Efficient Gene Editing for Heart Disease via ELIP-Based CRISPR Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Echogenic Liposomes (ELIP)
2.2. Preparation of ELIP-Containing NF-κB-FITC (ELIP-NF-κB-FITC)
2.3. Preparation of ELIP for CRISPR Complex
2.4. Characterization of Loading Efficiency and Ultrasound-Triggered Release
2.5. Isolation and Culture of Primary Ventricular Myocytes
2.6. Treatment of Cultured Mouse Neonatal Cardiomyocytes
2.7. In Vivo Delivery of ELIP-FITC-Labeled Oligonucleotides
2.8. Determination of Gene Editing Efficiency
2.9. Design of gRNA and Primers
2.10. Statistical Analysis
3. Results
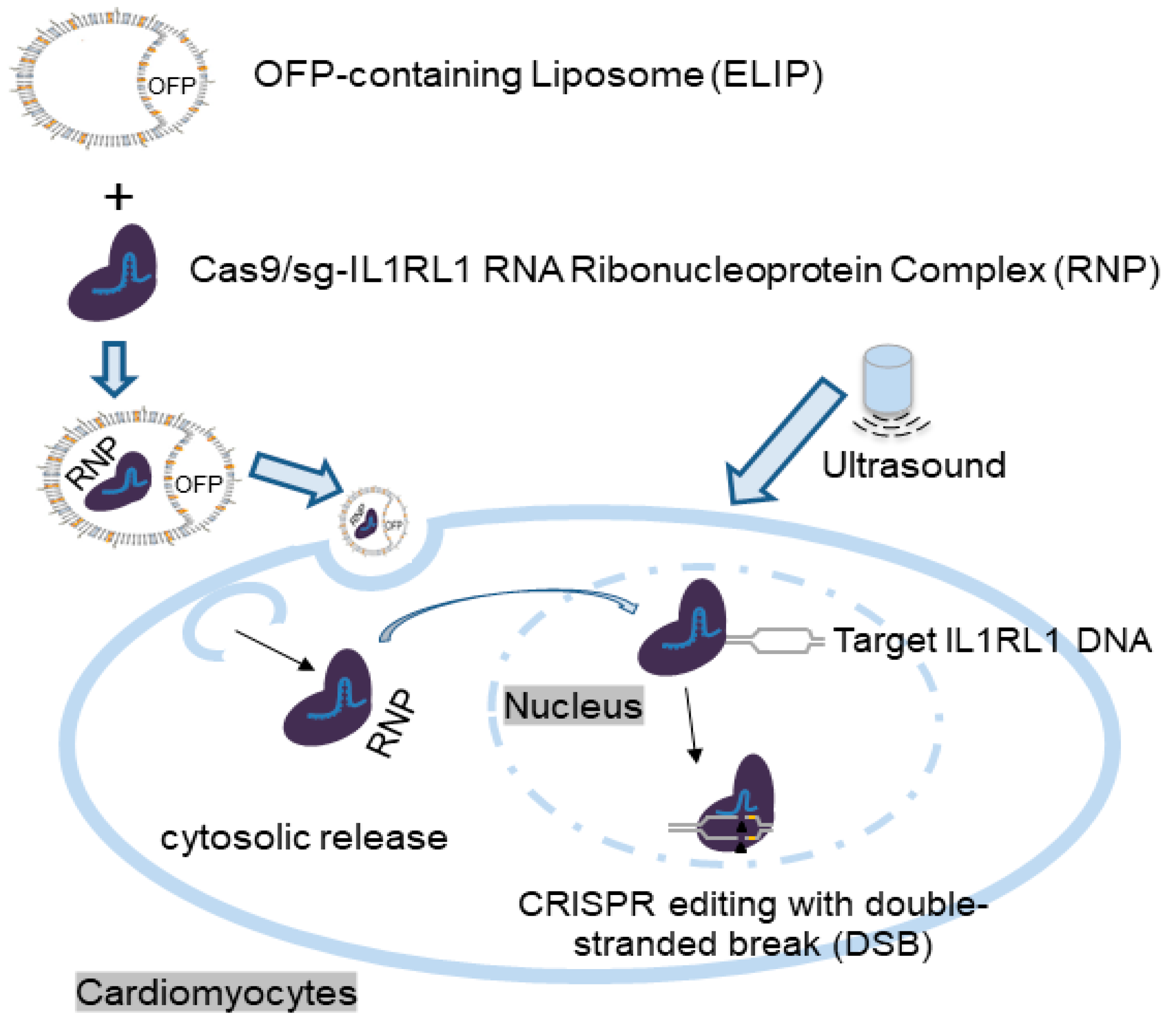
3.1. Design and Preparation of Gas-Containing Ionizable Liposomes and Experimental Setup
3.2. Octafluoropropane Enhances the Echogenic Properties of ELIP
3.3. Enhanced Delivery of NF-κB-FITC to Cultured Mouse Neonatal Cardiomyocytes In Vitro and Rat Heart In Vivo Using ELIP and Ultrasound
3.4. Characterization of ELIP-CRISPR Complex
3.5. Therapeutic Efficiency in Terms of Gene Disruption of Cas9/sg-IL1RL1 RNA Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Nishiyama, T.; Bassel-Duby, R.; Olson, E.N. Toward CRISPR Therapies for Cardiomyopathies. Circulation 2021, 144, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, C.; Bassel-Duby, R.; Olson, E.N. Myoediting: Toward Prevention of Muscular Dystrophy by Therapeutic Genome Editing. Physiol. Rev. 2018, 98, 1205–1240. [Google Scholar] [CrossRef] [PubMed]
- Koblan, L.W.; Erdos, M.R.; Wilson, C.; Cabral, W.A.; Levy, J.M.; Xiong, Z.M.; Tavarez, U.L.; Davison, L.M.; Gete, Y.G.; Mao, X.; et al. In vivo base editing rescues Hutchinson-Gilford progeria syndrome in mice. Nature 2021, 589, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, S.; Chen, X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: Challenges and opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef]
- Britton, G.L.; Kim, H.; Kee, P.H.; Aronowski, J.; Holland, C.K.; McPherson, D.D.; Huang, S.L. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation 2010, 122, 1578–1587. [Google Scholar] [CrossRef]
- Huang, S.L.; Kee, P.H.; Kim, H.; Moody, M.R.; Chrzanowski, S.M.; Macdonald, R.C.; McPherson, D.D. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J. Am. Coll. Cardiol. 2009, 54, 652–659. [Google Scholar] [CrossRef]
- Kee, P.H.; Moody, M.R.; Huang, S.L.; Kim, H.; Yin, X.; Peng, T.; Laing, S.T.; Klegerman, M.E.; Rahbar, M.H.; Vela, D.; et al. Stabilizing Peri-Stent Restenosis Using a Novel Therapeutic Carrier. JACC Basic Transl. Sci. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Huang, S.L.; Moody, M.R.; Yin, X.; McPherson, D.D.; Kim, H. Co-Delivery of Therapeutics and Bioactive Gas Using a Novel Liposomal Platform for Enhanced Treatment of Acute Arterial Injury. Biomolecules 2023, 13, 861. [Google Scholar] [CrossRef]
- Yin, X.; Harmancey, R.; McPherson, D.D.; Kim, H.; Huang, S.L. Liposome-Based Carriers for CRISPR Genome Editing. Int. J. Mol. Sci. 2023, 24, 12844. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.D.; Huang, S.L.; Kim, H.; McPherson, D.D.; MacDonald, R.C. Encapsulation of NF-κB decoy oligonucleotides within echogenic liposomes and ultrasound-triggered release. J. Control. Release 2010, 141, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Won, E.J.; Lee, H.A.R.; Kim, J.H.; Hui, E.; Kim, H.P.; Yoon, T.J. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials 2020, 232, 119736. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Sun, L.; Pu, Y.; Yu, J.; Feng, W.; Dong, C.; Zhou, B.; Du, D.; Zhang, Y.; Chen, Y.; et al. Ultrasound-Controlled CRISPR/Cas9 System Augments Sonodynamic Therapy of Hepatocellular Carcinoma. ACS Cent. Sci. 2021, 7, 2049–2062. [Google Scholar] [CrossRef] [PubMed]
- Jbara-Agbaria, D.; Blondzik, S.; Burger-Kentischer, A.; Agbaria, M.; Nordling-David, M.M.; Giterman, A.; Aizik, G.; Rupp, S.; Golomb, G. Liposomal siRNA Formulations for the Treatment of Herpes Simplex Virus-1: In Vitro Characterization of Physicochemical Properties and Activity, and In Vivo Biodistribution and Toxicity Studies. Pharmaceutics 2022, 14, 633. [Google Scholar] [CrossRef]
- Liang, C.; Li, F.; Wang, L.; Zhang, Z.K.; Wang, C.; He, B.; Li, J.; Chen, Z.; Shaikh, A.B.; Liu, J.; et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials 2017, 147, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Moody, M.R.; Hebert, V.; Klegerman, M.E.; Geng, Y.J.; Dugas, T.R.; McPherson, D.D.; Kim, H.; Huang, S.L. Oral delivery of xenon for cardiovascular protection. Sci. Rep. 2019, 9, 14035. [Google Scholar] [CrossRef]
- Nahire, R.; Haldar, M.K.; Paul, S.; Mergoum, A.; Ambre, A.H.; Katti, K.S.; Gange, K.N.; Srivastava, D.K.; Sarkar, K.; Mallik, S. Polymer-coated echogenic lipid nanoparticles with dual release triggers. Biomacromolecules 2013, 14, 841–853. [Google Scholar] [CrossRef][Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Gong, J.; Wang, H.X.; Lao, Y.H.; Hu, H.; Vatan, N.; Guo, J.; Ho, T.C.; Huang, D.; Li, M.; Shao, D.; et al. A Versatile Non-viral Delivery System for Multiplex Gene-Editing in the Liver. Adv. Mater. 2020, 32, e2003537. [Google Scholar] [CrossRef] [PubMed]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Batchelor, D.V.B.; Loadman, P.M.; McLaughlan, J.R.; Markham, A.F.; Evans, S.D.; Coletta, P.L. A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles. Pharmaceutics 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; Geers, B.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Mol. Ther. 2010, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Nahire, R.; Mallik, S.; Sarkar, K. Encapsulated microbubbles and echogenic liposomes for contrast ultrasound imaging and targeted drug delivery. Comput. Mech. 2014, 53, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, P.; Jashari, F.; Bajraktari, G.; Wester, P.; Henein, M.Y. Ultrasound assessment of carotid plaque echogenicity response to statin therapy: A systematic review and meta-analysis. Int. J. Mol. Sci. 2015, 16, 10734–10747. [Google Scholar] [CrossRef]
- Chai, A.C.; Cui, M.; Chemello, F.; Li, H.; Chen, K.; Tan, W.; Atmanli, A.; McAnally, J.R.; Zhang, Y.; Xu, L.; et al. Base editing correction of hypertrophic cardiomyopathy in human cardiomyocytes and humanized mice. Nat. Med. 2023, 29, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.L.; Bassel-Duby, R.; Olson, E.N. CRISPR Correction of Duchenne Muscular Dystrophy. Annu. Rev. Med. 2019, 70, 239–255. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Rashid, S.; Curtis, D.E.; Garuti, R.; Anderson, N.N.; Bashmakov, Y.; Ho, Y.K.; Hammer, R.E.; Moon, Y.A.; Horton, J.D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA 2005, 102, 5374–5379. [Google Scholar] [CrossRef]
- Price, R.J.; Skyba, D.M.; Kaul, S.; Skalak, T.C. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation 1998, 98, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Vancraeynest, D.; Havaux, X.; Pouleur, A.C.; Pasquet, A.; Gerber, B.; Beauloye, C.; Rafter, P.; Bertrand, L.; Vanoverschelde, J.L. Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur. Heart J. 2006, 27, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, K.; Liu, J.; Xie, M.; Wang, X.; Lu, Q.; Zhang, J.; Fang, L. Enhancement of survivin gene downregulation and cell apoptosis by a novel combination: Liposome microbubbles and ultrasound exposure. Med. Oncol. 2009, 26, 491–500. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liang, K.; Qiu, R.X.; Luo, L.P. Ultrasound- and liposome microbubble-mediated targeted gene transfer to cardiomyocytes in vivo accompanied by polyethylenimine. J. Ultrasound Med. 2011, 30, 1247–1258. [Google Scholar] [CrossRef]
- Feril, L.B., Jr.; Ogawa, R.; Kobayashi, H.; Kikuchi, H.; Kondo, T. Ultrasound enhances liposome-mediated gene transfection. Ultrason. Sonochem. 2005, 12, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Komamura, K.; Tatsumi, R.; Tsujita-Kuroda, Y.; Onoe, T.; Matsumoto, K.; Nakamura, T.; Miyazaki, J.; Horio, T.; Sugimachi, M. Cellular Injury of Cardiomyocytes during Hepatocyte Growth Factor Gene Transfection with Ultrasound-Triggered Bubble Liposome Destruction. J. Drug Deliv. 2011, 2011, 453619. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sonoda, S.; Suzuki, R.; Arimura, N.; Tachibana, K.; Maruyama, K.; Sakamoto, T. A novel bubble liposome and ultrasound-mediated gene transfer to ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp. Eye Res. 2007, 85, 741–748. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Kwon, Y.S.; Cho, H.S.; Heo, S.H.; Park, K.S.; Park, S.G.; Lee, S.H.; Hwang, S.I.; Kim, Y.I.; Jae, H.J.; et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics 2014, 4, 1133–1144. [Google Scholar] [CrossRef]






| Target | Sequence |
|---|---|
| IL1RL1-targeting sgRNA1 | ACUUGUAGGUAAAUCGUCCU |
| IL1RL1-targeting sgRNA2 | CUUGUAGGUAAAUCGUCCUG |
| IL1RL1-targeting sgRNA3 | AGGUAAAUCGUCCUGGGGUC |
| IL1RL1-targeting sgRNA4 | AGCCUCAUUUUCCAGACCCC |
| Target | Primers (Amplicon Size: 976 bp) |
|---|---|
| IL1RL1 | fwd: CCGACAAGCAGTCTCTAAGTTC |
| IL1RL1 | rev: CGGCTCATTCCCTTCCTAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Harmancey, R.; Frierson, B.; Wu, J.G.; Moody, M.R.; McPherson, D.D.; Huang, S.-L. Efficient Gene Editing for Heart Disease via ELIP-Based CRISPR Delivery System. Pharmaceutics 2024, 16, 343. https://doi.org/10.3390/pharmaceutics16030343
Yin X, Harmancey R, Frierson B, Wu JG, Moody MR, McPherson DD, Huang S-L. Efficient Gene Editing for Heart Disease via ELIP-Based CRISPR Delivery System. Pharmaceutics. 2024; 16(3):343. https://doi.org/10.3390/pharmaceutics16030343
Chicago/Turabian StyleYin, Xing, Romain Harmancey, Brion Frierson, Jean G. Wu, Melanie R. Moody, David D. McPherson, and Shao-Ling Huang. 2024. "Efficient Gene Editing for Heart Disease via ELIP-Based CRISPR Delivery System" Pharmaceutics 16, no. 3: 343. https://doi.org/10.3390/pharmaceutics16030343
APA StyleYin, X., Harmancey, R., Frierson, B., Wu, J. G., Moody, M. R., McPherson, D. D., & Huang, S.-L. (2024). Efficient Gene Editing for Heart Disease via ELIP-Based CRISPR Delivery System. Pharmaceutics, 16(3), 343. https://doi.org/10.3390/pharmaceutics16030343







