Overcoming MRSA Antibiotic Resistance Through Losartan Repurposing with Carbon Dot–Conjugated Cerosomal Nanocarriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization of Losartan-Loaded Cerosomes
2.3.1. Determination of Entrapment Efficiency (EE%) and Drug Loading Capacity (LC%)
2.3.2. Determination of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
2.3.3. D-Optimal Mixture Design and Selection of the Optimum LOS-CERs
2.4. Synthesis of Carbon Dots
2.5. Preparation of Carbon Dot-Functionalized Cerosomes
2.6. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.7. Effect of Storage
2.8. Morphological Examination by Transmission Electron Microscopy (TEM)
2.9. Determination of Drug Release
2.10. Ex Vivo Permeation Studies
2.11. Confocal Laser Scanning Microscopy (CLSM) for Skin Penetration and Distribution Studies
2.12. In Vivo MRSA Skin Infection Model
2.13. Histopathological Examination
2.14. Statistical Analysis
3. Results and Discussion
3.1. Optimization of LOS-CERs Using D-Optimal Mixture Design
3.2. Effect of Formulation Variables on the EE% (Y1)
- (1)
- Cosurfactant type (glycerol versus phytantriol):
- (2)
- Cosurfactant concentration:
- (3)
- Amount of ceramide:
3.3. Effect of Formulation Variables on PS (Y2)
- (1)
- Type of cosurfactant (glycerol versus phytantriol):
- (2)
- Amount of ceramide:
3.4. Evaluation of the PDI
3.5. Effect of Formulation Variables on ZP (Y3)
- (1)
- Type of cosurfactant (glycerol versus phytantriol):
- (2)
- Amount of ceramide:
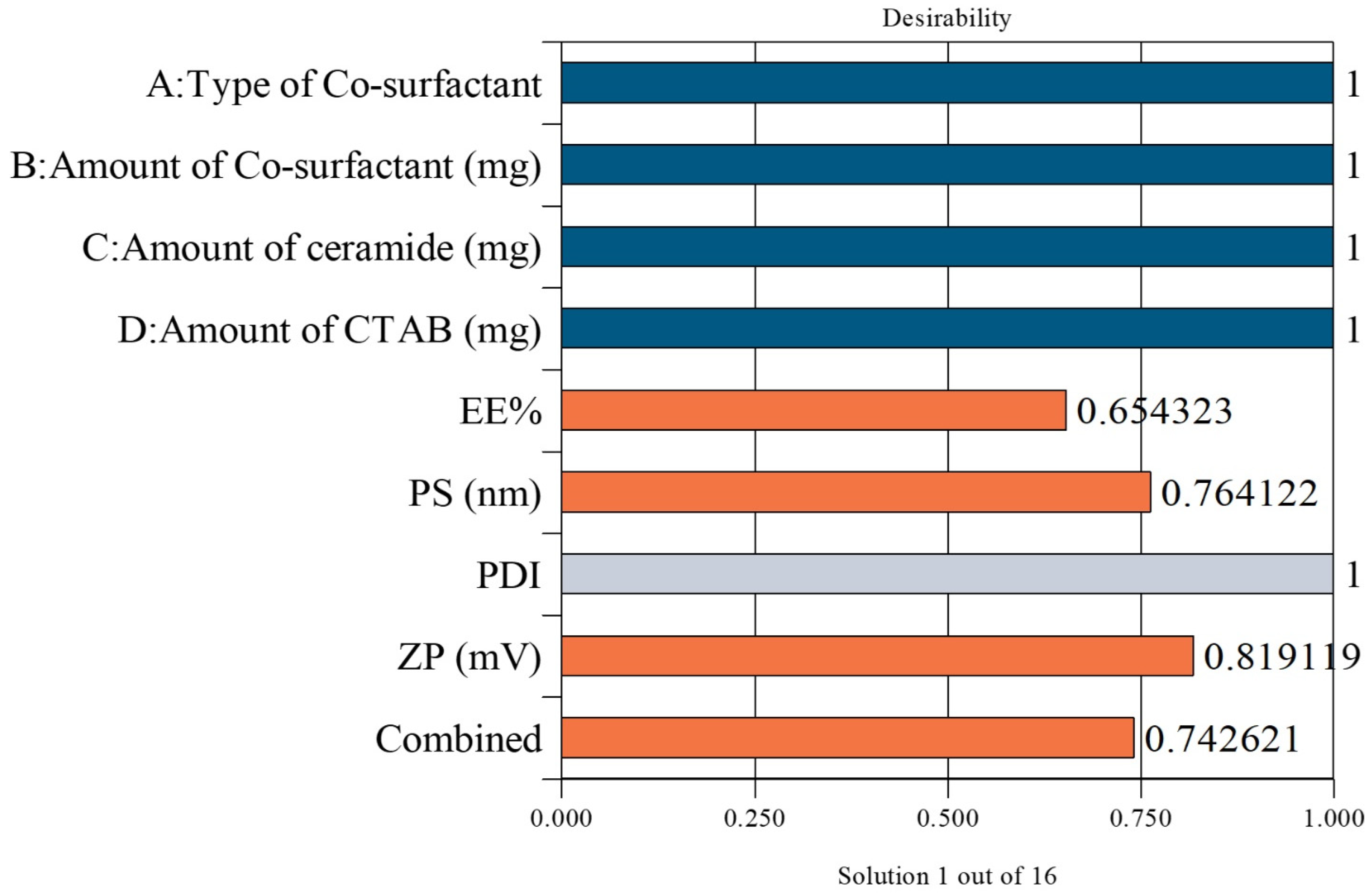
3.6. Selection of the LOS-CERs
3.7. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.8. Effect of Storage
3.9. Morphological Evaluation by Transmission Electron Microscopy (TEM)
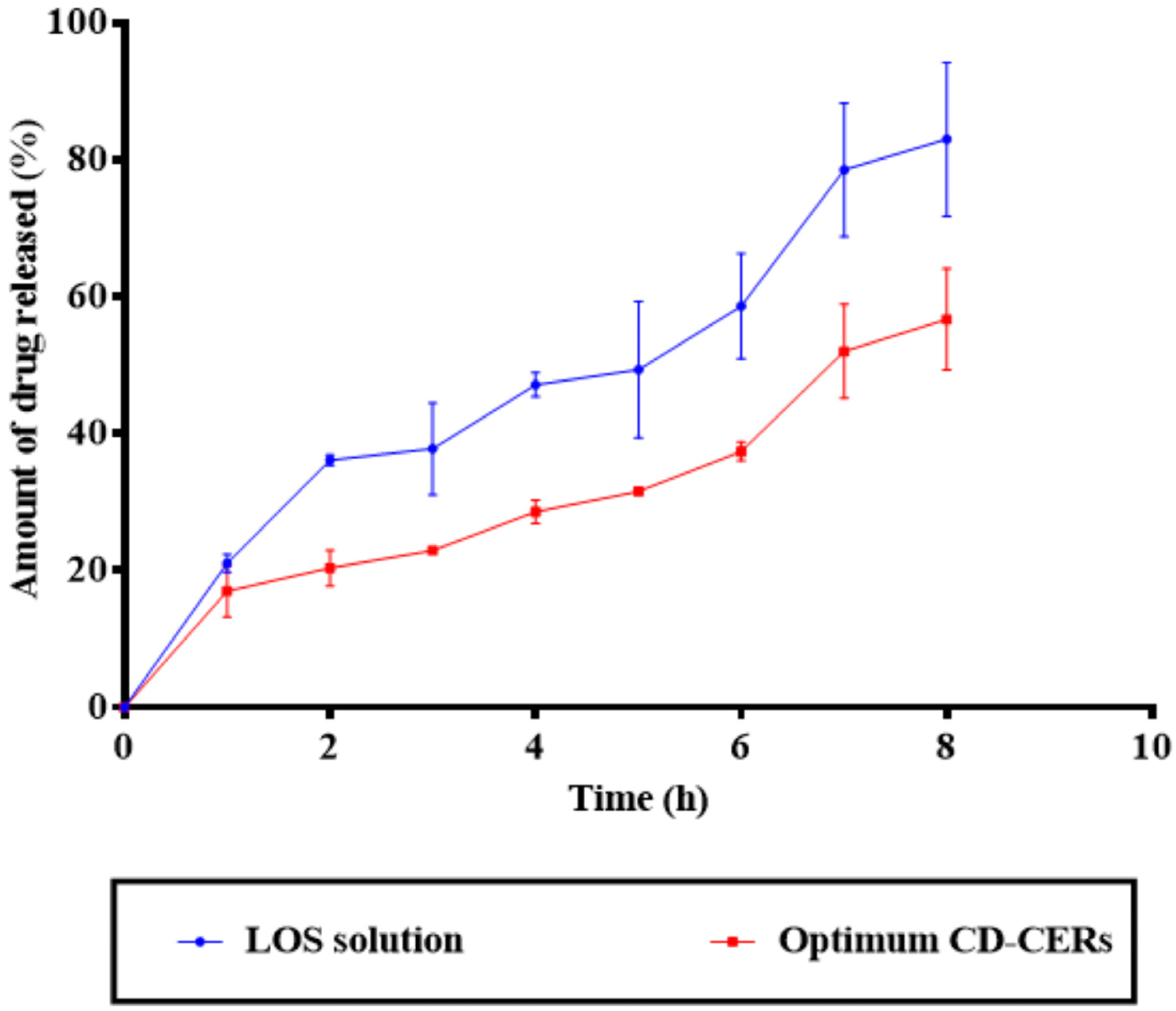
3.10. Drug Release Results
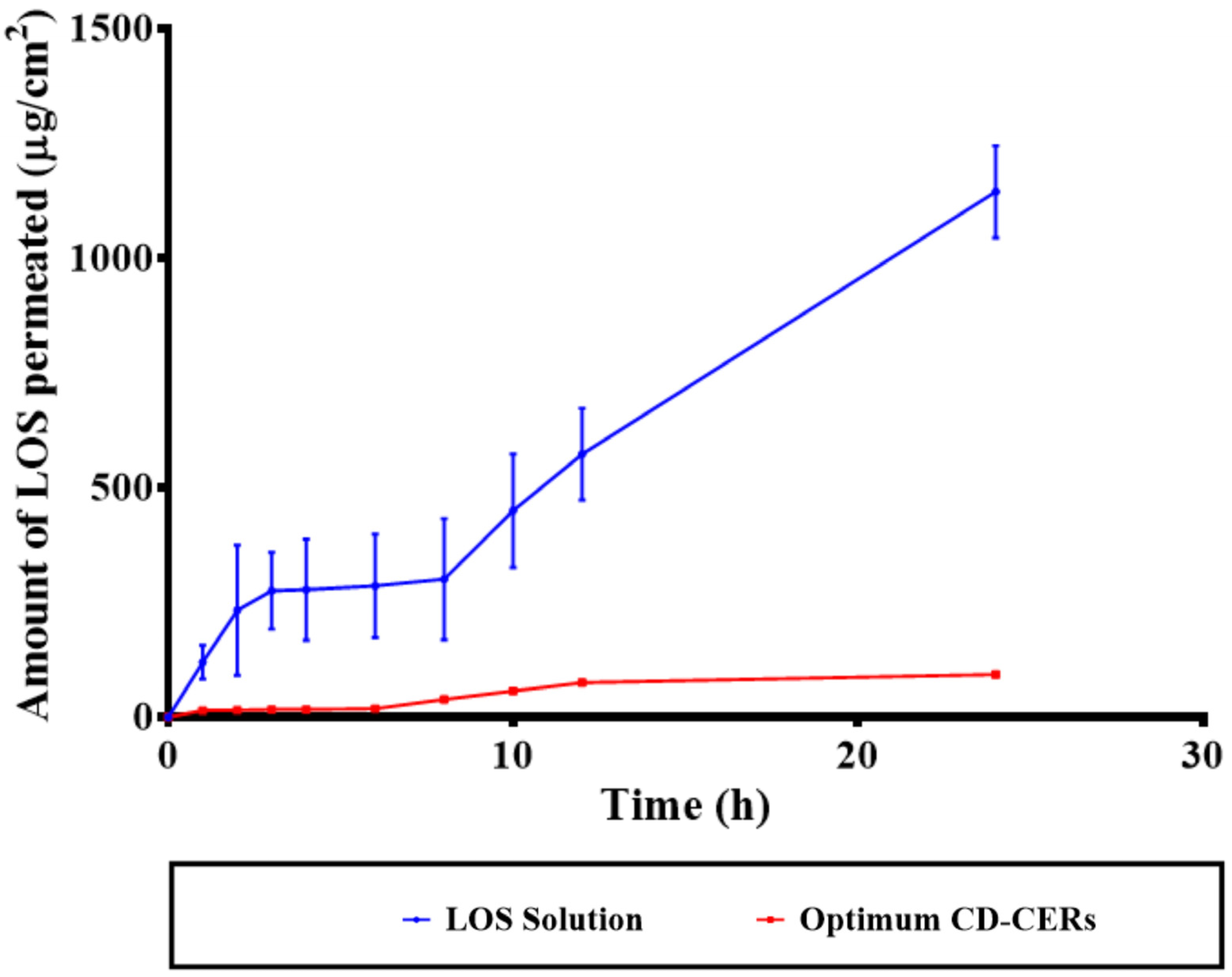
3.11. Ex Vivo Permeation Results
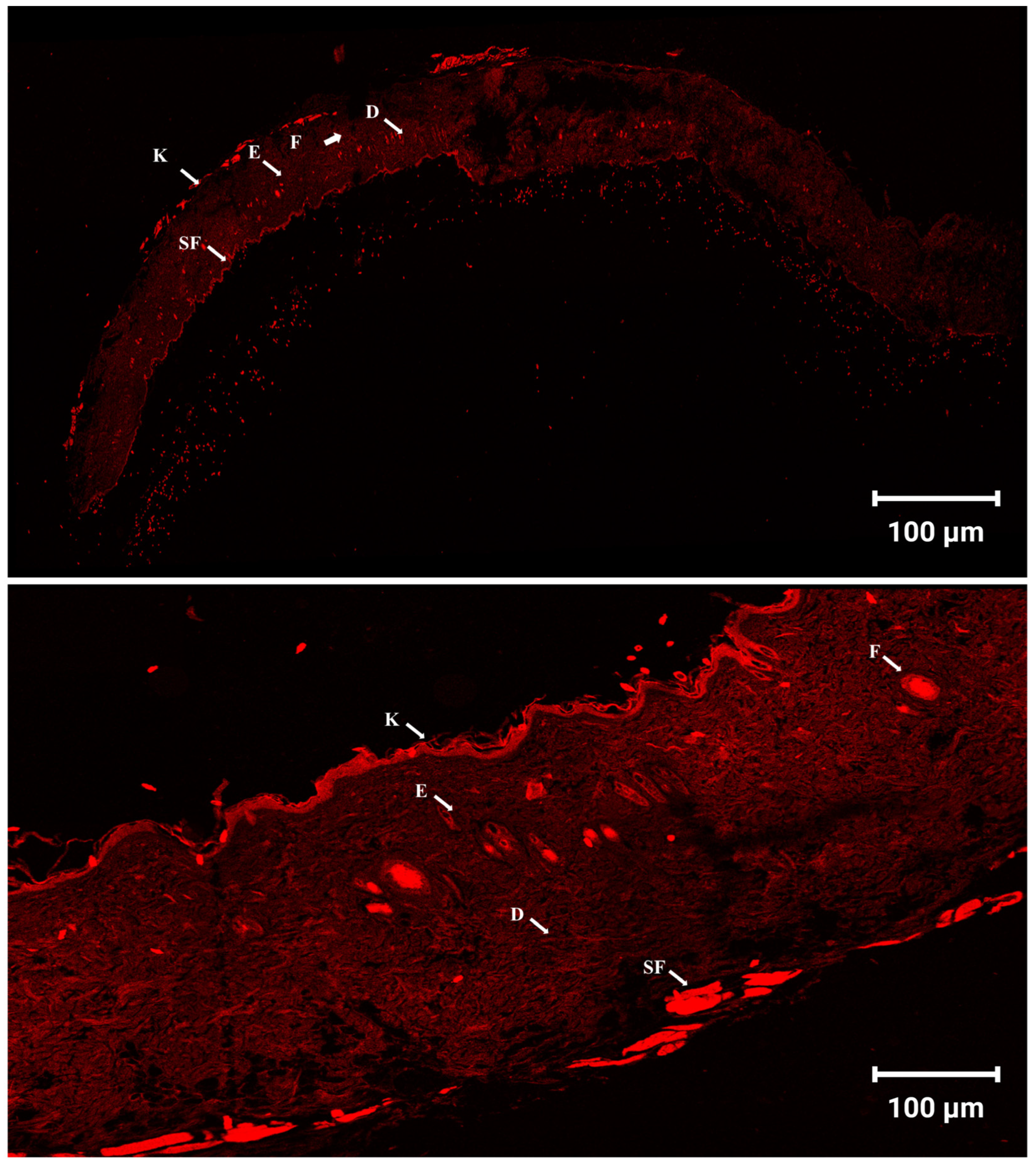
3.12. Skin Penetration and Distribution Observed by Confocal Laser Scanning Microscopy (CLSM)
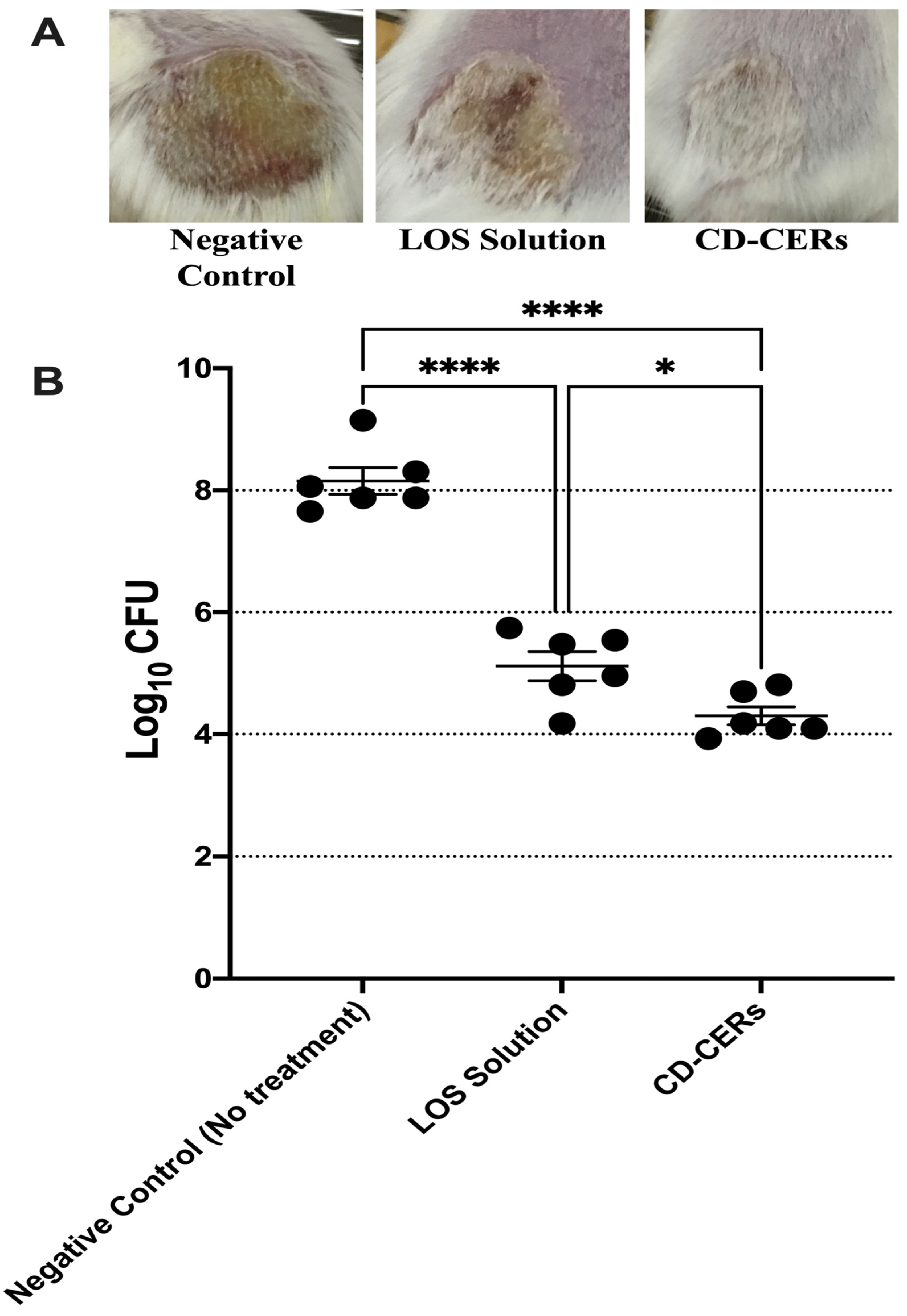
3.13. In Vivo Antibacterial Efficacy Against MRSA Skin Infection
3.14. Histopathological Evaluation of Skin Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahmasebi, H.; Arjmand, N.; Monemi, M.; Babaeizad, A.; Alibabaei, F.; Alibabaei, N.; Bahar, A.; Oksenych, V.; Eslami, M. From cure to crisis: Understanding the evolution of antibiotic-resistant bacteria in human microbiota. Biomolecules 2025, 15, 93. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; He, J.; Shen, J.; Ren, Q. Rapid release of high-valent silver ions from water-soluble porphyrin complexes to enhance the direct killing of Methicillin-Resistant Staphylococcus aureus. Acta Biomater. 2025, 192, 419–430. [Google Scholar] [CrossRef]
- Lei, D.; Dong, X.; Yang, T.; Jin, Y.; Zhou, W. Clade-specific adaptation and global spread of Staphylococcus aureus ST188 with emergence of a multidrug-resistant MRSA sublineage. Msystems 2025, e00848-25, online ahead of print. [Google Scholar] [CrossRef]
- Konreddy, A.K.; Rani, G.U.; Lee, K.; Choi, Y. Recent drug-repurposing-driven advances in the discovery of novel antibiotics. Curr. Med. Chem. 2019, 26, 5363–5388. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Morisaki, J.H.; et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, E.; Kaykın, M.; Şahin Bektay, H.; Güngör, S. Recent advances on topical application of ceramides to restore barrier function of skin. Cosmetics 2019, 6, 52. [Google Scholar] [CrossRef]
- Gaur, P.K.; Purohit, S.; Kumar, Y.; Mishra, S.; Bhandari, A. Ceramide-2 nanovesicles for effective transdermal delivery: Development, characterization and pharmacokinetic evaluation. Drug Dev. Ind. Pharm. 2014, 40, 568–576. [Google Scholar] [CrossRef]
- Oliveira, E.G.d.L.; de Oliveira, H.P.; Gomes, A.S.L. Metal nanoparticles/carbon dots nanocomposites for SERS devices: Trends and perspectives. SN Appl. Sci. 2020, 2, 1491. [Google Scholar] [CrossRef]
- Mou, C.; Wang, X.; Teng, J.; Xie, Z.; Zheng, M. Injectable self-healing hydrogel fabricated from antibacterial carbon dots and ɛ-polylysine for promoting bacteria-infected wound healing. J. Nanobiotechnol. 2022, 20, 368. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Pandey, S.; Mewada, A.; Patil, V.; Khade, M.; Goshi, E.; Sharon, M. Antibiotic conjugated fluorescent carbon dots as a theranostic agent for controlled drug release, bioimaging, and enhanced antimicrobial activity. J. Drug Deliv. 2014, 2014, 282193. [Google Scholar] [CrossRef]
- Albash, R.; Hassan, M.; Agiba, A.M.; Mohamed, H.W.; Hassan, M.S.; Ali, R.M.; Shalabi, Y.E.; Omran, H.M.A.; Eltabeeb, M.A.; Alamoudi, J.A.; et al. Advanced Vaginal Nanodelivery of Losartan Potassium via PEGylated Zein Nanoparticles for Methicillin-Resistant Staphylococcus aureus. Pharmaceutics 2025, 17, 1344. [Google Scholar] [CrossRef]
- Ahmed, S.; Aziz, D.E.; Sadek, M.A.; Tawfik, M.A. Capped flexosomes for prominent anti-inflammatory activity: Development, optimization, and ex vivo and in vivo assessments. Drug Deliv. Transl. Res. 2024, 14, 2474–2487. [Google Scholar] [CrossRef]
- Albash, R.; Ali, S.K.; Abdelmonem, R.; Agiba, A.M.; Aldhahri, R.; Saleh, A.; Kassem, A.B.; Abdellatif, M.M. Electrospun nanofiber-scaffold-loaded levocetirizine dihydrochloride cerosomes for combined management of atopic dermatitis and methicillin-resistant Staphylococcus Aureus (MRSA) skin infection: In vitro and in vivo studies. Pharmaceuticals 2025, 18, 633. [Google Scholar] [CrossRef]
- Agiba, A.M.; Rodríguez Huerta, L.G.; Ulloa-Castillo, N.A.; Sierra-Valdez, F.J.; Beigi-Boroujeni, S.; Lozano, O.; Aguirre-Soto, A. Fusion of polymer-coated liposomes and centrifugally spun microfibers as hybrid materials to enhance sustained release. Nanoscale Adv. 2025, 7, 1009–1017. [Google Scholar] [CrossRef]
- Seedad, R.; Ratanawimarnwong, N.; Jittangprasert, P.; Mantim, T.; Songsrirote, K. Carbon dots prepared from citric acid and urea by microwave-assisted irradiation as a turn-on Carbon fluorescent probe for allantoin determination. New J. Chem. 2021, 45, 22424–22431. [Google Scholar]
- Alzahrani, A.; Alsulami, T.; Salamatullah, A.M.; Ahmed, S.R. Non-spherical gold nanoparticles enhanced fluorescence of carbon dots for norovirus-like particles detection. J. Biol. Eng. 2023, 17, 33. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbari, M.A.; Elbesh, R.M.; Khaleel, E.F.; Badi, R.M.; Eldehna, W.M.; Elkaeed, E.B.; El Hassab, M.A.; Ahmed, S.M.; Mosallam, S. Sonophoresis mediated diffusion of caffeine loaded Transcutol® enriched cerosomes for topical management of cellulite. Eur. J. Pharm. Sci. 2024, 201, 106875. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Eita, A.S.; Makky, A.M.; Anter, A.; Khalil, I.A. Foamable pluroleosomes system loaded with amlodipine as a repurposed antibacterial topical formulation against MRSA-induced infection; optimization, in-vitro, ex-vivo, and in-vivo studies. Int. J. Pharm. X 2025, 10, 100406. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Eraqi, W.A.; Georghiou, P.E.; Zakaria, M.Y. Luteolin loaded PEGylated cerosomes: A novel treatment for MRSA skin infections. BMC Microbiol. 2025, 25, 182. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. (Eds.) Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; American Pharmacists Association: Washington, DC, USA, 2009. [Google Scholar]
- Patel, H.; Kundawala, A.; Patel, N. Effect of lipid and surfactant concentration on physicochemical properties and entrapment efficiency of niosomes prepared for topical delivery of erythromycin. Int. J. Drug Deliv. Technol. 2009, 1, 42–45. [Google Scholar]
- Boyd, B.J.; Khoo, S.M.; Whittaker, D.V.; Davey, G.; Porter, C.J.H. Nanostructured lipid dispersions formed from phytantriol—Templating of cubic lyotropic liquid crystalline phases. Int. J. Pharm. 2009, 347, 44–53. [Google Scholar]
- Abdallah, M.H.; Shahien, M.M.; El-Horany, H.E.S.; Ahmed, E.H. Modified Phospholipid Vesicular Gel for Transdermal Drug Delivery: The Influence of Glycerin and/or Ethanol on Their Lipid Bilayer Fluidity and Penetration Characteristics. Gels 2025, 11, 358. [Google Scholar] [CrossRef]
- Imura, T.; Sakai, H.; Yamauchi, H.; Kaise, C.; Kozawa, K.; Yokoyama, S.; Abe, M. Preparation of liposomes containing Ceramide 3 and their membrane characteristics. Colloids Surf. B Biointerfaces 2001, 20, 1–8. [Google Scholar] [CrossRef]
- El Hassab, M.A.; Ibrahim, M.H.; Abdel Mageed, S.S.; Mahmoud, A.M.A.; Othman Ahmed, Z.S.; Mosallam, S.; Abdelbari, M.A.; Khaleel, E.F.; El Hasab, M.A.; Elsayyad, A.; et al. Correction: Formulation of zein nanoparticles for augmenting the anti-inflammatory activity of dexketoprofen. Front. Pharmacol. 2025, 16, 1658349. [Google Scholar] [CrossRef]
- Tawfik, M.A.; Ahmed, S.; El-Dahmy, R.M.; Aziz, D.E. Oleic acid Enriched Leciplexes as Novel Mucoadhesive Cationic Nanocarriers of Agomelatine for Glaucoma Treatment. AAPS PharmSciTech 2025, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.-R.A.; Assiri, E.; Khalil, N.Y.; Abdel-Aziz, H.A. Losartan: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2015, 40, 159–194. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, M.; Smaranda, I.; Zgura, I.; Ganea, P.; Chivu, M.; Chiricuta, B.; Baibarac, M. Degradation of losartan potassium highlighted by correlated studies of photoluminescence, infrared absorption spectroscopy and dielectric spectroscopy. Pharmaceutics 2022, 14, 2419. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, Y.; Wang, M.; Wang, Y.; Wang, W.; Pang, M.; Xu, Y. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int. J. Pharm. 2021, 605, 120826. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Abdelaal, N.; Al-Zuhairy, S.A.S.; Mohamed Elrefai, M.F.; Khalifa, M.M.; Khasawneh, M.A.; Elsaid Hamdan, A.M.; Mohie, P.M.; Gad, R.A.; Kabil, S.L.; et al. Revolutionizing psoriasis topical treatment: Enhanced efficacy through ceramide/phospholipid composite cerosomes co-delivery of cyclosporine and dithranol: In-vitro, ex-vivo, and in-vivo studies. Int. J. Nanomed. 2024, 19, 1163–1187. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Zhou, W.Z.; Panyam, J.; Labhasetwar, V. Size-dependency of nanoparticle-mediated gene transfection: Studies with fractionated nanoparticles. Int. J. Pharm. 2002, 244, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.; Ghorab, D.; Refai, H.; Teba, H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int. J. Pharm. 2016, 506, 57–67. [Google Scholar] [CrossRef]
- Ahmed, S.; Ibrahim, M.M.; Balkhi, B.; Attia, H.; Aziz, D.E. From Bench to Biology: Unraveling the Efficiency of novel Brijosomes for Trans-Tympanic Drug Delivery. J. Drug Deliv. Sci. Technol. 2025, 114, 107493. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]




| Factors (Independent Variables) | Levels | |
|---|---|---|
| Low (−1) | High (+1) | |
| X1: Type of cosurfactant | Glycerol | Phytantriol |
| X2: Amount of cosurfactant (mg) | 10 | 20 |
| X3: Amount of ceramide (mg) | 15 | 30 |
| X4: Amount of CTAB (mg) | 10 | 20 |
| Responses (Dependent variables) | Constraints | |
| Y1: EE (%) | Maximize | |
| Y2: PS (nm) | Minimize | |
| Y3: ZP (mV) | Maximize | |
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors |
|---|---|---|---|---|---|
| EE% | 0.999 | 0.999 | 0.998 | 330.835 | X1, X2, X3, X4 |
| PS (nm) | 1 | 0.999 | 0.999 | 730.89 | X1, X2, X3, X4 |
| ZP (mV) | 0.893 | 0.842 | 0.758 | 12.87 | X2, X3, X4 |
| EE% | PS (nm) | PDI | ZP (mV) | ||
| Predicted values for the selected LOS-CERs | 76.90 | 223.69 | 0.517 | +40.15 | |
| Observed values for the selected LOS-CERs | 77.11 | 223.50 | 0.52 | +45.95 |
| Formulation Code | Type of Co-Surfactant (X1) | Amount of Cosurfactant (mg) (X2) | Amount of Ceramide (mg) (X3) | Amount of CTAB (mg) (X4) | EE (%) | LC (%) | PS (nm) | PDI | ZP (mV) |
|---|---|---|---|---|---|---|---|---|---|
| F1 | Glycerol | 10 | 30 | 10 | 58.82 ± 0.12 | 29.20 ± 0.0002 | 442.50 ± 0.50 | 0.421 ± 0.013 | +22.80 ± 0.20 |
| F2 | Glycerol | 10 | 15 | 10 | 60.67 ± 0.02 | 31.20 ± 0.001 | 357.50 ± 0.50 | 0.470 ± 0.001 | +16.40 ± 0.10 |
| F3 | Glycerol | 10 | 15 | 20 | 71.80 ± 0.20 | 53.60 ± 0.003 | 369.50 ± 0.50 | 0.580 ± 0.001 | +33.39 ± 0.29 |
| F4 | Glycerol | 10 | 30 | 20 | 73.45 ± 0.45 | 34.30 ± 0.001 | 291.50 ± 0.50 | 0.520 ± 0.01 | +35.50 ± 0.50 |
| F5 | Glycerol | 20 | 30 | 10 | 38.80 ± 0.20 | 18.20 ± 0.0004 | 442.50 ± 0.50 | 0.480 ± 0.03 | +31.68 ± 0.48 |
| F6 | Glycerol | 20 | 15 | 10 | 41.50 ± 0.39 | 21.60 ± 0.001 | 170.50 ± 0.50 | 0.440 ± 0.05 | +20.90 ± 0.10 |
| F7 | Glycerol | 20 | 15 | 20 | 59.36 ± 0.36 | 31.40 ± 0.002 | 156.50 ± 0.50 | 0.490 ± 0.01 | +33.40 ± 0.10 |
| F8 | Glycerol | 20 | 30 | 20 | 60.94 ± 0.06 | 27.10 ± 0.002 | 265.50 ± 0.50 | 0.500 ± 0.04 | +47.36 ± 0.56 |
| F9 | Phytantriol | 10 | 15 | 10 | 59.15 ± 0.04 | 44.70 ± 0.003 | 267.50 ± 0.50 | 0.54 ± 0.001 | +7.04 ± 0.04 |
| F10 | Phytantriol | 10 | 15 | 20 | 62.25 ± 0.25 | 32.40 ± 0.001 | 392.50 ± 0.50 | 0.51 ± 0.01 | +30.83 ± 0.13 |
| F11 | Phytantriol | 10 | 30 | 10 | 69.35 ± 0.35 | 34.80 ± 0.001 | 262.00 ± 1.00 | 0.500 ± 0.04 | +19.00 ± 0.70 |
| F12 | Phytantriol | 10 | 30 | 20 | 77.11 ± 0.10 | 36.40 ± 0.002 | 223.50 ± 0.50 | 0.52 ± 0.01 | +45.95 ± 0.45 |
| F13 | Phytantriol | 20 | 15 | 10 | 72.55 ± 0.50 | 37.60 ± 0.001 | 254.50 ± 0.50 | 0.490 ± 0.01 | +21.05 ± 0.45 |
| F14 | Phytantriol | 20 | 30 | 10 | 82.30 ± 0.30 | 39.00 ± 0.001 | 436.50 ± 0.50 | 0.42 ± 0.001 | +24.63 ± 0.53 |
| F15 | Phytantriol | 20 | 15 | 20 | 83.01 ± 0.01 | 40.40 ± 0.002 | 353.85 ± 0.05 | 0.54 ± 0.05 | +37.83 ± 1.33 |
| F16 | Phytantriol | 20 | 30 | 20 | 97.07 ± 0.07 | 43.00 ± 0.002 | 372.50 ± 0.50 | 0.54 ± 0.02 | +33.24 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmahboub, Y.; Albash, R.; Agiba, A.M.; Hassan, M.; Mohamed, H.W.; Hassan, M.S.; Ali, R.M.; Shalabi, Y.E.; Omran, H.M.A.; Alaa-Eldin, A.A.; et al. Overcoming MRSA Antibiotic Resistance Through Losartan Repurposing with Carbon Dot–Conjugated Cerosomal Nanocarriers. Pharmaceutics 2025, 17, 1483. https://doi.org/10.3390/pharmaceutics17111483
Elmahboub Y, Albash R, Agiba AM, Hassan M, Mohamed HW, Hassan MS, Ali RM, Shalabi YE, Omran HMA, Alaa-Eldin AA, et al. Overcoming MRSA Antibiotic Resistance Through Losartan Repurposing with Carbon Dot–Conjugated Cerosomal Nanocarriers. Pharmaceutics. 2025; 17(11):1483. https://doi.org/10.3390/pharmaceutics17111483
Chicago/Turabian StyleElmahboub, Yasmina, Rofida Albash, Ahmed M. Agiba, Mariam Hassan, Haneen Waleed Mohamed, Mohamed Safwat Hassan, Roaa Mohamed Ali, Yara E. Shalabi, Hend Mahmoud Abdelaziz Omran, Ahmed Adel Alaa-Eldin, and et al. 2025. "Overcoming MRSA Antibiotic Resistance Through Losartan Repurposing with Carbon Dot–Conjugated Cerosomal Nanocarriers" Pharmaceutics 17, no. 11: 1483. https://doi.org/10.3390/pharmaceutics17111483
APA StyleElmahboub, Y., Albash, R., Agiba, A. M., Hassan, M., Mohamed, H. W., Hassan, M. S., Ali, R. M., Shalabi, Y. E., Omran, H. M. A., Alaa-Eldin, A. A., Alamoudi, J. A., Saleh, A., Kassem, A. B., & Eltabeeb, M. A. (2025). Overcoming MRSA Antibiotic Resistance Through Losartan Repurposing with Carbon Dot–Conjugated Cerosomal Nanocarriers. Pharmaceutics, 17(11), 1483. https://doi.org/10.3390/pharmaceutics17111483









