Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Antigen Content
2.2. Analysis of Antigenicity of Vaccine
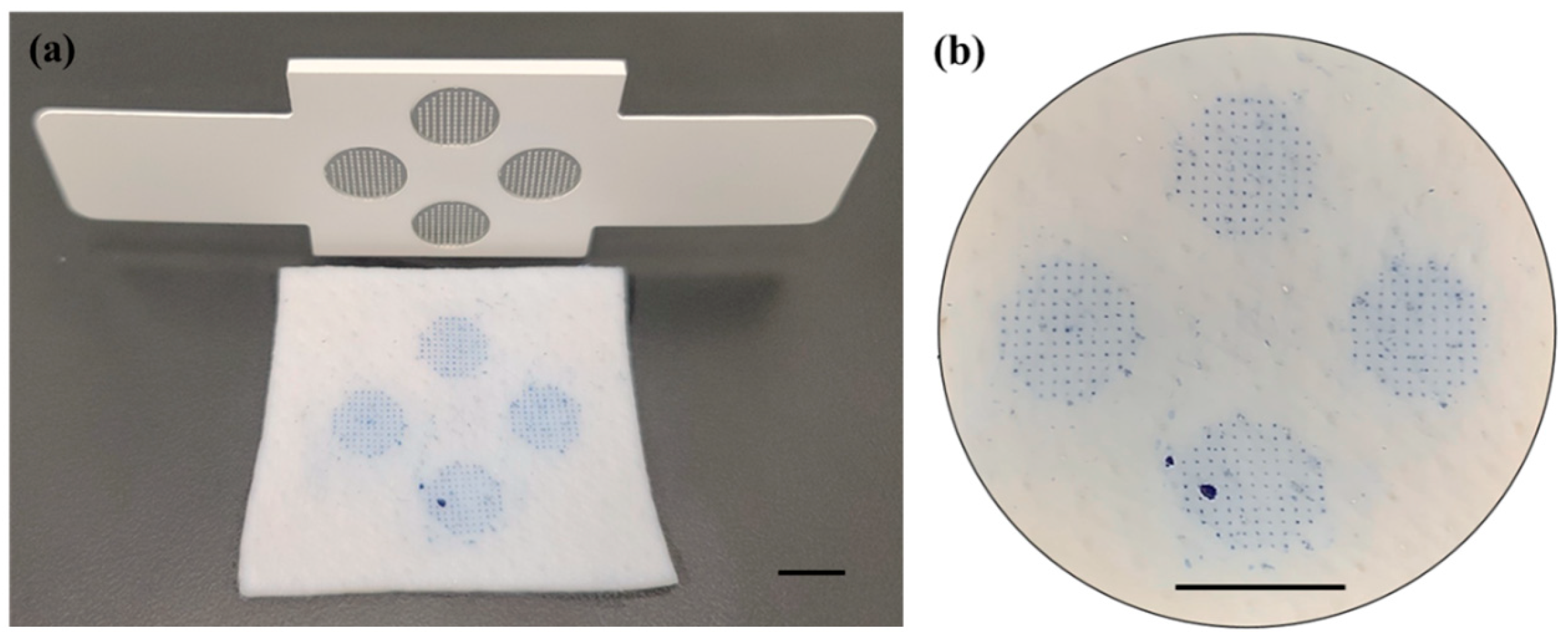
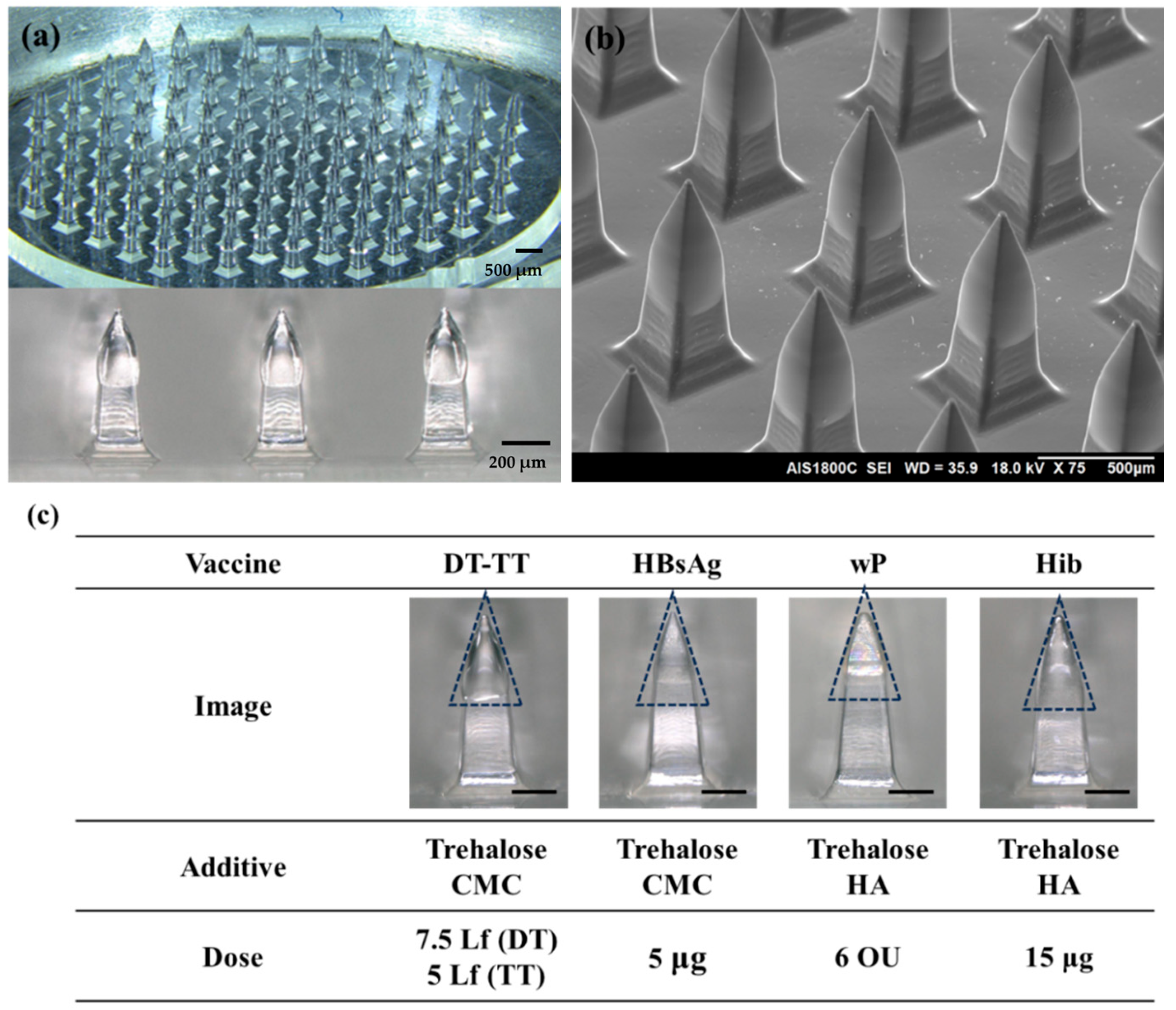
2.3. Fabrication of Vaccine Microneedle Array of DT-TT, wP, Hib, and HBsAg Vaccines
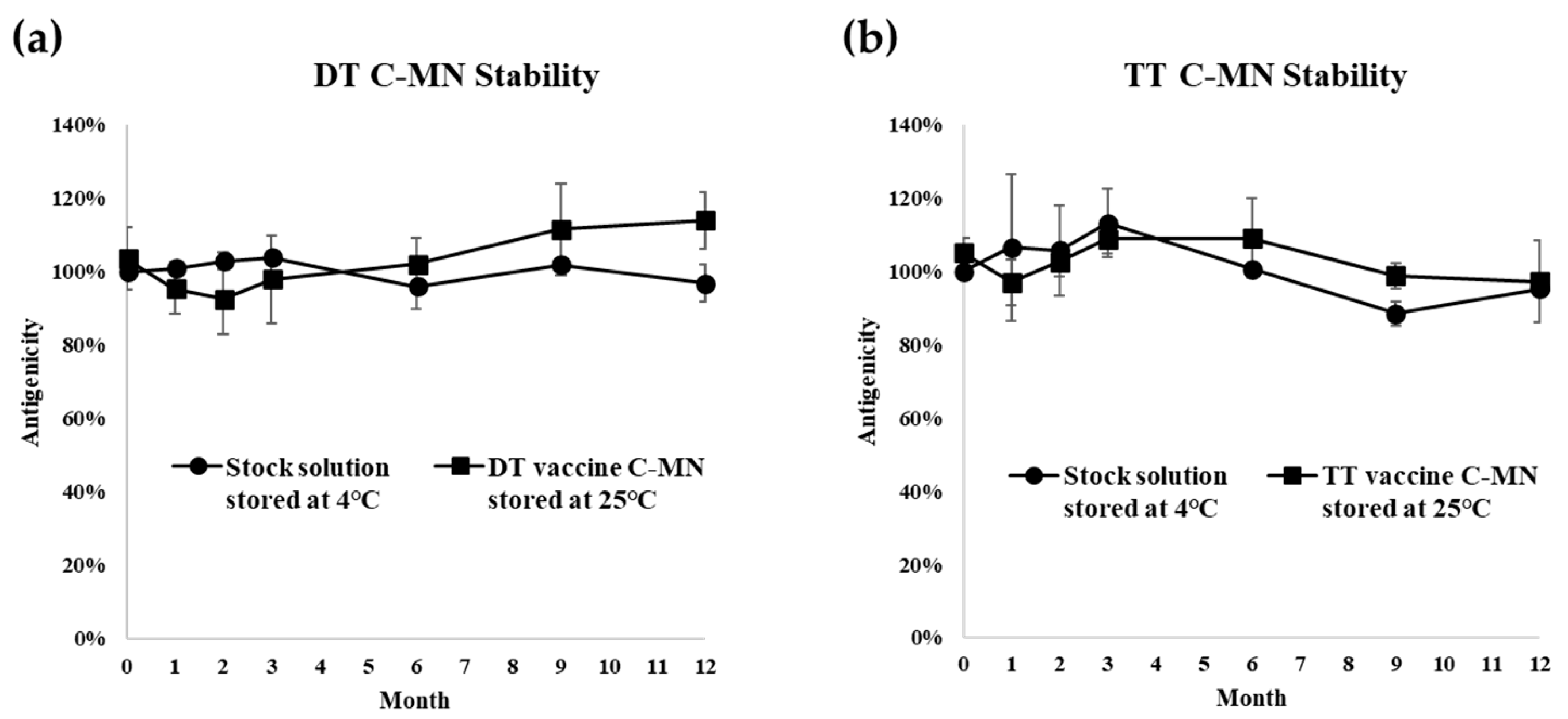
2.4. Stability Tests of Combination Vaccines for Each Vaccine
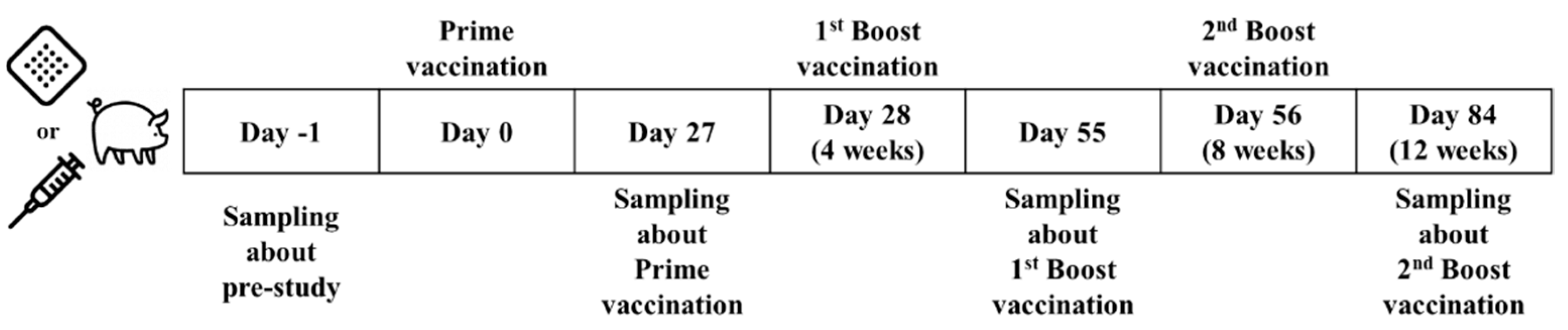
2.5. Vaccination in Animal Models
2.5.1. Mouse Study: Evaluation of Immunogenicity
Effect of Diphtheria Toxoid and Tetanus Toxoid Vaccine Combination MN Array in Mouse
Efficacy of 5-in-1 Vaccine Microneedle Patch in Mouse
2.5.2. Minipig Study: Evaluation of Immunogenicity
2.6. Analysis of Antigen-Specific Antibody Responses
Analysis of Antibody Titer
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Five-in-One Vaccine Microneedle Patch
3.1.1. Physical Properties of Five-in-One Vaccine Microneedle Patch
3.1.2. Formulation of Coating Solutions
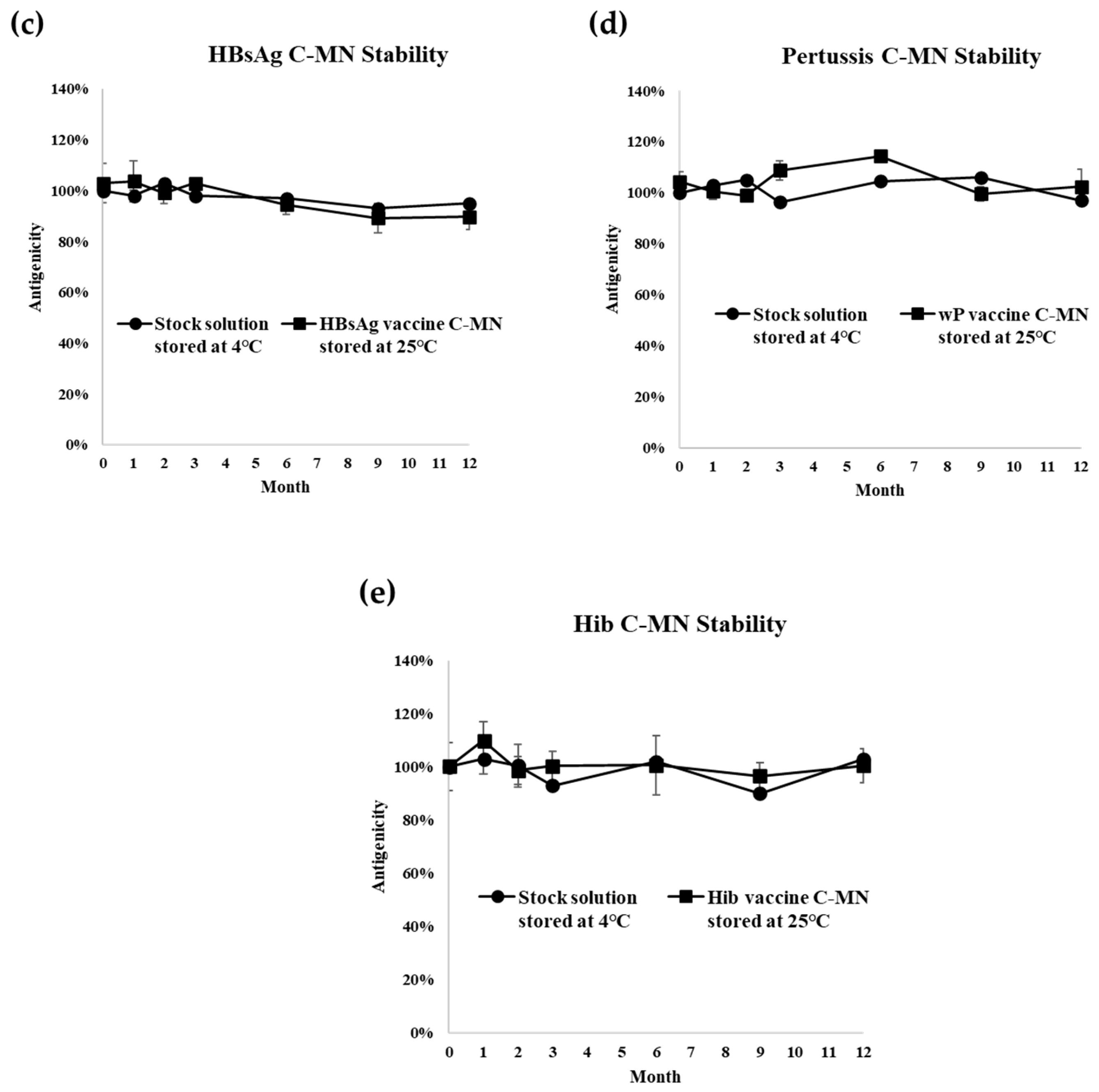
3.2. Long-Term Stability Test
3.3. Animal Study: Comprehensive Evaluation of Vaccine Efficacy and Immunogenicity in Mice and Pigs
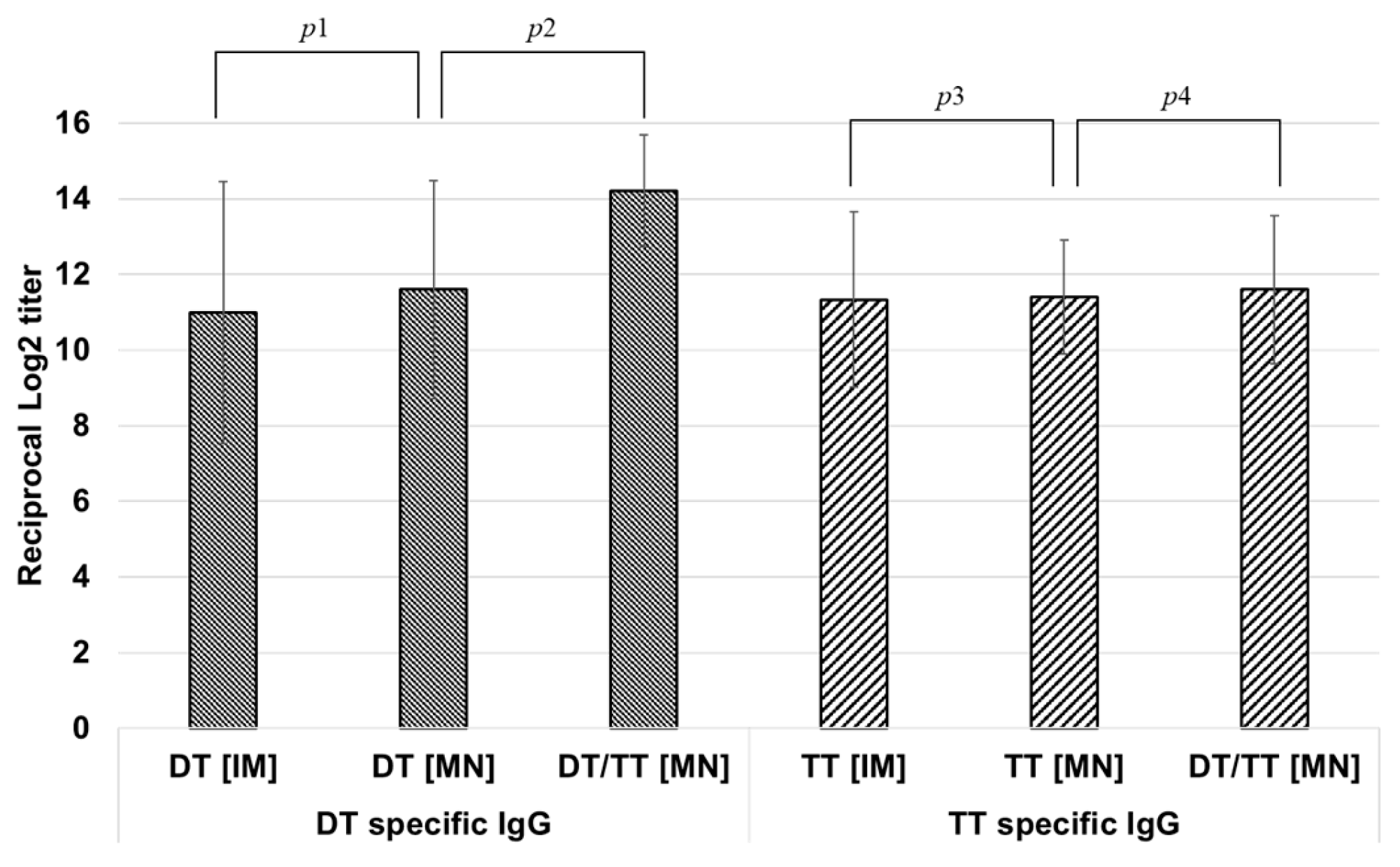
3.3.1. Antibody Response of DT-TT Vaccine C-MN Array in Mice
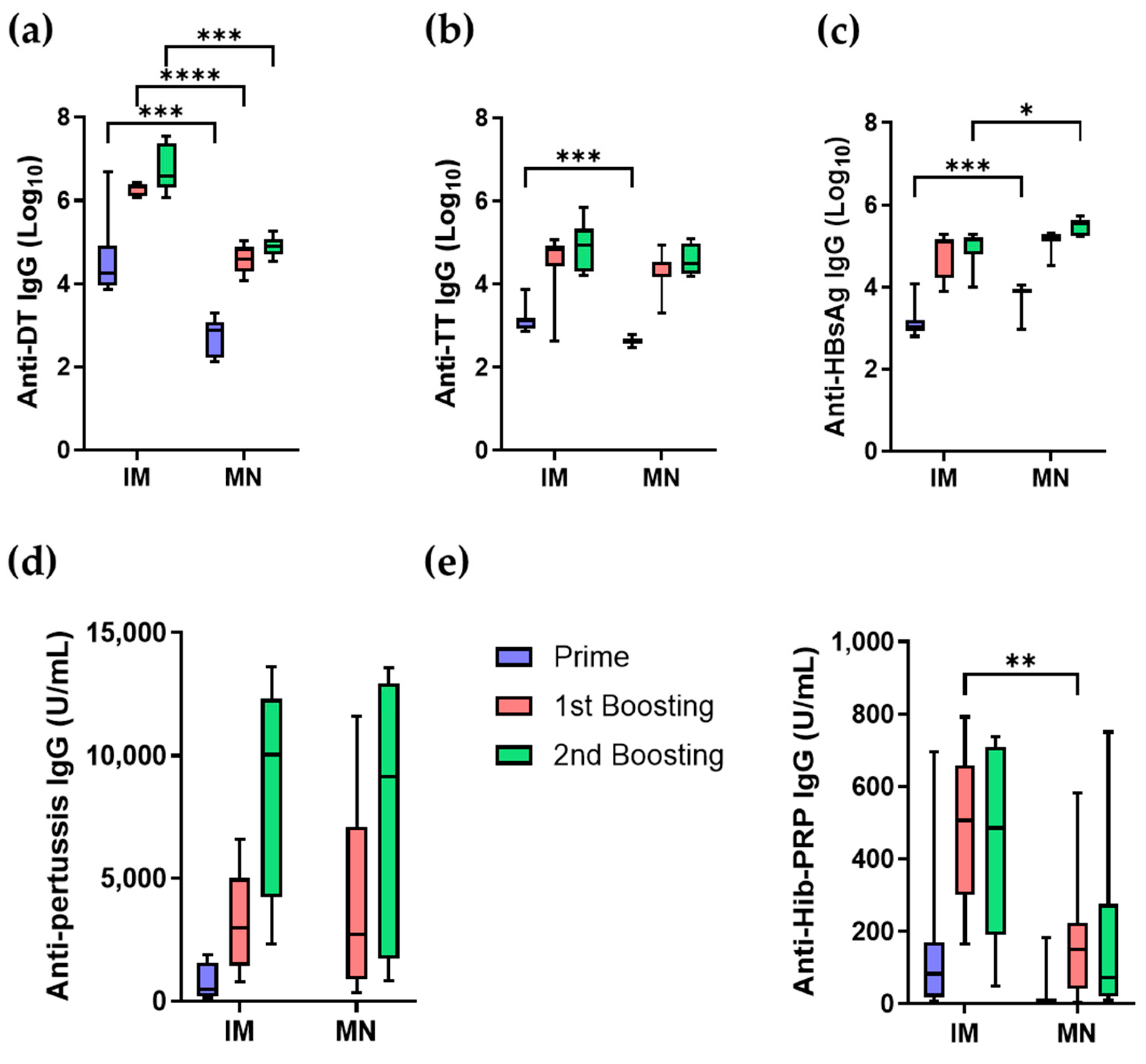
3.3.2. Antibody Responses of Five-in-One MN Patch in Mice
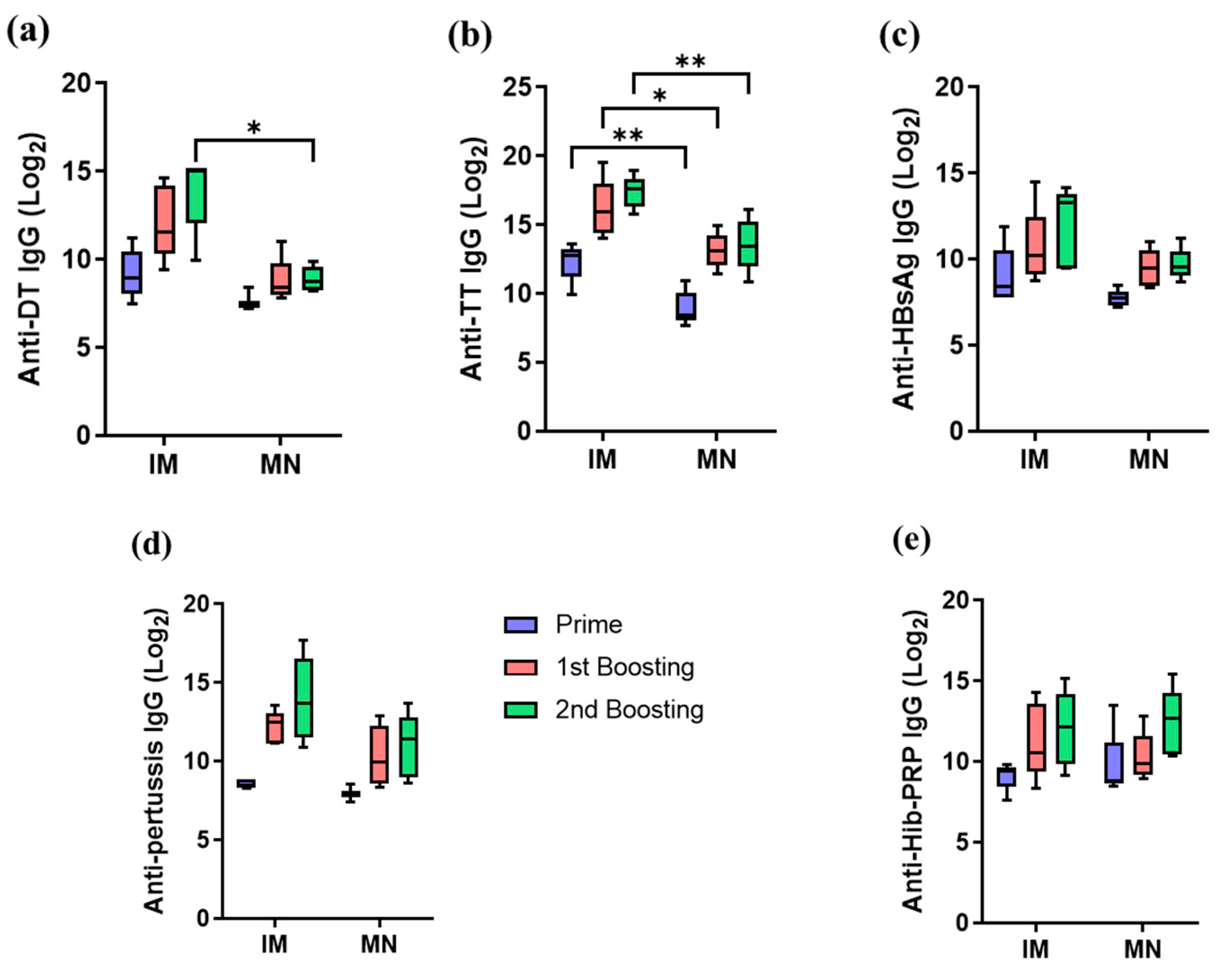
3.3.3. Antibody Responses of Five-in-One MN Patch in Minipigs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giersing, B.; Shah, N.; Kristensen, D.; Amorij, J.-P.; Kahn, A.-L.; Gandrup-Marino, K.; Jarrahian, C.; Zehrung, D.; Menozzi-Arnaud, M. Strategies for vaccine-product innovation: Creating an enabling environment for product development to uptake in low-and middle-income countries. Vaccine 2021, 39, 7208–7219. [Google Scholar] [CrossRef] [PubMed]
- Susarla, S.K.; Gupta, M.; Mantan, M.; Dhongade, R.; Bhave, S.; Das, R.K.; Ray, R.K.; Babu, T.R.; Ravi, M.; Krishnamurthy, B. Immunogenicity and safety of a liquid pentavalent (DTwP-Hb-Hib) combination vaccine manufactured by human biologicals institute in 6–8 weeks old healthy infants: A phase III, randomized, single blind, non-inferiority study. Vaccine 2019, 37, 5452–5459. [Google Scholar] [CrossRef] [PubMed]
- Charania, N.A.; Gaze, N.; Kung, J.Y.; Brooks, S. Interventions to reduce the burden of vaccine-preventable diseases among migrants and refugees worldwide: A scoping review of published literature, 2006–2018. Vaccine 2020, 38, 7217–7225. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, R.; Deb, A.K.; Bhattacharya, S.K.; Bose, A.; John, J.; Balraj, V.; Ganguly, N.; Kant, L.; Kapoor, A.N.; et al. Multi-center surveillance for pneumonia & meningitis among children (<2 yr) for Hib vaccine probe trial preparation in India. Indian J. Med. Res. 2010, 131, 649–658. [Google Scholar]
- Verma, R.; Khanna, P.; Chawla, S. Pentavalent DTP vaccine: Need to be incorporated in the vaccination program of India. Hum. Vaccines Immunother. 2013, 9, 1497–1499. [Google Scholar] [CrossRef][Green Version]
- Weniger, B.G. Combination vaccines for childhood immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP). MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 1–14. [Google Scholar]
- Plotkin, S.A.; Orenstein, W.; Offit, P.A. Vaccines E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2012. [Google Scholar]
- Maman, K.; Zöllner, Y.; Greco, D.; Duru, G.; Sendyona, S.; Remy, V. The value of childhood combination vaccines: From beliefs to evidence. Hum. Vaccines Immunother. 2015, 11, 2132–2141. [Google Scholar] [CrossRef]
- Kroger, A.T.; Atkinson, W.L.; Marcuse, E.K.; Pickering, L.K. General recommendations on immunization: Recommendations of the advisory committee on immunization practices (ACIP). MMWR. Recomm. Rep. 2006, 55, 1–48. [Google Scholar]
- Pichichero, M.E. New combination vaccines. Pediatr. Clin. N. Am. 2000, 47, 407–426. [Google Scholar] [CrossRef]
- Choo, S.; Finn, A. Pediatric combination vaccines. Curr. Opin. Pediatr. 1999, 11, 14–20. [Google Scholar] [CrossRef]
- Peter, G.; des Vignes-Kendrick, M.; Eickhoff, T.; Fine, A.; Galvin, V.; Levine, M.; Maldonado, Y.; Marcuse, E.; Monath, T.; Osborn, J. Lessons learned from a review of the development of selected vaccines. National Vaccine Advisory Committee. Pediatrics 1999, 104, 942–950. [Google Scholar] [PubMed]
- Ellis, R.W. Development of combination vaccines. Vaccine 1999, 17, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Halsey, N.A. A perspective on combination vaccines. Pediatr. Infect. Dis. J. 1998, 17, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Eko, F.O.; Witte, A.; Huter, V.; Kuen, B.; Fürst-Ladani, S.; Haslberger, A.; Katinger, A.; Hensel, A.; Szostak, M.; Resch, S.; et al. New strategies for combination vaccines based on the extended recombinant bacterial ghost system. Vaccine 1999, 17, 1643–1649. [Google Scholar] [CrossRef]
- Gilsdorf, J.R. Hib vaccines: Their impact on Haemophilus influenzae type b disease. J. Infect. Dis. 2021, 224, S321–S330. [Google Scholar] [CrossRef]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Okada, N.; Nakagawa, S. Frontiers of transcutaneous vaccination systems: Novel technologies and devices for vaccine delivery. Vaccine 2013, 31, 2403–2415. [Google Scholar] [CrossRef]
- Kang, S.-M.; Song, J.-M.; Kim, Y.-C. Microneedle and mucosal delivery of influenza vaccines. Expert Rev. Vaccines 2012, 11, 547–560. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of vaccines–Recent progress and critical issues. Biomed. Pharmacother. 2016, 83, 1080–1088. [Google Scholar] [CrossRef]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.-W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A new generation vaccine delivery system. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-J.; Kang, A.; Ahn, M.-H.; Jun, H.; Baek, S.-K.; Park, J.-H.; Na, W.; Choi, S.-O. Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J. Control. Release 2018, 286, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Choi, J.-A.; Park, H.; Yang, E.; Noh, S.; Kim, J.-S.; Kim, M.-J.; Song, M.; Park, J. Pharmaceutical and Immunological Evaluation of Cholera Toxin A1 Subunit as an Adjuvant of Hepatitis B Vaccine Microneedles. Pharm. Res. 2023, 40, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-J.; Na, W.; Kang, A.; Ahn, M.-H.; Yeom, M.; Kim, H.-O.; Lim, J.-W.; Choi, S.-O.; Baek, S.-K.; Song, D.; et al. Patchless administration of canine influenza vaccine on dog’s ear using insertion-responsive microneedles (IRMN) without removal of hair and its in vivo efficacy evaluation. Eur. J. Pharm. Biopharm. 2020, 153, 150–157. [Google Scholar] [CrossRef]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef]
- Chu, L.Y.; Ye, L.; Dong, K.; Compans, R.W.; Yang, C.; Prausnitz, M.R. Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm. Res. 2016, 33, 868–878. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef]
- Ray, S.; Wirth, D.M.; Ortega-Rivera, O.A.; Steinmetz, N.F.; Pokorski, J.K. Dissolving microneedle delivery of a prophylactic HPV vaccine. Biomacromolecules 2022, 23, 903–912. [Google Scholar] [CrossRef]
- Hassan, J.; Haigh, C.; Ahmed, T.; Uddin, M.J.; Das, D.B. Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics 2022, 14, 1066. [Google Scholar] [CrossRef]
- Peyraud, N.; Zehrung, D.; Jarrahian, C.; Frivold, C.; Orubu, T.; Giersing, B. Potential use of microarray patches for vaccine delivery in low-and middle-income countries. Vaccine 2019, 37, 4427–4434. [Google Scholar] [CrossRef]
- Doshi, J.; Ravetkar, S.; Ghole, V.; Rehani, K. Comparative quantitation for the protein content of diphtheria and tetanus toxoids by DC protein assay and Kjeldahl method. Biologicals 2003, 31, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, D.N.; Razzaghi, A.M.; Nofeli, M.; Zolfagharian, H.; Shahcheraghi, F. Study on toxicity reduction and potency induction in whole-cell pertussis vaccine by developing a new optimal inactivation condition processed on Bordetella pertussis. Jundishapur J. Microbiol. 2016, 9, e34153. [Google Scholar] [CrossRef] [PubMed]
- Prasath, C.S.; Manikandan, N.; Regin, B.; Prakash, S. Lab scale production of pertussis vaccine in B2 medium with the different concentration of bactocasamino acid, yeast Extract & soluble starch. Int. J. Biotechnol. Biochem. 2010, 6, 147–157. [Google Scholar]
- World Health Organization. Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Choi, I.-J.; Cha, H.-R.; Hwang, S.J.; Baek, S.-K.; Lee, J.M.; Choi, S.-O. Live vaccinia virus-coated microneedle array patches for smallpox vaccination and stockpiling. Pharmaceutics 2021, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Baek, S.-K.; Park, J.-H.; Betz, A.R.; Choi, S.-O. Fabrication of circular obelisk-type multilayer microneedles using micro-milling and spray deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Choi, J.-a.; Kim, J.S.; Park, H.; Yang, E.; Lee, W.J.; Baek, S.-K.; Song, M.; Park, J.-H. Skin immunization with third-generation hepatitis B surface antigen using microneedles. Vaccine 2019, 37, 5954–5961. [Google Scholar] [CrossRef]
- Pattarabhiran, S.P.; Saju, A.; Sonawane, K.R.; Manimaran, R.; Bhatnagar, S.; Roy, G.; Kulkarni, R.B.; Venuganti, V.V.K. Dissolvable microneedle-mediated transcutaneous delivery of tetanus toxoid elicits effective immune response. Aaps PharmSciTech 2019, 20, 257. [Google Scholar] [CrossRef]
- Zheng, Y.; Tesar, D.; Benincosa, L.; Birnböck, H.; Boswell, C.; Bumbaca, D.; Cowan, K.; Danilenko, D.; Daugherty, A.; Fielder, P.J.; et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs 2012, 4, 243–255. [Google Scholar] [CrossRef]
- Flisikowska, T.; Egli, J.; Flisikowski, K.; Stumbaum, M.; Küng, E.; Ebeling, M.; Schmucki, R.; Georges, G.; Singer, T.; Kurome, M.; et al. A humanized minipig model for the toxicological testing of therapeutic recombinant antibodies. Nat. Biomed. Eng. 2022, 6, 1248–1256. [Google Scholar] [CrossRef]


| Vaccine | Vaccine | Trehalose | CMC | HA |
|---|---|---|---|---|
| DT vaccine coating solution | 1% (w/v) | 15% (w/v) | 1% (w/v) | - |
| TT vaccine coating solution | 1% (w/v) | 15% (w/v) | 1% (w/v) | - |
| HBsAg vaccine coating solution | 1% (w/v) | 15% (w/v) | 1% (w/v) | - |
| wP vaccine coating solution | 4.5% (w/v) | 1.5% (w/v) | - | 1.5% (w/v) |
| Hib vaccine coating solution | 1% (w/v) | 15% (w/v) | - | 1% (w/v) |
| Antigen | Mouse | Minipig |
|---|---|---|
| Diphtheria toxoid | 62.5 ng/mL | 0.1 μg/mL |
| Tetanus toxoid | 62.5 ng/mL | 0.1 μg/mL |
| Hepatitis B surface antigen | 2 μg/mL | 1 μg/mL |
| Pertussis-PRN | N/A | 0.25 μg/mL |
| Hib-PRP | N/A | 2 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, I.-J.; Cha, H.-R.; Kwon, D.; Kang, A.; Kim, J.S.; Kim, J.; Choi, J.-E.; Chung, H.W.; Park, S.; Shim, D.H.; et al. Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability. Pharmaceutics 2024, 16, 1631. https://doi.org/10.3390/pharmaceutics16121631
Choi I-J, Cha H-R, Kwon D, Kang A, Kim JS, Kim J, Choi J-E, Chung HW, Park S, Shim DH, et al. Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability. Pharmaceutics. 2024; 16(12):1631. https://doi.org/10.3390/pharmaceutics16121631
Chicago/Turabian StyleChoi, In-Jeong, Hye-Ran Cha, Danbi Kwon, Aram Kang, Ji Seok Kim, Jooyoung Kim, Jeong-Eun Choi, Hyeon Woo Chung, Sunghoon Park, Doo Hee Shim, and et al. 2024. "Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability" Pharmaceutics 16, no. 12: 1631. https://doi.org/10.3390/pharmaceutics16121631
APA StyleChoi, I.-J., Cha, H.-R., Kwon, D., Kang, A., Kim, J. S., Kim, J., Choi, J.-E., Chung, H. W., Park, S., Shim, D. H., Kim, T.-H., Baek, S.-K., Na, W.-S., Lee, J. M., & Park, J.-H. (2024). Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability. Pharmaceutics, 16(12), 1631. https://doi.org/10.3390/pharmaceutics16121631






