Mechanistic Insights into Sphingomyelin Nanoemulsions as Drug Delivery Systems for Non-Small Cell Lung Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
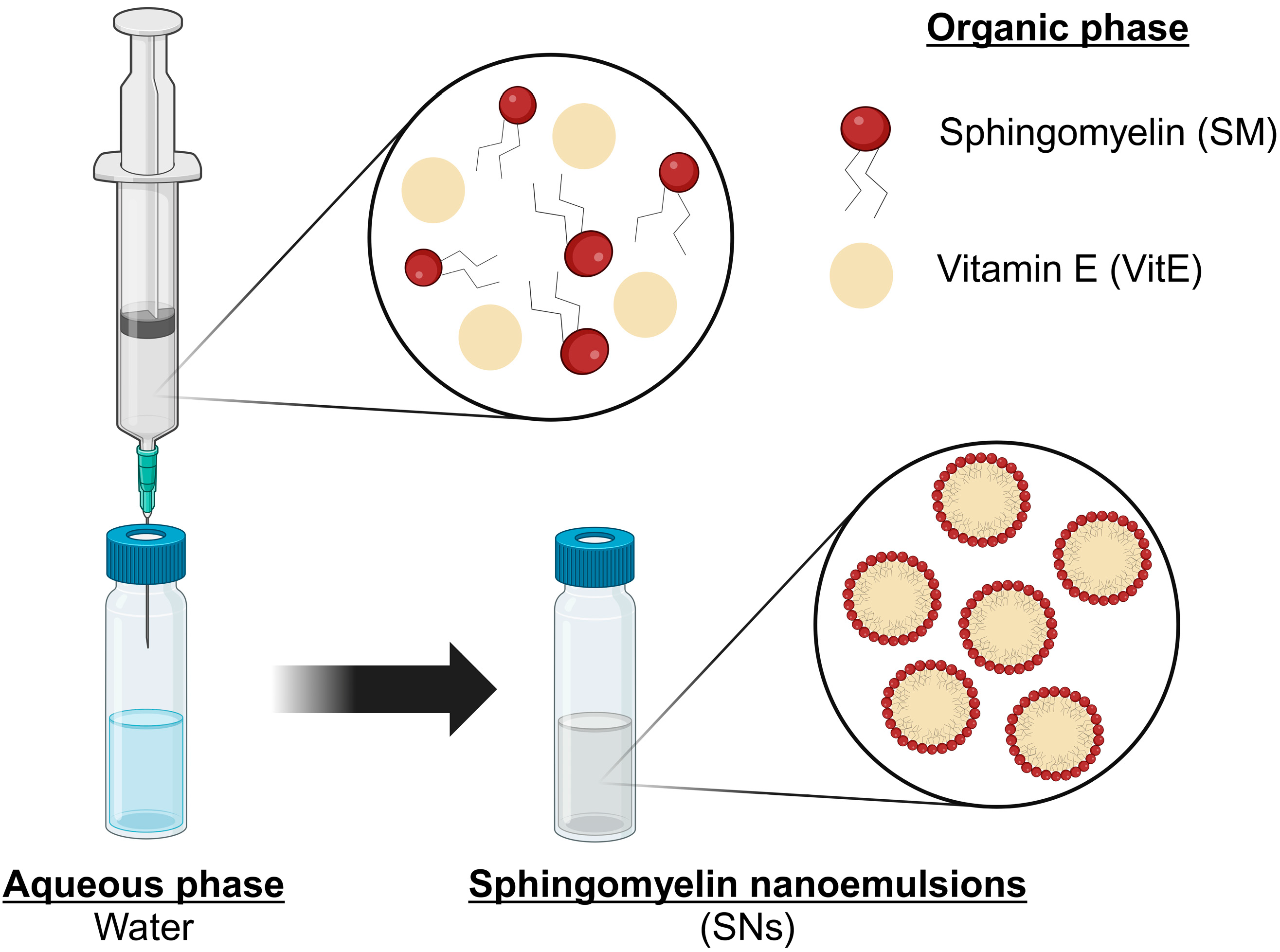
2.2. Preparation and Characterization of SNs
2.3. Preparation of Fluorescent-Labeled SNs
2.4. Preparation of RSV-Loaded SNs (RSV-SNs)
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Cell Death Study: Annexin V and Propidium Iodide (PI)
2.8. Measurement of Caspases 3 and 7 Activation
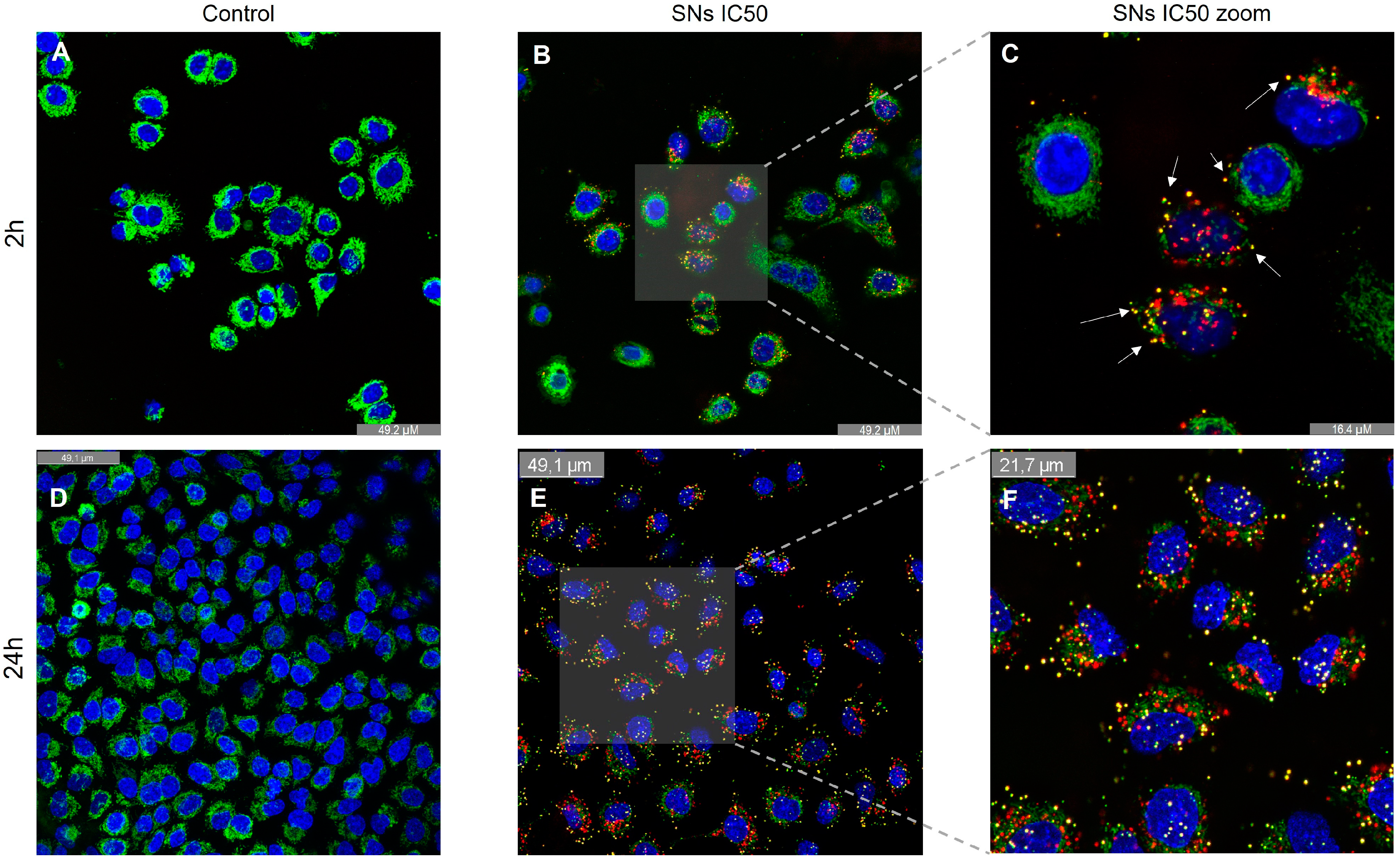
2.9. Determination of SNs Uptake by Confocal Microscopy
2.10. Determination of Lysosomal Co-Localization
2.11. Analysis of Acidic Lysosome
2.12. Analysis of Autophagosome Formation
2.13. Measurement of Mitochondrial Co-Localization
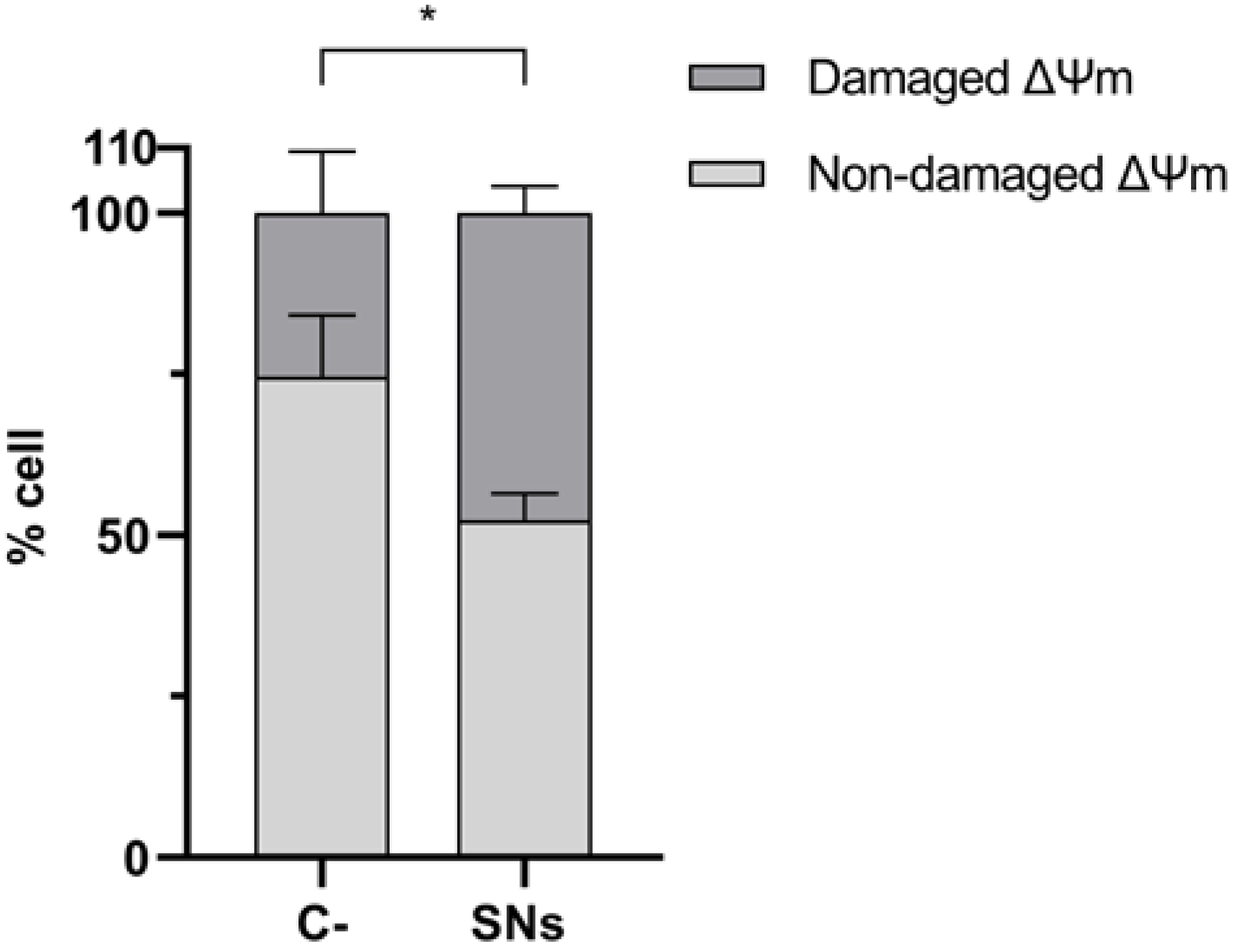
2.14. Analysis of Mitochondrial Membrane Potential (ψm) Integrity
2.15. Cell Uptake by Energy-Dependent Pathways Determination
2.16. Statistical Analysis
3. Results
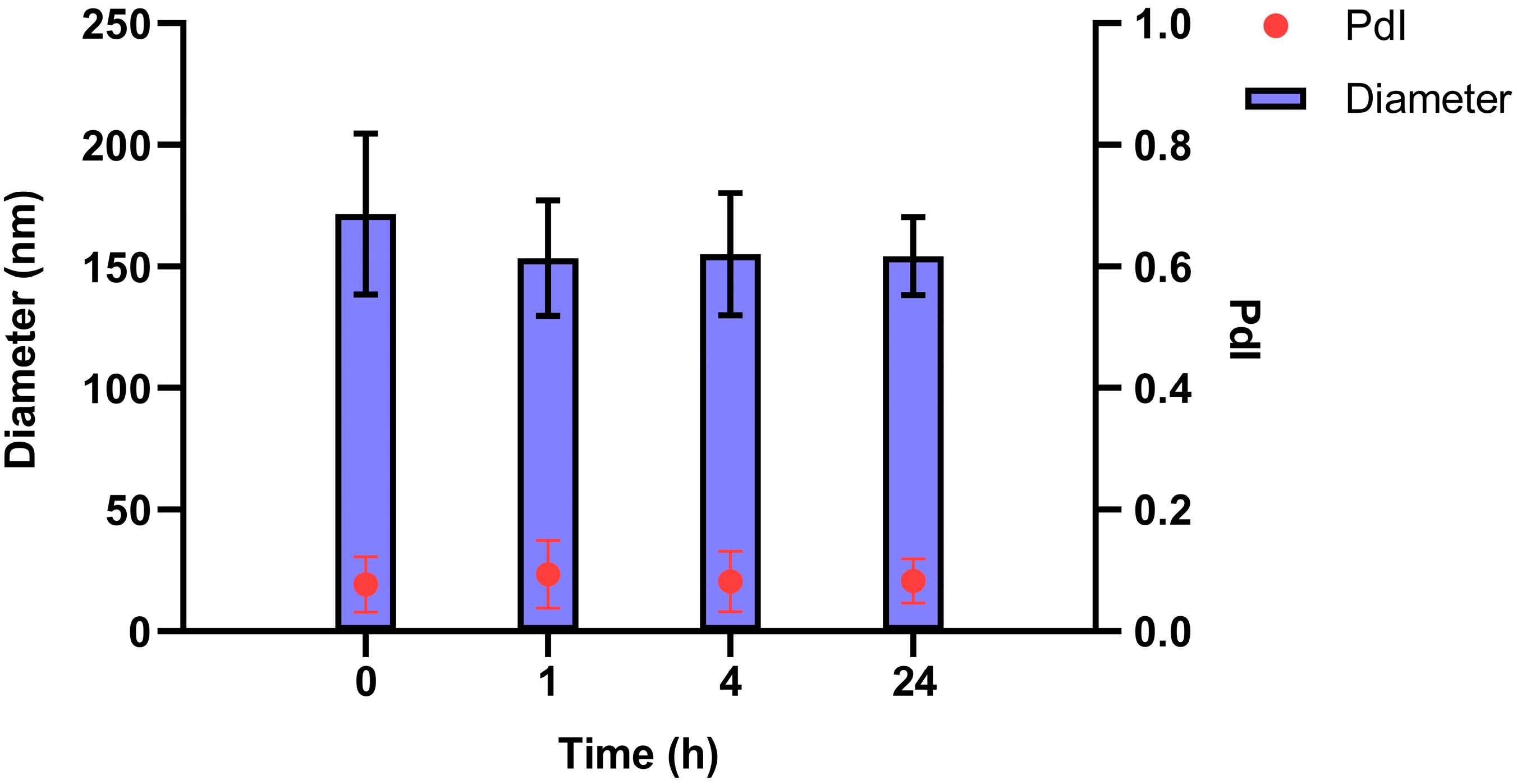
3.1. Preparation and Characterization of SNs
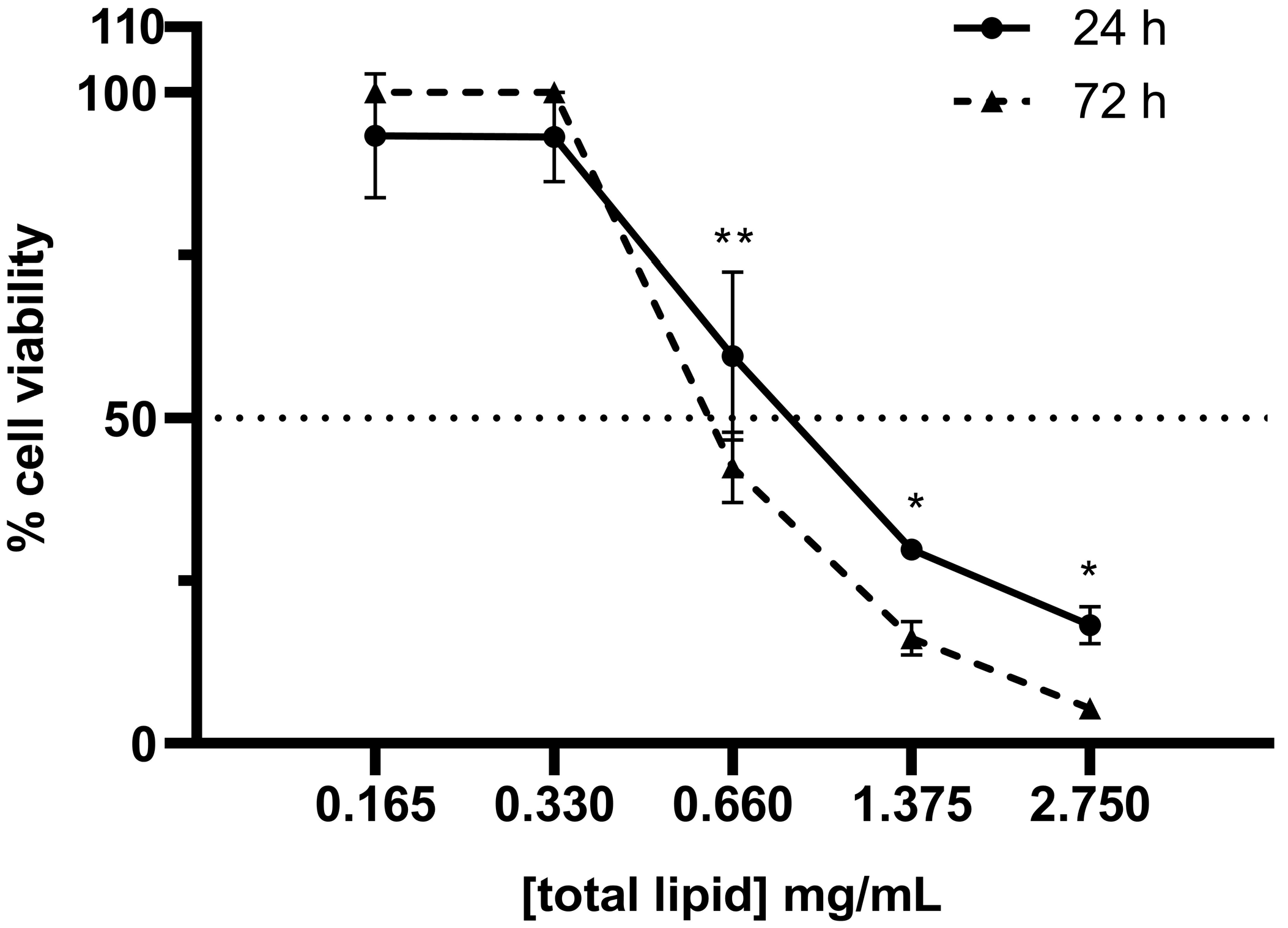
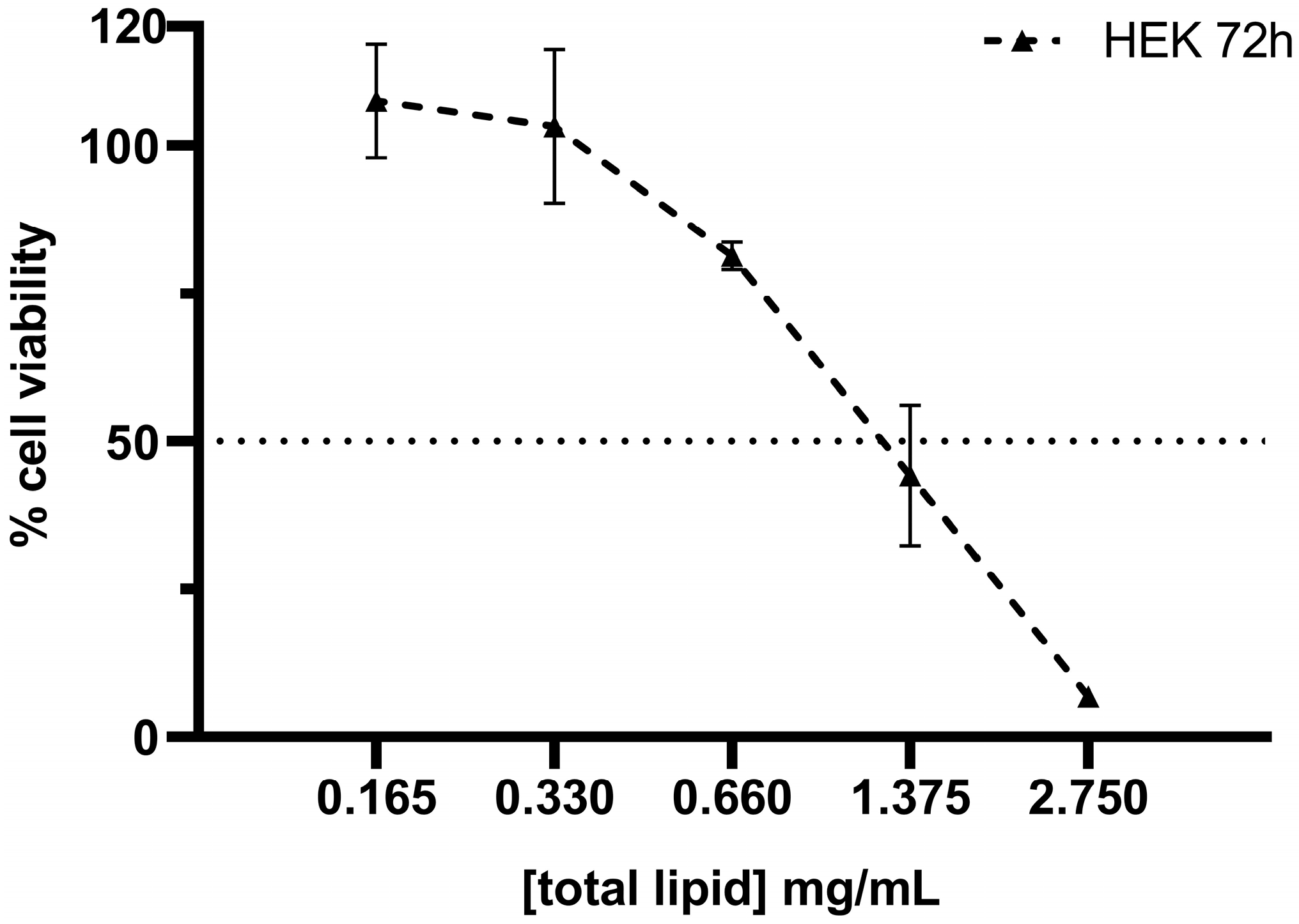
3.2. Cytotoxicity of SNs in the A549 Cell Line
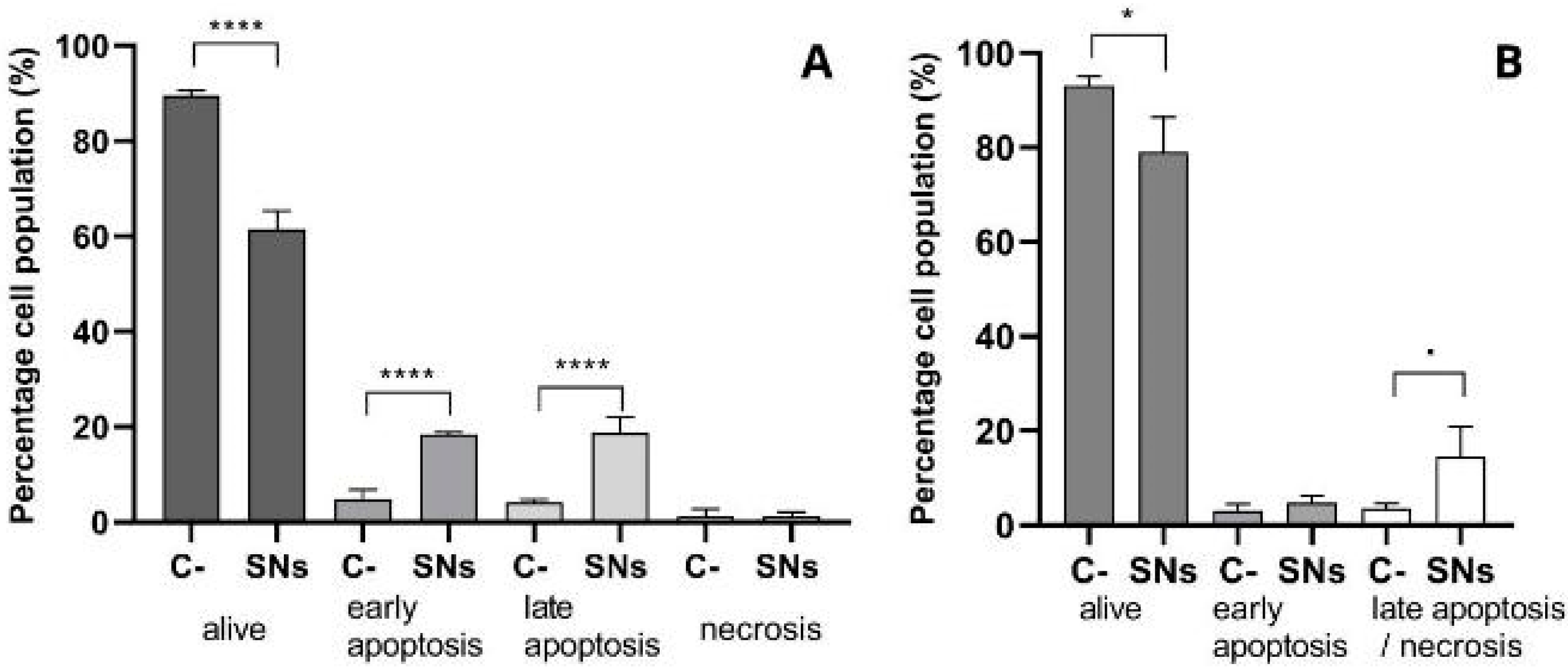
3.3. Mechanisms of Cell Death
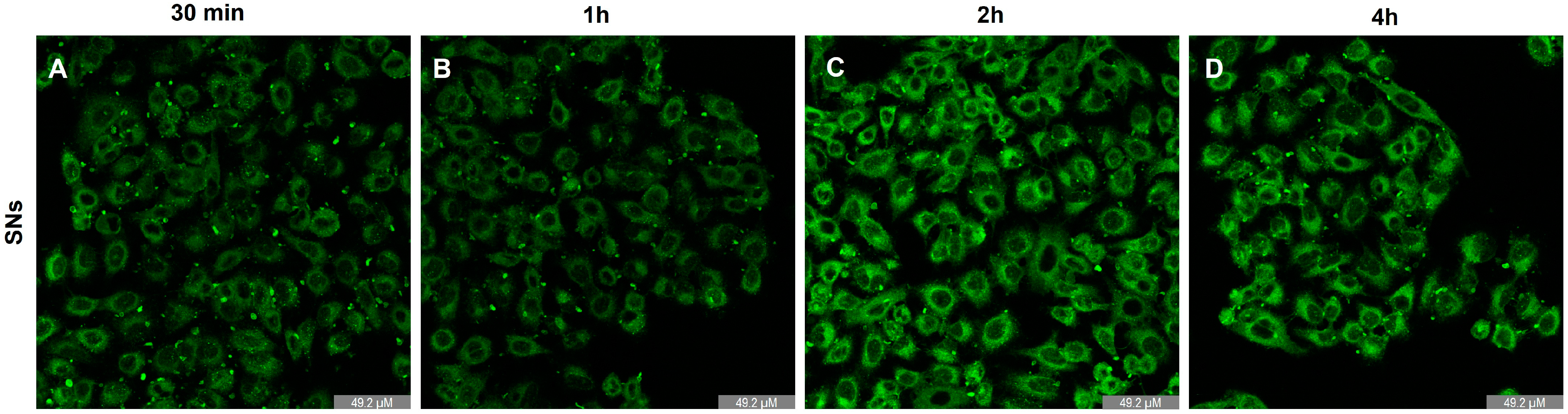
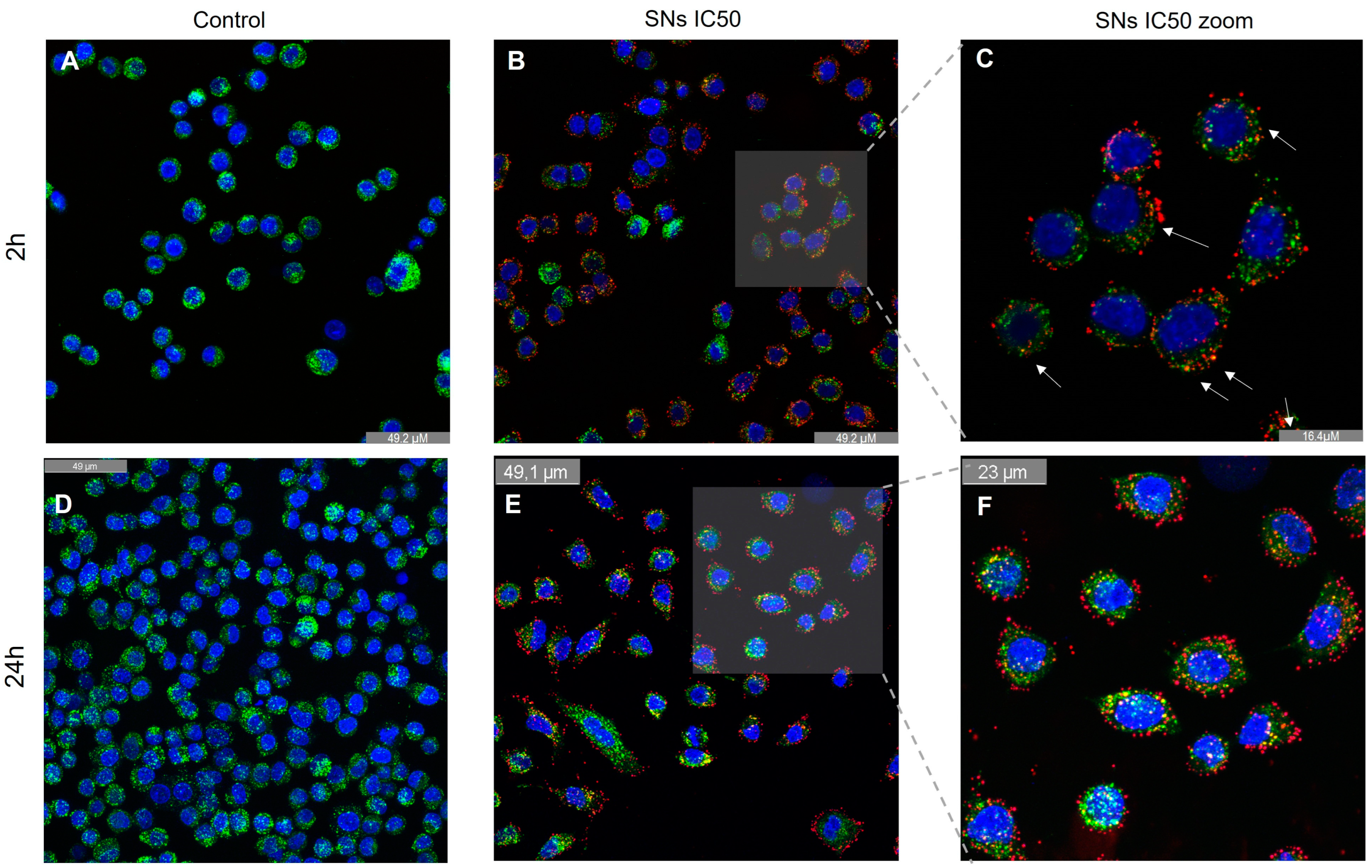
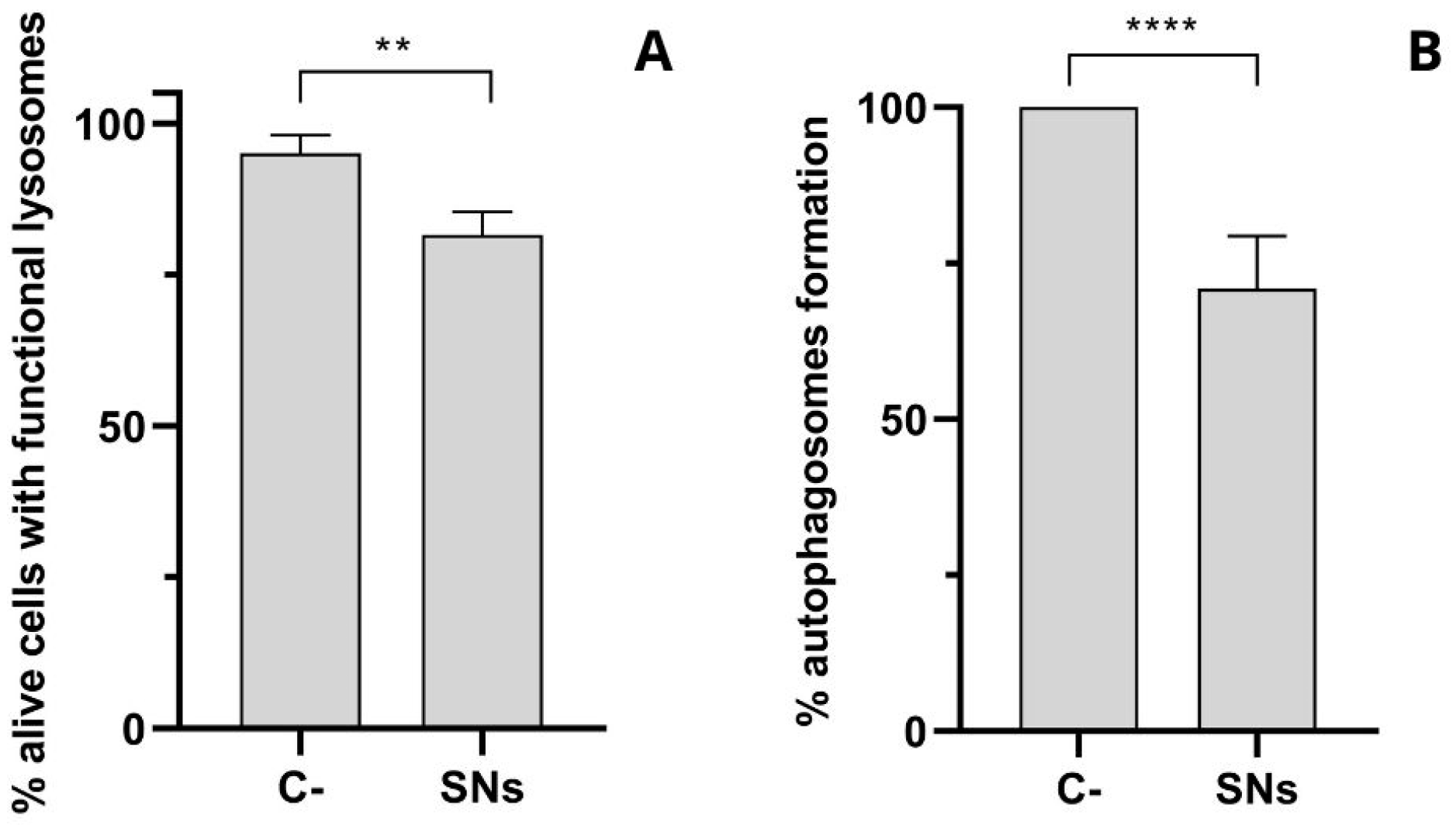
3.4. Intracellular Trafficking and Cell Uptake by Energy-Dependent Pathways Determination
3.5. Mitochondrial Impact
3.6. RSV Encapsulation in SNs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent Progress in Nanomedicine for Enhanced Cancer Chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar]
- Martins, J.P.; das Neves, J.; de la Fuente, M.; Celia, C.; Florindo, H.; Günday-Türeli, N.; Popat, A.; Santos, J.L.; Sousa, F.; Schmid, R.; et al. The Solid Progress of Nanomedicine. Drug Deliv. Transl. Res. 2020, 10, 726–729. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current Approaches of Nanomedicines in the Market and Various Stage of Clinical Translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A.; Navarro-Marchal, S.A.; Galisteo-González, F. Lipid-Core Nanoparticles: Classification, Preparation Methods, Routes of Administration and Recent Advances in Cancer Treatment. Adv. Colloid. Interface Sci. 2023, 314, 102871. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Pahwa, R.; Sharma, G.; Chhabra, J.; Haider, T.; Anitha, K.; Mishra, N. Nanoemulsion Therapy: A Paradigm Shift in Lung Cancer Management. J. Drug Deliv. Sci. Technol. 2024, 101, 106227. [Google Scholar] [CrossRef]
- Nagachinta, S.; Bouzo, B.L.; Vazquez-Rios, A.J.; Lopez, R.; de la Fuente, M. Sphingomyelin-Based Nanosystems (SNs) for the Development of Anticancer MiRNA Therapeutics. Pharmaceutics 2020, 12, 189. [Google Scholar] [CrossRef]
- Díez-Villares, S.; Ramos-Docampo, M.A.; da Silva-Candal, A.; Hervella, P.; Vázquez-Ríos, A.J.; Dávila-Ibáñez, A.B.; López-López, R.; Iglesias-Rey, R.; Salgueiriño, V.; de la Fuente, M. Manganese Ferrite Nanoparticles Encapsulated into Vitamin E/Sphingomyelin Nanoemulsions as Contrast Agents for High-Sensitive Magnetic Resonance Imaging. Adv. Healthc. Mater. 2021, 10, e2101019. [Google Scholar] [CrossRef]
- Díez-Villares, S.; Pellico, J.; Gómez-Lado, N.; Grijalvo, S.; Alijas, S.; Eritja, R.; Herranz, F.; Aguiar, P.; de la Fuente, M. Biodistribution of 68/67Ga-Radiolabeled Sphingolipid Nanoemulsions by Pet and Spect Imaging. Int. J. Nanomed. 2021, 16, 5923–5935. [Google Scholar] [CrossRef] [PubMed]
- Bouzo, B.L.; Lores, S.; Jatal, R.; Alijas, S.; Alonso, M.J.; Conejos-Sánchez, I.; de la Fuente, M. Sphingomyelin Nanosystems Loaded with Uroguanylin and Etoposide for Treating Metastatic Colorectal Cancer. Sci. Rep. 2021, 11, 17213. [Google Scholar] [CrossRef]
- Lores, S.; Gámez-Chiachio, M.; Cascallar, M.; Ramos-Nebot, C.; Hurtado, P.; Alijas, S.; López López, R.; Piñeiro, R.; Moreno-Bueno, G.; de la Fuente, M. Effectiveness of a Novel Gene Nanotherapy Based on Putrescine for Cancer Treatment. Biomater. Sci. 2023, 11, 4210–4225. [Google Scholar] [CrossRef]
- García-Fernández, J.; Rivadulla Costa, L.; Pinto-Díez, C.; Elena Martín, M.; González, V.M.; de la Fuente Freire, M. Chemical Conjugation of Aptamer-Sphingomyelin Nanosystems and Their Potential as Inhibitors of Tumour Cell Proliferation in Breast Cancer Cells. Nanoscale 2023, 15, 19110–19127. [Google Scholar] [CrossRef]
- Bouzo, B.L.; Calvelo, M.; Martín-Pastor, M.; García-Fandiño, R.; De La Fuente, M. In Vitro- In Silico Modeling Approach to Rationally Designed Simple and Versatile Drug Delivery Systems. J. Phys. Chem. B 2020, 124, 5788–5800. [Google Scholar] [CrossRef]
- Jatal, R.; Mendes Saraiva, S.; Vázquez-Vázquez, C.; Lelievre, E.; Coqueret, O.; López-López, R.; de la Fuente, M. Sphingomyelin Nanosystems Decorated with TSP-1 Derived Peptide Targeting Senescent Cells. Int. J. Pharm. 2022, 617, 121618. [Google Scholar] [CrossRef]
- Strachan, J.B.; Dyett, B.P.; Nasa, Z.; Valery, C.; Conn, C.E. Toxicity and Cellular Uptake of Lipid Nanoparticles of Different Structure and Composition. J. Colloid. Interface Sci. 2020, 576, 241–251. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Cascallar, M.; Hurtado, P.; Lores, S.; Pensado-López, A.; Quelle-Regaldie, A.; Sánchez, L.; Piñeiro, R.; de la Fuente, M. Zebrafish as a Platform to Evaluate the Potential of Lipidic Nanoemulsions for Gene Therapy in Cancer. Front. Pharmacol. 2022, 13, 1007018. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Gutiérrez-Lovera, C.; Martínez-Val, J.; Lores, S.; Bouzo, B.L.; Díez-Villares, S.; Alijas, S.; Pensado-López, A.; Vázquez-Ríos, A.J.; Sánchez, L.; et al. Edelfosine Nanoemulsions Inhibit Tumor Growth of Triple Negative Breast Cancer in Zebrafish Xenograft Model. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-Mimetic Nanoplatforms for Targeted Cancer Drug Delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef]
- De la Fuente Freire, M.; López López, R.; López Bouzo, B.; Vázquez Ríos, A.J.; Alonso Nocelo, M. Nanosystems as Selective Vehicles. European Patent Application Ep 3510995 A1, 19 July 2019. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Vazquez Ríos, A.J.; Bertolini, G.; Alonso Nocelo, M.; Calabuig-Fañanas, S.; Groba de Antas, S.; Rivadulla Costa, L.; Alijas Pérez, S.; Centonze, G.; Murianni, F.; Milione, M.; et al. Taste Receptor Type 1 Member 3 (TAS1R3) Is Enriched in Lung Cancer Stem Cells and Involved in Metastatic Progression of Non-Small Cell Lung Cancer. Cancer Lett. 2025; Submitted. [Google Scholar]
- Garcia-Fernandez, J.; Diez-Villares, S.; Cascallar, M.; Rivadulla Costa, L.; Martins, A.S.; Groba de Antas, S.; Tarasco, M.C.; Pantano, E.; de la Fuente, M. Targeted Delivery of Therapeutics to Metastatic Lung Cancer Cells Using Aptamer-Conjugated Nanoemulsions. J. Control. Release, 2025; Submitted. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar]
- Szwed, M.; Torgersen, M.L.; Kumari, R.V.; Yadava, S.K.; Pust, S.; Iversen, T.G.; Skotland, T.; Giri, J.; Sandvig, K. Biological Response and Cytotoxicity Induced by Lipid Nanocapsules. J. Nanobiotechnol. 2020, 18, 5. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. Pharmacol. 2004, 25. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef]
- Rodrigues, T.; Ferraz, L.S. Therapeutic Potential of Targeting Mitochondrial Dynamics in Cancer. Biochem. Pharmacol. 2020, 182, 114282. [Google Scholar] [CrossRef]
- Mirhadi, E.; Roufogalis, B.D.; Banach, M.; Barati, M.; Sahebkar, A. Resveratrol: Mechanistic and Therapeutic Perspectives in Pulmonary Arterial Hypertension. Pharmacol. Res. 2021, 163, 105287. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, H.; Wang, C.; He, F.; Shi, Z.; Tu, H.; Ning, N.; Duan, S.; Zhao, Y. The Anti-Cancer Effects of Mitochondrial-Targeted Triphenylphosphonium–Resveratrol Conjugate on Breast Cancer Cells. Pharmaceuticals 2022, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Tsujioka, T.; Sasaki, D.; Takeda, A.; Harashima, H.; Yamada, Y. Resveratrol-Encapsulated Mitochondria-Targeting Liposome Enhances Mitochondrial Respiratory Capacity in Myocardial Cells. Int. J. Mol. Sci. 2021, 23, 112. [Google Scholar] [CrossRef]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef]
- Nassimi, M.; Schleh, C.; Lauenstein, H.D.; Hussein, R.; Lbbers, K.; Pohlmann, G.; Switalla, S.; Sewald, K.; Müller, M.; Krug, N.; et al. Low Cytotoxicity of Solid Lipid Nanoparticles in in Vitro and Ex Vivo Lung Models. Inhal. Toxicol. 2009, 21 (Suppl. 1), 104–109. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Mi, S.N.; Woo, M.; Kim, B.G.; Lee, K.H.; Sohn, B.H.; Park, S.B.; Lee, J.K.; et al. Cellular Uptake of Magnetic Nanoparticle Is Mediated through Energy-Dependent Endocytosis in A549 Cells. J. Vet. Sci. 2006, 7, 321. [Google Scholar] [CrossRef]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef]
- Adler, J.; Parmryd, I. Quantifying Colocalization by Correlation: The Pearson Correlation Coefficient Is Superior to the Mander’s Overlap Coefficient. Cytom. Part A 2010, 77, 733–742. [Google Scholar] [CrossRef]
- Syama, K.; Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Development of Lipid Nanoparticles and Liposomes Reference Materials (II): Cytotoxic Profiles. Sci. Rep. 2022, 12, 18071. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Hervella, P.; Alonso-Sande, M.; Ledo, F.; Lucero, M.L.; Alonso, M.J.; Garcia-Fuentes, M.; T6d1, E.C.; Barcelona, A.; Vida, C. Pegylated Lipid Nanocapsules with Improved Drug Encapsulation and Controlled Release Properties. Curr. Top. Med. Chem. 2014, 14, 115–1123. [Google Scholar]
- Suffness, M. Assays Related to Cancer Drug Discovery. Methods PlantBiochem. Assays Bioactivity 1990, 6, 71–133. [Google Scholar]
- Sun, Y.; Chen, Y.; Ma, X.; Yuan, Y.; Liu, C.; Kohn, J.; Qian, J. Mitochondria-Targeted Hydroxyapatite Nanoparticles for Selective Growth Inhibition of Lung Cancer in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 25680–25690. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.H.; Hulett, M.D.; Parish, C.R. Molecular Mechanisms of Late Apoptotic/Necrotic Cell Clearance. Cell Death Differ. 2010, 17, 381–397. [Google Scholar]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 953–957. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Lafont, E.; Milhas, D.; Carpentier, S.; Garcia, V.; Jin, Z.X.; Umehara, H.; Okazaki, T.; Schulze-Osthoff, K.; Levade, T.; Benoist, H.; et al. Caspase-Mediated Inhibition of Sphingomyelin Synthesis Is Involved in FasL-Triggered Cell Death. Cell Death Differ. 2010, 17, 642–654. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar]
- Carmona-Ule, N.; Abuín-Redondo, C.; Costa, C.; Piñeiro, R.; Pereira-Veiga, T.; Martínez-Pena, I.; Hurtado, P.; López-López, R.; de la Fuente, M.; Dávila-Ibáñez, A.B. Nanoemulsions to Support Ex Vivo Cell Culture of Breast Cancer Circulating Tumor Cells. Mater. Today Chem. 2020, 16. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, H.-J.; Wen, H.-Y.; Wang, Z.-G.; Liu, S.-L. Engineered Lipidic Nanomaterials Inspired by Sphingomyelin Metabolism for Cancer Therapy. Molecules 2023, 28, 5366. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief Update on Endocytosis of Nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The Endocytosis and Intracellular Fate of Nanomedicines: Implication for Rational Design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Cifuentes, J.; Puentes, P.R.; Quezada, V.; Garcia-Brand, A.J.; Cruz, J.C.; Reyes, L.H. Assessing Cellular Internalization and Endosomal Escape Abilities of Novel BUFII-Graphene Oxide Nanobioconjugates. Front. Chem. 2022, 10, 974218. [Google Scholar] [CrossRef]
- Uzhytchak, M.; Smolková, B.; Lunova, M.; Frtús, A.; Jirsa, M.; Dejneka, A.; Lunov, O. Lysosomal Nanotoxicity: Impact of Nanomedicines on Lysosomal Function. Adv. Drug Deliv. Rev. 2023, 197, 114828. [Google Scholar] [CrossRef]
- Liu, C.G.; Han, Y.H.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. 2020, 15, 675–704. [Google Scholar]
- Elimam, H.; El-Say, K.M.; Cybulsky, A.V.; Khalil, H. Regulation of Autophagy Progress via Lysosomal Depletion by Fluvastatin Nanoparticle Treatment in Breast Cancer Cells. ACS Omega 2020, 5, 15476–15486. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Tian, Y.; Ma, W.; Sun, Y. Effects of Polymer Carriers on the Occurrence and Development of Autophagy in Drug Delivery. Nanoscale Adv. 2022, 4, 3676–3688. [Google Scholar]
- Allemailem, K.S.; Almatroudi, A.; Alsahli, M.A.; Aljaghwani, A.; El-Kady, A.M.; Rahmani, A.H.; Khan, A.A. Novel Strategies for Disrupting Cancer-Cell Functions with Mitochondria-Targeted Antitumor Drug–Loaded Nanoformulations. Int. J. Nanomed. 2021, 16, 3907. [Google Scholar] [CrossRef]
- Yamada, Y.; Akita, H.; Kamiya, H.; Kogure, K.; Yamamoto, T.; Shinohara, Y.; Yamashita, K.; Kobayashi, H.; Kikuchi, H.; Harashima, H. MITO-Porter: A Liposome-Based Carrier System for Delivery of Macromolecules into Mitochondria via Membrane Fusion. Biochim. Biophys. Acta Biomembr. 2008, 1778, 423–432. [Google Scholar] [CrossRef]
- Knupp, J.; Martinez-Montañés, F.; Van Den Bergh, F.; Cottier, S.; Schneiter, R.; Beard, D.; Chang, A. Sphingolipid Accumulation Causes Mitochondrial Dysregulation and Cell Death. Cell Death Differ. 2017, 24, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Thuy, L.T.; Lee, S.; Dongquoc, V.; Choi, J.S. Nanoemulsion Composed of α-Tocopherol Succinate and Dequalinium Shows Mitochondria-Targeting and Anticancer Effects. Antioxidants 2023, 12, 437. [Google Scholar] [CrossRef]
- Chen, Q.; Fang, H.; Shao, X.; Tian, Z.; Geng, S.; Zhang, Y.; Fan, H.; Xiang, P.; Zhang, J.; Tian, X.; et al. A Dual-Labeling Probe to Track Functional Mitochondria–Lysosome Interactions in Live Cells. Nat. Commun. 2020, 11, 6290. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.F.; Li, X.; Wang, Y.; Huang, Q.; Ma, X.; Liang, X.J. Nanoparticle-Driven Controllable Mitochondrial Regulation through Lysosome-Mitochondria Interactome. ACS Nano 2022, 16, 12553–12568. [Google Scholar] [CrossRef]
- Sola, F.; Montanari, M.; Fiorani, M.; Barattini, C.; Ciacci, C.; Burattini, S.; Lopez, D.; Ventola, A.; Zamai, L.; Ortolani, C.; et al. Fluorescent Silica Nanoparticles Targeting Mitochondria: Trafficking in Myeloid Cells and Application as Doxorubicin Delivery System in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3069. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In Vitro and in Vivo Antitumor Potential of Carvacrol Nanoemulsion against Human Lung Adenocarcinoma A549 Cells via Mitochondrial Mediated Apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef]
- Meghani, N.; Patel, P.; Kansara, K.; Ranjan, S.; Dasgupta, N.; Ramalingam, C.; Kumar, A. Formulation of Vitamin D Encapsulated Cinnamon Oil Nanoemulsion: Its Potential Anti-Cancerous Activity in Human Alveolar Carcinoma Cells. Colloids Surf. B Biointerfaces 2018, 166, 349–357. [Google Scholar] [CrossRef]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and Mitochondrial Dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-MTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Javanmardi, S.; Moradi-Ozarlou, M.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S.; Garg, M. Natural Products and Phytochemical Nanoformulations Targeting Mitochondria in Oncotherapy: An Updated Review on Resveratrol. Biosci. Rep. 2020, 40, BSR20200257. [Google Scholar] [CrossRef]
- Kong, F.; Xie, C.; Zhao, X.; Zong, X.; Bu, L.; Zhang, B.; Tian, H.; Ma, S. Resveratrol Regulates PINK1/Parkin -Mediated Mitophagy via the LncRNA ZFAS1-MiR-150-5p-PINK1 Axis, and Enhances the Antitumor Activity of Paclitaxel against Non-Small Cell Lung Cancer. Toxicol. Res. 2022, 11, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Syu, J.J.; Chen, J.C.; Wang, T.J.; Chang, P.Y.; Chen, C.Y.; Jian, Y.T.; Jian, Y.J.; Lin, Y.W. Resveratrol Enhances Etoposide-Induced Cytotoxicity through Down-Regulating ERK1/2 and AKT-Mediated X-Ray Repair Cross-Complement Group 1 (XRCC1) Protein Expression in Human Non-Small-Cell Lung Cancer Cells. Basic. Clin. Pharmacol. Toxicol. 2015, 117, 383–391. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the Mitochondria: From Triggering the Intrinsic Apoptotic Pathway to Inducing Mitochondrial Biogenesis, a Mechanistic View. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-Encapsulation of α-Tocopherol and Resveratrol within Zein Nanoparticles: Impact on Antioxidant Activity and Stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]

| Condition | Control | 4 °C | 37 °C |
|---|---|---|---|
| Fluorescent cells (%) | 0.63 ± 0.42 | 0.53 ± 0.35 | 45.37 ± 17.10 |
| Condition | RSV (Free Drug) | SNs-RSV (Nanoencapsulated) |
|---|---|---|
| IC50 (µM) | 519.34 ± 22.52 | 375.64 ± 17.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Docampo, E.; García-Fernández, J.; Mármol, I.; Morín-Jiménez, I.; Iglesias Baleato, M.; de la Fuente Freire, M. Mechanistic Insights into Sphingomyelin Nanoemulsions as Drug Delivery Systems for Non-Small Cell Lung Cancer Therapy. Pharmaceutics 2025, 17, 461. https://doi.org/10.3390/pharmaceutics17040461
Ramos Docampo E, García-Fernández J, Mármol I, Morín-Jiménez I, Iglesias Baleato M, de la Fuente Freire M. Mechanistic Insights into Sphingomyelin Nanoemulsions as Drug Delivery Systems for Non-Small Cell Lung Cancer Therapy. Pharmaceutics. 2025; 17(4):461. https://doi.org/10.3390/pharmaceutics17040461
Chicago/Turabian StyleRamos Docampo, Emma, Jenifer García-Fernández, Inés Mármol, Irene Morín-Jiménez, Maria Iglesias Baleato, and María de la Fuente Freire. 2025. "Mechanistic Insights into Sphingomyelin Nanoemulsions as Drug Delivery Systems for Non-Small Cell Lung Cancer Therapy" Pharmaceutics 17, no. 4: 461. https://doi.org/10.3390/pharmaceutics17040461
APA StyleRamos Docampo, E., García-Fernández, J., Mármol, I., Morín-Jiménez, I., Iglesias Baleato, M., & de la Fuente Freire, M. (2025). Mechanistic Insights into Sphingomyelin Nanoemulsions as Drug Delivery Systems for Non-Small Cell Lung Cancer Therapy. Pharmaceutics, 17(4), 461. https://doi.org/10.3390/pharmaceutics17040461






