1. Introduction
Glaucoma refers to a group of eye disorders with different clinical indications. Glaucoma causes progressive optic nerve neuropathy, leading to the loss of the visual field and, if not treated, blindness [
1]. Generally, this occurs due to high pressure inside the eye, which can damage the optic nerve and retina by causing the loss of retinal ganglion cells [
2]. However, it is also possible for glaucoma to occur in people with normal eye pressure, which typically ranges from 10 to 21 mmHg [
3]. Among those, about 36 million people are blind, and an estimated 217 million experience significant visual impairment, ranging from moderate to severe [
4]. In the UK, glaucoma impacts around 2% of individuals aged over 40 and increases to 10% among those older than 75, especially within the African-Caribbean population [
5]. Treating ocular hypertension can be achieved by either decreasing the production of aqueous humor or increasing the outflow of aqueous humor.
Rho-kinase (ROCK) inhibitors are a novel class of anti-glaucoma medicines [
6,
7]. Unlike other medicines, ROCK inhibitors offer advantages beyond lowering intraocular pressure; they enhance blood flow to eye tissues and protect optic nerve cells from oxidative stress, resulting in improved neuron survival and regeneration [
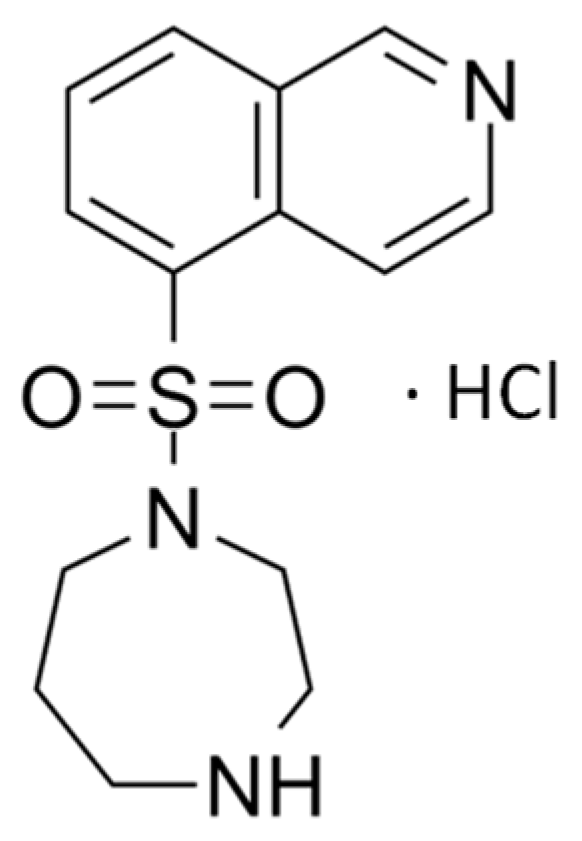
8]. Fasudil, depicted as the hydrochloride salt in
Figure 1, is a selective ROCK inhibitor that has demonstrated promising results in the treatment of glaucoma [
7,
9]. It works by inhibiting the ROCK pathway, which plays a crucial role in regulating the trabecular meshwork and canal of Schlemm’s aqueous humor outflow. Fasudil works by decreasing the formation of stress fibers and actomyosin contractility and reducing the expression of extracellular matrix proteins [
10]. This leads to an increase in the aqueous outflow, leading to a decrease in IOP. In addition to its IOP-lowering effect, Fasudil has been shown to improve blood flow to ocular tissues and protect optic nerve cells against oxidative stress, promoting neuron survival and regeneration [
11]. However, due to its hydrophilic nature (log P 0.16) and low molecular weight (MW 327.83 g/mL), attempts to formulate Fasudil using drug carriers like poly (lactic-co-glycolic acid) microspheres [
10] and liposomes [
12] have encountered issues, notably low entrapment efficiency and drug leakage. These limitations translate to low ocular bioavailability and pose a challenge for effective delivery of the drug to target ocular tissues. On the other hand, nanoparticles with mucoadhesive properties were found to be useful in modifying the pharmacokinetics and bioavailability of small hydrophilic molecules [
13].
Nanoparticles have a large surface area-to-volume ratio, allowing for better entrapment efficiency [
14]. They can prolong ocular drug residence time, sustain drug release, and potentially enhance ocular drug bioavailability [
15]. However, when nanoparticles lack mucoadhesive properties, they are susceptible to rapid drainage from the ocular surface into the nasolacrimal duct, resulting in reduced effectiveness. Conversely, mucoadhesive nanoparticles for topical administration to the eyes were shown to prolong the precorneal retention of the topically applied formulation, hence enhancing drug bioavailability and reducing adverse effects associated with frequent dosing [
16].
Chitosan (Cs) is one of the most abundant natural polysaccharides, primarily sourced from marine crustaceans [
17]. Cs exhibits many advantages when employed in the formulation of nanoparticles for drug delivery, including biocompatibility and biodegradability. Moreover, the positive charge present on the surface of chitosan grants it mucoadhesive properties, rendering it a promising candidate for ocular drug delivery [
18]. Cs nanoparticles interact with the negatively charged residues of sialic acid in the corneal and conjunctival mucosa, prolonging the drug’s presence and enhancing its bioavailability in the ocular region [
19]. Additionally, Cs enhances ocular drug permeation by disrupting tight cell junctions in the corneal and conjunctival epithelial cell surfaces [
20,
21]. Sonaje et al.’s study strongly affirms the capability of chitosan to transiently and reversibly open these junctions, as indicated by their results [
22].
Cs NPs have been used for delivering various drugs used in the treatment of eye conditions. These drugs include diclofenac [
23], a non-steroidal anti-inflammatory agent; daptomycin, an anti-bacterial agent [
24]; brimonidine, an anti-glaucoma agent [
25]; and dexamethasone, a corticosteroid effective in managing ocular inflammatory disorders [
26]. Cs is a promising drug delivery material with numerous advantages, but concerns have emerged regarding its potential ocular surface toxicity. Despite receiving approval from the US-FDA and EU for dietary and wound-dressing applications due to its biocompatibility and biodegradability, it has yet to achieve FDA approval for drug delivery applications [
27]. Additionally, there have been reports indicating that cationic polymers can display toxicity due to their positive charge [
28]. Therefore, one aspect of this article is to investigate chitosan’s ocular cytotoxicity to address some of these concerns.
Different methods have been reported for preparing Cs nanoparticles. These include ionic gelation, emulsion crosslinking, coacervation–precipitation, and spray drying [
29]. Ionic gelation remains one of the most reported methods of preparation due to its simplicity and cost-effectiveness. It does not require the use of organic solvents, thus reducing the risk of toxicity due to residues [
30]. This method is based on the electrostatic interactions that occur in the presence of oppositely charged groups; for example, those between protonated amino groups of Cs (positive charge) and negatively charged groups of polyanions, such as sodium tripolyphosphate (TPP), sodium alginate, and hyaluronic acid. Nanoparticles are produced due to the formation of crosslinks in between (intramolecular) and within (intermolecular) molecules [
31].
Ocular surface absorption of topically applied drugs happens via the corneal or conjunctival/scleral routes. Elucidating the absorption pathway of a topically applied ocular drug is important to inform and guide further formulation development. To the best of our knowledge, no such studies have been conducted on Fasudil. Hence, the objectives of this study are to:
Investigate the ocular absorption pathway of Fasudil hydrochloride using an appropriate ex vivo model.
Develop a chitosan nanoparticulate system using the ionic gelation method and investigate (using statistical experimental design) the parameters that affect the characteristics of chitosan nanoparticles.
Conduct in vitro release and ex vivo permeation studies of Fasudil from the prepared chitosan nanoparticles and assess their ocular (conjunctival and corneal) tolerability.
Evaluate the cytotoxic effect of the formulated system on a human lens epithelial cell line using the neutral red uptake assay.
2. Materials and Methods
2.1. Materials
Fasudil hydrochloride was obtained from Biosynth Ltd., Compton, UK. Low-molecular-weight chitosan (deacetylation degree 75–85%) (Cs), sodium tripolyphosphate (TPP), fetal bovine serum (FBS), Trypan blue, sterile phosphate-buffered saline, Dimethyl sulfoxide (DMSO), penicillin–streptomycin, 0.25% trypsin-EDTA, and neutral red were all purchased from Sigma Aldrich Chemical Co., London, UK. Glacial acetic acid, Industrial Methylated Spirit (IMS), and absolute ethanol were purchased from Fisher Scientific, Leicestershire, UK. Eagle’s Minimum Essential Medium (EMEM) and Human Lens Epithelial Cell line (HLEC line-B3) were obtained from the American Type Culture Collection (ATCC), London, UK. All other solvents and buffer salts were obtained from Sigma Aldrich Chemical Co., London, UK; they were of analytical grade and used as received.
2.2. High-Performance Liquid Chromatography (HPLC) Analysis of Fasudil
Fasudil was separated and quantified by liquid chromatographic analysis using an Agilent HPLC Infinity system (Agilent, Waldbronn, Germany) coupled with a photodiode array detector (PDA). Separation was achieved using the Phenomenex® Gemini 5 µm C6-Pheny column (150 × 4.6 mm) at room temperature (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 90/10 (v/v) purified water/acetonitrile, with the addition of 0.1% trifluoroacetic acid by volume to both phases. Prior to use, the mobile phases were degassed using bath ultrasonication to eliminate any trapped gas bubbles. The flow rate was set at 1 mL/min, and the injection volume was 10 μL. When analyzed at a λmax of 235 nm, Fasudil exhibited a retention time of 3.5 min. The validation of the method was carried out in accordance with FDA guidelines. A calibration curve was established within concentration ranges of 2–75 μg/mL, exhibiting a coefficient of determination value of 0.999 and a curve equation (y = 40.889X + 2.5458). The method demonstrated high accuracy and selectivity, with a low limit of detection (LLOD) of 0.22 µg/mL and a low limit of quantification (LLOQ) of 0.65 µg/mL.
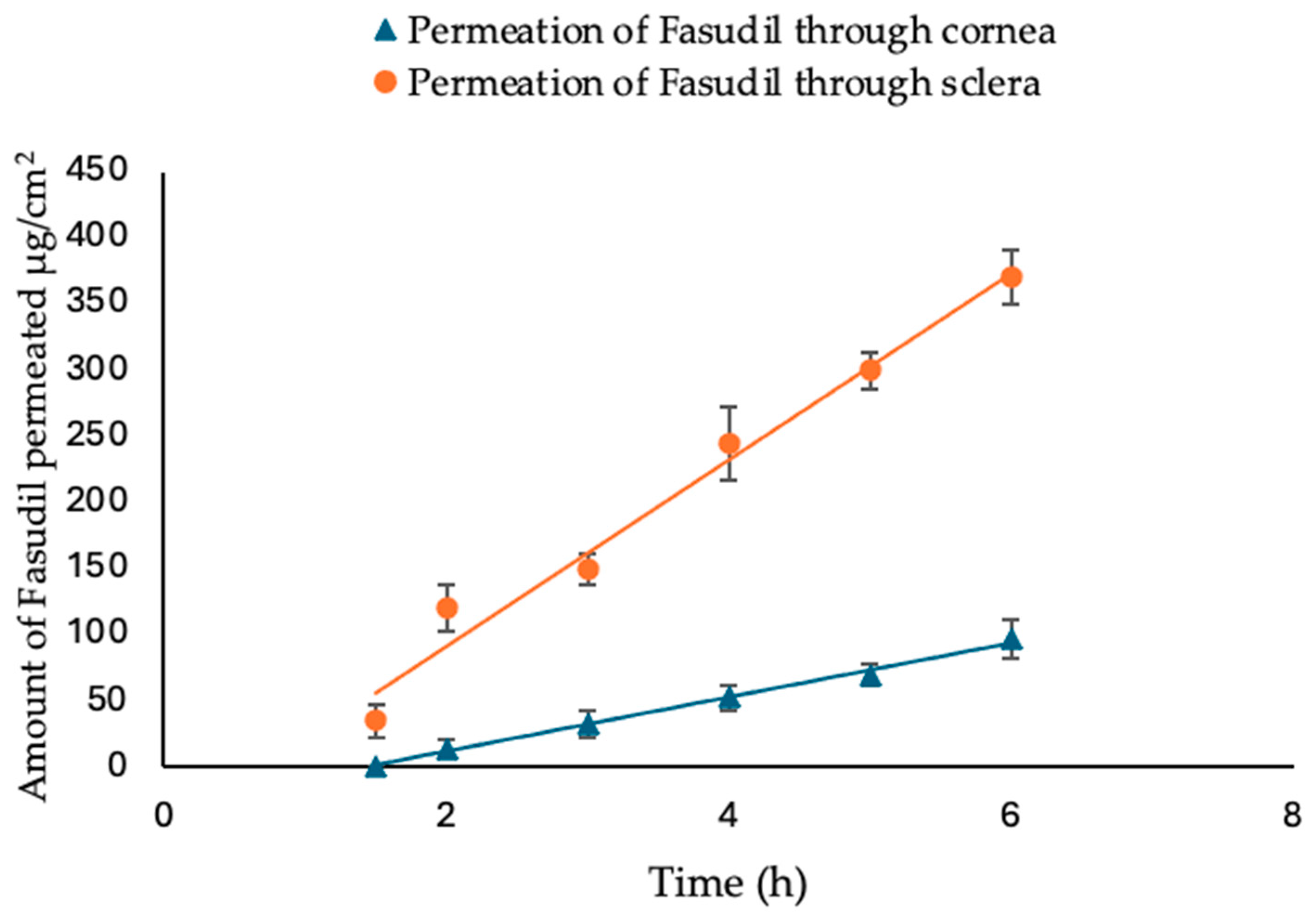
2.3. Ex Vivo Permeation of Fasudil Hydrochloride through Excised Bovine Cornea and Sclera
In this ex vivo trans-corneal/scleral permeation study, freshly excised bovine corneas and scleras were used. The experiments were conducted with a Franz diffusion cell apparatus (FDC-6, Logan Instrument Corp., Somerset, NJ, USA), which provided a diffusion area of 1.7 cm
2. The bovine eyes were obtained from a local slaughterhouse (ABP Food Group, Guildford, UK) and transported to the laboratory in cold saline (8–10 °C). Prior to dissection, the eyes were examined for corneal damage. The excised bovine ocular membrane (cornea or sclera) separated the two compartments in such a way that the epithelial surface of the cornea/conjunctival side of the sclera faced the donor chamber. The receptor chamber (12 mL) was filled with simulated tear fluid (STF). STF was prepared by dissolving 0.67 g NaCl, 0.21 g NaHCO
3, and 0.008 g CaCl
2.2H
2O in 100 mL of purified water and adjusted to pH 7.4 [
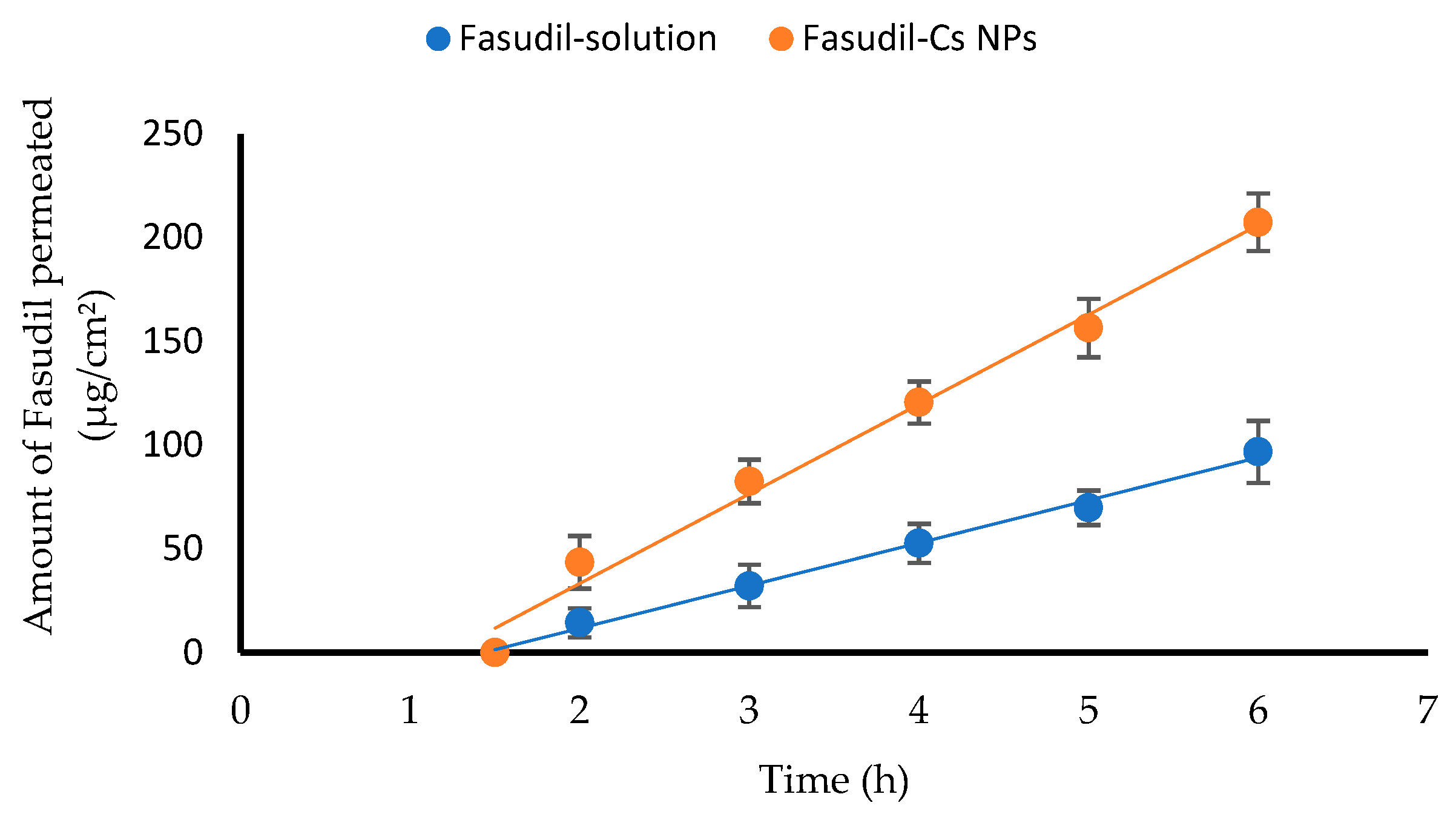
32]. The temperature of the cell was maintained at 35 ± 0.5 °C with continuous stirring. Samples of the Fasudil solution (2 mL, 1 mg/mL) were placed into the donor compartment. At predetermined time intervals (every hour), a sample (0.4 mL) of the receptor medium was taken and immediately replaced with an equivalent volume of fresh STF. The quantity of Fasudil permeated was subsequently measured using HPLC. A graph was created by plotting the cumulative amount of Fasudil permeated vs. time, and this graph was then used to calculate the apparent permeability coefficient (Papp, cm/s) as follows:
where ΔQ/Δt indicates the permeability flux rate of Fasudil (μg/cm
2.h) through the excised bovine corneas, calculated as the slope from the graph plotting the amount of Fasudil permeated vs. time; A is the exposed area (cm
2) of the corneal/scleral surface; C0 represents the initial concentration (μg/mL) of Fasudil; and 3600 is the conversion constant from hours to seconds. The lag time (tL) calculation involves using the intercept and slope values from a regression line of permeation studies.
2.4. Statistical Experimental (Factorial) Design
A two-level full factorial design was utilized to investigate the effects of three independent variables (Cs concentration, Cs:TPP mass ratio, and sonication time) on the desired responses (dependent variables), namely, particle size, polydispersity index (PDI), zeta potential, and entrapment efficiency (EE) (
Table 1). The design of 8 experimental runs and one center point was developed using Minitab software, version 21.4 (Minitab, Inc., State College, PA, USA). All experiments were carried out in triplicate (
Table 2). Response surface plots were generated to demonstrate how independent variables affect the response.
2.5. Preparation of Chitosan Nanoparticles (Cs NPs)
Cs NPs were prepared via the ionic gelation method as previously reported by Calvo et al. [
33], with minor modifications. Briefly, Cs solutions at concentrations of 1.2, 3.7, and 6.2 mg/mL were prepared by dissolving appropriate amounts of Cs (with low molecular weight and degree of deacetylation ranging between 75 and 85%) in 8 mL of acetic acid (1%
v/
v, pH 4). Next, 1 mL of Fasudil aqueous solution (0.5%
w/
v) was added to the Cs solution and stirred for 10 min. To prepare the nanoparticles, 1 mL of aqueous TPP solution (
Table 2) was added dropwise to the Cs solutions by means of sonication over an ice bath using a probe sonicator (150 plus, MSE, London, UK) at 16% amplitude. The nanoparticles were separated by centrifugation at a speed of 40,000×
g for 45 min at 4 °C. Then, the nanoparticles were washed by dispersing the formed pellet in distilled water and recentrifuged at the same previous conditions; then, the washed pellet was lyophilized, collected, and stored for further characterization.
2.6. Characterization of Fasudil-Cs NPs
2.6.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential Measurements
The particle size and PDI were analyzed at 25 °C using dynamic light scattering with a Malvern Zetasizer (Malvern Instruments Ltd., Malvern, UK). Freshly prepared Cs NP samples were used for these measurements. For particle size analysis, a square disposable cuvette was employed, and all samples were diluted with deionized water at a ratio of 1:10 (v/v). The zeta potential of the nanoparticles was also determined using the same instrument. In this analysis, a folded capillary cell was used, and all samples were diluted with deionized water at a ratio of 1:100 (v/v). Electrophoretic mobility analysis was conducted at 25 °C with a scattering angle of 90°. Each experiment was conducted in triplicate, and the results are presented as the mean ± SD. These measurements were performed with a 120 s interval between each sample run. Data were obtained by measuring the random changes in the intensity of light scattered from the moving nanoparticles.
2.6.2. Scanning Electron Microscopy (SEM)
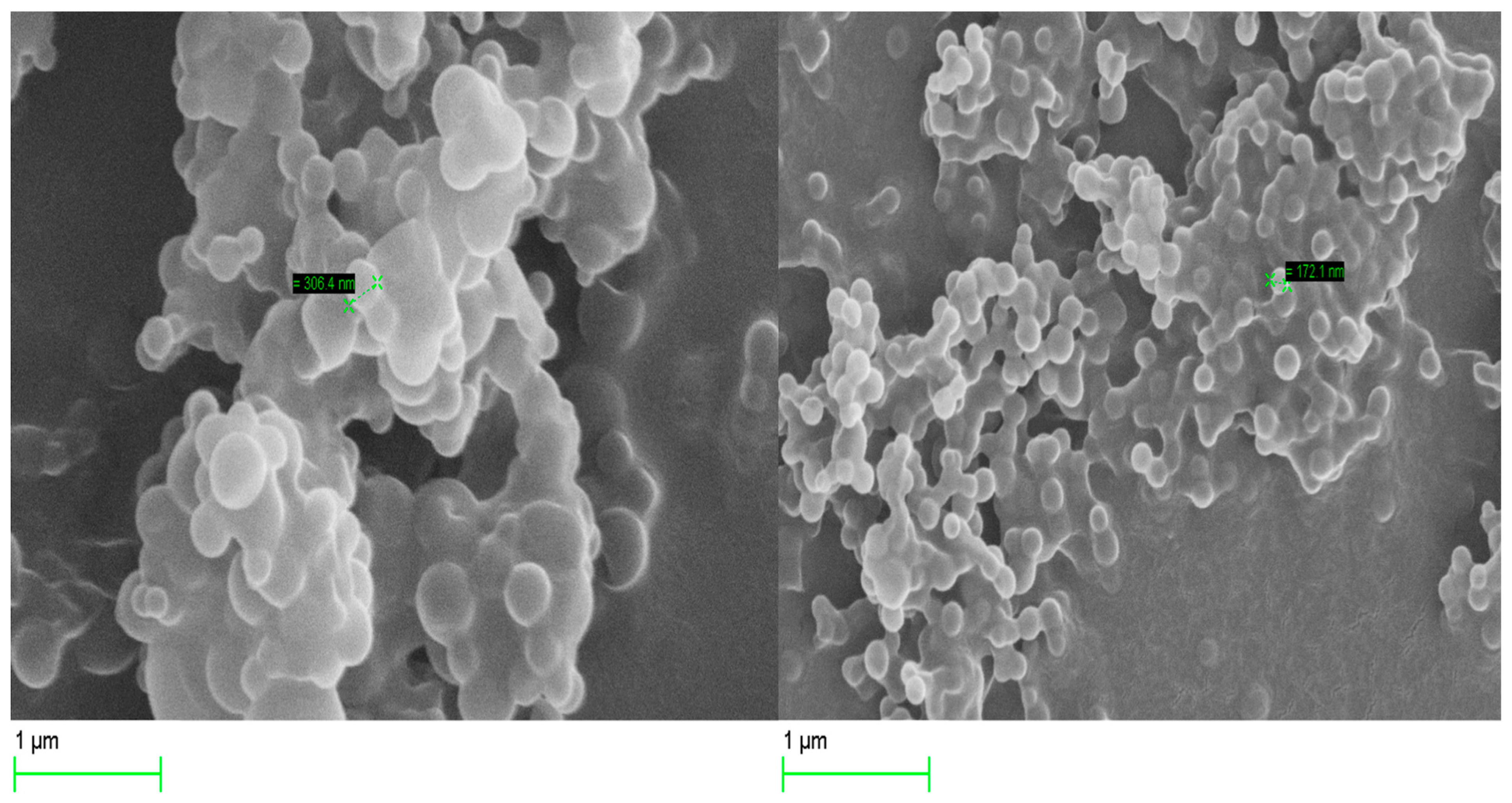
The surface morphology of the optimized Cs NPs was examined by scanning electron microscopy (SEM, Zeiss Evo50-Oxford instrument, Cambridge, UK). A drop of the nanoparticle suspension was placed over a slide cover and left to dry at room temperature before being attached to an aluminum stud using carbon double-sided tape. The samples were coated with a layer of gold using a sputter coater. Nanoparticle morphology was determined at 20.00 kV.
2.6.3. Entrapment Efficiency (EE) determination
The amount of Fasudil entrapped within the nanoparticles was determined using an indirect method by calculating the amount of unentrapped drug (free drug) in the supernatant. Following the previously mentioned process, the nanosuspension was centrifuged, and the clear supernatant containing the free, unentrapped drug was carefully collected and diluted with deionized water. The amounts of Fasudil in the Cs NPs were determined using the validated HPLC method mentioned above. The percentage of drug entrapment efficiency (EE%) was then calculated by using the following equation:
2.7. In Vitro Drug Release Study
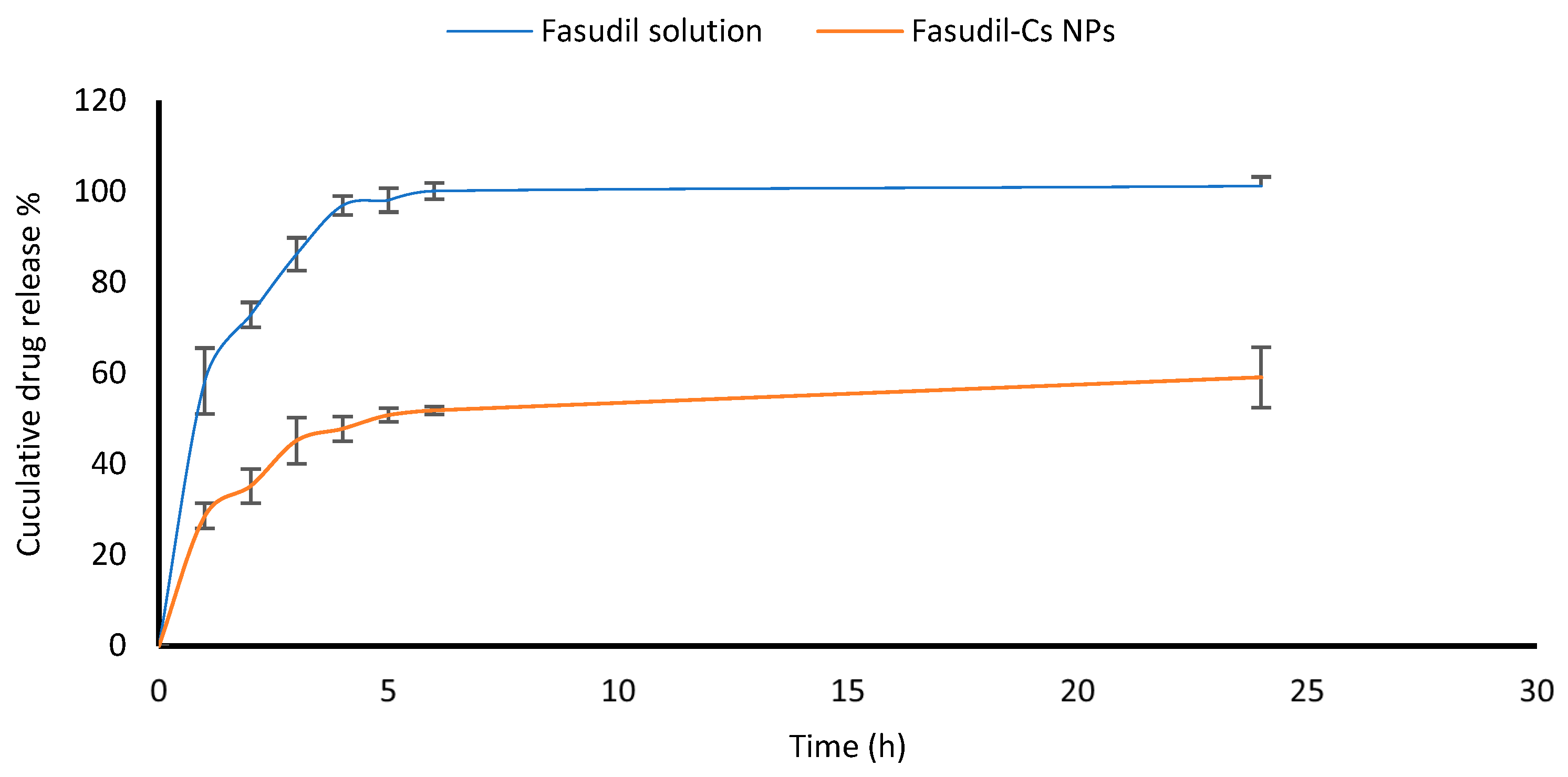
An in vitro release study of Fasudil from Cs NPs was conducted using a standard Franz diffusion cell (Copley scientific limited, Colwick, UK). Receptor chambers (11 mL volume) were filled with freshly prepared STF and constantly stirred using small magnetic bars. Lyophilized Cs nanoparticles (20 mg) were dispersed in two milliliters of PBS (pH 7.4) and transferred into the donor chamber before sealing it with Parafilm. The two chambers of Franz cells, a donor and a receptor, were separated by a dialysis membrane (12,000–14,000 Dalton molecular weight cut-off). The membrane was previously pre-soaked overnight in the receptor medium. The Franz cells were set at 35 ± 0.5 °C in order to mimic the ocular surface temperature. Care was taken to eliminate air bubble entrapment at the membrane/liquid interface. Samples (0.4 mL each) were withdrawn at different time intervals during the running experiment and replaced with fresh receptor medium. The samples were analyzed using the developed HPLC method to measure the amount of Fasudil released from nanoparticles. The experiments were performed in triplicate.
2.8. Ex Vivo Trans-Corneal Permeation of Fasudil from Fasudil-Cs NPs
A modified Franz diffusion cell apparatus (FDC-6, Logan Instruments Corp., Somerset, NJ, USA) was used for the ex vivo permeation study of Fasudil from both chitosan nanoparticles and Fasudil solution across freshly excised bovine corneas. See
Section 2.3 for the detailed experimental procedure. In the donor compartment of the apparatus, 2 mL each of both the Fasudil solution and the Fasudil-Cs NPs dispersion, was placed. The samples withdrawn from the receptor compartment were analyzed using the developed HPLC method to quantify the amount of Fasudil permeated. Samples were measured in triplicate, and the results were presented as the mean value ± SD. A graph was created by plotting the amount of Fasudil that permeated vs. time. The permeation flux, apparent permeability coefficient, and lag time were calculated.
2.9. Conjunctival Irritation Test
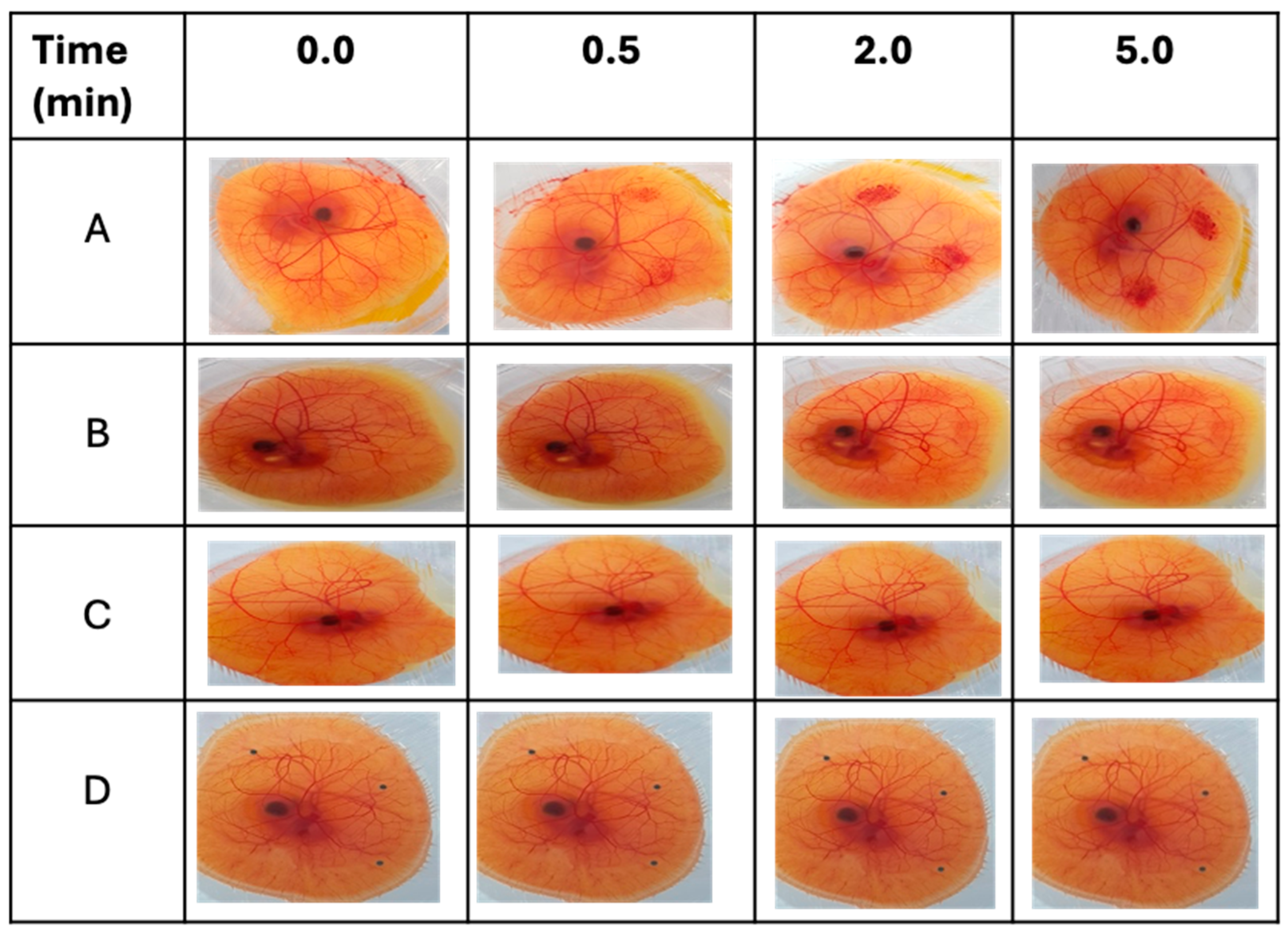
The conjunctival tolerability of the Cs NPs was evaluated by a modified HET-CAM test, as reported previously [
34]. Freshly collected fertilized hen’s eggs were incubated for 3 days at 37.5 ± 0.5 °C and 65 ± 5% relative humidity. During the incubation period, eggs were manually rotated 4–5 times a day and left in a horizontal position to ensure the correct development and viability of the embryo. On day three, the eggshells were sterilized with 70% IMS and opened by cracking at the edge of the growing chamber. The contents of the egg were poured into the fabricated growing chamber. Each egg was examined, and only viable embryos with intact chorioallantoic membranes (CAMs) and yolk sacs were further incubated at the same conditions after being covered with a Petri dish. On day 10 of incubation, 0.2 mL of each test formulation and control solution was gently dropped onto the surface of the CAM. Sodium hydroxide (0.5 M) was used as a strong irritant, propylene glycol as a moderate irritant, and normal saline as a negative control. After treatment, the CAMs and blood vessels, including the capillary system, were visually observed at three time points over 5 min (0.5, 2, and 5 min) to monitor the following irritant effects (responses): hyperemia, hemorrhage, and clotting. A time-dependent numerical score was assigned to every response, as indicated in
Table 3. The cumulative score obtained for all three irritant responses provides a single numerical value (cumulative score). This numerical score serves as an interpretation of the conjunctival irritation potential of the respective test substance (
Table 4). In the evaluation of each substance, three CAMs were employed for each test sample.
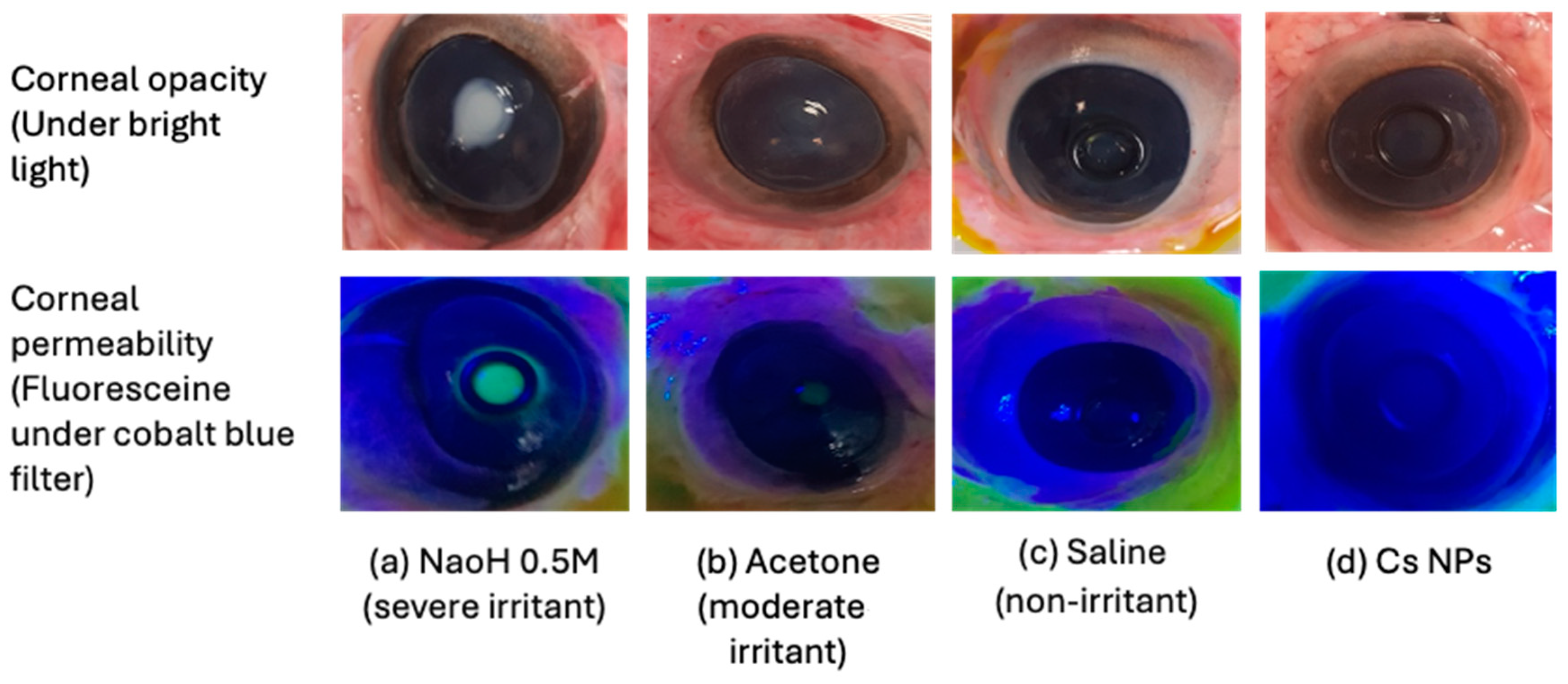
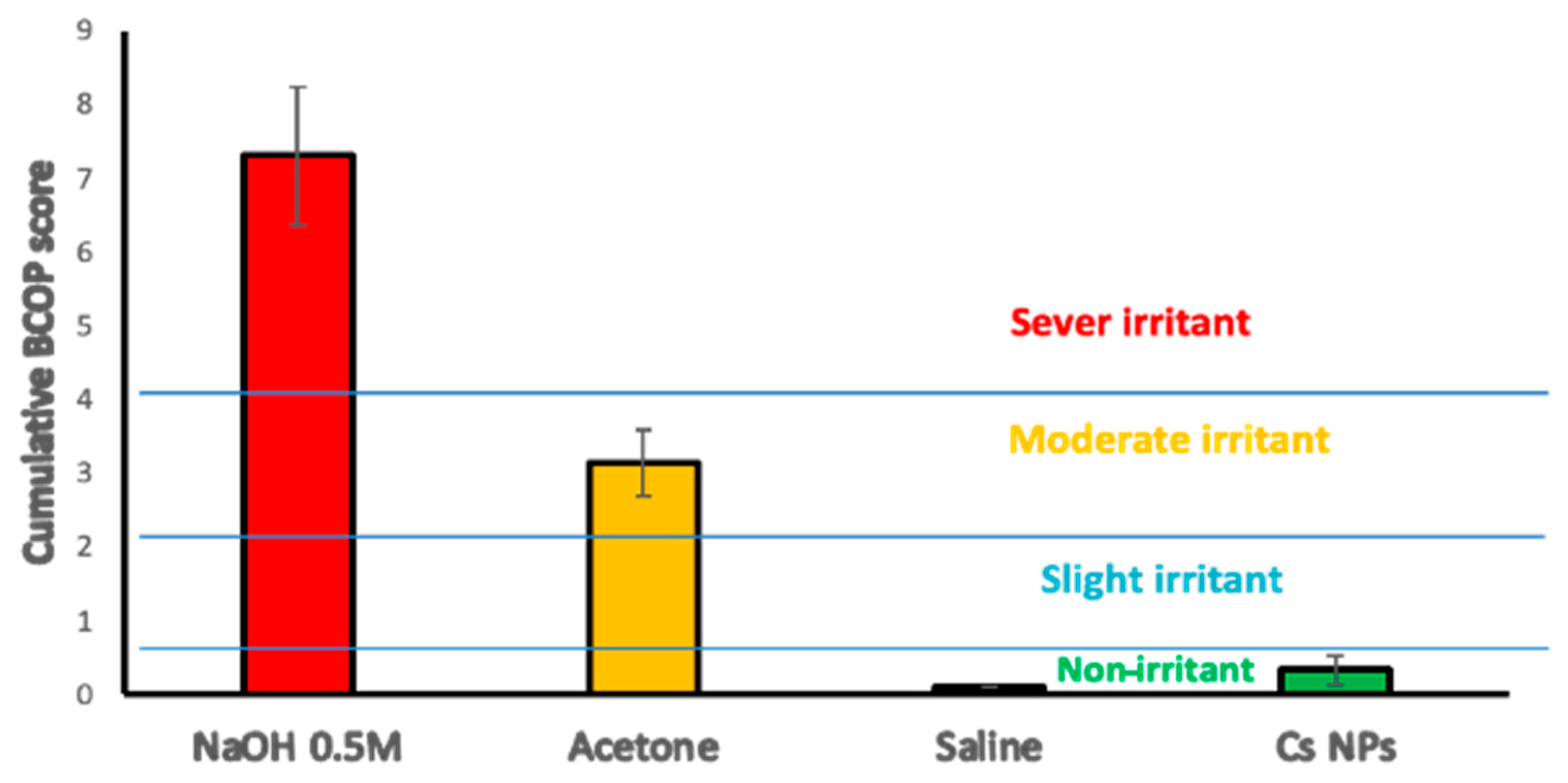
2.10. Bovine Corneal Opacity and Permeability (BCOP) Assay
Freshly excised bovine eyes were carefully examined for epithelial integrity and corneal damage, and those presenting defects, such as neovascularization, pigmentation, or scratches, were discarded. Plastic weighing boats were used to hold the eyes with the cornea facing upwards in the humid atmosphere of a closed shaking water bath shaken for 10 min at 37 ± 0.5 °C. Silicone O-shaped rings were placed on the center of the cornea in order to localize the effect of the testing materials. The positive and negative controls used were NaOH (0.5 M) and NaCl (0.9%
w/
v), respectively. A drop of saline was applied to the corneal surface before incubating for 5 min. Testing materials and controls (0.1 mL) were applied to the corneal surface for 30 s before the eyes were rinsed with 10 mL of normal saline and left in incubation for 10 min. Then, the degree of corneal damage was visually determined from the extent of opacification and was further assessed for corneal epithelium integrity using sodium fluorescein staining (2%
w/
v, pH 7.4) under a cobalt blue light. The observations were graded according to individual numerical scores in terms of opacity, epithelial integrity, and epithelial detachment, as reported previously [
35]. The cumulative scores were calculated, and the mean of 3 independent scores was used to interpret the corneal irritation potential for the test materials (
Table 5).
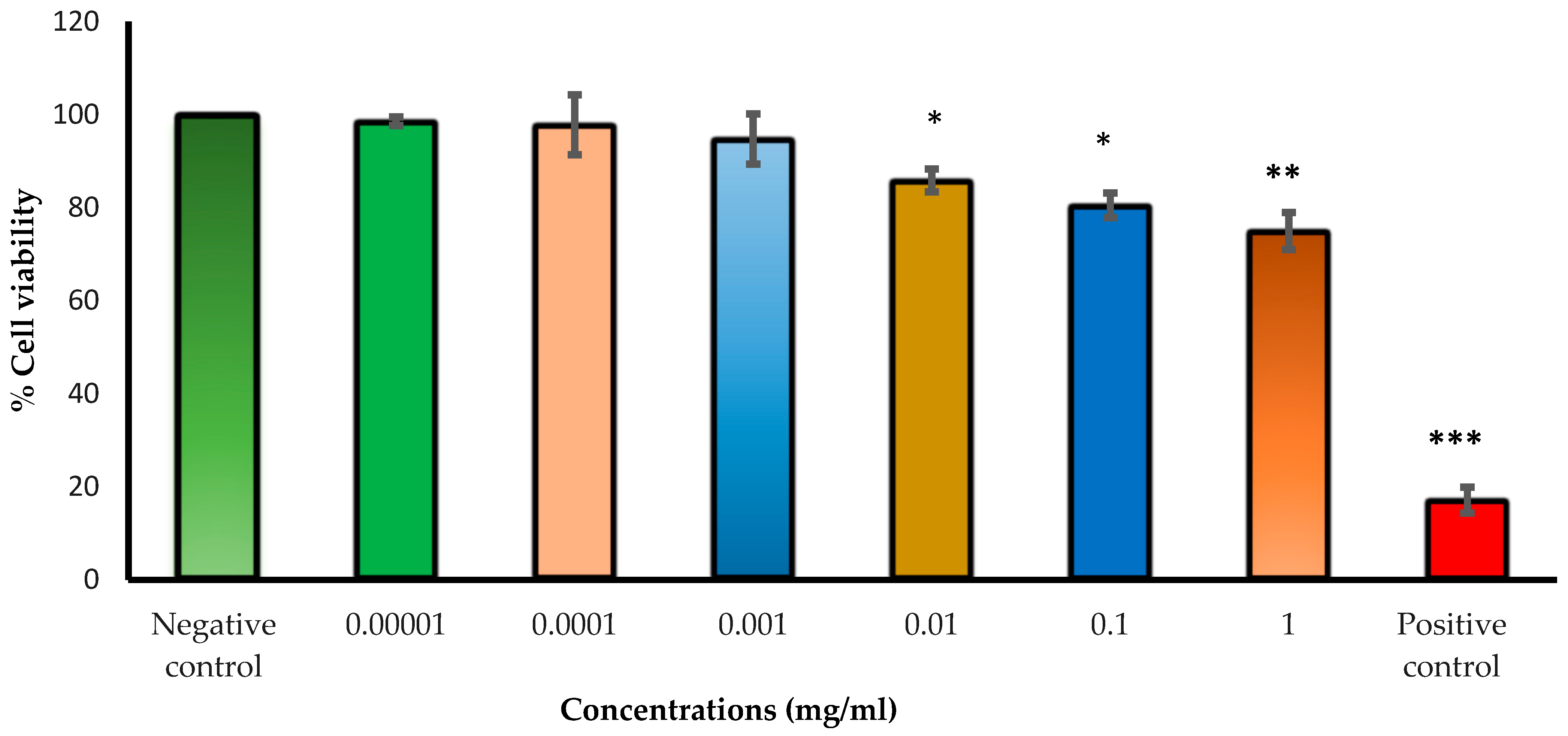
2.11. Human Lens Epithelial Cell Viability Investigation Using the Neutral Red Uptake Assay
Cell viability upon exposure to varying concentrations of Cs NPs was evaluated by providing a quantitative estimation of the number of live cells in a culture. Neutral red uptake assays were carried out on human lens epithelial cells (HLECs B-3) to assess the cytotoxicity of the studied Cs NPs. The assay depends on the ability of living cells (with functional lysosomes) to take up and bind the basic dye neutral red (which is a pH indicator), while dead cells cannot take up the dye. Liquid nitrogen-stored HLEC B-3 cells were thawed and grown in a humidified incubator at 37 °C in an atmosphere of 5% CO
2 and 95% air. All cell lines were cultured in cell complete medium (EMEM) supplemented with 20% (
v/
v) fetal bovine serum and 1% (
v/
v) penicillin–streptomycin. Cells were passaged by trypsinization using trypsin-EDTA (0.25%) and seeded in a cell culture flask for adherent cells. A neutral red assay was conducted as previously described, with slight modifications [
36,
37]. In brief, cells were seeded into 96-well plates at a seeding of 8 × 10
3 cells in a total complete medium volume of 200 µL per well and incubated at 37 °C with 5% CO
2 for 24 h. Then, the complete medium (EMEM + 20% FBS + 1% penicillin–streptomycin) was discarded, and cells were exposed to different concentrations of Cs NPs dispersed in treatment medium (EMEM + 5% FBS + 1% penicillin–streptomycin) and incubated for 24 h. After medium aspiration, the cells were washed with 150 μL of phosphate-buffered saline (PBS) and 100 μL of complete medium supplemented with neutral red (40 μg/mL), then added to the cells and incubated for three hours at the same conditions. Later, the cells were again washed with PBS, and an NR de-staining solution (50% ethanol, 49% deionized water, and 1% glacial acetic acid) was added to the cells. The plates were shaken for 10 min to extract the NR. The optical density (OD) was measured at 540 nm using a plate reader to calculate the cell survival rate.
2.12. Statistical Analysis
All experiments were conducted in triplicate, and the results were presented as the mean value ± standard deviation (SD). A one-way analysis of variance (ANOVA) was performed using Minitab® software, version 21.4, (Minitab, Inc, State College, PA, USA). The resulting data were deemed statistically significant when the p-value was less than 0.05.