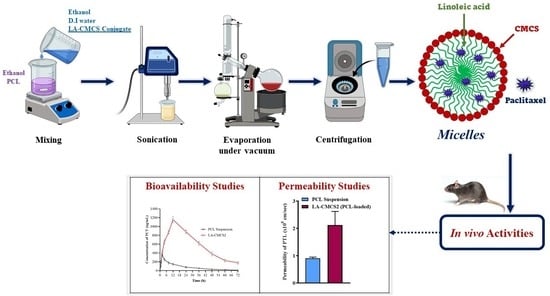
Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
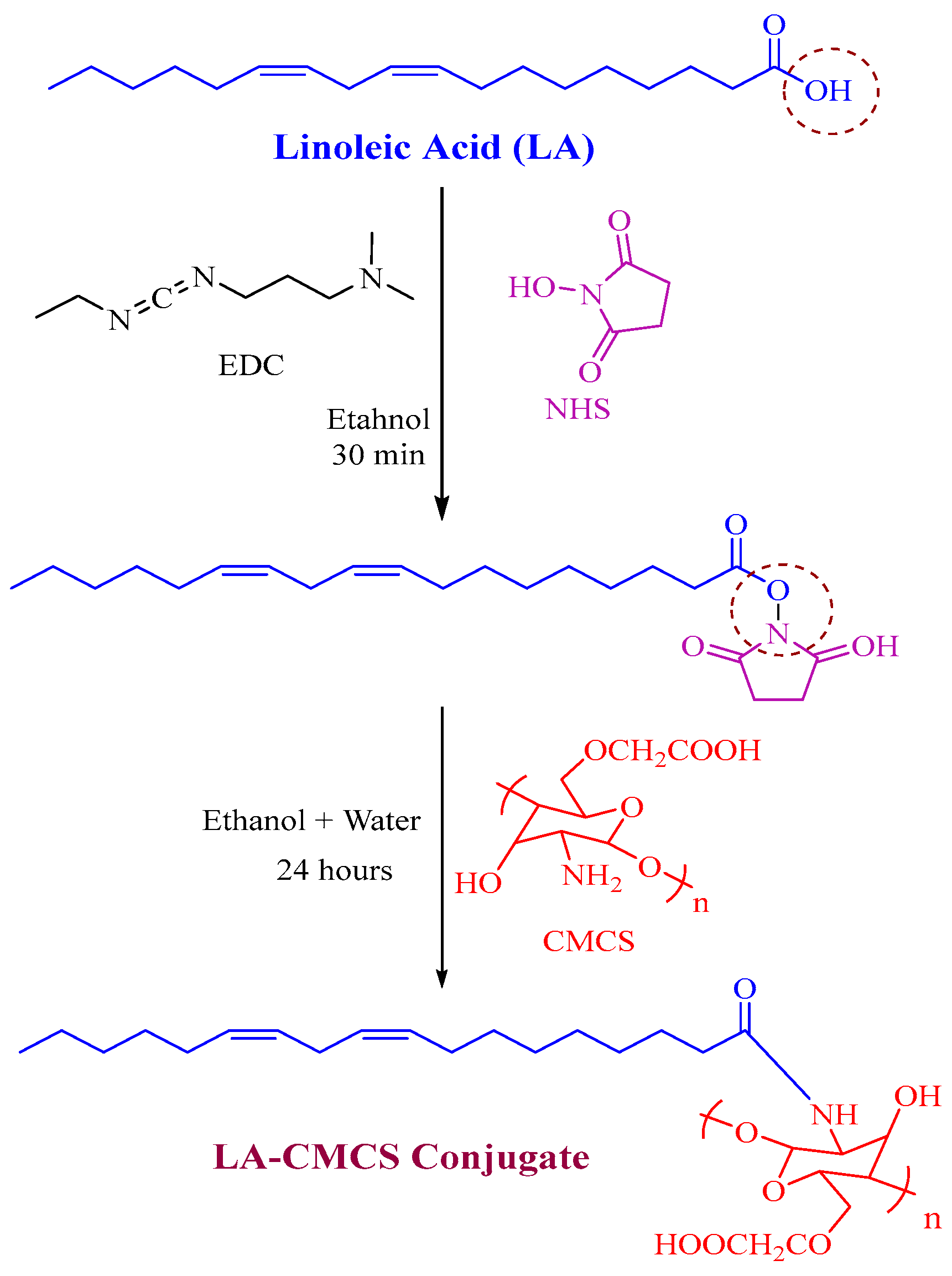
2.2. Synthesis of LA-CMCS Conjugate
2.3. Characterisation of LA-CMCS Conjugate by 1H-NMR
2.4. Solubility Studies of PCL
2.5. Formulation of Polymeric Micelles
2.6. Critical Micelle Concentration (CMC)Measurement
2.7. Characterisation
2.7.1. Particle Size and Zeta Potential
2.7.2. Fourier Transforms Infrared Spectroscopy (FTIR)
2.7.3. DSC and TGA Analysis
2.7.4. Transmission Electron Microscopy (TEM)
2.8. Drug Loading and Entrapment Efficiency
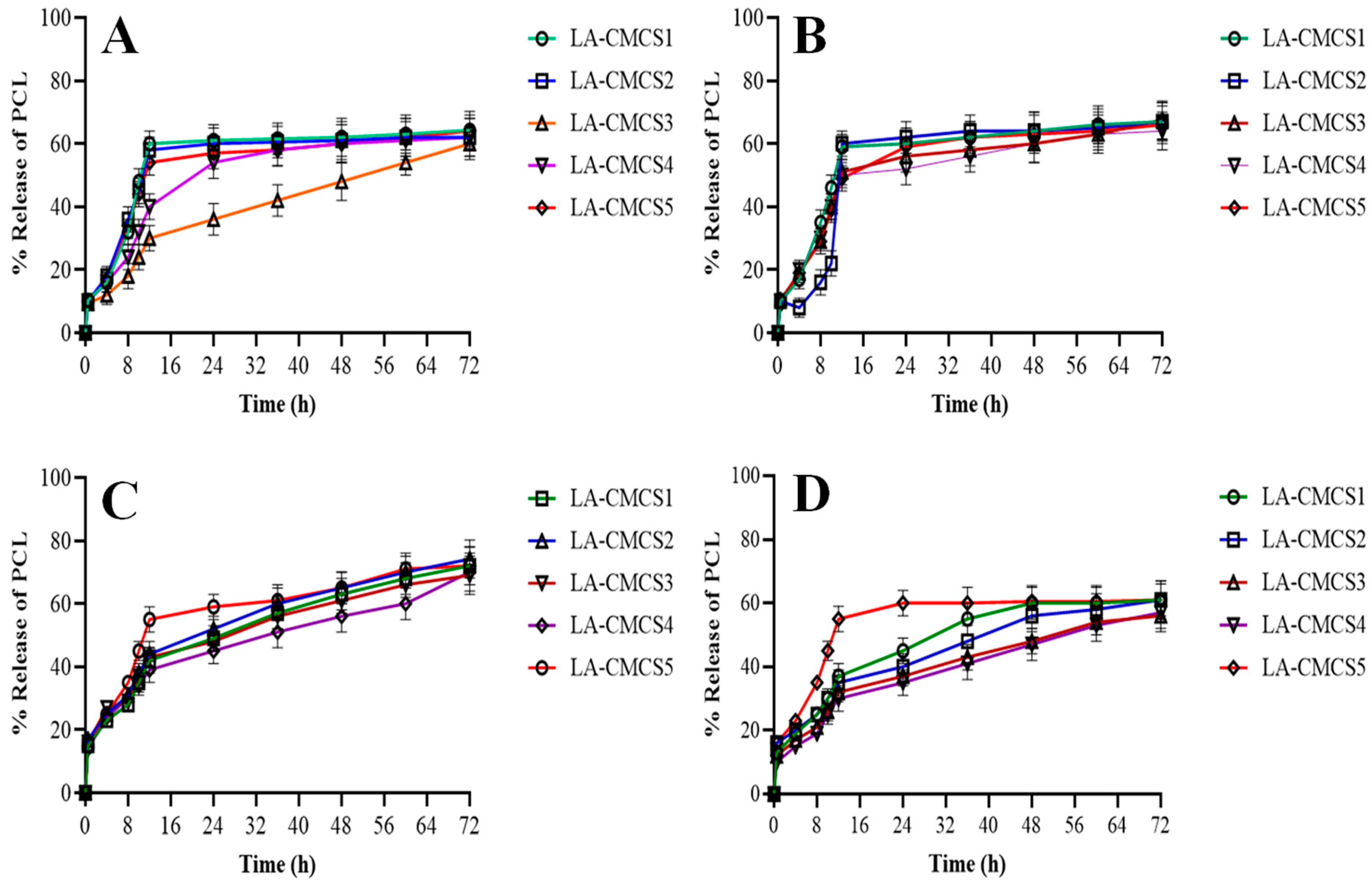
2.9. In Vitro Release Studies
2.10. Stability of Micelles in Gastrointestinal Fluids
2.11. Storage Stability of Micelles
2.12. Intestinal Permeability
2.13. Biological Studies
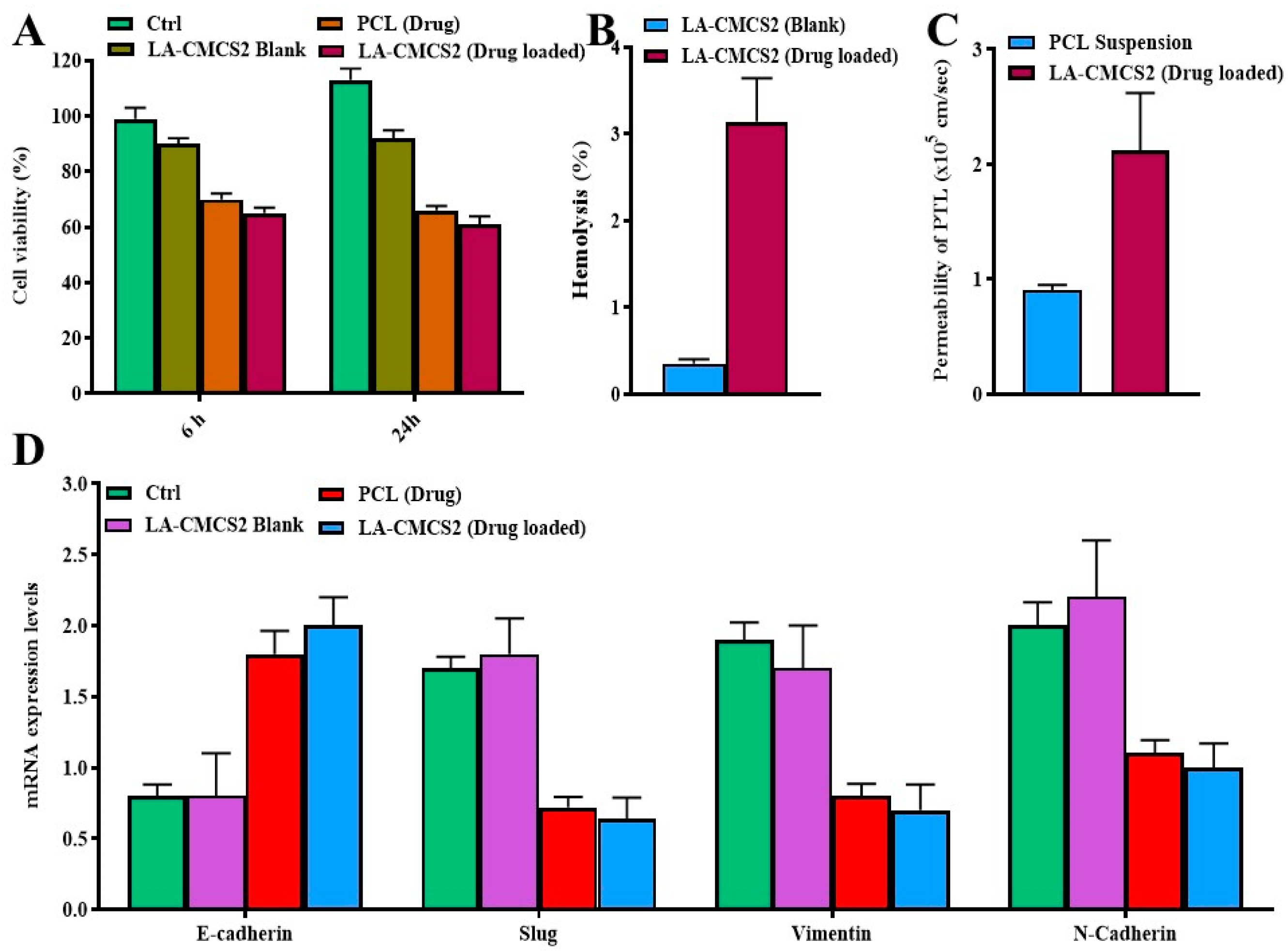
2.13.1. Cell Viability Assay
2.13.2. Haemolysis Test
2.13.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.13.4. Western Blot Analysis
2.13.5. Pharmacokinetic Study
2.13.6. Tissue Distribution Study
3. Results and Discussion
3.1. Synthesis and Characterisation of LA-CMCS Conjugate
3.2. Solubility Studies
3.3. Formulation of Polymeric Micelles and Critical Micelle Concentration Measurement
3.4. Characterisation
3.4.1. Particle Size and Zeta Potential
3.4.2. Fourier Transforms Infrared Spectroscopy (FTIR)
3.4.3. DSC and TGA Analysis
3.4.4. Transmission Electron Microscopy (TEM)
3.5. Drug Loading and Entrapment Efficiency
3.6. Release Studies and Kinetics of PCL
3.7. Stability of Micelles in Gastrointestinal Fluids
3.8. Storage Stability of Micelles
3.9. Intestinal Permeability
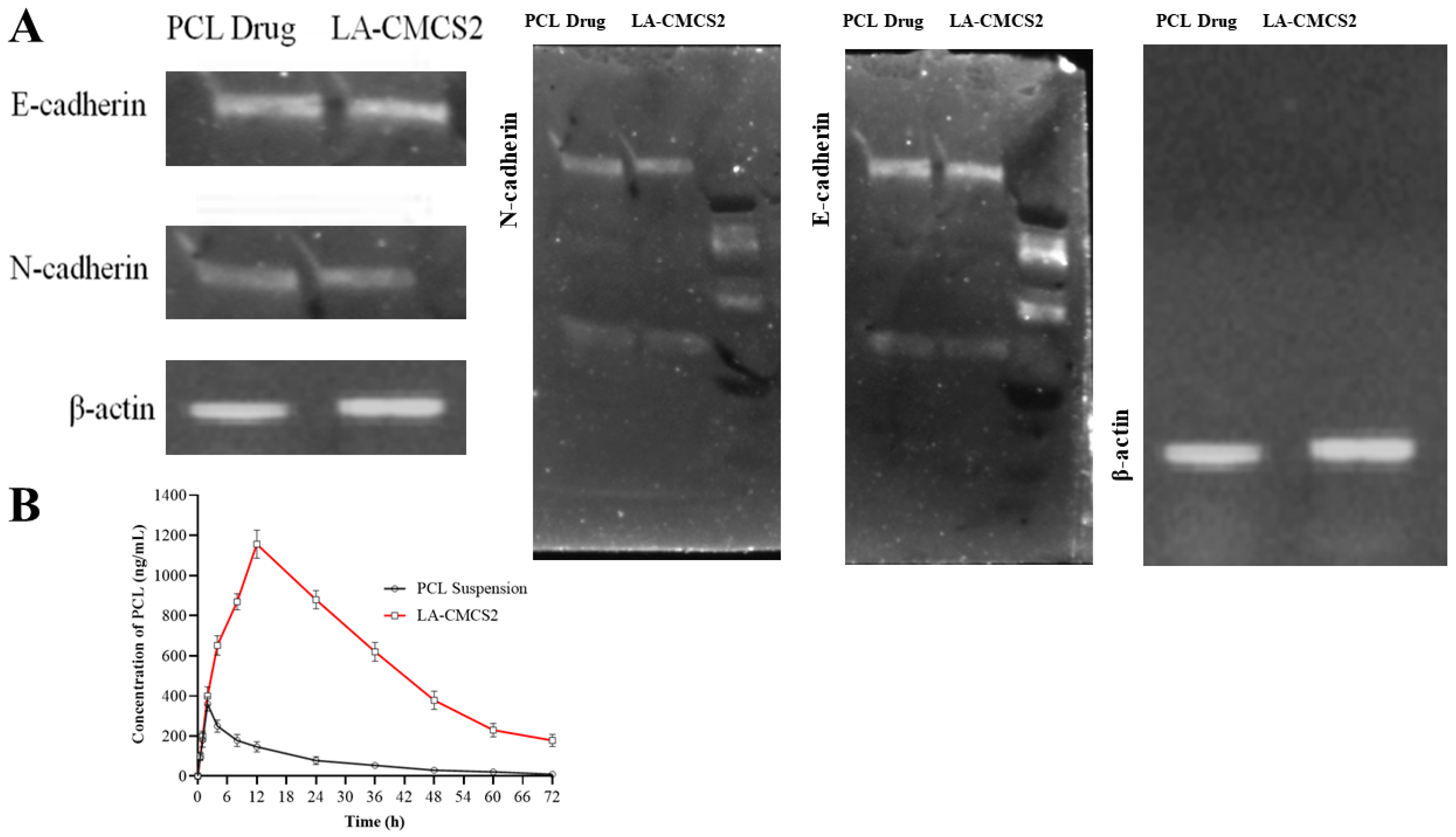
3.10. Cell Viability, qPCR, Western Blot, and Haemolysis Study
3.11. Pharmacokinetic Study
3.12. Tissue Distribution Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pangeni, R.; Choi, J.U.; Panthi, V.K.; Byun, Y.; Park, J.W. Enhanced oral absorption of pemetrexed by ion-pairing complex formation with deoxycholic acid derivative and multiple nanoemulsion formulations: Preparation, characterization, and in vivo oral bioavailability and anticancer effect. Int. J. Nanomed. 2018, 13, 3329–3351. [Google Scholar] [CrossRef] [PubMed]
- Malingré, M.M.; Beijnen, J.H.; Schellens, J.H. Oral delivery of taxanes. Investig. New Drugs 2001, 19, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J. Control. Release 2009, 140, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles of amphiphilic graft copolymer of α-tocopherol succinate-g-carboxymethyl chitosan for tamoxifen delivery: Synthesis, characterization and in vivo pharmacokinetic study. Carbohydr. Polym. 2016, 151, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Chai, Y.; Yang, F.; Cao, M.; Dai, W.-L. In situ growth of g-C3N4 on hexangular flowerlike FeWO4 microcrystals: Highly efficient catalyst and the crucial roles of Fe3+/Fe2+ couple in the photoassisted oxidation and reduction reactions. J. Phys. Chem. C 2018, 122, 12900–12912. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Renukuntla, J. Thermo-and pH dual responsive polymeric micelles and nanoparticles. Chem.-Biol. Interact. 2018, 295, 20–37. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, L. The history of chito/chitin oligosaccharides and its monomer. In Oligosaccharides of Chitin and Chitosan: Bio-Manufacture and Applications; Springer: Singapore, 2019; pp. 3–14. [Google Scholar]
- Yang, Y.; Chen, Y.; Li, D.; Lin, S.; Chen, H.; Wu, W.; Zhang, W. Linolenic acid conjugated chitosan micelles for improving the oral absorption of doxorubicin via fatty acid transporter. Carbohydr. Polym. 2023, 300, 120233. [Google Scholar] [CrossRef]
- Sakhi, M.; Khan, A.; Khan, I.; Khan, S.A.; Khan, S.I.; Khattak, M.A.; Uddin, M.N.; Kazi, M.; Nasir, F. Effect of polymeric stabilizers on the size and stability of PLGA paclitaxel nanoparticles. Saudi Pharm. J. 2023, 31, 101697. [Google Scholar] [CrossRef]
- Bilensoy, E.; Gürkaynak, O.; Doğan, A.L.; Hıncal, A.A. Safety and efficacy of amphiphilic ß-cyclodextrin nanoparticles for paclitaxel delivery. Int. J. Pharm. 2008, 347, 163–170. [Google Scholar] [CrossRef]
- Yerlikaya, F.; Ozgen, A.; Vural, I.; Guven, O.; Karaagaoglu, E.; Khan, M.A.; Capan, Y. Development and evaluation of paclitaxel nanoparticles using a quality-by-design approach. J. Pharm. Sci. 2013, 102, 3748–3761. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.M.B.; Rasul, A.; Shah, S.; Abbas, G.; Mahmood, A. Disulfide Bridged Nanoparticles of Thiolated Sodium Alginate and Eudragit RS100 for Oral Delivery of Paclitaxel: In Vitro and In Vivo Evaluation. ACS Omega 2023, 8, 9662–9672. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Schramm, M.P. Measuring critical micelle concentration as a function of cavitand additives using surface tension and dye micellization. Ronald E. McNair Postbaccalaureate Achiev. Program 2010, 14, 155. [Google Scholar]
- Zhai, Y.; Guo, S.; Liu, C.; Yang, C.; Dou, J.; Li, L.; Zhai, G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 119105. [Google Scholar]
- Muhoza, B.; Zhang, Y.; Xia, S.; Cai, J.; Zhang, X.; Su, J. Improved stability and controlled release of lutein-loaded micelles based on glycosylated casein via Maillard reaction. J. Funct. Foods 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Hong, J.W.; Lee, I.-H.; Kwak, Y.H.; Park, Y.T.; Sung, H.C.; Kwon, I.C.; Chung, H. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol. Cancer Ther. 2007, 6, 3239–3247. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Gulshan, S.; Shah, S.; Shah, P.A.; Irfan, M.; Saadullah, M.; Abbas, G.; Hanif, M.; Rasul, A.; Ahmad, N.; Mahmood, A. Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate. Pharmaceutics 2023, 15, 2259. [Google Scholar] [CrossRef]
- Shen, T.; Guan, S.; Gan, Z.; Zhang, G.; Yu, Q. Polymeric micelles with uniform surface properties and tunable size and charge: Positive charges improve tumor accumulation. Biomacromolecules 2016, 17, 1801–1810. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bhatia, S.R. Effect of anti-inflammatories on Pluronic® F127: Micellar assembly, gelation and partitioning. Int. J. Pharm. 2004, 278, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Man, D.K.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F.; Sze, S.C.; Leung, G.P.; Lam, J.K.; Kwok, P.C. Oleanolic acid loaded PEGylated PLA and PLGA nanoparticles with enhanced cytotoxic activity against cancer cells. Mol. Pharm. 2015, 12, 2112–2125. [Google Scholar] [CrossRef]
- Shin, H.-C.; Alani, A.W.; Rao, D.A.; Rockich, N.C.; Kwon, G.S. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J. Control. Release 2009, 140, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lai, K.L.; Chan, P.S.; Leung, S.C.; Li, H.Y.; Fang, Y.; To, K.K.; Choi, C.H.J.; Gao, Q.Y.; Lee, T.W. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Colloids Surf. B Biointerfaces 2016, 140, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Dizaji, B.F.; Azerbaijan, M.H.; Sheisi, N.; Goleij, P.; Mirmajidi, T.; Chogan, F.; Irani, M.; Sharafian, F. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int. J. Biol. Macromol. 2020, 164, 1461–1474. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.; Xue, M.; Cao, Y.; Zhang, W.; Li, D. Liquid handling properties of carboxymethyl modified chitosan nonwovens for medical dressings. J. Mol. Struct. 2023, 1292, 136118. [Google Scholar] [CrossRef]
- Wu, D.; Chen, X.; Shi, P.; Wang, S.; Feng, F.; He, Y. Determination of α-linolenic acid and linoleic acid in edible oils using near-infrared spectroscopy improved by wavelet transform and uninformative variable elimination. Anal. Chim. Acta 2009, 634, 166–171. [Google Scholar] [CrossRef]
- Martins, K.F.; Messias, A.D.; Leite, F.L.; Duek, E.A. Preparation and characterization of paclitaxel-loaded PLDLA microspheres. Mater. Res. 2014, 17, 650–656. [Google Scholar] [CrossRef]
- Patale, R.L.; Patravale, V.B. O,N-carboxymethyl chitosan–zinc complex: A novel chitosan complex with enhanced antimicrobial activity. Carbohydr. Polym. 2011, 85, 105–110. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. DSC study of linolenic acid autoxidation inhibited by BHT, dehydrozingerone and olivetol. J. Therm. Anal. Calorim. 2007, 88, 781–785. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of films produced using nanoemulsions stabilized by carboxymethyl chitosan-peptide conjugates and application in blueberry preservation. Int. J. Biol. Macromol. 2022, 202, 26–36. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zheng, L.S.; Guo, X.D.; Qian, Y.; Zhang, L.J. pH-sensitive micelles self-assembled from amphiphilic copolymer brush for delivery of poorly water-soluble drugs. Biomacromolecules 2011, 12, 116–122. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, L.-J.; Du, Y.-Z.; Hu, F.-Q. Stearic acid-g-chitosan polymeric micelle for oral drug delivery: In vitro transport and in vivo absorption. Mol. Pharm. 2011, 8, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, Y.; Dai, X.; Shi, L.; Mckinley, D.; Tan, C. Reduction-responsive crosslinked micellar nanoassemblies for tumor-targeted drug delivery. Pharm. Res. 2015, 32, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zou, Y.; He, C.; Zhou, Y.; Jin, Y.; Deng, Y.; Wang, Z.; Li, X.; Zhou, Y.; Liu, Y. Improved intestinal absorption of paclitaxel by mixed micelles self-assembled from vitamin E succinate-based amphiphilic polymers and their transcellular transport mechanism and intracellular trafficking routes. Drug Deliv. 2018, 25, 210–225. [Google Scholar] [CrossRef]
- Gu, W.; Chen, J.; Patra, P.; Yang, X.; Gu, Q.; Wei, L.; Acker, J.P.; Kong, B. Nanoformulated water-soluble paclitaxel to enhance drug efficacy and reduce hemolysis side effect. J. Biomater. Appl. 2017, 32, 66–73. [Google Scholar] [CrossRef]
- Han, Y.; Liang, N.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. A chitosan-based micellar system as nanocarrier for the delivery of paclitaxel. Polymers 2020, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Zhao, Y.; Li, L.; Xu, W. Preparation and in vitro evaluation of amphiphilic paclitaxel small molecule prodrugs and enhancement of oral absorption. Eur. J. Med. Chem. 2021, 215, 113276. [Google Scholar] [CrossRef]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: In vitro and in vivo characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.T.; Giaccone, G.; Van Warmerdam, L.; Rosing, H.; Bakker, P.; Vermorken, J.B.; Postmus, P.E.; van Zandwijk, N.; Koolen, M.; ten Bokkel Huinink, W.W. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer. The European Cancer Centre. J. Clin. Oncol. 1997, 15, 317–329. [Google Scholar] [CrossRef] [PubMed]

| Code | Particle Size (nm) before Loading of Drug | Particle Size (nm) after Loading of Drug | PDI | Particle Size (nm) after Lyophilisation of PCL-Loaded Micelles | Zeta Potential (mV) |
|---|---|---|---|---|---|
| LA-CMCS1 | 99 ± 13 | 100 ± 18 | 0.198 ± 0.01 | 99.5 ± 13 | −18 ± 2 |
| LA-CMCS2 | 93 ± 11 | 94 ± 20 | 0.023 ± 0.01 | 94 ± 11 | −29 ± 2 |
| LA-CMCS3 | 101 ± 18 | 100 ± 21 | 0.208 ± 0.02 | 100.5 ± 14 | −16 ± 2 |
| LA-CMCS4 | 119 ± 12 | 121 ± 17 | 0.308 ± 0.03 | 120 ± 12 | −24 ± 1 |
| LA-CMCS5 | 108 ± 19 | 108 ± 21 | 0.287 ± 0.03 | 107.5 ± 15 | −23 ± 3 |
| Code | PCL:LA-CMCS | %DL | %EE |
|---|---|---|---|
| LA-CMCS1 | 1:2 | 65 ± 2 | 60 ± 2 |
| LA-CMCS2 | 1:1 | 67 ± 3 | 61 ± 3 |
| LA-CMCS3 | 1:4 | 64 ± 4 | 59 ± 2 |
| LA-CMCS4 | 1:6 | 63 ± 3 | 57 ± 2 |
| LA-CMCS5 | 1:8 | 62 ± 3 | 56 ± 4 |
| Code | First Order | Zero Order | Higuchi | Korsmeyer–Peppas | ||
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | n | R2 | k | |
| LA-CMCS1 | 0.567 | 0.995 | 0.876 | 0.33 | 0.993 | 0.53 |
| LA-CMCS2 | 0.598 | 0.999 | 0.898 | 0.21 | 0.998 | 0.49 |
| LA-CMCS3 | 0.745 | 0.990 | 0.766 | 0.24 | 0.981 | 0.44 |
| LA-CMCS4 | 0.609 | 0.991 | 0.821 | 0.38 | 0.989 | 0.42 |
| LA-CMCS5 | 0.798 | 0.996 | 0.859 | 0.32 | 0.990 | 0.39 |
| Conditions | Size of Micelles (nm) | PDI | Surface Charge (mV) |
|---|---|---|---|
| SIF with pancreatin pH 6.8 | 108 ± 7 | 0.025 ± 0.02 | +7 ± 1 |
| SIF with pancreatin pH 6.8 | 107 ± 3 | 0.023 ± 0.03 | +8 ± 2 |
| SGF with pepsin pH 1.2 | 94 ± 5 | 0.024 ± 0.01 | +7 ± 1 |
| SGF without pepsin pH 1.2 | 94 ± 6 | 0.022 ± 0.02 | +8 ± 2 |
| PBS pH 5 | 99 ± 7 | 0.023 ± 0.03 | +9 ± 3 |
| Code | Size of Micelles after 4 Weeks | %DL of PCL after 4 Weeks |
|---|---|---|
| LA-CMCS1 | 99 ± 12 | 63 ± 3 |
| LA-CMCS2 | 93 ± 07 | 65 ± 2 |
| LA-CMCS3 | 98 ± 08 | 62 ± 3 |
| LA-CMCS4 | 119 ± 13 | 61 ± 2 |
| LA-CMCS5 | 106 ± 11 | 60 ± 3 |
| Parameters | Units | PCL Suspension | LA-CMCS Micelles |
|---|---|---|---|
| Cmax | ng/mL | 360 ± 86 | 1156 ± 18 |
| Tmax | h | 2 ± 1 | 12 ± 2 |
| t1/2 | h | 9 ± 2 | 50 ± 10 |
| t1/2a | h | 10.82 ± 3 | 7.5 ± 3 |
| t1/2b | h | 15.4 ± 4 | 17.7 ± 4 |
| AUC0–t | ng·h/mL | 3960 ± 23 | 12,716 ± 78 |
| AUC0–ꝏ | ng·h/mL | 4312 ± 24 | 14,512 ± 82 |
| Samples | Concentration of PCL in ng/mL | |
|---|---|---|
| LA-CMCS2 Micelles | Pure PCL | |
| Blood | 1158 ± 28 | 359 ± 46 |
| Heart | 2012 ± 31 | 634 ± 41 |
| Lung | 2324 ± 87 | 734 ± 32 |
| Kidney | 4532 ± 102 | 1231 ± 51 |
| Liver | 3345 ± 54 | 978 ± 59 |
| Spleen | 2139 ± 62 | 634 ± 21 |
| Stomach | 5234 ± 208 | 1765 ± 104 |
| Jejunum | 5039 ± 108 | 1345 ± 87 |
| Ileum | 9564 ± 234 | 1643 ± 106 |
| Colon | 9786 ± 298 | 1845 ± 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubeen, I.; Abbas, G.; Shah, S.; Assiri, A.A. Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics 2024, 16, 342. https://doi.org/10.3390/pharmaceutics16030342
Mubeen I, Abbas G, Shah S, Assiri AA. Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics. 2024; 16(3):342. https://doi.org/10.3390/pharmaceutics16030342
Chicago/Turabian StyleMubeen, Iqra, Ghulam Abbas, Shahid Shah, and Abdullah A Assiri. 2024. "Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel" Pharmaceutics 16, no. 3: 342. https://doi.org/10.3390/pharmaceutics16030342
APA StyleMubeen, I., Abbas, G., Shah, S., & Assiri, A. A. (2024). Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics, 16(3), 342. https://doi.org/10.3390/pharmaceutics16030342