Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Granules
2.3. Preparation of Tablets
2.4. Particle Size Distribution
2.5. Evaluation of Granules
2.5.1. API Content Uniformity
2.5.2. Friability Analysis
2.5.3. Particle Size Analysis
2.6. Evaluation of Tablets
2.6.1. Tensile Strength Analysis
2.6.2. Dissolution Testing
3. Results and Discussion
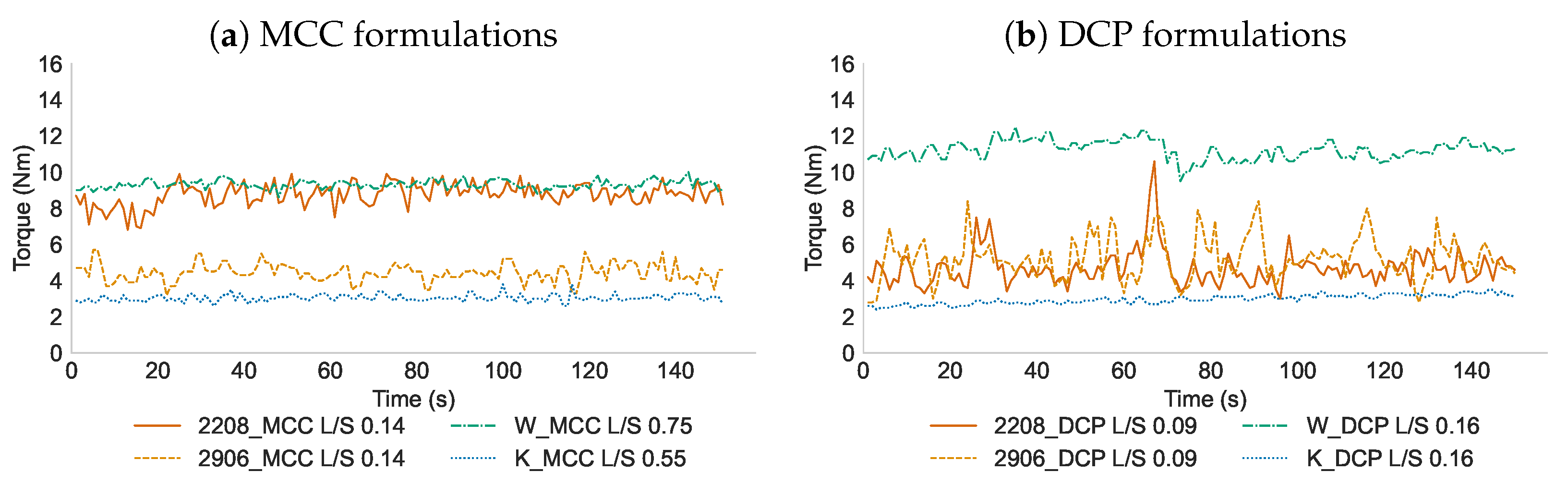
3.1. Evaluation of Granulation Process
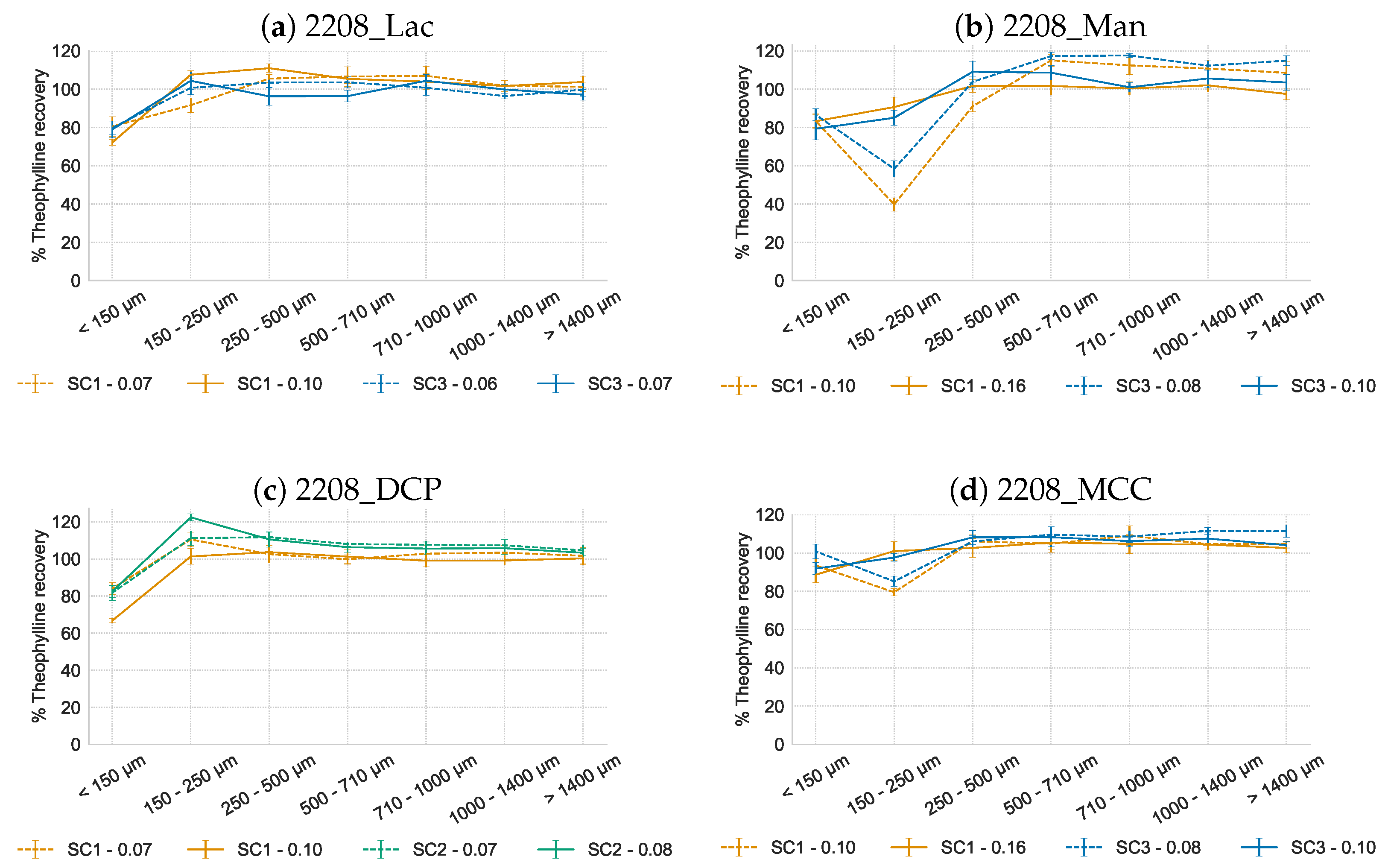
3.2. Influence of L/S-Ratio, Screw Configuration and Filler Solubility on API Homogeneity of Granules Containing HPMC Type 2208
3.3. Influence of Matrix Former Types on API Homogeneity of CR Granules
3.3.1. Without Matrix Former
3.3.2. Hydrophilic Matrix Formers HPMC Type 2906 and 2208
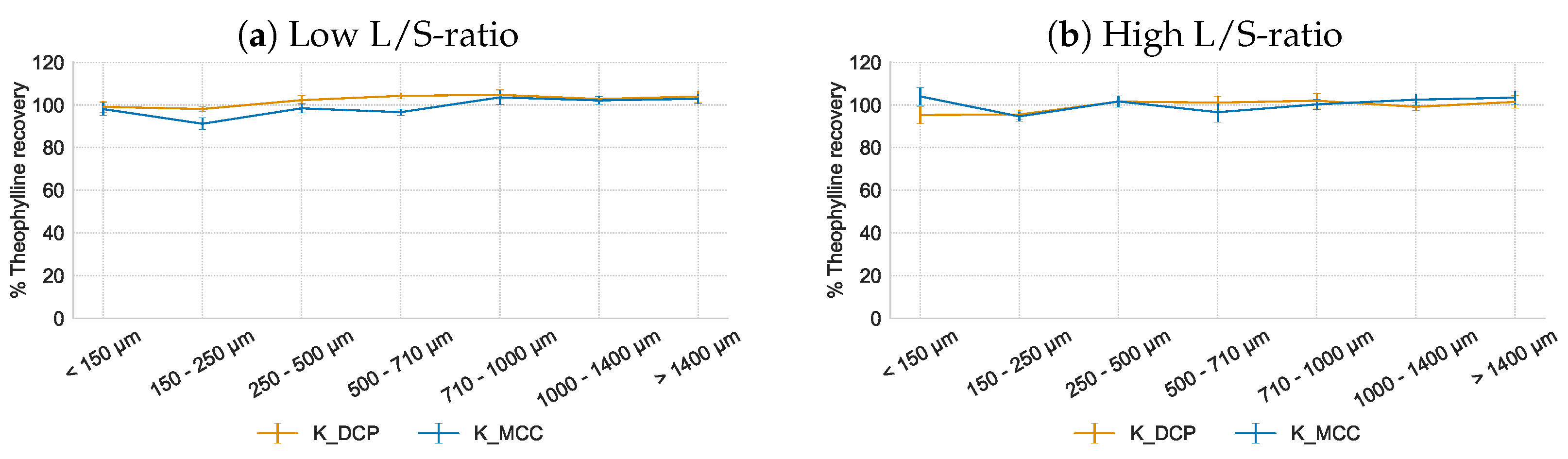
3.3.3. Hydrophobic Matrix Former Kollidon SR
3.4. Influence of Formulation Variables on Tablet Quality and Dissolution Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ummadi, S.; Shavrani, B.; Raghavendra Rao, N.G.; Srikanth Reddy, M.; Sanjeev Nayak, B. Overview on Controlled Release Dosage Form. Int. J. Pharm. Sci. 2013, 3, 13. [Google Scholar]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Viridén, A.; Wittgren, B.; Larsson, A. Investigation of critical polymer properties for polymer release and swelling of HPMC matrix tablets. Eur. J. Pharm. Sci. 2009, 36, 297–309. [Google Scholar] [CrossRef]
- Herder, J.; Adolfsson, Å.; Larsson, A. Initial studies of water granulation of eight grades of hypromellose (HPMC). Int. J. Pharm. 2006, 313, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Jayaswal, S.R.; Felix Joe, V.; Viswanath, B.A. Formulation and evaluation of sustained release matrix tablets of glibenclamide. Int. J. Pharm. Technol. 2014, 6, 6572–6586. [Google Scholar]
- Pani, N.R.; Nath, L.K. Development of controlled release tablet by optimizing HPMC: Consideration of theoretical release and RSM. Carbohydr. Polym. 2014, 104, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Moussa, E.; Siepmann, F.; Flament, M.P.; Benzine, Y.; Penz, F.; Siepmann, J.; Karrout, Y. Controlled release tablets based on HPMC:lactose blends. J. Drug Deliv. Sci. Technol. 2019, 52, 607–617. [Google Scholar] [CrossRef]
- Cao, Q.R.; Choi, J.S.; Liu, Y.; Xu, W.J.; Yang, M.; Lee, B.J.; Cui, J.H. A formulation approach for development of HPMC-based sustained release tablets for tolterodine tartrate with a low release variation. Drug Dev. Ind. Pharm. 2013, 39, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Brahma, C.; Nandi, S.; Parida, K. Formulation and design of sustained release matrix tablets of metformin hydrochloride: Influence of hypromellose and polyacrylate polymers. Int. J. Appl. Basic Med. Res. 2013, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, K.M.; Cho, C.H.; Nguyen, T.T.; Seok, S.H.; Hwang, K.M.; Kim, J.Y.; Park, C.W.; Rhee, Y.S.; Park, E.S. Application of continuous twin screw granulation for the metformin hydrochloride extended release formulation. Int. J. Pharm. 2017, 529, 410–422. [Google Scholar] [CrossRef]
- Seem, T.C.; Rowson, N.A.; Ingram, A.; Huang, Z.; Yu, S.; de Matas, M.; Gabbott, I.; Reynolds, G.K. Twin screw granulation—A literature review. Powder Technol. 2015, 276, 89–102. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms. Int. J. Pharm. 2020, 579, 119194. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Thompson, M.R.; O’Donnell, K.P. “Rolling” phenomenon in twin screw granulation with controlled-release excipients. Drug Dev. Ind. Pharm. 2015, 41, 482–492. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vanbillemont, B.; Vercruysse, J.; De Leersnyder, F.; Gomes, P.; De Beer, T.; Remon, J.P.; Vervaet, C. Development of a controlled release formulation by continuous twin screw granulation: Influence of process and formulation parameters. Int. J. Pharm. 2016, 505, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef]
- EDQM. Theophyllinum. In European Pharmacopoeia, 11th ed.; EDQM: Strasbourg, France, 2017; p. 4183. [Google Scholar]
- EDQM. Hypromellose. In European Pharmacopoeia, 11th ed.; EDQM: Strasbourg, France, 2022; pp. 3055–3057. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In vitro dissolution profile comparison- Statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effects of properties of granulation liquid. Powder Technol. 2012, 229, 126–136. [Google Scholar] [CrossRef]
- Vercruysse, J.; Córdoba Díaz, D.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Perspectives on the Wetting of Solids in Pharmaceutical Systems. Pharm. Res. 2023, 40, 3099–3118. [Google Scholar] [CrossRef]
- Portier, C.; Vigh, T.; Di Pretoro, G.; Leys, J.; Klingeleers, D.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Impact of microcrystalline cellulose batch-to-batch variability during granulation and drying—A QbD approach. Int. J. Pharm. X 2021, 3, 100077. [Google Scholar] [CrossRef] [PubMed]
- Willecke, N.; Szepes, A.; Wunderlich, M.; Remon, J.P.; Vervaet, C.; De Beer, T. Identifying overarching excipient properties towards an in-depth understanding of process and product performance for continuous twin-screw wet granulation. Int. J. Pharm. 2017, 522, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dries, K.; De Vegt, O.M.; Girard, V.; Vromans, H. Granule breakage phenomena in a high shear mixer; influence of process and formulation variables and consequences on granule homogeneity. Powder Technol. 2003, 133, 228–236. [Google Scholar] [CrossRef]
- Reynolds, G.K.; Fu, J.S.; Cheong, Y.S.; Hounslow, M.J.; Salman, A.D. Breakage in granulation: A review. Chem. Eng. Sci. 2005, 60, 3969–3992. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression physics in the formulation development of tablets. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 1–65. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, L.; Lin, X.; Shen, L. An update on microcrystalline cellulose in direct compression: Functionality, critical material attributes, and co-processed excipients. Carbohydr. Polym. 2022, 278, 118968. [Google Scholar] [CrossRef]
- Badawy, S.I.; Gray, D.B.; Hussain, M.A. A study on the effect of wet granulation on microcrystalline cellulose particle structure and performance. Pharm. Res. 2006, 23, 634–640. [Google Scholar] [CrossRef]
- Westermarck, S.; Juppo, A.M.; Kervinen, L.; Yliruusi, J. Microcrystalline cellulose and its microstructure in pharmaceutical processing. Eur. J. Pharm. Biopharm. 1999, 48, 199–206. [Google Scholar] [CrossRef]
- Staniforth, J.N.; Baichwal, A.R.; Hart, J.P.; Heng, P.W. Effect of addition of water on the rheological and mechanical properties of microcrystalline celluloses. Int. J. Pharm. 1988, 41, 231–236. [Google Scholar] [CrossRef]
- Bendgude, T.; Iyer, R.; Sushi, S. The effects of lactose, microcrystalline cellulose and dicalcium phosphate on swelling and erosian of compressed HPMC matrix tablets. Iran. J. Pharm. Res. 2010, 9, 349–358. [Google Scholar]
- Ali, J.; Saigal, N.; Baboota, S.; Ahuja, A. Microcrystalline cellulose as a versatile excipient in drug research. J. Young Pharm. 2009, 1, 6. [Google Scholar] [CrossRef]
- Hauschild, K.; Picker-Freyer, K.M. Evaluation of tableting and tablet properties of Kollidon SR: The influence of moisture and mixtures with theophylline monohydrate. Pharm. Dev. Technol. 2006, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
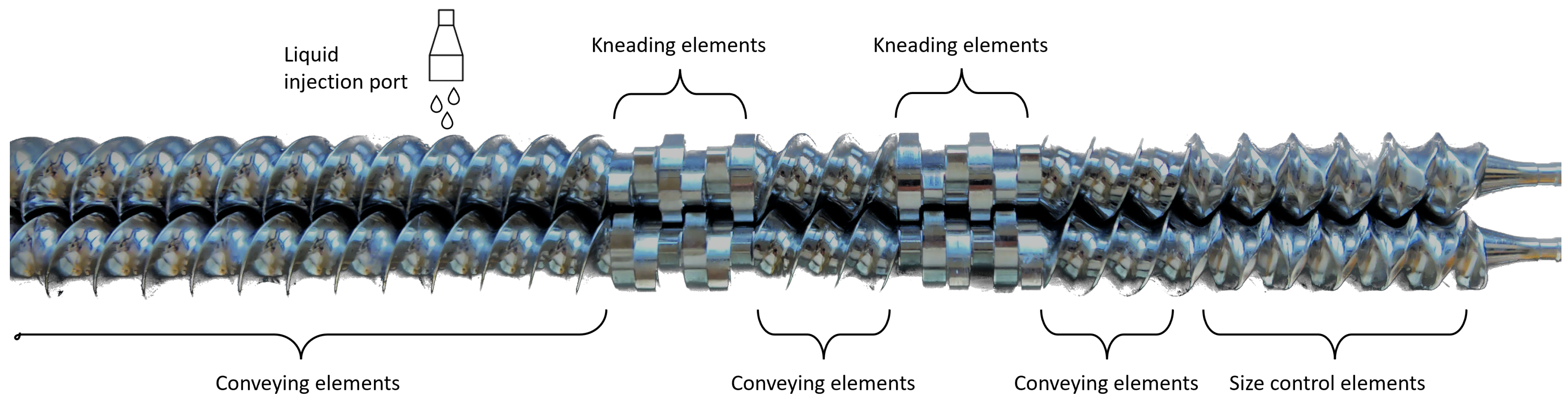



| Theophylline | Pharmatose 200M | Parteck M200 | Emcompress Anhydrous Powder | Avicel PH101 | |
|---|---|---|---|---|---|
| Dv_10 (µm) | 7.91 ± 0.02 | 7.58 ± 0.08 | 59.07 ± 0.88 | 1.57 ± 0.03 | 26.07 ± 0.12 |
| Dv_50 (µm) | 30.90 ± 0.08 | 46.13 ± 0.05 | 135.33 ± 2.62 | 14.53 ± 0.33 | 69.93 ± 0.09 |
| Dv_90 (µm) | 88.97 ± 0.21 | 119.33 ± 0.47 | 337.33 ± 9.53 | 32.90 ± 0.22 | 147.33 ± 0.47 |
| Formulation (a) | Theophylline (%) | Matrix Former (%) | Filler (%) | L/S-Ratio (Low–High) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPMC 90SH- 4000SR | HPMC 65SH- 4000SR | Kollidon SR | Pharmatose 200M | Parteck M200 | Emcompress Anhydrous Powder | Avicel PH101 | SC1 12KE60° | SC2 10KE60° 2KE90° | SC3 8KE60° 4KE90° | ||
| 2208_Lac | 20 | 20 | - | - | 60 | - | - | - | 0.07–0.10 | - | 0.06–0.07 |
| 2208_Man | 20 | 20 | - | - | - | 60 | - | - | 0.10–0.16 | - | 0.08–0.10 |
| 2208_DCP | 20 | 20 | - | - | - | - | 60 | - | 0.07–0.10 | 0.07–0.08 | NA |
| 2208_MCC | 20 | 20 | - | - | - | - | - | 60 | 0.10–0.16 | - | 0.08–0.10 |
| W_Lac | 20 | - | - | - | 80 | - | - | - | 0.10–0.14 | - | - |
| W_Man | 20 | - | - | - | - | 80 | - | - | 0.14–0.20 | - | - |
| W_DCP | 20 | - | - | - | - | - | 80 | - | 0.10–0.16 | - | - |
| W_MCC | 20 | - | - | - | - | - | - | 80 | 0.75–0.90 | - | - |
| 2906_DCP | 20 | - | 20 | - | - | - | 60 | - | 0.09–0.13 | - | - |
| 2906_MCC | 20 | - | 20 | - | - | - | - | 60 | 0.14–0.20 | - | - |
| K_DCP | 20 | - | - | 20 | - | - | 60 | - | 0.14–0.16 | - | - |
| K_MCC | 20 | - | - | 20 | - | - | - | 60 | 0.55–0.85 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denduyver, P.; Vervaet, C.; Vanhoorne, V. Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters. Pharmaceutics 2024, 16, 341. https://doi.org/10.3390/pharmaceutics16030341
Denduyver P, Vervaet C, Vanhoorne V. Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters. Pharmaceutics. 2024; 16(3):341. https://doi.org/10.3390/pharmaceutics16030341
Chicago/Turabian StyleDenduyver, Phaedra, Chris Vervaet, and Valérie Vanhoorne. 2024. "Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters" Pharmaceutics 16, no. 3: 341. https://doi.org/10.3390/pharmaceutics16030341
APA StyleDenduyver, P., Vervaet, C., & Vanhoorne, V. (2024). Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters. Pharmaceutics, 16(3), 341. https://doi.org/10.3390/pharmaceutics16030341







