A Design of Experiments Approach to Identify Critical Processing Parameters for Manufacture of an Autologous Platelet Gel for Diabetic Foot Ulcer
Abstract
1. Introduction
2. Materials and Methods
2.1. Fresh Human Blood
2.2. Standard Protocol for RAPID Biodynamic Haematogel Manufacture
2.3. Development of a Design of Experiments (DoE) Approach
2.4. RAPID Gel Quality Attributes
2.5. Human Thrombin–Antithrombin Complexes
2.6. Statistical Analysis
3. Results and Discussion
3.1. Gel Manufacture
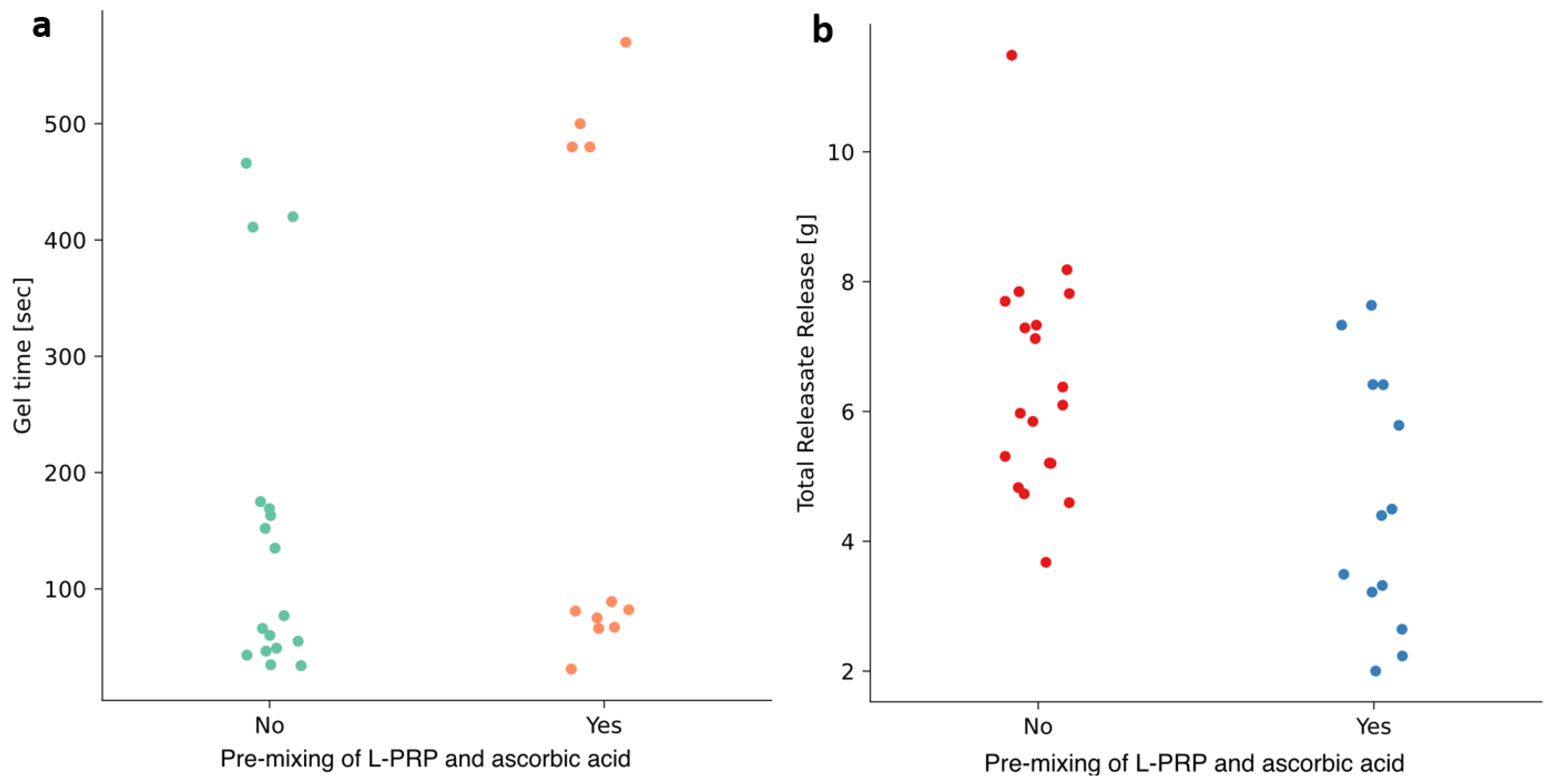
3.2. Effects of Temperature and Pre-Mixing of L-PRP with Ascorbic Acid
3.3. Statistical Evaluation of Processing Parameter Influences and Interactions
3.3.1. Exudation of Releasate
3.3.2. Time to Gel
3.4. Discretized Response Modeling for ‘Successful’ Gel Formation
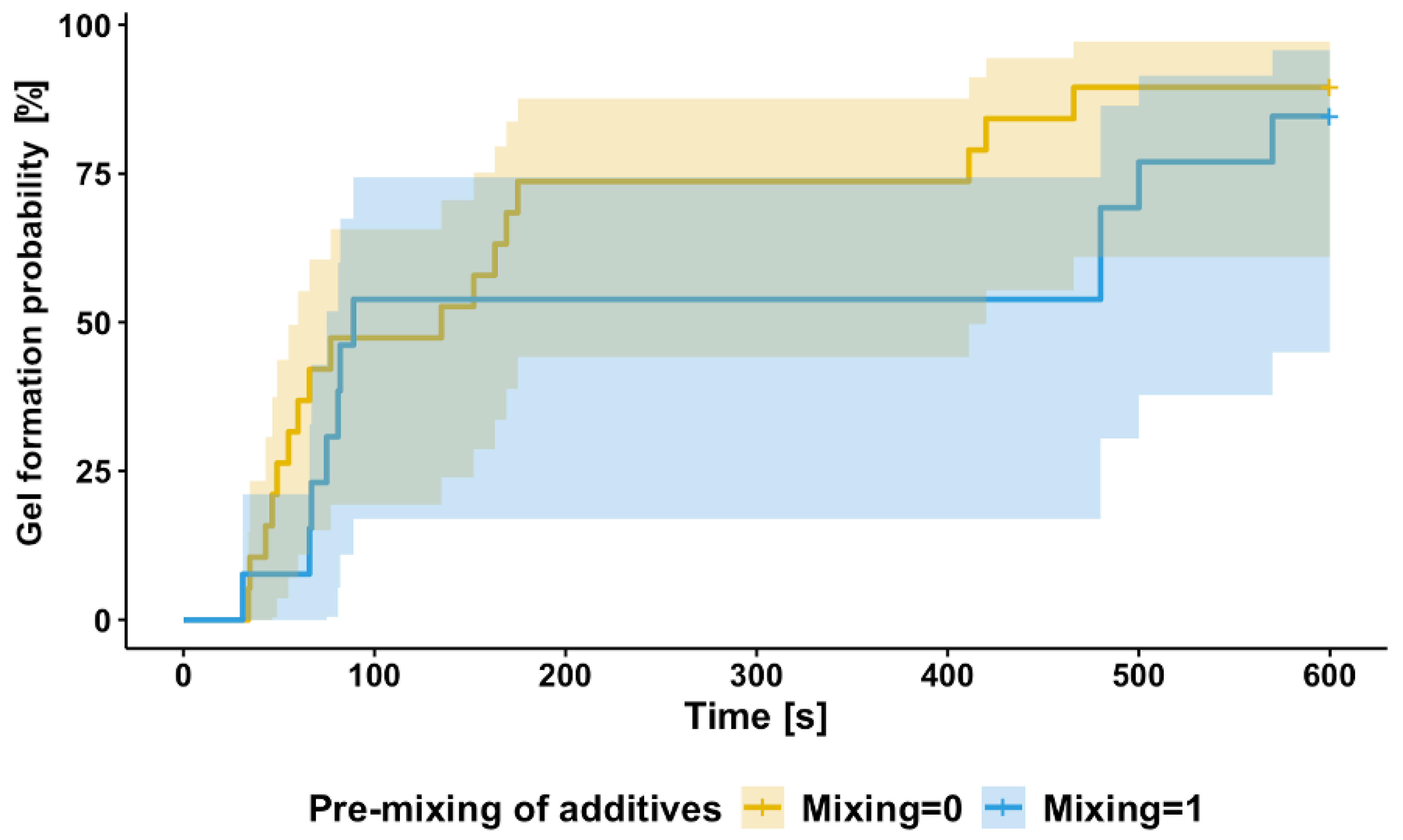
3.5. Time-to-Gel Analysis: Survival Modeling
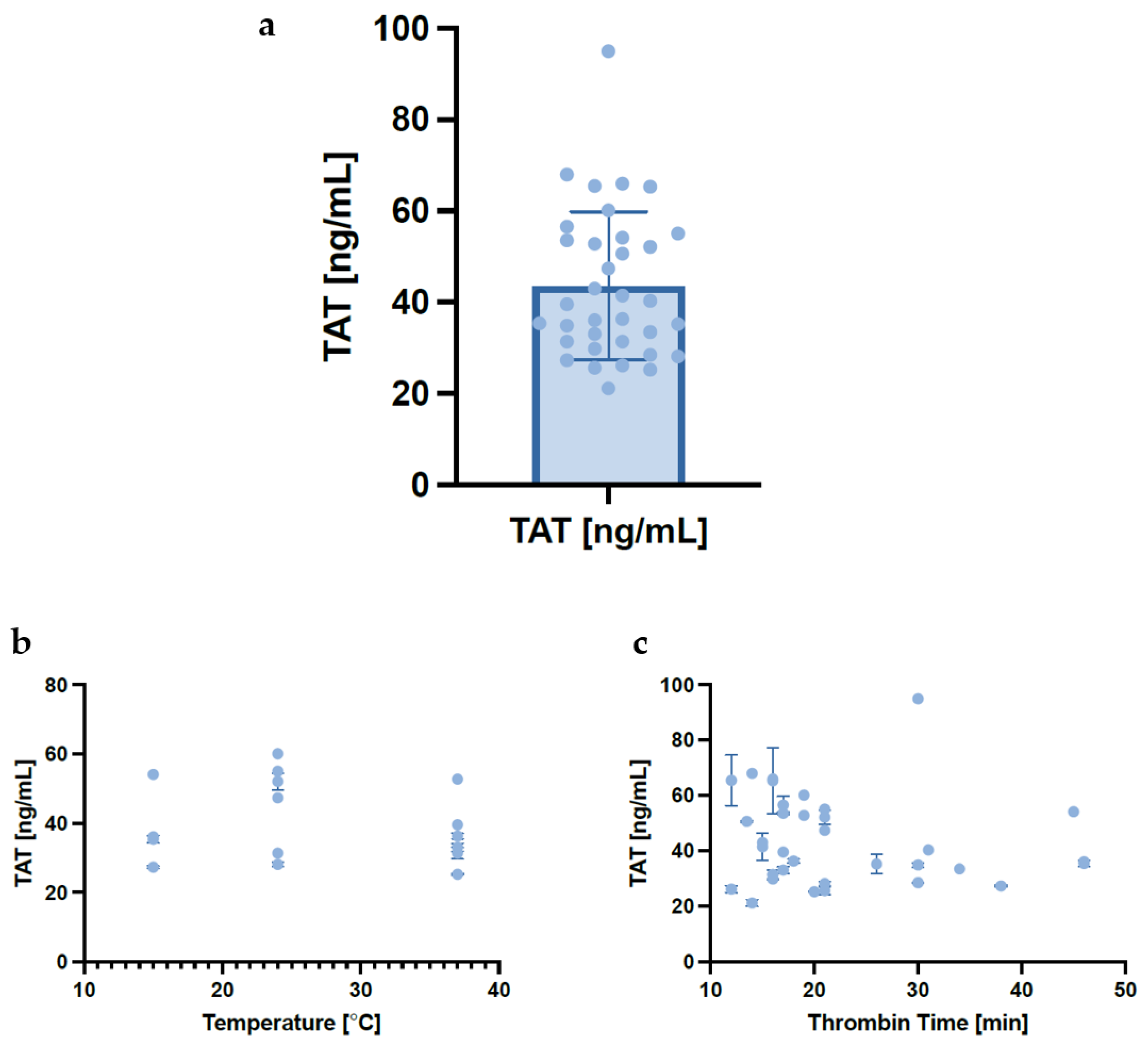
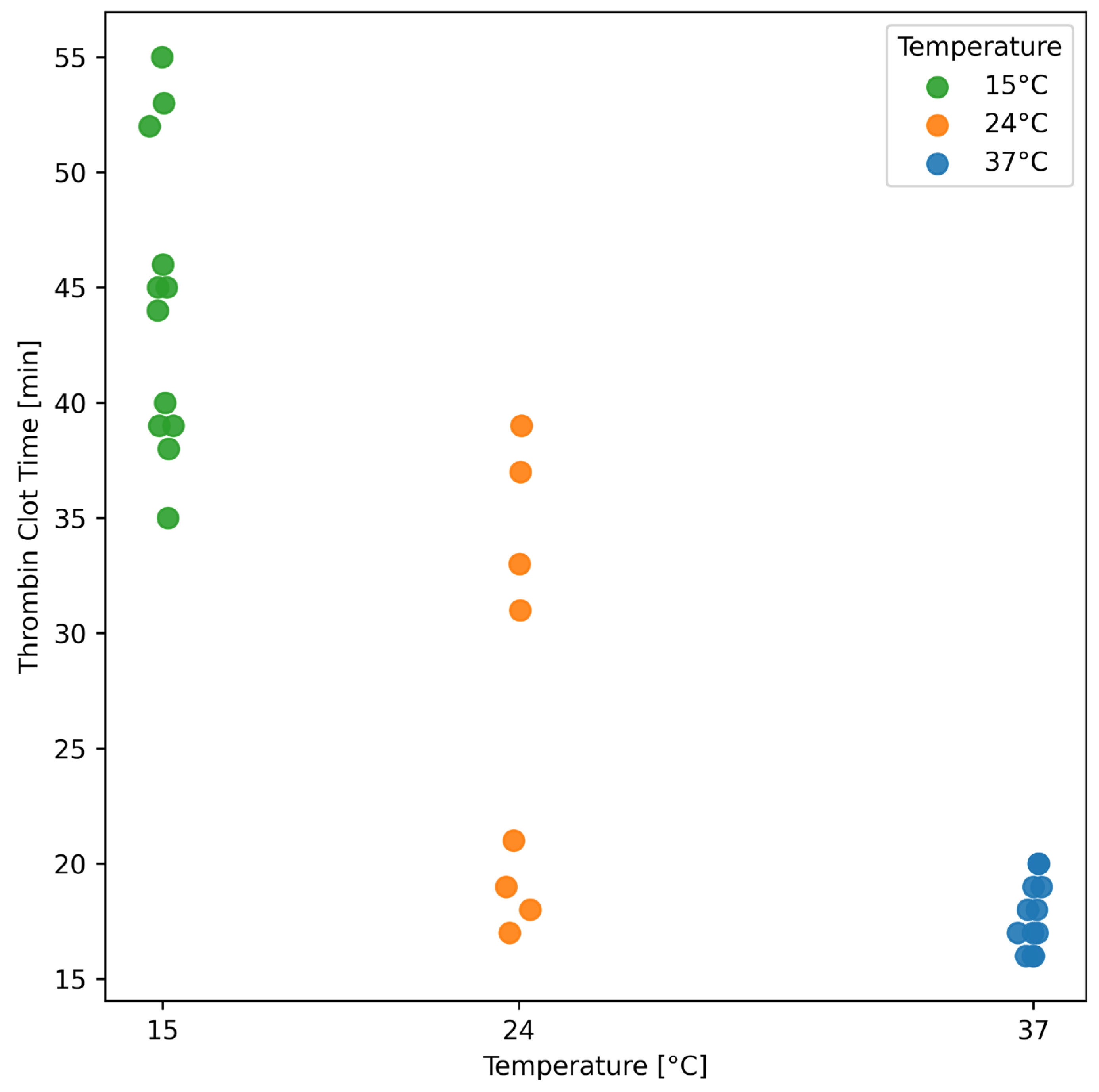
3.6. Effect of Temperature on Thrombin–Antithrombin Complexes and Thrombin Clot Time
4. Discussion
4.1. Time to Gel Is Affected by Mixing During Manufacture
4.2. Exudation of Releasate Is Affected by Manufacturing Temperature and Mixing
4.3. Design of Experiments Approach Is a Cost-Effective and Efficient Methodology for Pharmaceutical Product Development and Design
4.4. RAPID Gel Manufacturing Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort Study Evaluating the Burden of Wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2022, 46, 209–221. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhao, G.; Liu, R.; Du, Y.; Cai, Z.; Luan, J.; Shen, Y.; Chen, B. Current Applications of Platelet Gels in Wound Healing—A Review. Wound Repair Regen. 2021, 29, 370–379. [Google Scholar] [CrossRef] [PubMed]
- ICH Q8 (R2) Pharmaceutical Development-Scientific Guideline|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline (accessed on 28 June 2025).
- Kumar, V.; Madsen, T.; Zhu, H.; Semple, E. Stability of Human Thrombin Produced From 11 Ml of Plasma Using the Thrombin Processing Device. J. Extra. Corpor. Technol. 2005, 37, 390–395. [Google Scholar] [CrossRef]
- Mihalko, E.; Brown, A.C. Clot Structure and Implications for Bleeding and Thrombosis. Semin. Thromb. Hemost. 2020, 46, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.D.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) Applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of Experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Otava, M.; Mylona, K. Split-Plot Experiments with Replicated Runs in Pharmaceutical Synthesis. Qual. Reliab. Eng. Int. 2024, 41, 641–651. [Google Scholar] [CrossRef]
- Oberleitner, T.; Zahel, T.; Kunzelmann, M.; Thoma, J.; Herwig, C. Incorporating Random Effects in Biopharmaceutical Control Strategies. AAPS Open 2023, 9, 4. [Google Scholar] [CrossRef]
- Diaz, F.J.; Yeh, H.-W.; de Leon, J. Role of Statistical Random-Effects Linear Models in Personalized Medicine. Curr. Pharmacogenomics Pers. Med. 2012, 10, 22–32. [Google Scholar] [CrossRef]
- Olszewska, A.; Duan, J.; Javorovic, J.; Chan, K.L.A.; Rickard, J.; Pitchford, S.; Forbes, B. Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers. Gels 2024, 10, 572. [Google Scholar] [CrossRef]
- Letsinger, J.D.; Myers, R.H.; Lentner, M. Response Surface Methods for Bi-Randomization Structures. J. Qual. Technol. 1996, 28, 381–397. [Google Scholar] [CrossRef]
- Gilmour, S.G.; Trinca, L.A. Optimum Design of Experiments for Statistical Inference. J. R. Stat. Soc. Ser. C Appl. Stat. 2012, 61, 345–401. [Google Scholar] [CrossRef]
- Trinca, L.A.; Gilmour, S.G. Improved Split-Plot and Multistratum Designs. Technometrics 2015, 57, 145–154. [Google Scholar] [CrossRef]
- de Oliveira, H.M.; de Oliveira, C.B.A.; Gilmour, S.G.; Trinca, L.A. Compound Optimality Criteria and Graphical Tools for Designs for Prediction. Qual. Reliab. Eng. Int. 2022, 38, 3543–3558. [Google Scholar] [CrossRef]
- Egorova, O.; Gilmour, S.G. Optimal Response Surface Designs for Detection and Minimization of Model Contamination. Technometrics 2025, 64, 632–642. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Terry, M. Therneau Coxme: Mixed Effects Cox Models. CRAN Repos. 2022. [Google Scholar]
- Haematology Reference Ranges. Available online: https://www.gloshospitals.nhs.uk/our-services/services-we-offer/pathology/haematology/haematology-reference-ranges/ (accessed on 17 January 2024).
- Olszewska, A.; Egorova, O.; Gaggia, G.; Mylona, K.; Pitchford, S.; Rickard, J.; Forbes, B. Controlling Point-of-Care Manufacture of an Autologous Platelet-Based Wound Healing Gel. In Proceedings of the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology (PBP), Vienna, Austria, 18–22 March 2024. Poster Presentation number 137. [Google Scholar]
- Cox, D.R. The Regression Analysis of Binary Sequences. J. R. Stat. Soc. Ser. B Methodol. 1958, 20, 215–232. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; Wiley Series in Probability and Statistics, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-0-470-58247-3. [Google Scholar]
- Clark, T.G.; Bradburn, M.J.; Love, S.B.; Altman, D.G. Survival Analysis Part I: Basic Concepts and First Analyses. Br. J. Cancer 2003, 89, 232–238. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Godoi, T.T.F.; Rodrigues, B.L.; Huber, S.C.; Santana, M.H.A.; Fonseca, L.F.d.; Santos, G.S.; Azzini, G.O.M.; Mosaner, T.; Paulus-Romero, C.; Lana, J.F.S.D. Platelet-Rich Plasma Gel Matrix (PRP-GM): Description of a New Technique. Bioengineering 2022, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Risman, R.A.; Belcher, H.A.; Ramanujam, R.K.; Weisel, J.W.; Hudson, N.E.; Tutwiler, V. Comprehensive Analysis of the Role of Fibrinogen and Thrombin in Clot Formation and Structure for Plasma and Purified Fibrinogen. Biomolecules 2024, 14, 230. [Google Scholar] [CrossRef]
- Burnouf, T. Platelet Gels. ISBT Sci. Ser. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Chen, J.-L.; Cheng, W.-J.; Chen, C.-C.; Huang, S.-C.; Chen, C.P.C.; Suputtitada, A. Thermal Oscillation Changes the Liquid-Form Autologous Platelet-Rich Plasma into Paste-Like Form. BioMed Res. Int. 2022, 2022, 6496382. [Google Scholar] [CrossRef]
- Carmona, J.U.; López, C. Effects of Temperature and Time on the Denaturation of Transforming Growth Factor Beta-1 and Cytokines from Bovine Platelet-Rich Gel Supernatants. Gels 2024, 10, 583. [Google Scholar] [CrossRef]
- Etulain, J.; Mena, H.A.; Meiss, R.P.; Frechtel, G.; Gutt, S.; Negrotto, S.; Schattner, M. An Optimised Protocol for Platelet-Rich Plasma Preparation to Improve Its Angiogenic and Regenerative Properties. Sci. Rep. 2018, 8, 1513. [Google Scholar] [CrossRef]
- Whelihan, M.F.; Kiankhooy, A.; Brummel-Ziedins, K.E. Thrombin Generation and Fibrin Clot Formation under Hypothermic Conditions: An in Vitro Evaluation of Tissue Factor Initiated Whole Blood Coagulation. J. Crit. Care 2014, 29, 24–30. [Google Scholar] [CrossRef]
- Mitrophanov, A.Y.; Rosendaal, F.R.; Reifman, J. Computational Analysis of the Effects of Reduced Temperature on Thrombin Generation: The Contributions of Hypothermia to Coagulopathy. Anesth. Analg. 2013, 117, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fok, M.R.; Pelekos, G.; Jin, L.; Tonetti, M.S. In Vitro and Ex Vivo Kinetic Release Profile of Growth Factors and Cytokines from Leucocyte- and Platelet-Rich Fibrin (L-PRF) Preparations. Cells 2022, 11, 2089. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. JBJS 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of Concentration Yields in Commercially Available Platelet-Rich Plasma (PRP) Systems: A Call for PRP Standardization. Reg. Anesth. Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP-The Role of Monocyte/Macrophage Function in the Healing Cascade. J. Clin. Orthop. Trauma 2019, 10, S7. [Google Scholar] [CrossRef]
- Al-Amer, O.M. The Role of Thrombin in Haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145. [Google Scholar] [CrossRef] [PubMed]
- Tarandovskiy, I.D.; Surov, S.S.; Parunov, L.A.; Liang, Y.; Jankowski, W.; Sauna, Z.E.; Ovanesov, M.V. Investigation of Thrombin Concentration at the Time of Clot Formation in Simultaneous Thrombin and Fibrin Generation Assays. Sci. Rep. 2024, 14, 9225. [Google Scholar] [CrossRef]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and Thrombin Generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef]





| Factor | Process Parameter | Range of Values | Levels | Scaled Levels |
|---|---|---|---|---|
| Temperature [°C] | 15–37 °C | {15, 24, 37} | {−1, −0.18, +1} | |
| Mixing time [rotations] | 0–30 rotations | {0, 15, 30} | {−1, 0, +1} | |
| WBC content [%] | 2–8% | {2, 5, 8} | {−1, 0, +1} | |
| Time-to-thrombin use [min] | 5–20 min | {5, 12.5, 20} | {−1, 0, +1} | |
| Filtration of thrombin | No, Yes | {0, 1} | {−1, +1} | |
| Pre-mixing of additives | No, Yes | {0, 1} | {−1, +1} |
| Day/Donor | Run | Temperature [°C] | Mixing Time [Rotations] | HCT [%] | Time-to-Thrombin Use [min] | Filtering of Thrombin | Pre-Mixing Ascorbic Acid/L-PRP |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 15 | 15 | 8 | 20 | No | No |
| 1 | 2 | 15 | 30 | 8 | 5 | Yes | No |
| 1 | 3 | 15 | 0 | 2 | 5 | No | No |
| 1 | 4 | 15 | 0 | 5 | 20 | Yes | Yes |
| 2 | 5 | 37 | 0 | 5 | 20 | Yes | No |
| 2 | 6 | 37 | 30 | 2 | 20 | Yes | No |
| 2 | 7 | 37 | 0 | 8 | 5 | Yes | No |
| 2 | 8 | 37 | 30 | 2 | 5 | No | No |
| 3 | 9 | 37 | 0 | 2 | 20 | Yes | No |
| 3 | 10 | 37 | 30 | 8 | 20 | Yes | No |
| 3 | 11 | 37 | 0 | 5 | 20 | No | No |
| 3 | 12 | 37 | 30 | 8 | 5 | Yes | No |
| 4 | 13 | 15 | 30 | 2 | 20 | No | Yes |
| 4 | 14 | 15 | 30 | 2 | 5 | Yes | No |
| 4 | 15 | 15 | 15 | 8 | 12.5 | Yes | No |
| 4 | 16 | 15 | 30 | 8 | 5 | No | No |
| 5 | 17 | 15 | 30 | 5 | 12.5 | No | No |
| 5 | 18 | 15 | 0 | 2 | 5 | Yes | No |
| 5 | 19 | 15 | 0 | 8 | 5 | Yes | No |
| 5 | 20 | 15 | 0 | 2 | 20 | Yes | No |
| 6 | 21 | 24 | 30 | 8 | 20 | Yes | No |
| 6 | 22 | 24 | 15 | 2 | 20 | Yes | Yes |
| 6 | 23 | 24 | 0 | 5 | 20 | No | No |
| 6 | 24 | 24 | 0 | 8 | 20 | No | Yes |
| 7 | 25 | 24 | 0 | 2 | 12.5 | Yes | No |
| 7 | 26 | 24 | 30 | 8 | 20 | Yes | Yes |
| 7 | 27 | 24 | 30 | 2 | 20 | No | No |
| 7 | 28 | 24 | 15 | 5 | 5 | No | Yes |
| 8 | 29 | 37 | 30 | 8 | 5 | Yes | No |
| 8 | 30 | 37 | 0 | 8 | 5 | No | No |
| 8 | 31 | 37 | 0 | 2 | 5 | Yes | No |
| 8 | 32 | 37 | 0 | 8 | 20 | Yes | No |
| Parameter | Mean | STD | Min | Max |
|---|---|---|---|---|
| WBC | 5.43 × 106 | 1.50 × 106 | 2.72 × 106 | 8.14 × 106 |
| WBC IN L-PRP | 3.32 × 106 | 2.44 × 106 | 9.38 × 105 | 8.63 × 106 |
| INCREASE FROM BASAL | 0.66 | 0.43 | 0.14 | 1.45 |
| PLT | 2.54 × 108 | 4.34 × 107 | 1.99 × 108 | 3.20 × 108 |
| PLT IN L-PRP | 1.03 × 109 | 7.03 × 108 | 4.40 × 108 | 2.24 × 109 |
| INCREASE FROM BASAL | 3.81 | 1.94 | 1.89 | 7.25 |
| (a) | |||||
| Parameter | Value | Standard Error | Degrees of Freedom | t-Value | p-Value |
| Intercept, | 6.4499 | 0.3957 | 8.8723 | 16.236 | 0.0000 |
| Temperature, | 1.3260 | 0.4057 | 5.8767 | 3.268 | 0.0176 |
| Time-to-Thrombin use, | 0.3917 | 0.2470 | 24.2309 | 1.586 | 0.1257 |
| Pre-mixing of additives, | −1.6888 | 0.4611 | 22.9054 | −3.662 | 0.0013 |
| Temperature x Time-to-Thrombin use, | 0.7144 | 0.2650 | 21.8555 | 2.696 | 0.0133 |
| (b) | |||||
| Parameter | Value | Standard Error | Degrees of Freedom | t-Value | p-Value |
| Intercept, | 4.62513 | 0.14513 | 11.30670 | 31.868 | 0.0000 |
| Temperature, | −0.05217 | 0.16551 | 10.65287 | −0.315 | 0.7587 |
| Mixing time, | −0.75937 | 0.13010 | 22.71603 | −5.837 | 0.0000 |
| Pre-mixing of additives, | 0.55067 | 0.24328 | 22.06006 | 2.264 | 0.0338 |
| Temperature x Pre-mixing of additives, | 0.64610 | 0.29638 | 22.92721 | 2.180 | 0.0398 |
| Parameter | Value | Standard Error | z-Value | p-Value |
|---|---|---|---|---|
| Intercept, | 0.8869 | 0.4704 | 1.885 | 0.0594 |
| Mixing time, | 1.2973 | 0.5107 | 2.540 | 0.0111 |
| Parameter | Value | Exp (Value) | Standard Error | z-Value | p-Value |
|---|---|---|---|---|---|
| Temperature, | −1.03690 | 0.35455 | 0.31834 | −3.26 | 0.001 |
| Time-to-Thrombin use, | −0.61069 | 0.54298 | 0.2429487 | −2.51 | 0.012 |
| Pre-mixing of additives, | −1.02978 | 0.35709 | 0.4541926 | −2.27 | 0.023 |
| Mixing time, | 1.42476 | 4.15684 | 0.3452564 | 4.13 | 0.000 |
| Temperature x Time-to-Thrombin use, | −0.81193 | 0.44400 | 0.2822142 | −2.88 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, A.; Egorova, O.; Gaggia, G.; Mylona, K.; Pitchford, S.; Rickard, J.; Forbes, B. A Design of Experiments Approach to Identify Critical Processing Parameters for Manufacture of an Autologous Platelet Gel for Diabetic Foot Ulcer. Pharmaceutics 2025, 17, 1482. https://doi.org/10.3390/pharmaceutics17111482
Olszewska A, Egorova O, Gaggia G, Mylona K, Pitchford S, Rickard J, Forbes B. A Design of Experiments Approach to Identify Critical Processing Parameters for Manufacture of an Autologous Platelet Gel for Diabetic Foot Ulcer. Pharmaceutics. 2025; 17(11):1482. https://doi.org/10.3390/pharmaceutics17111482
Chicago/Turabian StyleOlszewska, Aleksandra, Olga Egorova, Gabriella Gaggia, Kalliopi Mylona, Simon Pitchford, James Rickard, and Ben Forbes. 2025. "A Design of Experiments Approach to Identify Critical Processing Parameters for Manufacture of an Autologous Platelet Gel for Diabetic Foot Ulcer" Pharmaceutics 17, no. 11: 1482. https://doi.org/10.3390/pharmaceutics17111482
APA StyleOlszewska, A., Egorova, O., Gaggia, G., Mylona, K., Pitchford, S., Rickard, J., & Forbes, B. (2025). A Design of Experiments Approach to Identify Critical Processing Parameters for Manufacture of an Autologous Platelet Gel for Diabetic Foot Ulcer. Pharmaceutics, 17(11), 1482. https://doi.org/10.3390/pharmaceutics17111482







