Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy
Abstract
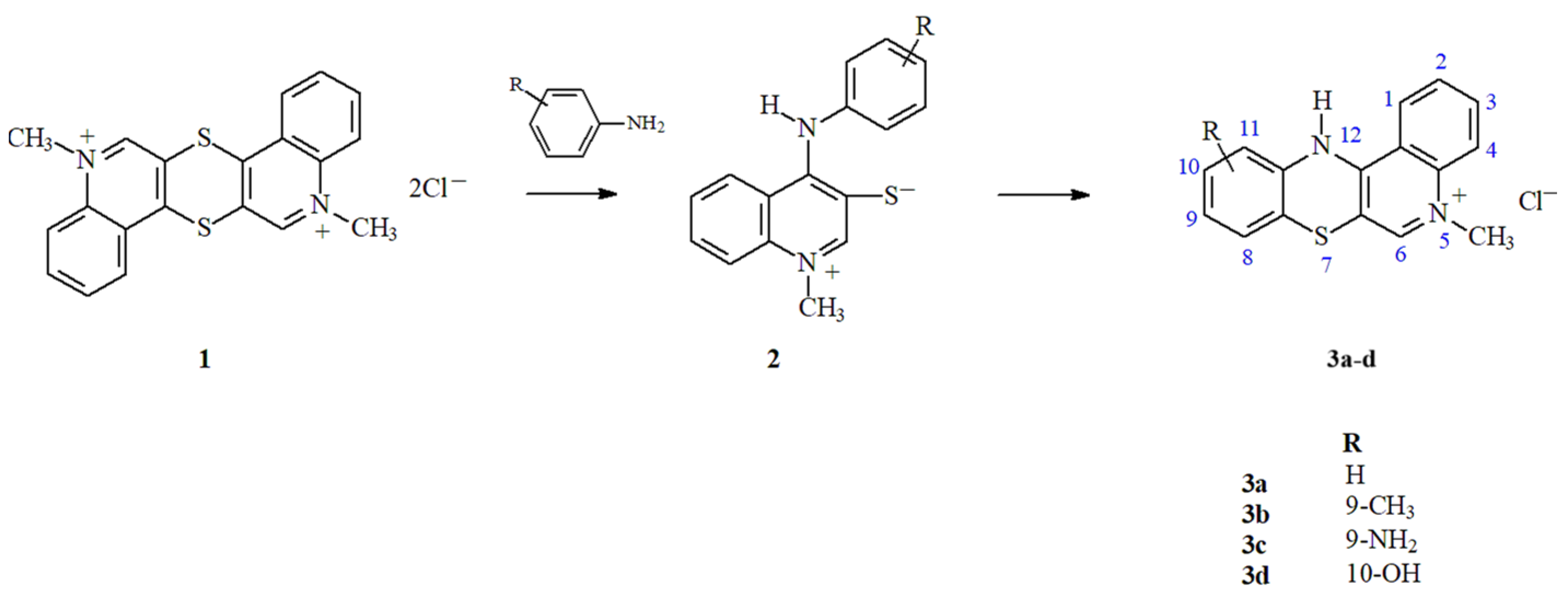
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles
2.1.1. Preparation of Nanospheres
2.1.2. Preparation of Micelles
2.2. Microscopic Analysis
2.3. Drug Encapsulation Properties and In Vitro Release Study
2.3.1. Drug Release from NS
2.3.2. Drug Release from Micelles
2.4. Cytotoxicity Study
2.4.1. CCK-8 Assay
2.4.2. Sulforhodamine B Assay
2.4.3. LDH Assay
2.4.4. BrdU Assay
2.5. Hemolysis Assay
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the NPs
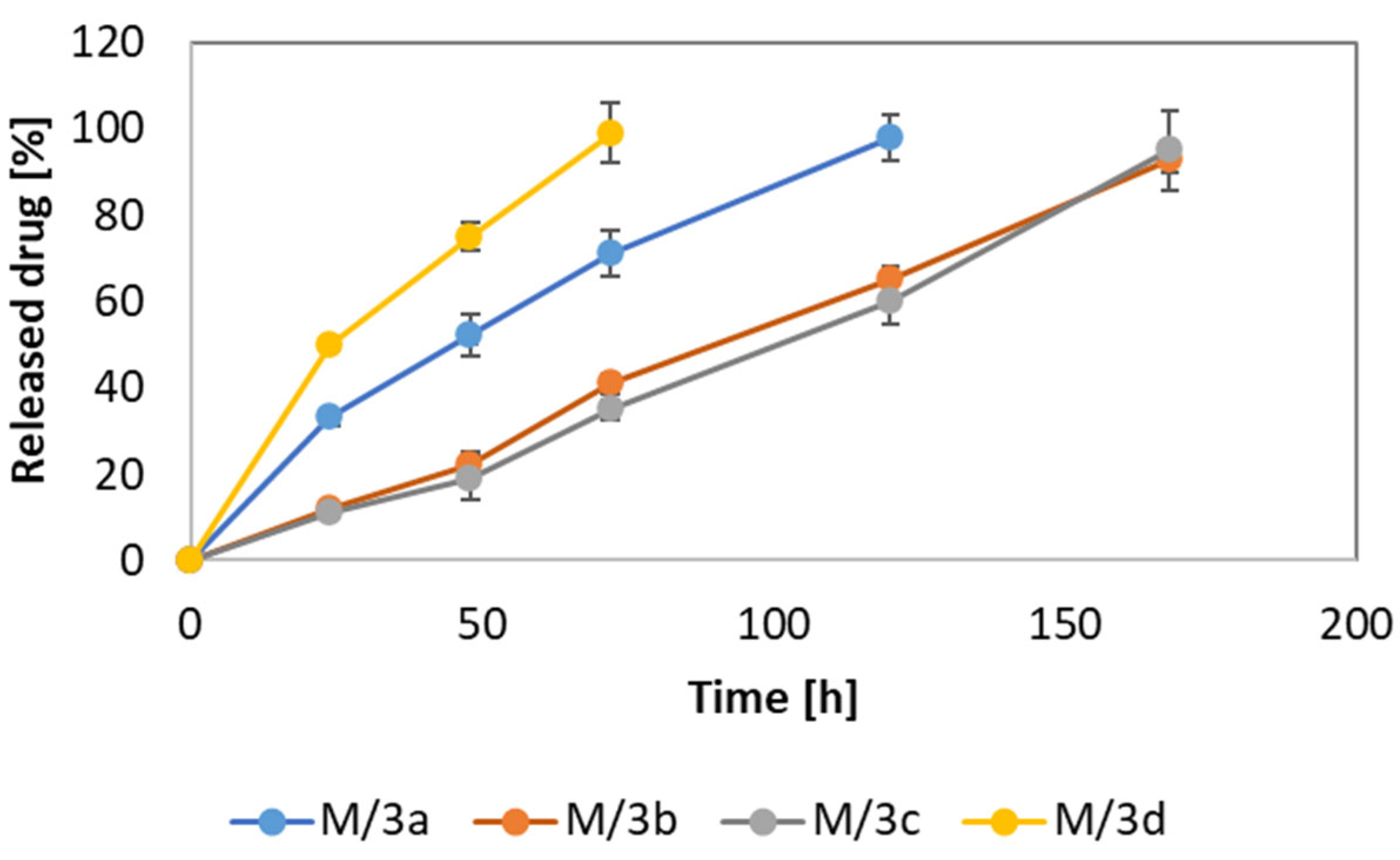
3.2. Drug-Loading and Release Properties
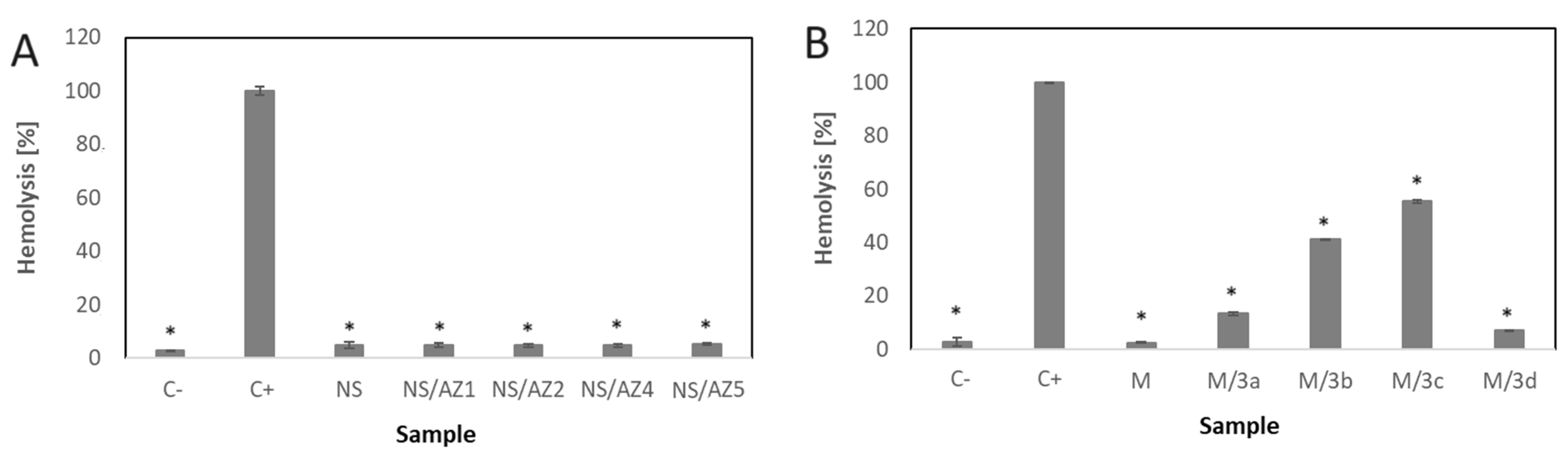
3.3. Hemolytic Effect
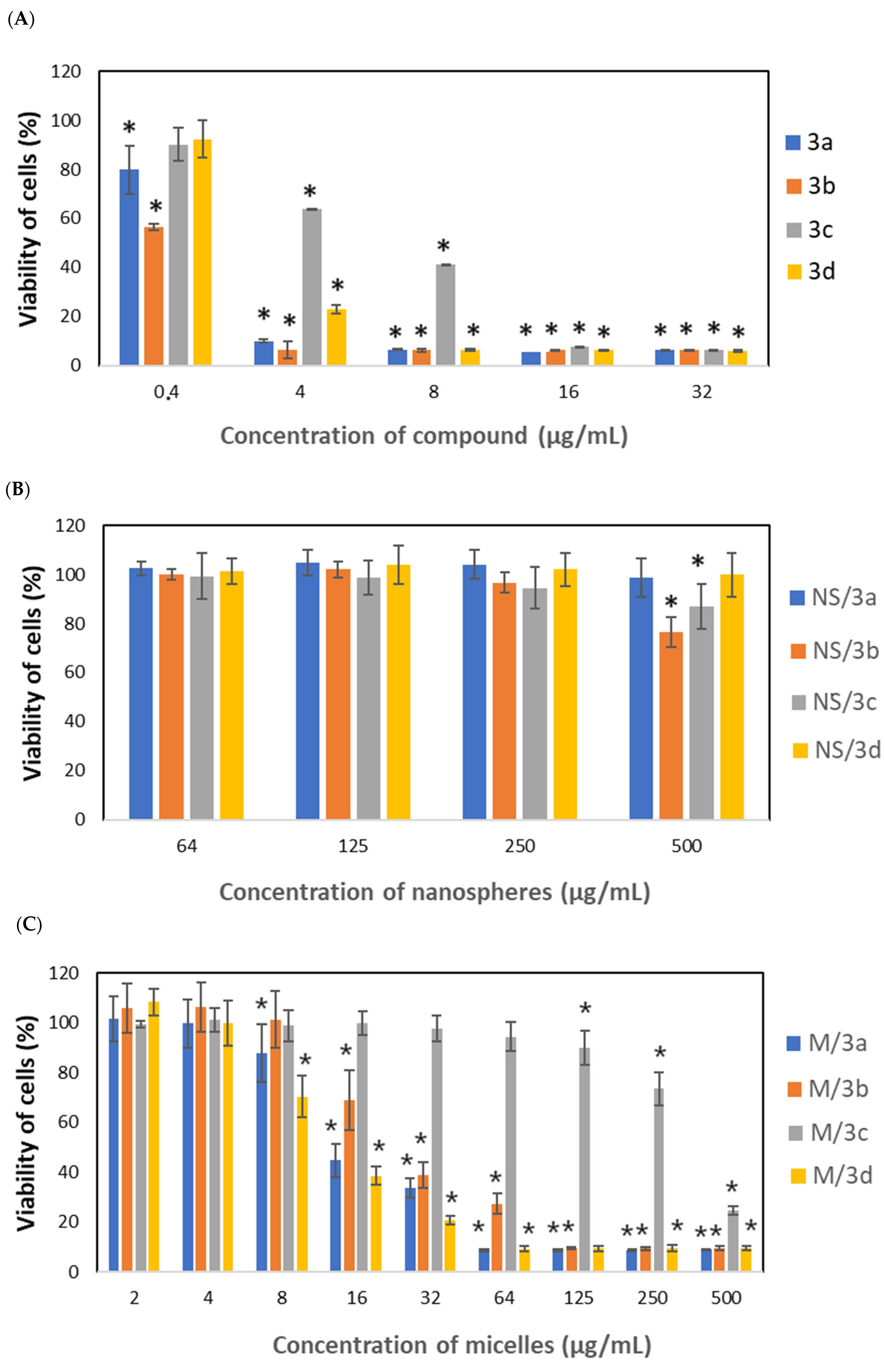
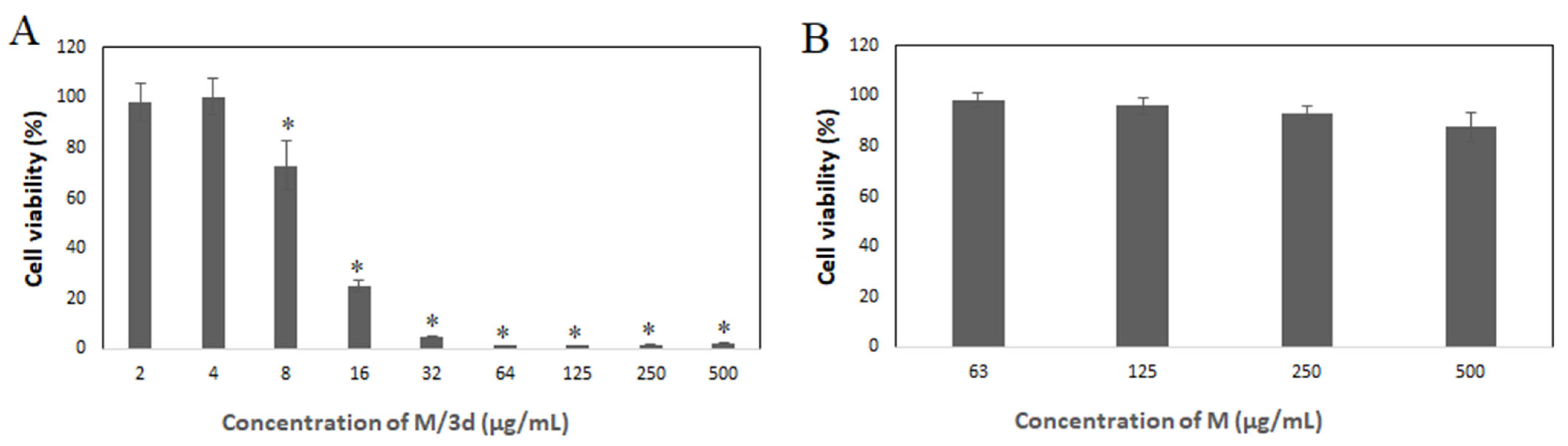
3.4. Cytotoxic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morak-Młodawska, B.; Jeleń, M.; Pluta, K. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Sochanik, A.; Szurko, A.; Rams, M.; Mrozek, A.; Cmoch, P. Synthesis and in vitro antiproliferative activity of 5-alkyl-12(H)-quino[3,4-b][1,4]benzothiazinium salts. Eur. J. Med. Chem. 2010, 45, 4733–4739. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent advances in long-acting drug delivery systems for anticancer drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Kozik, V.; Pentak, D.; Paździor, M.; Zięba, A.; Bąk, A. From Design to Study of Liposome-Driven Drug Release Part 1: Impact of Temperature and pH on Environment. Int. J. Mol. Sci. 2023, 24, 11686. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Jelonek, K.; Zajdel, A.; Wilczok, A.; Kaczmarczyk, B.; Musiał-Kulik, M.; Hercog, A.; Foryś, A.; Pastusiak, M.; Kasperczyk, J. Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells. Pharmaceutics 2021, 13, 1881. [Google Scholar] [CrossRef]
- Jurczyk, M.; Musiał-Kulik, M.; Foryś, A.; Godzierz, M.; Kaczmarczyk, B.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Comparison of PLLA-PEG and PDLLA-PEG micelles for co-encapsulation of docetaxel and resveratrol. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35318. [Google Scholar] [CrossRef]
- Jelonek, K.; Karpeta, P.; Jaworska, J.; Pastusiak, M.; Włodarczyk, J.; Kasperczyk, J.; Dobrzyński, P. Comparison of extraction methods of sirolimus from polymeric coatings of bioresorbable vascular scaffolds. Mater. Lett. 2018, 214, 220–223. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Kuru, M.M.; Dalgakiran, E.A.; Kacar, G. Investigation of morphology, micelle properties, drug encapsulation and release behavior of self-assembled PEG-PLA-PEG block copolymers: A coarse-grained molecular simulations study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127445. [Google Scholar] [CrossRef]
- Bentrivedi, A.; Kitabatake, N.; Doi, E. Toxicity of Dimethyl-Sulfoxide as a Solvent in Bioassay System with Hela-Cells Evaluated Colorimetrically with 3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyl-tetrazolium Bromide. Agric. Biol. Chem. 1990, 54, 2961–2966. [Google Scholar]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Shah, K.G.; Idrovo, J.P.; Nicastro, J.; McMullen, H.F.; Molmenti, E.P.; Coppa, G. A retrospective analysis of the incidence of hemolysis in type and screen specimens from trauma patients. Int. J. Angiol. 2009, 18, 182–183. [Google Scholar] [CrossRef][Green Version]
- Lee, M.M.-L.; Chan, B.D.; Wong, W.-Y.; Leung, T.-W.; Qu, Z.; Huang, J.; Zhu, L.; Lee, C.-S.; Chen, S.; Tai, W.C.-S. Synthesis and Evaluation of Novel Anticancer Compounds Derived from the Natural Product Brevilin A. ACS Omega 2020, 5, 14586–14596. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Tavakoli, M.M.; Safaeinejad, F.; Jafari, A. Integrating natural compounds and nanoparticle-based drug delivery systems: A novel strategy for enhanced efficacy and selectivity in cancer therapy. Cancer Med. 2024, 13, e7010. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (lactic-co-glycolic acid) and Progress of Poly(lactic-co-glycolic acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(ethylene glycol)-polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 202. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef] [PubMed]
- López-Estevez, A.M.; Gref, R.; Alonso, M.J. A journey through the history of PEGylated drug delivery nanocarriers. Drug Deliv. Transl. Res. 2024, 14, 2026–2031. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef]
- Parvanova, B.; Tacheva, B.; Ivanov, I. Prehemolytic impact of phenothiazine drugs on the attachment of spectrin network in red blood cells. Folia Med. 2023, 65, 783–787. [Google Scholar] [CrossRef]
- Santos-Martinez, J.; Aviles, T.A.; Laboy-Torres, J.A. The hemolytic effect of some phenothiazine derivatives in vitro and in vivo. Arch. Int. Pharmacodyn. Ther. 1972, 196, 83–92. [Google Scholar]
- Aki, H.; Yamamoto, M. Biothermodynamic characterization of erythrocyte hemolysis induced by phenothiazine derivatives and anti-inflammatory drugs. Biochem. Pharmacol. 1990, 39, 396–398. [Google Scholar] [CrossRef]





| Concentration of Drug-Loaded NS or M (µg/mL) | NS/3a | NS/3b | NS/3c | NS/3d | M/3a | M/3b | M/3c | M/3d |
|---|---|---|---|---|---|---|---|---|
| 2 | N/A | N/A | N/A | N/A | 0.42 | 0.36 | 0.41 | 0.42 |
| 4 | N/A | N/A | N/A | N/A | 0.84 | 0.72 | 0.82 | 0.83 |
| 8 | N/A | N/A | N/A | N/A | 1.69 | 1.44 | 1.65 | 1.67 |
| 16 | N/A | N/A | N/A | N/A | 3.39 | 2.89 | 3.30 | 3.34 |
| 31 | N/A | N/A | N/A | N/A | 6.79 | 5.78 | 6.60 | 6.68 |
| 63 | 0.16 | 1.71 | 1.13 | 1.92 | 13.58 | 11.56 | 13.21 | 13.37 |
| 125 | 0.31 | 3.42 | 2.27 | 3.74 | 27.17 | 23.13 | 26.43 | 26.75 |
| 250 | 0.62 | 6.85 | 4.55 | 7.67 | 54.35 | 46.27 | 52.87 | 53.50 |
| 500 | 1.25 | 13.70 | 9.10 | 15.35 | 108.70 | 92.55 | 105.75 | 107.00 |
| Name of Drug Delivery System | LC (%) |
|---|---|
| NS/3a | 0.75 ± 0.07 |
| NS/3b | 2.74 ± 0.13 |
| NS/3c | 1.82 ± 0.10 |
| NS/3d | 3.07 ± 0.17 |
| M/3a | 21.74 ± 1.34 |
| M/3b | 18.51 ± 0.49 |
| M/3c | 21.15 ± 0.45 |
| M/3d | 21.40 ± 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelonek, K.; Musiał-Kulik, M.; Pastusiak, M.; Foryś, A.; Zięba, A.; Kasperczyk, J. Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy. Pharmaceutics 2024, 16, 1597. https://doi.org/10.3390/pharmaceutics16121597
Jelonek K, Musiał-Kulik M, Pastusiak M, Foryś A, Zięba A, Kasperczyk J. Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy. Pharmaceutics. 2024; 16(12):1597. https://doi.org/10.3390/pharmaceutics16121597
Chicago/Turabian StyleJelonek, Katarzyna, Monika Musiał-Kulik, Małgorzata Pastusiak, Aleksander Foryś, Andrzej Zięba, and Janusz Kasperczyk. 2024. "Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy" Pharmaceutics 16, no. 12: 1597. https://doi.org/10.3390/pharmaceutics16121597
APA StyleJelonek, K., Musiał-Kulik, M., Pastusiak, M., Foryś, A., Zięba, A., & Kasperczyk, J. (2024). Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy. Pharmaceutics, 16(12), 1597. https://doi.org/10.3390/pharmaceutics16121597








