers for targeted breast cancer treatment. Betulinic acid effectively inhibits cancer cell proliferation and induces apoptosis by blocking the PI3K/AKT and MAPK signaling pathways. | Breast cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Rao, R.; Nair, A.B. Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications. Pharmaceutics 2025, 17, 464. https://doi.org/10.3390/pharmaceutics17040464
Jacob S, Kather FS, Boddu SHS, Rao R, Nair AB. Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications. Pharmaceutics. 2025; 17(4):464. https://doi.org/10.3390/pharmaceutics17040464
Chicago/Turabian StyleJacob, Shery, Fathima Sheik Kather, Sai H. S. Boddu, Rekha Rao, and Anroop B. Nair. 2025. "Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications" Pharmaceutics 17, no. 4: 464. https://doi.org/10.3390/pharmaceutics17040464
APA StyleJacob, S., Kather, F. S., Boddu, S. H. S., Rao, R., & Nair, A. B. (2025). Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications. Pharmaceutics, 17(4), 464. https://doi.org/10.3390/pharmaceutics17040464






