Unlocking the Potential of Ganoderma lucidum (Curtis): Botanical Overview, Therapeutic Applications, and Nanotechnological Advances
Abstract
1. Introduction
2. Review Methodology
3. Ganoderma lucidum: Botanical Overview, Characterization, Uses in Traditional Medicine, and Chemical Studies
3.1. Botanical Overview and Characterization
- (1)
- Variety or Strain
- (2)
- Growing Substrate
- (3)
- Environmental Conditions
- (4)
- Age and Maturity
- (5)
- Genetic Expression
3.2. Uses in Traditional Medicine
3.3. Chemical Studies
4. Pharmacological and Toxicological Properties
4.1. Pharmacological Properties
4.1.1. Polysaccharides
4.1.2. Triterpenes
4.2. Toxicological Properties
4.3. Dosage Forms and Posology
- Capsules or Tablets: GL is commonly available in the form of capsules or tablets. The recommended dosage may vary depending on the concentration of GL extract or powder in each capsule/tablet. A common dosage range is 1–3 capsules/tablets per day, taken with water or as directed by a healthcare professional [21,83].
- Powder: GL powder can be mixed with water, juice, smoothies, or other beverages. Dosage may fluctuate depending on the particular product and the desired effects. Generally, a typical dosage range is 1–3 g of GL powder per day [21,83]. It is advisable to start with a lower dosage, and gradually increase if needed, based on individual tolerance and response.
- Tea or Decoction: GL can be brewed as a tea or decoction. Dried GL slices or powder can be simmered in water for a certain period to extract the bioactive compounds. The dosage of GL tea or decoction can vary depending on the concentration, brewing time, and individual preferences [19,21]. It is recommended to start with a small amount, and adjust the dosage based on taste and individual response.
5. Application of Ganoderma lucidum in Cancer Therapy
- Inducing Apoptosis: GL may promote apoptosis (programmed cell death) in cancer cells, inhibiting their uncontrolled growth and survival.
- Modulating the Immune System: GL is known for its immunomodulatory effects, enhancing the activity of immune cells pivotal in identifying and eradicating cancer cells.
- Reducing Inflammation: Chronic inflammation has been linked to cancer development and progression. GL’s anti-inflammatory properties may contribute to controlling tumor growth.
- Inhibiting Angiogenesis: GL’s compounds may help inhibit the formation of new blood vessels that supply tumors, limiting their nutrient supply.
5.1. Triple-Negative Breast Cancer
- Anticancer effects: In vitro studies focused on MDA-MB-231 breast cancer cells highlighted the potential of GL extracts for inhibiting the adhesion and further migration of cancer cells through the interruption of phosphatidylinositol 3-kinase (Pl3K). Another study observed that inhibition of interleukin 8 (IL-8) secretion by GL extracts was associated with oxidative-stress suppression. In addition, ganoderic acids have also shown promising results in avoiding the progression of these invasive cells. This was due to the inhibition of activator protein-1 (APA-1) and nuclear factor kB (NF-kB) [96,97,98]. Regarding in vivo studies, one underscored the effectiveness of GL extracts in impairing tumor growth. The animal model (mice) was injected with CD44+/CD24− breast cancer stem-like cells and the results confirmed a significant reduction in tumor weight. Another in vivo study in a mice model focused on targeting the signal transducer and activator of transcription 3 (STAT3) signaling, which plays a major role in cancer stem cells maintenance [32,99].
- Immunomodulation: TNBC is distinguished by its aggressive nature and the absence of specific targeted treatment options. Immunomodulatory properties of GL may be relevant in the context of TNBC, as they can potentially enhance the body’s immune response against cancer cells [96,97,98]. Some studies suggest that GL can modulate immune cells, such as natural killer cells (NK cells) and T-lymphocytes, and enhance their activity against cancer cells.
- Chemopreventive potential: In the case of TNBC, which lacks targeted therapies, chemopreventive strategies may be particularly valuable. Some studies have suggested that GL extracts or their bioactive compounds may help inhibit the initiation or progression of breast cancer, potentially reducing the risk of developing TNBC [96,97,98].
5.2. Colon Rectal Cancer
- Anticancer effects: Some in vitro studies observed the anticancer potential of GL extracts by using human colorectal cancer cells. One was performed on an SW 480 human colorectal cancer cell line and proceeded to analyze the effectiveness of GL extract 1 (GLE-1), with high content in polysaccharides, and GL extract 2 (GLE-2), with treterpenoids, on the inhibition of cell proliferation. While both fractions showed great results in this regard, it was observed that GLE-2 had a significantly higher inhibitory activity. In addition, an in vitro study in LoVo human colon cancer cells described the promising activity of GLPs in avoiding cell migration, apoptosis induction, and activation of caspases-3, -8, and -9. Regarding in vivo studies, one performed in a mice model investigated the effect of GLP on AOM/DSS-induced colorectal cancer, and the results were very satisfactory, with decreased tumor size and cancer cells, and an extra-functional gut barrier [82,102,103].
- Immunomodulation: GL is known for its immunomodulatory effects, meaning it can modulate the immune system. Enhancing the immune response may be relevant in the context of colon rectal cancer, as the immune system plays a crucial role in identifying and eliminating cancer cells. Some studies suggest that GL can modulate immune cells, and enhance their activity against cancer cells, potentially supporting the body’s immune response to colon rectal cancer [82,102,103].
- Chemopreventive potential: In the case of colon rectal cancer, chemopreventive strategies may be valuable, especially in individuals at high risk or with a history of precancerous polyps. Some studies have suggested that GL extracts or its bioactive compounds may help to inhibit the initiation or progression of colon rectal cancer, potentially reducing the risk of developing this disease [82,102,103].
5.3. Other Types of Cancer
5.3.1. Lung Cancer
- Anticancer Properties: According to the literature, to date, many in vivo and in vitro studies have been developed in order to understand the impact of GL extracts in cancer treatment. Regarding in vivo studies, mainly performed in animal models (mice), the results highlight the activity of GL extracts in the suppression of tumor growth, angiogenesis, and interfering with cell adhesion. Similar results were observed in in vitro studies, predominantly in human lung cancer cell lines, such as A549. One underscored the potential of ganoderic acids to trigger mitochondria apoptosis in cancer cells. In addition, GLPs have shown great inhibitory activity regarding the proliferation of vascular endothelial cells and stimulation of vascular endothelial growth factor (VEGF) production in lung cancer cells [77,78,79].
- Anti-Inflammatory Effects: Chronic inflammation has been linked to the development and progression of lung cancer. GL’s anti-inflammatory properties may help reduce inflammation in the lungs, potentially impacting cancer growth and progression [80].
5.3.2. Prostate Cancer
- Anticancer Properties: GL’s bioactive compounds may exhibit antitumor effects, including inhibiting cancer cell growth and promoting apoptosis in prostate cancer cells [105]. Some in vitro studies highlight the effectiveness of GL extracts on dihydrotestoterone (DHT) inhibition and impairing cell proliferation. In addition, an in vivo study performed in an animal model (mice) verified increased mitigation of tumor cells [105,106].
6. Application of Ganoderma lucidum in Nanotechnology
6.1. Nanoparticle Synthesis
- Nanocarriers for drug delivery: GL extracts or their components have been incorporated into nanocarriers for drug delivery purposes. By encapsulating therapeutic agents within nanoscale systems, such as liposomes or nanoparticles, it is possible to enhance drug stability, improve bioavailability, and target specific tissues or cells [115].
- Antimicrobial nanomaterials: GL extracts have shown antimicrobial activity against various microorganisms. Researchers have explored incorporating these extracts into nanomaterials, such as coatings or films, to create antimicrobial surfaces. Such surfaces could find applications in medical devices, food packaging, and other areas where preventing microbial growth is crucial [116].
- Biosensors: GL extracts have demonstrated potential for use in biosensing applications. By immobilizing the mushroom extract or its bioactive compounds into nanomaterials, it is possible to create biosensors capable of detecting specific targets, such as biomarkers or pollutants, with high sensitivity and selectivity [49]. It is worth noting that the research and development of GL in nanotechnology are still in their early stages, and further studies are needed to explore the full potential of this mushroom in various nanotechnological applications. The synthesis of GL nanoparticles involves the utilization of extracts or components derived from the mushroom to produce nanoparticles with unique properties. The general steps involved in the synthesis process of nanoparticles from GL are the preparation of GL extract, reduction and stabilization of nanoparticles, and characterization and functionalization [42,111,112,116,117,118]. Techniques such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), dynamic light scattering (DLS), and Infrared Spectroscopy (FTIR) are commonly used for nanoparticle characterization [110].
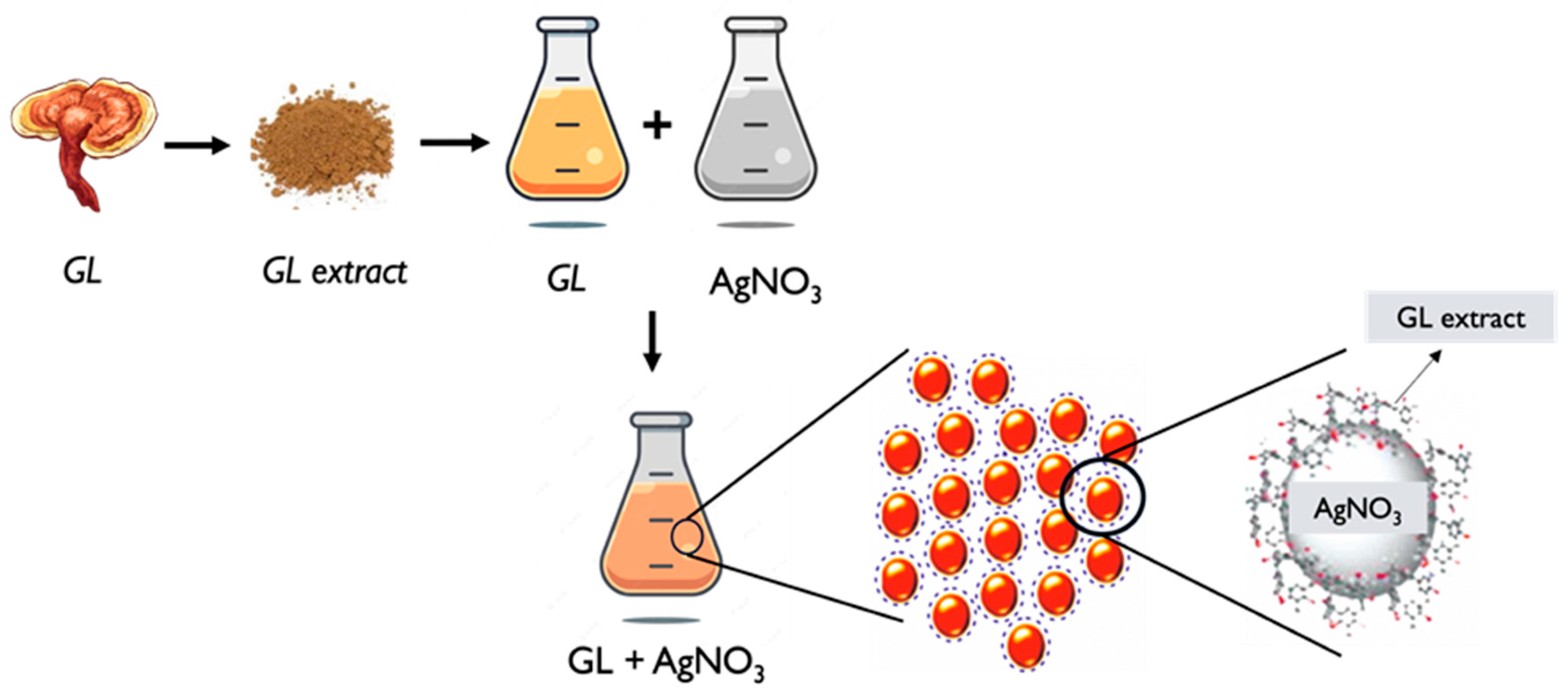
6.2. Silver Nanoparticles
- GL extract preparation: Similar to the general process described earlier, an extract is obtained from GL. The extraction can be performed using solvents such as water or ethanol. The extract contains bioactive compounds that will serve as both reducing and stabilizing agents in the process of nanoparticle synthesis [116,117].
- Characterization: The synthesized GL AgNPs are then characterized to determine their size, shape, distribution, morphology, and other properties. Techniques such as TEM, SEM, XRD, UV–Vis and FTIR spectroscopy, and DLS can be employed to analyze the nanoparticles and assess their characteristics [42,110,117,118].
- Functionalization: If desired, the GL AgNPs can be further functionalized by modifying their surface. This involves the attachment of specific molecules called ligands or coatings to enhance their stability and biocompatibility, or targeting cellular capabilities for particular applications [54,120]. The specific synthesis methods and conditions may vary among different studies and researchers. The concentration of GL extract, silver precursor, reaction time, and temperature can all influence the size and properties of the resulting silver nanoparticles (Figure 4). It is worth noting that further research and optimization are ongoing to explore the potential applications and benefits of GL AgNPs in various fields, including biomedicine, catalysis, and environmental remediation.
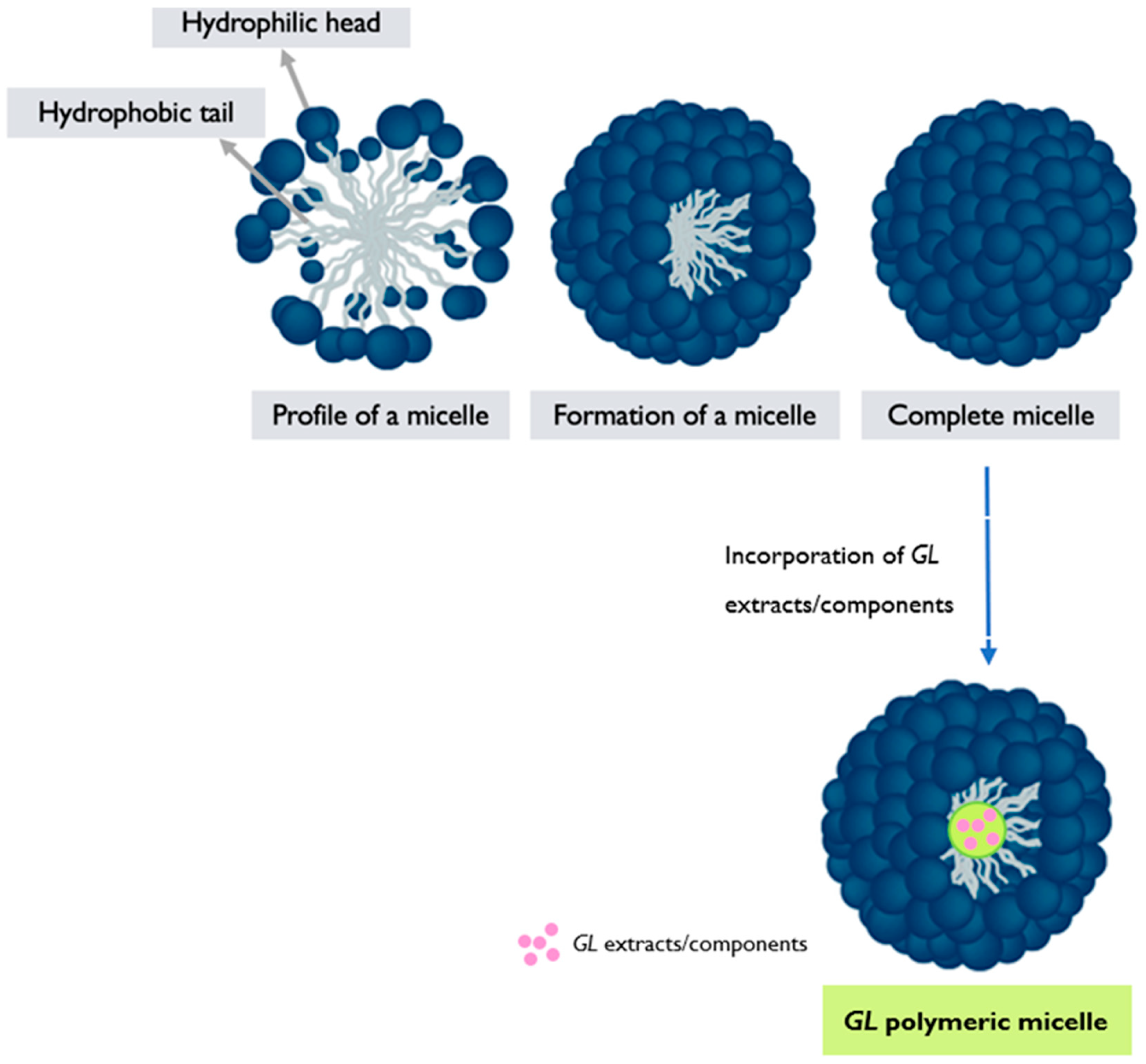
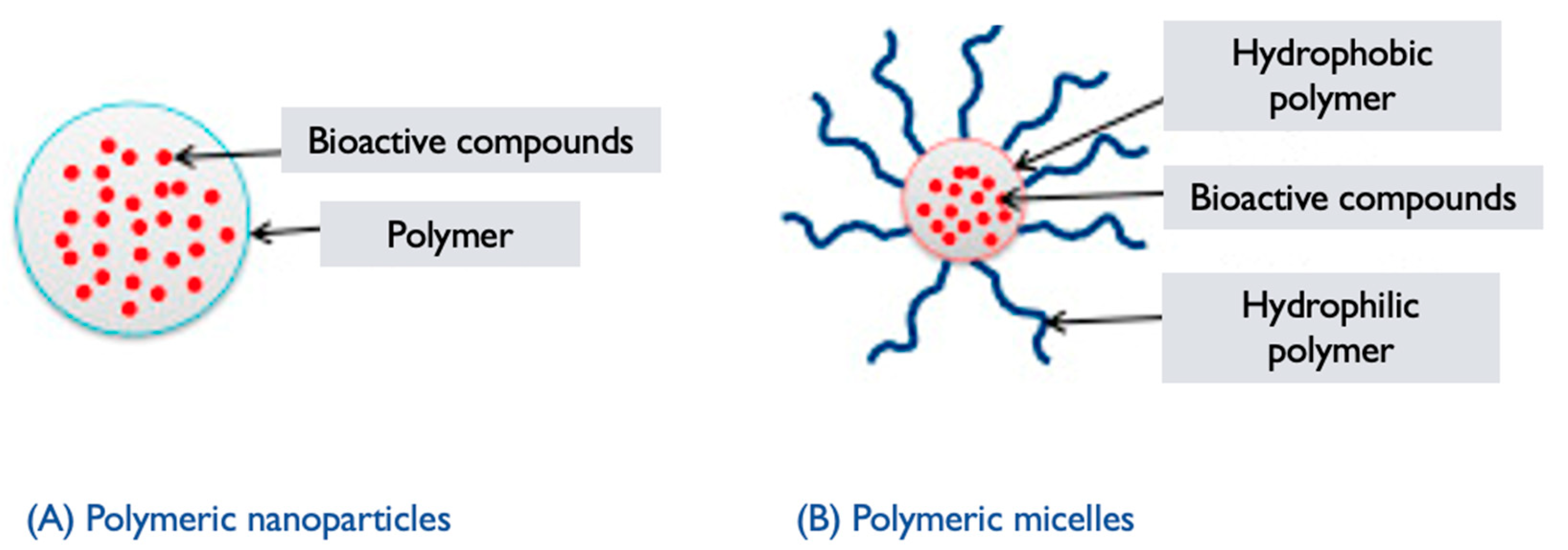
6.3. Polymeric Micelles
- Polymer synthesis: The selected polymers are synthesized using appropriate techniques, such as polymerization or modification reactions [122]. The hydrophilic and hydrophobic blocks are incorporated into the polymer structure, resulting in an amphiphilic copolymer.
- Micelle formation: The synthesized amphiphilic copolymer is then dissolved in a suitable solvent, typically aqueous solution. Due to the amphiphilic nature of the polymer, it self-assembles into micellar structures in the solution. The hydrophilic segments of the polymer form the outer shell of the micelle, while the hydrophobic segments aggregate, forming the core, and encapsulating hydrophobic drugs or other cargo [122,123,124] (Figure 5).
- Characterization and functionalization: The resulting GL PMs are characterized to assess their size, morphology, stability, critical micelle concentration (CMC), drug-loading capacity, and efficiency of encapsulation. Techniques such as DLS, TEM, and drug release studies are commonly employed [54,120,123]. The micelles can also be further functionalized by modifying the surface with targeting ligands or other functional moieties to enhance their specificity and therapeutic efficacy. GL PMs hold promise for targeted drug delivery systems, as the bioactive compounds from the mushroom may contribute to additional therapeutic effects. However, the research and development of GL PMs are still ongoing, and further studies are needed to explore their full potential and optimize their performance in drug delivery applications.
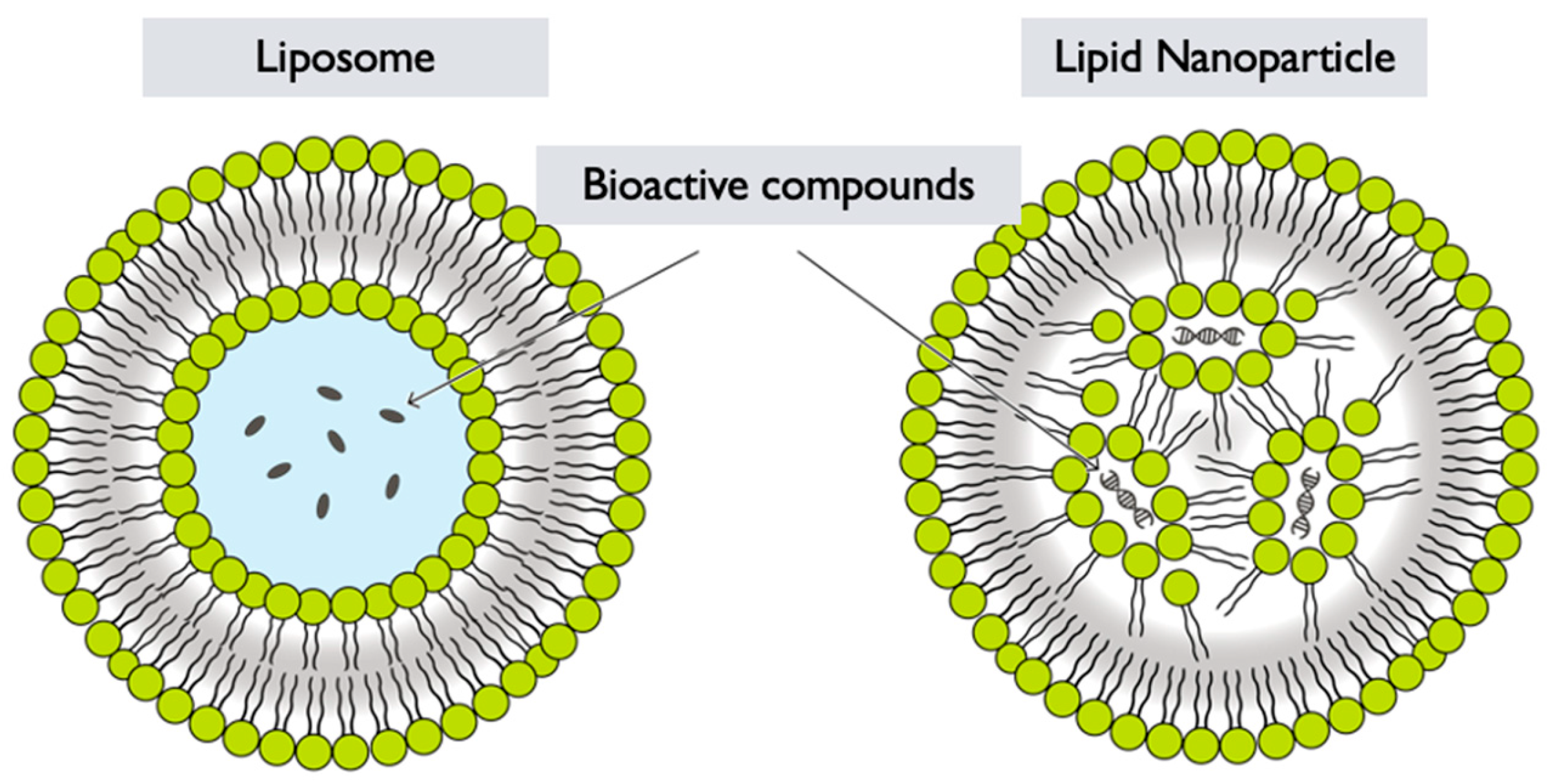
6.4. Lipid Nanoparticles
- Selection of lipids: Lipids are chosen based on their biocompatibility, stability, and ability to form nanoparticles. Common lipids used in lipid nanoparticle formulations include phospholipids, such as phosphatidylcholine or phosphatidylglycerol, and other lipid-based materials like solid lipids or oils [119,127] (Figure 6).
- Emulsification: The lipid solution containing GL extracts is then emulsified with an aqueous phase, typically a buffer or water. This can be achieved through techniques like ultrasonication, high-pressure homogenization, or microfluidics, resulting in the formation of small droplets [128].
- Nanoparticle formation: After emulsification, the organic solvent is removed by evaporation or other methods, leading to the formation of LNPs encapsulating GL extracts [128]. The removal of the organic solvent allows the lipids to solidify and stabilize, forming nanoparticles with the GL components entrapped within.
- Functionalization: Depending on the desired application, the surface of the GL LNPs can be further functionalized with targeting ligands, polymers, or other surface modifications to improve their specificity, stability, or targeting properties. GL LNPs have the potential to enhance the delivery and bioavailability of GL bioactive compounds. However, specific formulation strategies and optimization processes may vary depending on the desired application and intended use of the LNPs. More research and development are needed to exploit the full potential of GL LNPs in various fields, including pharmaceuticals, nutraceuticals, and cosmetics.
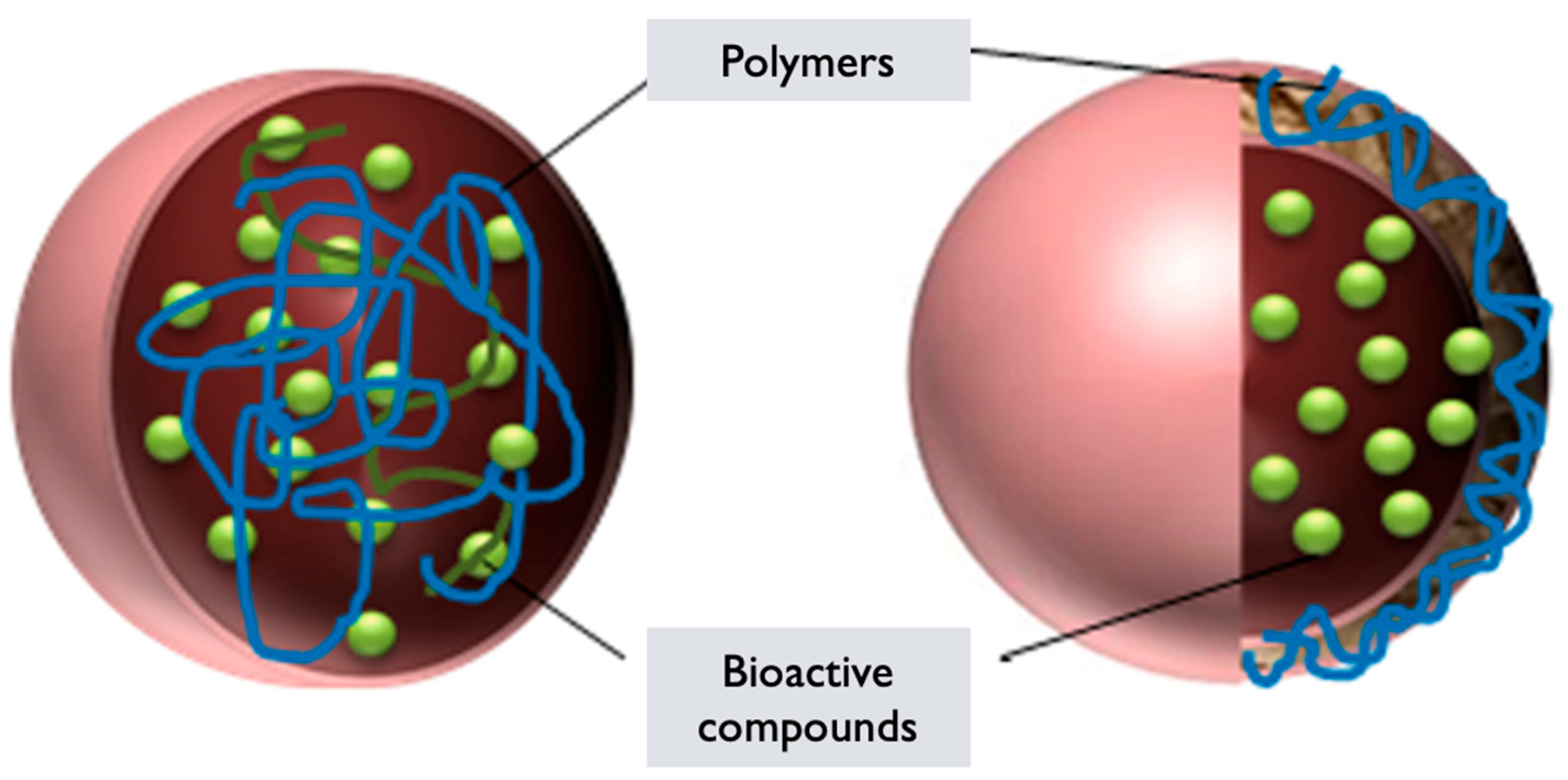
6.5. Polymeric Nanoparticles
- Polymer selection: Polymers derived from GL or incorporating extracts/components from the mushroom are chosen based on their biocompatibility, stability, and ability to self-assemble into nanoparticles [129]. These polymers can include GL-derived polysaccharides, proteins, or modified polymers with incorporated mushroom extracts.
- Polymer synthesis or modification: The selected polymers are synthesized or modified to incorporate the desired properties for nanoparticle formation. This can involve polymerization techniques or chemical modifications to introduce hydrophilic and hydrophobic segments within the polymer structure, which are essential for self-assembly into nanoparticles [130].
- Nanoparticle formation: The synthesized or modified GL polymers are dissolved in an appropriate solvent to form a polymer solution. Self-assembly of the polymers occurs spontaneously due to the establishment of hydrophilic and hydrophobic interactions [123]. This results in the formation of PNPs encapsulating GL components or in the components integrating within the polymer matrix.
- Characterization: The GL PNPs are characterized to determine their size, morphology, stability, drug-loading capacity, and efficiency of encapsulation. Techniques such as DLS, TEM, SEM, and UV–Vis and FTIR spectroscopy can be employed to assess these properties [49].
- Functionalization: Depending on the desired application, the surface of the GL PNPs can be further functionalized with targeting ligands, polymers, or other surface modifications to improve their specificity, stability, or targeting properties. Surface modifications can also enable the attachment of imaging agents or other functionalities.
- Enhanced Drug Delivery: Nanoparticles loaded with GL bioactive compounds have been investigated for improved drug delivery in cancer therapy. In one study, polymeric nanoparticles loaded with GLPs exhibited enhanced cellular uptake and cytotoxicity against cancer cells, when compared with free polysaccharides [23,65]. The resulting NPs demonstrated sustained release of the bioactive compounds, resulting in prolonged anticancer effects.
- Targeted Therapy: Targeted delivery of GL bioactive compounds to cancer cells has been achieved using functionalized nanoparticles. In a preclinical study, folate-conjugated NPs encapsulating GLTs selectively targeted folate receptor-expressing cancer cells [23,65]. This targeted delivery approach improved the efficacy of the bioactive compounds and reduced toxicity to healthy cells.
- Synergistic Effects: Nanotechnology has been employed to combine GL bioactive compounds with other therapeutic agents, leading to synergistic effects. For example, in a preclinical study, co-encapsulation of GLTs and some chemotherapeutic drugs (e.g., Paclitaxel, Doxorubicin, Cisplatin, 5-Fluorouracil, Gemcitabine, Etoposide, and Vinblastine) within nanoparticles resulted in enhanced cytotoxicity against cancer cells compared to the individual treatments alone [23,65]. The combination therapy demonstrated improved antitumor activity and reduced drug resistance.
- Immunomodulation: Nanotechnology-based formulations incorporating GL bioactive compounds have shown potential for immunomodulatory effects. In a preclinical study, nanocarriers loaded with GLPs effectively stimulated immune responses, and enhanced the activation of immune cells, leading to improved anticancer immune responses [23,65]. The nanotechnology-mediated delivery facilitated the targeted modulation of the immune system.
- Theranostics: GL-based nanomaterials have been explored for theranostic applications, combining therapy, and diagnostics. In a preclinical study, multifunctional nanoparticles loaded with GL bioactive compounds were developed as theranostic agents for simultaneous cancer therapy and imaging technology [23,65]. The NPs exhibited selective tumor accumulation, efficient tumor regression, and imaging capabilities for real-time monitoring of treatment response.
7. Regulatory Issues and Clinical Trials
- Safety and Toxicity Assessment: Regulatory bodies require a thorough evaluation of the safety profile of GL-based nanotechnological products. This includes assessing potential adverse effects, toxicity, and interactions with other treatments or medications.
- Standardization and Quality Control: Ensuring the consistency and quality of GL-derived nanoparticles or formulations is crucial. Regulatory agencies often require standardized processes and rigorous quality control measures to maintain product integrity.
- Clinical Trial Authorization: Clinical trials involving GL in nanotechnology applications typically require authorization from regulatory bodies such as the United States or the EMA in Europe. Obtaining these approvals involves providing detailed documentation on the product, its manufacturing process, and preclinical data.
- Data Integrity and Reporting: Regulatory agencies expect accurate and complete reporting of clinical trial data. This includes transparency in reporting both positive and negative results, adverse events, and patient outcomes.
- Good Clinical Practice (GCP): Adherence to GCP guidelines is essential. GCP ensures that clinical trials are conducted ethically, with patient safety in mind, and that the data collected are reliable and credible.
- Post-Market Surveillance: After clinical trials, regulatory agencies may require post-market surveillance to continue monitoring the safety and efficacy of GL-based nanotechnological products once they are in use by the general population.
7.1. Preclinical Studies
7.2. Clinical Studies
- Cancer Therapy: Clinical trials have evaluated the efficacy and safety of GL in cancer patients. These studies have explored its potential as an adjuvant therapy to conventional cancer treatments, such as chemotherapy or radiation therapy [23,58,65]. While some studies have reported positive outcomes, including improved quality of life, immune system modulation, and enhanced treatment response, the overall evidence is limited, and more rigorous studies are needed.
- Immunomodulation: Clinical investigations have explored the immunomodulatory effects of GL in various populations, including healthy individuals and patients with chronic diseases. These studies have explored the impact of GL on immune parameters, such as cytokine levels, immune cell activity, and antioxidant status [23,65,69,138,139,140,141,142]. Results have indicated potential immunomodulatory effects, but further research is needed to establish clear clinical recommendations.
- Liver Health: GL has been studied in clinical trials focusing on liver health, particularly in patients with hepatitis B or hepatitis C. These studies have assessed its potential hepatoprotective effects, antiviral activity, and impact on liver function. While some studies have reported positive outcomes, the evidence is still limited and larger, well-controlled trials are needed to confirm these findings [23,65,141].
- Cardiovascular Health: Cardiovascular health markers, such as blood pressure, cholesterol levels, and oxidative stress, have been assessed in clinical studies. Some trials have reported potential benefits, including improved lipid profiles and antioxidant status [17,65,142]. However, more robust clinical trials are required to establish the efficacy and safety of GL in cardiovascular health management.
7.3. Critical Assessments of the Pharmacological Activities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 20 November 2024).
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Kang, S.-M.; Kim, A.-Y.; Imran, M.; Aaqil Khan, M.; Lee, I.-J. Current Knowledge of Medicinal Mushrooms Related to Anti-Oxidant Properties. Sustainability 2021, 13, 7948. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Fu, J.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Nanocarriers for combating biofilms: Advantages and challenges. J. Appl. Microbiol. 2022, 133, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Mathur, G. Cancer: An overview. Acad. J. Cancer Res. 2015, 8, 1–9. [Google Scholar]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and Beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K.; Khajuria, R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): A bitter mushroom with amazing health benefits. Int. J. Med. Mushrooms 2013, 15, 127–143. [Google Scholar] [CrossRef]
- Magro, M.; Venerando, A.; Macone, A.; Canettieri, G.; Agostinelli, E.; Vianello, F. Nanotechnology-Based Strategies to Develop New Anticancer Therapies. Biomolecules 2020, 10, 735. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, X.; Chen, G.; Su, L.; Liu, Z.; Zhou, P.; Weng, J.; Min, Y. Advances of functional nanomaterials for magnetic resonance imaging and biomedical engineering applications. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2022, 14, e1800. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Karsten, P.A. Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Rev. Mycol. 1881, 3, 16–19. [Google Scholar]
- Murrill, W.A. The Polyporaceae of North America. I. The Genus Ganoderma. Bull. Torrey Bot. Club 1902, 29, 599–608. [Google Scholar] [CrossRef]
- Patrouillard, N. Le genre Ganoderma. Bull. De La Société Mycol. De Fr. 1889, 5, 64–80. [Google Scholar]
- Hong, S.G.; Jung, H.S. Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences. Mycologia 2004, 96, 742–755. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Wachtel-Galor, S. (Eds.) Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar] [PubMed]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; AlHudaithi, N.; AlHebshi, A.; Bukhari, A. Extraction and UHPLC-DAD detection of undeclared substances in market-available dietary supplements and slimming products in Eastern region, Saudi Arabia: An application of principal component analysis. Biomed. Chromatogr. 2020, 34, e4698. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Choeyklin, R.; Thummarukcharoen, T.; Rachtawee, P.; Sappan, M.; Srichomthong, K.; Fujii, R.; Kawashima, K.; Mori, S. Highly Modified Lanostane Triterpenes from the Wood-Rot Basidiomycete Ganoderma colossus: Comparative Chemical Investigations of Natural and Artificially Cultivated Fruiting Bodies and Mycelial Cultures. J. Nat. Prod. 2020, 83, 2066–2075. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, J.; Li, X.; Ye, L.; Jia, D.; Gan, B.; Tan, W. Temperature affects substrate-associated bacterial composition during Ganoderma lucidum hyphal growth. Can. J. Microbiol. 2021, 67, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.X.; Kong, X.M.; Huang, X.M.; Li, N.; Feng, N.; Xu, J.W. Breeding a new Ganoderma lucidum strain with increased contents of individual ganoderic acids by mono-mono crossing of genetically modified monokaryons. Front. Microbiol. 2024, 15, 1410368. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Viceconte, F.R.; Pereyra, M.T.; Bidegain, M.A.; Cubitto, M.A. DNA damaging potential of Ganoderma lucidum extracts. J. Ethnopharmacol. 2018, 217, 83–88. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging Roles of Ganoderma lucidum in Anti-Aging. Aging Dis. 2017, 8, 691–707. [Google Scholar] [CrossRef]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-cancer Activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, 210. [Google Scholar] [CrossRef]
- Teng, L.; Wang, C.; Cui, B.; Zhang, J.; Zhou, S.; Pan, X.; Pan, F.; Dai, Y.; Feng, N. Lanostane triterpenoids from mycelia-associated Ganoderma sinense and their anti-inflammatory activity. Phytochemistry 2023, 215, 113870. [Google Scholar] [CrossRef] [PubMed]
- Ganoderma applanatum. Available online: https://en.wikipedia.org/wiki/Ganoderma_applanatum#/media/File:Ganoderma_applanatum_2010_G1.jpg (accessed on 6 March 2025).
- Ganoderma orbiforme. Available online: https://en.wikipedia.org/wiki/Ganoderma_orbiforme#/media/File:2016-04-21_Ganoderma_orbiforme_(Fr.)_Ryvarden_616191.jpg (accessed on 6 March 2025).
- Ganoderma lucidum. Available online: https://pt.wikipedia.org/wiki/Ganoderma_lucidum#/media/Ficheiro:Ganoderma_lucidum_01.jpg (accessed on 6 March 2025).
- Peng, X.; Li, L.; Wang, X.; Zhu, G.; Li, Z.; Qiu, M. Antioxidant farnesylated hydroquinones from Ganoderma capense. Fitoterapia 2016, 111, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ganoderma tsugae. Available online: https://en.wikipedia.org/wiki/Ganoderma_tsugae#/media/File:Ganoderma_tsugae.jpg (accessed on 6 March 2025).
- Zhang, X.; Wu, D.; Tian, Y.; Chen, X.; Lan, J.; Wei, F.; Li, Y.; Luo, Y.; Sun, X. Ganoderma lucidum polysaccharides ameliorate lipopolysaccharide-induced acute pneumonia via inhibiting NRP1-mediated inflammation. Pharm. Biol. 2022, 60, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.-W.; Wang, J.-K.; Cheah, S.-C.; Naidu, M.; David, P.; Sabaratnam, V. A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Crit. Rev. Biotechnol. 2018, 38, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.F.; Zhu, L.F.; Wu, Z.; Wang, G.; Ahmad, Z.; Chang, M.W. Extraction of triterpenoid compounds from Ganoderma lucidum spore powder through a dual-mode sonication process. Drug Dev. Ind. Pharm. 2020, 46, 963–974. [Google Scholar] [CrossRef]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Cao, L.; Wei, X.; Xiao, H.; Wu, X. A review of Ganoderma lucidum polysaccharides: Health benefit, structure–activity relationship, modification, and nanoparticle encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Shao, Y.; Qiao, L.; Wu, L.; Sun, X.; Zhu, D.; Yang, G.; Zhang, X.; Mao, X.; Chen, W.; Liang, W.; et al. Structure Identification and Anti-Cancer Pharmacological Prediction of Triterpenes from Ganoderma lucidum. Molecules 2016, 21, 678. [Google Scholar] [CrossRef]
- Ding, L.; Shangguan, H.; Wang, X.; Liu, J.; Shi, Y.; Xu, X.; Xie, Y. Extraction, purification, structural characterization, biological activity, mechanism of action and application of polysaccharides from Ganoderma lucidum: A review. Int. J. Biol. Macromol. 2024, 288, 138575. [Google Scholar] [CrossRef]
- Du, Y.; Tian, L.; Wang, Y.; Li, Z.; Xu, Z. Chemodiversity, pharmacological activity, and biosynthesis of specialized metabolites from medicinal model fungi Ganoderma lucidum. Chin. Med. 2024, 19, 51. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Liu, Y. Supercritical carbon dioxide extraction of Ganoderma lucidum spore lipids. LWT 2016, 70, 16–23. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Advancement of Microwave-Assisted Biosynthesis for Preparing Au Nanoparticles Using Ganoderma lucidum Extract and Evaluation of Their Catalytic Reduction of 4-Nitrophenol. ACS Omega 2021, 6, 32198–32207. [Google Scholar] [CrossRef]
- Karimi, M.; Raofie, F.; Karimi, M. Production Ganoderma lucidum extract nanoparticles by expansion of supercritical fluid solution and evaluation of the antioxidant ability. Sci. Rep. 2022, 12, 9904. [Google Scholar] [CrossRef]
- Li, S.; Hou, W.; Li, Y.; Liu, Z.; Yun, H.; Liu, Q.; Niu, H.; Liu, C.; Zhang, Y. Modeling and optimization of the protocol of complex chromatography separation of cyclooxygenase-2 inhibitors from Ganoderma lucidum spore. Phytochem. Anal. 2023, 34, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-b.; Wang, J.-l.; Zhong, J.-J. Enhanced recovery of four antitumor ganoderic acids from Ganoderma lucidum mycelia by a novel process of simultaneous extraction and hydrolysis. Process. Biochem. 2013, 48, 331–339. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Johansen, K.T.; Nyberg, N.T.; Jaroszewski, J.W. Direct 13C NMR Detection in HPLC Hyphenation Mode: Analysis of Ganoderma lucidum Terpenoids. J. Nat. Prod. 2012, 75, 876–882. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J.; He, M.; Duan, T.; Yao, W. Biomass-derived carbon from Ganoderma lucidum spore as a promising anode material for rapid potassium-ion storage. J. Colloid Interface Sci. 2020, 567, 256–263. [Google Scholar] [CrossRef]
- Shao, P.; Xuan, S.; Wu, W.; Qu, L. Encapsulation efficiency and controlled release of Ganoderma lucidum polysaccharide microcapsules by spray drying using different combinations of wall materials. Int. J. Biol. Macromol. 2019, 125, 962–969. [Google Scholar] [CrossRef]
- Taşkın, H.; Kafkas, E.; Çakıroğlu, Ö.; Büyükalaca, S. Determination of volatile aroma compounds of Ganoderma lucidum by gas chromatography mass spectrometry (HS-GC/MS). Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 353–355. [Google Scholar]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma lucidum Terpenoids and Polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Sliva, D.; Loganathan, J.; Jiang, J.; Jedinak, A.; Lamb, J.G.; Terry, C.; Baldridge, L.A.; Adamec, J.; Sandusky, G.E.; Dudhgaonkar, S. Mushroom Ganoderma lucidum prevents colitis-associated carcinogenesis in mice. PLoS ONE 2012, 7, e47873. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Yadava, U.; Panwar, A.; Lucas, S.J.; Pandey, A.; Kang, S.G. Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against Dengue virus NS2B-NS3 protease. Sci. Rep. 2019, 9, 19059. [Google Scholar] [CrossRef]
- Peng, G.; Xiong, C.; Zeng, X.; Jin, Y.; Huang, W. Exploring Nutrient Profiles, Phytochemical Composition, and the Antiproliferative Activity of Ganoderma lucidum and Ganoderma leucocontextum: A Comprehensive Comparative Study. Foods 2024, 13, 614. [Google Scholar] [CrossRef]
- Gao, X.; Huo, H.; Bao, H.; Wang, J.; Gao, D. Changes of Active Substances in Ganoderma lucidum during Different Growth Periods and Analysis of Their Molecular Mechanism. Molecules 2024, 29, 2591. [Google Scholar] [CrossRef]
- Ansari, M.H.R.; Khan, W.; Parveen, R.; Saher, S.; Ahmad, S. Pharmacokinetic, Metabolomic, and Stability Assessment of Ganoderic Acid H Based Triterpenoid Enriched Fraction of Ganoderma lucidum P. Karst. Metabolites 2022, 12, 97. [Google Scholar] [CrossRef]
- Guo, Y.; Ye, H.; Wang, H.; Wang, Q.; Fan, S.; Dou, H. Asymmetrical flow field-flow fractionation combined with ultrafiltration: A novel and high-efficiency approach for separation, purification, and characterization of Ganoderma lucidum polysaccharides. Talanta 2023, 253, 124053. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J.; Szyjka, A.; Moreira, H.; Barg, E. Anticancer and Antioxidant Activities in Ganoderma lucidum Wild Mushrooms in Poland, as Well as Their Phenolic and Triterpenoid Compounds. Int. J. Mol. Sci. 2022, 23, 9359. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Soccol, C.R.; Bissoqui, L.Y.; Rodrigues, C.; Rubel, R.; Sella, S.R.; Leifa, F.; de Souza Vandenberghe, L.P.; Soccol, V.T. Pharmacological Properties of Biocompounds from Spores of the Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2016, 18, 757–767. [Google Scholar] [CrossRef]
- Lian, W.; Yang, X.; Duan, Q.; Li, J.; Zhao, Y.; Yu, C.; He, T.; Sun, T.; Zhao, Y.; Wang, W. The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2023, 229, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Andrejč, D.C.; Knez, Ž.; Marevci, M.K. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef]
- Oke, M.A.; Afolabi, F.J.; Oyeleke, O.O.; Kilani, T.A.; Adeosun, A.R.; Olanbiwoninu, A.A.; Adebayo, E.A. Ganoderma lucidum: Unutilized natural medicine and promising future solution to emerging diseases in Africa. Front. Pharmacol. 2022, 13, 952027. [Google Scholar] [CrossRef]
- Sun, L.X.; Li, W.D.; Lin, Z.B.; Duan, X.S.; Li, X.F.; Yang, N.; Lan, T.F.; Li, M.; Sun, Y.; Yu, M.; et al. Protection against lung cancer patient plasma-induced lymphocyte suppression by Ganoderma lucidum polysaccharides. Cell. Physiol. Biochem. 2014, 33, 289–299. [Google Scholar] [CrossRef]
- Bade, B.C.; Cruz, C.S.D. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest. Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung cancer: Diagnosis and management. Am. Fam. Physician 2007, 75, 56–63. [Google Scholar]
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef]
- Petrella, F.; Rizzo, S.; Attili, I.; Passaro, A.; Zilli, T.; Martucci, F.; Bonomo, L.; Del Grande, F.; Casiraghi, M.; De Marinis, F.; et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr. Oncol. 2023, 30, 3160–3175. [Google Scholar] [CrossRef]
- Lin, T.Y.; Hsu, H.Y. Ling Zhi-8 reduces lung cancer mobility and metastasis through disruption of focal adhesion and induction of MDM2-mediated Slug degradation. Cancer Lett. 2016, 375, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Navgeet; Kumar, S. Ganoderma lucidum targeting lung cancer signaling: A review. Tumour Biol. 2017, 39, 1010428317707437. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.J.; Li, S.Q.; Lin, H. Preliminary Efficacy and Safety of Reishi & Privet Formula on Quality of Life Among Non-Small Cell Lung Cancer Patients Undergoing Chemotherapy: A Randomized Placebo-Controlled Trial. Integr. Cancer Ther. 2020, 19, 1534735420944491. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, R.; Hou, A.; Tan, S.; Liu, X.; Tan, P.; Huang, X.; Wang, J. Ganoderma triterpenoids attenuate tumour angiogenesis in lung cancer tumour-bearing nude mice. Pharm. Biol. 2020, 58, 1061–1068. [Google Scholar] [CrossRef]
- Chen, B.; Tian, J.; Zhang, J.; Wang, K.; Liu, L.; Yang, B.; Bao, L.; Liu, H. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia 2017, 120, 6–16. [Google Scholar] [CrossRef]
- Xie, J.T.; Wang, C.Z.; Wicks, S.; Yin, J.J.; Kong, J.; Li, J.; Li, Y.C.; Yuan, C.S. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp. Oncol. 2006, 28, 25–29. [Google Scholar]
- Pazzi, F.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; García-Gordillo, M.; Gusi, N.; Collado-Mateo, D. Effects of Ganoderma lucidum and Ceratonia siliqua on blood glucose, lipid profile, and body composition in women with fibromyalgia. Nutr. Hosp. 2021, 38, 139–145. [Google Scholar] [CrossRef]
- Pang, G.; Wei, S.; Zhao, J.; Wang, F.J. Improving nanochemoimmunotherapy efficacy by boosting “eat-me” signaling and downregulating “don’t-eat-me” signaling with Ganoderma lucidum polysaccharide-based drug delivery. J. Mater. Chem. B 2023, 11, 11562–11577. [Google Scholar] [CrossRef]
- Available online: https://www.foodicine.co.in/contract-manufacturing-ganoderma-lucidum-reishi-mushroom-extract-nutraceuticals (accessed on 6 February 2025).
- Available online: https://mountainroseherbs.com/reishi-mushroom?srsltid=AfmBOopSLXsAhTc5FLjLwo5gH6WX7h_u9bnR1zUtxzFfWzRFazWt6bYs (accessed on 6 February 2025).
- Available online: https://www.bio-botanica.com/product/reishi-ganoderma-lucidum-mushroom-extract/ (accessed on 6 February 2025).
- Available online: https://www.bulksupplements.com/pt-pt/products/podeextratodecogumeloreishi?srsltid=AfmBOooFiFIOLWGlOfTOaElH8NeFkeVzd4NgwOw_mf2-AEPyWBpaX8b_ (accessed on 6 February 2025).
- Available online: https://www.tri-k.com/solutions/p/naturepep-ganoderma (accessed on 6 February 2025).
- Wu, G.S.; Guo, J.J.; Bao, J.L.; Li, X.W.; Chen, X.P.; Lu, J.J.; Wang, Y.T. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—A review. Expert Opin. Investig. Drugs 2013, 22, 981–992. [Google Scholar] [CrossRef]
- Cancemi, G.; Caserta, S.; Gangemi, S.; Pioggia, G.; Allegra, A. Exploring the Therapeutic Potential of Ganoderma lucidum in Cancer. J. Clin. Med. 2024, 13, 1153. [Google Scholar] [CrossRef]
- Mahmoud, N.N. Colorectal Cancer: Preoperative Evaluation and Staging. Surg. Oncol. Clin. N. Am. 2022, 31, 127–141. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, X.T.; Qin, F.Y.; Zhang, H.X.; Cheng, Y.X. Gancochlearols E-I, meroterpenoids from Ganoderma cochlear against COX-2 and triple negative breast cancer cells and the absolute configuration assignment of ganomycin K. Bioorg. Chem. 2021, 109, 104706. [Google Scholar] [CrossRef]
- Zhong, C.; Li, Y.; Li, W.; Lian, S.; Li, Y.; Wu, C.; Zhang, K.; Zhou, G.; Wang, W.; Xu, H.; et al. Ganoderma lucidum extract promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem. Toxicol. 2023, 174, 113654. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. The in vitro and in vivo experimental evidences disclose the chemopreventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin. Exp. Metastasis 2010, 27, 361–369. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. BioMed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Yang, Y.; Zhang, J.; Xu, Y.; Lu, X. The Natural Products and Extracts: Anti-Triple-Negative Breast Cancer in Vitro. Chem. Biodivers 2021, 18, e2001047. [Google Scholar] [CrossRef]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget 2018, 9, 35907. [Google Scholar]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Sullivan, B.A.; Noujaim, M.; Roper, J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 177–194. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.E.; Yi, Y.J.; Guo, Y.T.; Wang, R.C.; Hu, Q.L.; Xiong, X.Y. Inhibition of migration and induction of apoptosis in LoVo human colon cancer cells by polysaccharides from Ganoderma lucidum. Mol. Med. Rep. 2015, 12, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H. Brief Overview of Prostate Cancer Statistics, Grading, Diagnosis and Treatment Strategies. Adv. Exp. Med. Biol. 2018, 1095, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, B.Z.; Wasser, S.P.; Nevo, E.; Mahajna, J. Coprinus comatus and Ganoderma lucidum interfere with androgen receptor function in LNCaP prostate cancer cells. Mol. Biol. Rep. 2008, 35, 107–117. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Guo, Y.; Lai, H.; Cheng, S.; Chen, Z.; Li, H.; Li, Q.; Mao, X. Water extract of sporoderm-broken spores of Ganoderma lucidum elicits dual antitumor effects by inhibiting p-STAT3/PD-L1 and promoting ferroptosis in castration-resistant prostate cancer. J. Funct. Foods 2024, 113, 106018. [Google Scholar] [CrossRef]
- Stanley, G.; Harvey, K.; Slivova, V.; Jiang, J.; Sliva, D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem. Biophys. Res. Commun. 2005, 330, 46–52. [Google Scholar] [CrossRef]
- Jiang, J.; Slivova, V.; Valachovicova, T.; Harvey, K.; Sliva, D. Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int. J. Oncol. 2004, 24, 1093–1099. [Google Scholar]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Li, N.; Hu, Y.L.; He, C.X.; Hu, C.J.; Zhou, J.; Tang, G.P.; Gao, J.Q. Preparation, characterisation and anti-tumour activity of Ganoderma lucidum polysaccharide nanoparticles. J. Pharm. Pharmacol. 2010, 62, 139–144. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Dhasarathan, P.; Ranjitsingh, A.J.A.; Al-Humaid, L.A. Ganoderma lucidum inspired silver nanoparticles and its biomedical applications with special reference to drug resistant Escherichia coli isolates from CAUTI. Saudi J. Biol. Sci. 2020, 27, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Kızılbey, K. Green Synthesis of ZnO Nanoparticles via Ganoderma Lucidum Extract: Structural and Functional Analysis in Polymer Composites. Gels 2024, 10, 576. [Google Scholar] [CrossRef]
- Zarrabi, A.; Abadi, M.A.A.; Khorasani, S.; Mohammadabadi, M.-R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, T.; He, J.; Zhang, Y.; Gu, P.; Qiu, T.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Adjuvanticity of Ganoderma lucidum polysaccharide liposomes on porcine circovirus type-II in mice. Int. J. Biol. Macromol. 2019, 141, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Răut, I.; Suica-Bunghez, R.; Firinca, C.; Radu, N.; Gurban, A.M.; Preda, S.; Alexandrescu, E.; Doni, M.; Jecu, L. Ganoderma lucidum-Mediated Green Synthesis of Silver Nanoparticles with Antimicrobial Activity. Materials 2023, 16, 4261. [Google Scholar] [CrossRef]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef]
- Karwa, A.; Gaikwad, S.; Rai, M.K. Mycosynthesis of silver nanoparticles using Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.:Fr.) P. Karst. and their role as antimicrobials and antibiotic activity enhancers. Int. J. Med. Mushrooms 2011, 13, 483–491. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Available online: https://www.exeleadbiopharma.com (accessed on 1 August 2024).
- Cheng, C.R.; Yang, M.; Guan, S.H.; Wu, X.H.; Pang, X.Y.; Wang, Y.; Yang, Y.; Ding, J.; Guo, D.A. Pharmacokinetics of ganoderic acid D and its main metabolite by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2013, 930, 1–6. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fourniols, T.; Labrak, Y.; Préat, V.; Beloqui, A.; des Rieux, A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 2022, 16, 7168–7196. [Google Scholar] [CrossRef] [PubMed]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Tsai, I.L.; Tsai, C.Y.; Kuo, L.L.; Woung, L.C.; Ku, R.Y.; Cheng, Y.H. PLGA nanoparticles containing Lingzhi extracts rescue corneal epithelial cells from oxidative damage. Exp. Eye Res. 2021, 206, 108539. [Google Scholar] [CrossRef]
- Available online: https://media.springernature.com/lw685/springer-static/image/chp%3A10.1007%2F978-3-319-99602-8_17/MediaObjects/460157_1_En_17_Fig1_HTML.png (accessed on 3 August 2024).
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F. Research Progress on the Anticancer Activities and Mechanisms of Polysaccharides from Ganoderma. Front. Pharmacol. 2022, 13, 891171. [Google Scholar] [CrossRef]
- Gao, X.; Homayoonfal, M. Exploring the anti-cancer potential of Ganoderma lucidum polysaccharides (GLPs) and their versatile role in enhancing drug delivery systems: A multifaceted approach to combat cancer. Cancer Cell Int. 2023, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhao, J.; Tao, Y.; Liu, J.; Wang, L.; He, J.; Lei, J.; Liu, K. pH and glutathione dual responsive nanoparticles based on Ganoderma lucidum polysaccharide for potential programmable release of three drugs. Chem. Eng. J. 2020, 389, 124418. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Yang, F.; Tan, W.; Li, X.; Jiao, C.; Huang, R.; Yang, B.B. The anti-cancer components of Ganoderma lucidum possesses cardiovascular protective effect by regulating circular RNA expression. Oncoscience 2016, 3, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Nayir, E.; Kirca, O.; Ozdogan, M. Ganoderma Lucidum (Reishi Mushroom) and cancer. J. Buon. 2016, 21, 792–798. [Google Scholar]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Zeyaullah, M.; Alsayegh, A.A.; Mahmood, S.E.; AlShahrani, A.M.; Khan, M.S.; Shama, E.; Hamouda, A.; Elbendary, E.Y.; et al. Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action. Nutrients 2023, 15, 1874. [Google Scholar] [CrossRef]
- Chan, S.W.; Tomlinson, B.; Chan, P.; Lam, C.W.K. The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm. Biol. 2021, 59, 1161–1171. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, J.; Li, Y.; Zhu, L.; Jin, M.; Wang, L.; Liu, J.; Lei, J.; Li, Z. Self-Assembled pH-Sensitive Nanoparticles Based on Ganoderma lucidum Polysaccharide-Methotrexate Conjugates for the Co-delivery of Anti-tumor Drugs. ACS Biomater. Sci. Eng. 2021, 7, 3764–3773. [Google Scholar] [CrossRef]
- Taléns-Visconti, R.; Díez-Sales, O.; de Julián-Ortiz, J.V.; Nácher, A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. Int. J. Mol. Sci. 2022, 23, 4249. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef] [PubMed]
- Krobthong, S.; Yingchutrakul, Y.; Visessanguan, W.; Mahatnirunkul, T.; Samutrtai, P.; Chaichana, C.; Papan, P.; Choowongkomon, K. Study of the Lipolysis Effect of Nanoliposome-Encapsulated Ganoderma lucidum Protein Hydrolysates on Adipocyte Cells Using Proteomics Approach. Foods 2021, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zhong, D.; Su, L.; Lin, Z.; Yang, B. Preventive and therapeutic effect of Ganoderma lucidum on kidney injuries and diseases. Adv. Pharmacol. 2020, 87, 257–276. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef]


| Extraction Methods | References | |
|---|---|---|
| Hot Water extraction | The dried mushroom or mycelium is boiled in water, and the water-soluble polysaccharides are extracted. After the extraction, the solution is concentrated and then dried to obtain the polysaccharide-rich extract. Most common method for extracting polysaccharides from GL. | [42,43,44] |
| Ethanol or Methanol extraction | The dried mushroom or mycelium is soaked in ethanol or methanol to solubilize the compounds of interest. The solvent is then evaporated to obtain the extract. Most common method for extracting triterpenoids, sterols, and other secondary metabolites. | [45,46] |
| Supercritical Fluid Extraction | Supercritical fluid extraction uses carbon dioxide (CO2) as a solvent at its supercritical state (a state where it exhibits both liquid and gas-like properties). Most common method for extracting triterpenoids and essential oils. | [46,47] |
| Enzyme-Assisted Extraction | Enzymes can be used to enhance the extraction of specific compounds from GL. Most common method for extracting β-glucans from cellulases. | [45,46] |
| Nanoparticle- Assisted Extraction | This method involves the use of nanoparticles such as liposomes or magnetic nanoparticles to extract bioactive compounds from GL. The nanoparticles can selectively capture and improve the bioavailability, stability, and controlled release of the active substances, which are crucial for enhancing their therapeutic effects. | [46,48,49] |
| Supercritical CO2 with Nanotechnology | This extraction technique combines the use of supercritical carbon dioxide (SCO2), a highly efficient and eco-friendly solvent, with nanotechnology to improve the purity and yield of bioactive compounds. The use of nanoparticles in this process helps isolate specific compounds more effectively, while maintaining the bioactivity of the extracted molecules. | [46,47,49] |
| Nanoencapsulation | Nanoencapsulation involves embedding the bioactive compounds within nanocarriers, such as lipid- or polymer-based nanoparticles. This method enhances the solubility, stability, and bioavailability of the compounds, ensuring their safe and efficient delivery to target areas, such as cancer cells or the immune system. | [46] |
| Magnetic Nanoparticle Extraction | In this approach, magnetic nanoparticles are used to capture bioactive molecules from GL extracts. The nanoparticles are then separated from the mixture using a magnetic field, making the extraction process more efficient and allowing for the easy isolation of specific bioactive compounds. | [43,46] |
| Separation methods | ||
| Solvent Extraction | A straightforward method where the dried mushroom material is soaked in a suitable solvent (such as water, ethanol, methanol, or a mixture of solvents) to extract the bioactive compounds. The solvent is then evaporated to obtain the extract. | [45,46] |
| Liquid–Liquid Extraction | Liquid–liquid extraction involves the partitioning of compounds between two immiscible solvents. This method can be useful for the extraction and concentration of specific compounds from the crude extract. | [50,51] |
| Solid-Phase Extraction (SPE) | SPE is a chromatographic technique that uses a solid-phase material (such as silica gel or other resins) to selectively adsorb and separate the target compounds from the extract. | [52] |
| Centrifugal Partition Chromatography (CPC) | CPC is a liquid–liquid chromatographic technique that uses a biphasic solvent system to separate compounds based on their partitioning between the two liquid phases. | [53] |
| High-Performance Liquid Chromatography (HPLC) | HPLC is a powerful analytical and preparative technique used to separate and purify compounds based on their chemical properties. | [45,54] |
| Gas Chromatography (GC) | GC is typically used for the analysis and separation of volatile compounds present in GL, such as essential oils. | [55] |
| Size-Exclusion Chromatography (SEC) | SEC is used to separate compounds based on their molecular size. It is particularly useful for the separation of polysaccharides from GL. | [45] |
| Purification methods | ||
| Chromatography | Column chromatography, HPLC, and flash chromatography can be employed to separate and isolate individual compounds or groups of compounds. | [45,54] |
| Fractionation | The chromatographic process often generates multiple fractions containing different compounds. Each fraction can be further analyzed and tested for bioactivity to identify the most promising fractions for further purification. | [45,46] |
| Crystallization | For some compounds, crystallization may be employed to obtain highly purified and well-defined crystals. | [47,54] |
| Centrifugation | Centrifugation can be used to separate solid particles or aggregates from the purified compounds. | [45,48] |
| Toxicological Properties | Potential Effects | References |
|---|---|---|
| Allergic responses | [7,65,72] | |
| Anticoagulants or antiplatelet medications | ↑ Anticoagulant effect ↑ Prothrombin time ↑ Effects of clotting factors | [23,65,73] |
| Gastrointestinal bleeding or gastric ulcers | ↑ Bleeding risk ↑ Gastric irritation | [23,65,72] |
| Hypoglycemia | ↓ Blood sugar levels | [23,65,74] |
| Liver function | Subchronic toxicity on the liver observed in rats given GL extract at doses exceeding 1.2 g per kilogram of body weight. | [23,65,72,73,74,75,76,77,78,79,80] |
| Toxic effects on cells | ↓ Cell viability at higher concentrations than those required for stimulatory results. | [23,65,74,75,77] |
| Antihypertensive effect | ↑ Non-rapid eye movement sleep, significantly in rats, potentially linked to tumor necrosis factor-α. ↑ Effects of antihypertension drugs. ↑ Hypotension in individuals with cardiac disorder. | [39,65,73,74,77,78,81] |
| Toxic and teratogenic effects | In a dose- and time-dependent manner in zebrafish embryos. | [23,65,79] |
| Anticancer agent | ↑ Toxicity when using it in conjunction with chemotherapy. | [23,57,65,72,73,74,75,76,77,78,79,80,82,83] |
| Antibacterial effect | ↑ Activity of some antibiotics | [23,57,65,72,73,74,75,76,77,78,79,80,82,83] |
| Dosage Forms | Posology |
|---|---|
Tablets or Capsules | 1–3 capsules/tablets of GL per day. |
Powder | 1–3 g of GL powder per day. |
Extracts | Can vary depending on the concentration and potency of the extract. |
Tea or Decoction | Can vary depending on the concentration, brewing time, and individual preferences. |
Topical formulations | May depend on the specific formulation and intended use. |
| Anticancer Activity | GL | GL with NPs | References |
|---|---|---|---|
| Induction of Apoptosis | Promotes apoptosis in cancer cells through bioactive compounds like polysaccharides and triterpenes. | Targeted delivery via nanoparticles enhances apoptotic efficacy in specific tumor cells. Example: in vivo study using AuNPs focused on the incorporation of GLPs to impair breast cancer growth and induce the apoptosis of cancer cells in a tumor-bearing animal model (mice). | [32,77,79,82,102,111,112,133,134] |
| Inhibition of Angiogenesis | Inhibits the formation of new blood vessels that supply tumors, limiting their growth. | Sustained delivery of GL compounds improves the inhibition of angiogenesis at tumor sites. Example: in vivo study was performed in a tumor-bearing model (mice) and it was observed that GL polymeric NPs (loaded with triterpenoids) induced a significant decrease in blood vessels formation, thus highlighting their potential effect in this regard. | [82,90,92,102,103,135] |
| Immune Modulation | Stimulates immune cells like T-lymphocytes and NK cells, enhancing the anticancer immune response. | Nanoparticles enable more effective immune modulation, concentrating effects at target tissues. Example: in vitro study using mice cell lines used AuNPs for GLP delivery and it was observed that these conjugations contributed to enhancing and improving immunoregulatory properties in cancer therapy. | [90,92,96,98,107,108,111,112,135] |
| Reduction in Inflammation | Reduces chronic inflammation, which is linked to cancer progression. | Nanotechnology amplifies anti-inflammatory effects with controlled release at target tissues. Example: In vitro study focused on evaluating the efficacy of selenium NPs loaded with GLPs in reducing inflammation and combating cancer. The study was performed in a murine cell line and results verified great anti-inflammatory response by monitoring the secretion of pro- and anti-inflammatory cytokines. | [90,92,105,107,111,112,135] |
| Tumor Specificity | Broad and nonspecific action, affecting both healthy and cancerous cells. | Functionalized nanoparticles ensure greater specificity to cancer cells, reducing collateral damage. Example: In vitro study performed in a simulated intracellular microenvironment of cancer cells used GLP-based polymeric NPs for drug delivery. Compared to usual physiological conditions, under tumor-simulating conditions, NPs obtained greater results regarding drug release, thus highlighting their promising application in increasing bioavailability in cancer therapy. | [105,107,111,112,136] |
| Bioavailability | Limited bioavailability due to instability and poor solubility of bioactive compounds. | Nanotechnology improves stability, solubility, and efficacy of compounds in biological environments. Example: Regarding the use of polymeric GLP-NPs, an in vivo study used them to target a tumor-bearing animal model (mice) and understand related anticancer properties. In consideration of this, results found that these GLP-NPs were mainly responsible for tumor growth impairment, thus suggesting higher tumor specificity and promising therapeutic application. | [54,115,119,120,136] |
| Therapeutic Effect | Action Mechanisms | Model | Reference |
|---|---|---|---|
| Anticancer | |||
| In vitro In vivo | ↑ CD47/CD8+ ratio ↑ Immune system activity ↑ Apoptosis ↑ Expression of Bax and caspase-3 ↑ mRNA expression ↑ Protein production ↑ Population of Tc-cells ↓ Activation of Akt and its downstream regulator | Cell lines related to melanoma, lung cancer, prostate cancer, colorectal cancer, breast cancer, osteosarcoma, and human prostate cancer. | [23,32,65,69,73,90,133,138] |
| ↓ Cellular levels; Activation of Akt and its downstream regulators; Inhibition of STAT3 signaling; cell viability, autophagy flux, Rac activity and downstream signaling pathway, osteosarcoma cell activity, and expression of anti-apoptotic proteins; ↑ Autophagy through Akt/TOR signaling, apoptosis with cell cycle arrest via NAG-1 induction, and autophagosome accumulation; ↓ Tumor volume; ↓ Growth; ↓ Metastasis; Progression and release of matrix metalloproteinases; ↑ Cytotoxicity; ↑ Apoptosis; ↑Immunomodulatory activity. | Breast cancer, mammary adenocarcinoma, ascitic tumor, cervical carcinoma, hepatoma, lung tumor, and glioma | ||
| Antibacterial | |||
| In vitro In vivo | ↑ Cell permeability and leakage; ↑ Polysaccharides binding to leukocyte surfaces; Activation of Th/NK/macrophages; Upregulation of IgA/RD-5, 6/TLR4 mRNA levels; Improved attachment and permeability, increased oxidative stress, and killing of pathogens. | [23,65,139] | |
| ↓ Firmicutes-to-Bacteroidetes ratio; ↓ Proteobacteria abundance; ↓ Levels of Aerococcus, Ruminococcus, and Corynebacterium. | Mice with dysbiosis and rats with type-2 diabetes | ||
| Anti-obesity | |||
| In vitro In vivo | ↓ mRNA expression of SREBP-1c, C/EBPa, and PPARy; Inhibition of MAPK pathway increases energy expenditure with the inhibition of 3T3-L1 pre-adipocytes proliferation and differentiation. | Murine pre-adipocyte cells; M. miehei lipase. | [23,65,140] |
| ↓ Body and liver weight; ↓ Subcutaneous fat; ↑ Microbiome–gut–liver and gut–brain axes; Regulate metabolism by modulating gut microbiota composition; ↑ Levels of Clostridiales, Lachnospiraceae, Oscillospira, and Ruminococcaceae; ↓ Levels of Lactobacillus, Bifidobacterium, and Roseburia. | High-fat diet-fed; MK-fat mice. | ||
| Hepatoprotective | |||
| In vivo | ↑ Antioxidant activity; ↓ Oxidative stress; Regulating key molecular pathways: FOXO4/mTOR/SIRT1; ↓ Expression of hepatic glucose regulatory enzymes, p-AMPK/AMPK, lipid peroxidation, protein oxidation, MDA, and heat shock proteins; ↓ Expression of inflammatory markers: iNOS, COX2, TNF-α, NF-KB, and IL-6; ↑ Superoxide dismutase activity, lipid peroxidation, and apoptosis; Inhibits fatty acid synthesis; ↓ Serum ALT levels indicating its potential in protecting liver health. | [23,65,141] | |
| Anti-dyslipidaemia | |||
| In vitro In vivo | ↓ 3T-L1 pre-adipocytes proliferation/differentiation; ↓ Key lipid-metabolizing enzymes. | [23,65,142] | |
| ↓ Haemorrhage/thrombosis; ↓ Stroke, cardiac necrosis; ↓ Atherosclerotic plaque; ↑ HDL-c; ↑ Total BAs. | |||
| Cardioprotective | |||
| In vitro In vivo: | ↓ Cardiomyocyte necrosis; Reperfusion contracture; Antioxidant effects; Activation of PI3K/AKT signaling pathway; Modulation of specific molecular targets. | [23,65,142] | |
| ↓ Haemorrhage/thrombosis; ↓ Stroke; ↓ Cardiac necrosis; ↓ Atherosclerotic plaque; ↑ Anti-angiogenic; ↑ Antioxidant properties. | |||
| Antidiabetic | |||
| In vitro In vivo | ↓ Hepatic PECK gene expression; ↓ Glucose level; ↓ SREBP1; ↓ FAS-mRNA expression; ↓ mRNA level for gluconeogenesis enzymes and H2O; | Human breast adenocarcinoma cell line (MCF-7/ADR) and HepG2 cells | [23,65,142] |
| ↑ Glucose uptake ↑ Insulin level ↑ Hepatic glycogen level ↑ Insulin sensitivity ↑ Glycogen synthesis ↑ Glucose transport via the PI3K/Akt pathway. | Mice and rat models | ||
| Immunomodulatory | |||
| In vitro In vivo | Upregulation of immunomodulators IL-12, IF-4, IL-2, IL-6, IL-4, IL-17, TNF-a, IFN-%, granulysin, perforin, and NKG2D/NCR cell surface receptors; ↑ Production of nitric oxide (NO); Activates ERK, JNK, and p38 signaling pathways. | Mice, rats, and pigs | [23,32,65,69,73,90,100,133,138,139,140,141,142] |
| Activates humoral and cellular immune responses; Promotes antigen-specific IgG production; Enhances haematopoiesis, macrophage phagocytosis, and proliferation of spleen lymphocytes and undifferentiated spleen cells; Stimulates the activity of T/B-cells, LAK cells, CD3+, CD4+, and CD8+ T-cells; Activation of NF-KB/MAPK, NK cells, NF cells, TNF activity, and cytokine secretion. | |||
| Anti-inflammatory | |||
| In vitro In vivo | ↓ Expression of NF-κB, MAPK, and AP-1; ↓ Activity of G-CSF, IL-1α, MCP-5, and MIP3α; ↓ mRNA expression of CHUK and NFκB1/p150; ↓ NO, MDA, TNF-α, IL-1β, and IL-6 levels; ↓ iNOS and COX-2 expression; ↑ level of SOD. | [17,65] | |
| Suppression of inflammatory mediators TNF-α, IFN-γ, IL-1β, IL-6, MCP1, and hydroxyproline; ↑ Expression of keratinocyte differentiation markers; ↓ Serum Ig-E level; ↑ SOD/TOAC level. | |||
| Neuroprotective | |||
| In vivo | Downregulating caspases-3, -8, and -9; Modulation of Bcl-2/Bax ratio; Protects DNA and cell membranes from the harmful effects of radiation; ↑ Cerebral blood flow; ↓ Neuronal damage and apoptosis; Promotes mitochondrial movement; Enhances the production of anti-inflammatory cytokines; Improves spatial learning and memory-related behavior; ↓ Production of pro-inflammatory cytokines induced by Aβ and oxidative stress induced by spinal cord injury; Inhibits apoptosis caused by hydrogen peroxide, lipid peroxidation, and GSH. | [21,65] | |
| Anti-epileptic | |||
| In vivo | ↓ Hippocampal neurons; ↓ Number of excitatory neurons and delays the onset of epilepsy; Prevents CA3 degeneration; ↓ Astrocytic reactivity; ↓ Levels of pro-inflammatory cytokines; ↑ Cytokines IL-1B and TNF-α; threshold for psychomotor seizures; ↑ Content of GABA; ↓ Seizures and convulsions. | [21,65] | |
| Sedative | |||
| In vivo | Inducing a hypnotic effect in rat and mice models; Promotes relaxation and sleep; Modulation of cytokines, specifically TNF-a; Sedative effects; Regulates sleep-related processes; ↓ Sleep latency; ↑ Sleep duration. | [21,65] | |
| Nootropic | |||
| In vivo | Improving cerebral blood flow, brain energy supply, memory-related neurotransmitters, and cognition; ↓ Brain cell apoptosis and ameliorates spatial memory deficits; Inhibits acetylcholinesterase activity; Antioxidant properties; Improves anterograde amnesia. | [23,65] | |
| Antidepressant | |||
| In vivo | Blocking 5-HT2A receptors; Inhibiting MAO; Antagonizing preganglionic 5-HT receptors; ↓ Depression-related activities. | [23,65] | |
| Anti-osteoporotic | |||
| In vivo | Promoting bone healing; Regeneration; ↑ Trabecular bone volume; Inhibits osteoclastogenesis and reverses bone loss; ↑ OPG/RANKL ratio; ↓ Bone differentiation; Formation of RANKL-induced osteoclast; Facilitates cross-talk between the Wnt/B-catenin and BMP/SMAD signaling pathways; Protective effects on bone. | [23,65] | |
| Anxiolytic | |||
| In vivo | ↓ Anxiety levels at doses ranging between 20 and 400 mg/kg. | Swiss Albino mice | [23,65] |
| Radioprotective | |||
| In vivo | Antioxidant and free radical scavenging properties; ↑ Levels of GSH; Protection against radiation-induced damage; ↓ Reactive oxygen species (ROS); Restoration of TNF-d production; Repair of damaged T-cells; Protection against gamma rays; Reducing DNA strand breaks and micronuclei formation; ↓ MDA levels; Promoting the recovery of SOD activity. | [23,65] | |
| Activity | Effect | References |
|---|---|---|
| Anticancer | ↑Mitogenic reactivity to concanavalin-A and phytohemagglutinin; Lymphocyte; CD3/CD4 and natural killer cells activity; CD3/CD4/CD8/CD56, IL-2 IL-6, IFN-Y, and NK activity. | [23,65,142,147,148] |
| Antioxidant and hepatoprotective | ↑Antioxidant activity ↓Thiobarbituric acid, 8-OH-dG. GOT and GPT levels; ↓Triglycerides; ↑HDL-c. | |
| Cardioprotective | ↓Blood pressure and atherosclerosis; Improve chest pain/palpitation/angina pectoris; ↓Diastolic/systolic pressure, TAG, MDA, CEC, EPC levels; ↑capillary loop diameter, density, RBC velocity, and HDL-cholesterol. | |
| Antidiabetic | ↓Cell resistance to insulin and HbA1c, FPG, and PPG values; The antiplatelet effect of GL, though contains a high level of adenosine; Lack of effect on platelets aggregation. | |
| Anti-histaminic | Most symptoms were relieved in hay fever patients due to restored normal balance between Th1 and Th2. | |
| Antiviral | Inhibition of virus replication in hepatitis-B and HIV patients; ↓HBeAg. HBV, DNA, and liver enzymes. | |
| Immunomodulatory | ↑CD3+, CD4+, CD8+ T cells. | |
| Anti-fibromyalgia | Aerobic endurance was improved along with lower body flexibility and velocity via the antioxidant effect of GL. | |
| Anti-Alzheimer’s | ↓Ab, 3, 4-methylenedioxyamphetamine, Fasl, caspase-3, and tau hyperphosphorylation. | |
| Anti-macular degeneration | Improvement of pre-ganglionic retinal elements in age-related macular degeneration patients with an increase in mfERG R1 and R2, and RADs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eira, A.; Gonçalves, M.B.S.; Fongang, Y.S.F.; Domingues, C.; Jarak, I.; Mascarenhas-Melo, F.; Figueiras, A. Unlocking the Potential of Ganoderma lucidum (Curtis): Botanical Overview, Therapeutic Applications, and Nanotechnological Advances. Pharmaceutics 2025, 17, 422. https://doi.org/10.3390/pharmaceutics17040422
Eira A, Gonçalves MBS, Fongang YSF, Domingues C, Jarak I, Mascarenhas-Melo F, Figueiras A. Unlocking the Potential of Ganoderma lucidum (Curtis): Botanical Overview, Therapeutic Applications, and Nanotechnological Advances. Pharmaceutics. 2025; 17(4):422. https://doi.org/10.3390/pharmaceutics17040422
Chicago/Turabian StyleEira, Ana, Maria Beatriz S. Gonçalves, Yannick Stéphane Fotsing Fongang, Cátia Domingues, Ivana Jarak, Filipa Mascarenhas-Melo, and Ana Figueiras. 2025. "Unlocking the Potential of Ganoderma lucidum (Curtis): Botanical Overview, Therapeutic Applications, and Nanotechnological Advances" Pharmaceutics 17, no. 4: 422. https://doi.org/10.3390/pharmaceutics17040422
APA StyleEira, A., Gonçalves, M. B. S., Fongang, Y. S. F., Domingues, C., Jarak, I., Mascarenhas-Melo, F., & Figueiras, A. (2025). Unlocking the Potential of Ganoderma lucidum (Curtis): Botanical Overview, Therapeutic Applications, and Nanotechnological Advances. Pharmaceutics, 17(4), 422. https://doi.org/10.3390/pharmaceutics17040422











