Quality by Design Approach for the Formulation and Evaluation of Stem Cells Derived Rosmarinic Acid-Loaded Nanofibers as an Anti-Wrinkle Patch: In Vitro and In Vivo Characterizations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Callus Culture from Shoot Tip Meristematic Stem Cells of Salvia miltiorrhiza
2.3. Statistical Optimization for Eliciation Process
2.4. Eliciation Treatment and Effect of Different Concentrations of Jasmonic and Salicylic Acid Elicitors on Rosmarinic Acid RA Concentration
2.5. Extraction of Rosmarinic Acid and Determination of Its Level in Salvia miltiorrhiza Callus
2.5.1. Ultrasonic-Assisted Extraction of Rosmarinic Acid RA
2.5.2. Determination of Rosmarinic Acid RA Levels Using UV Spectrophotometer
2.6. Electrospinning Nanofiber Formulation of Extracted Rosmarinic Acid
2.7. In-Vitro Characterization of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
2.7.1. Drug Content Percentage
2.7.2. In Vitro Release of Rosmarinic Acid-Loaded Nanofiber Patch
2.7.3. Swelling Degree of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
2.7.4. Mechanical Strength of the Rosmarinic Acid-Loaded Nanofiber Patch
2.7.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.7.6. Impact of Storage on the Prepared Electrospun Nanofiber Patch on Drug Content and In Vitro Release
2.7.7. Scanning Electron Microscope (SEM) and Surface Roughness
2.8. In Vivo Studies
2.8.1. Animals
2.8.2. Groups and Induction
2.8.3. Tissue Extract
2.9. In Vivo Characterization of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
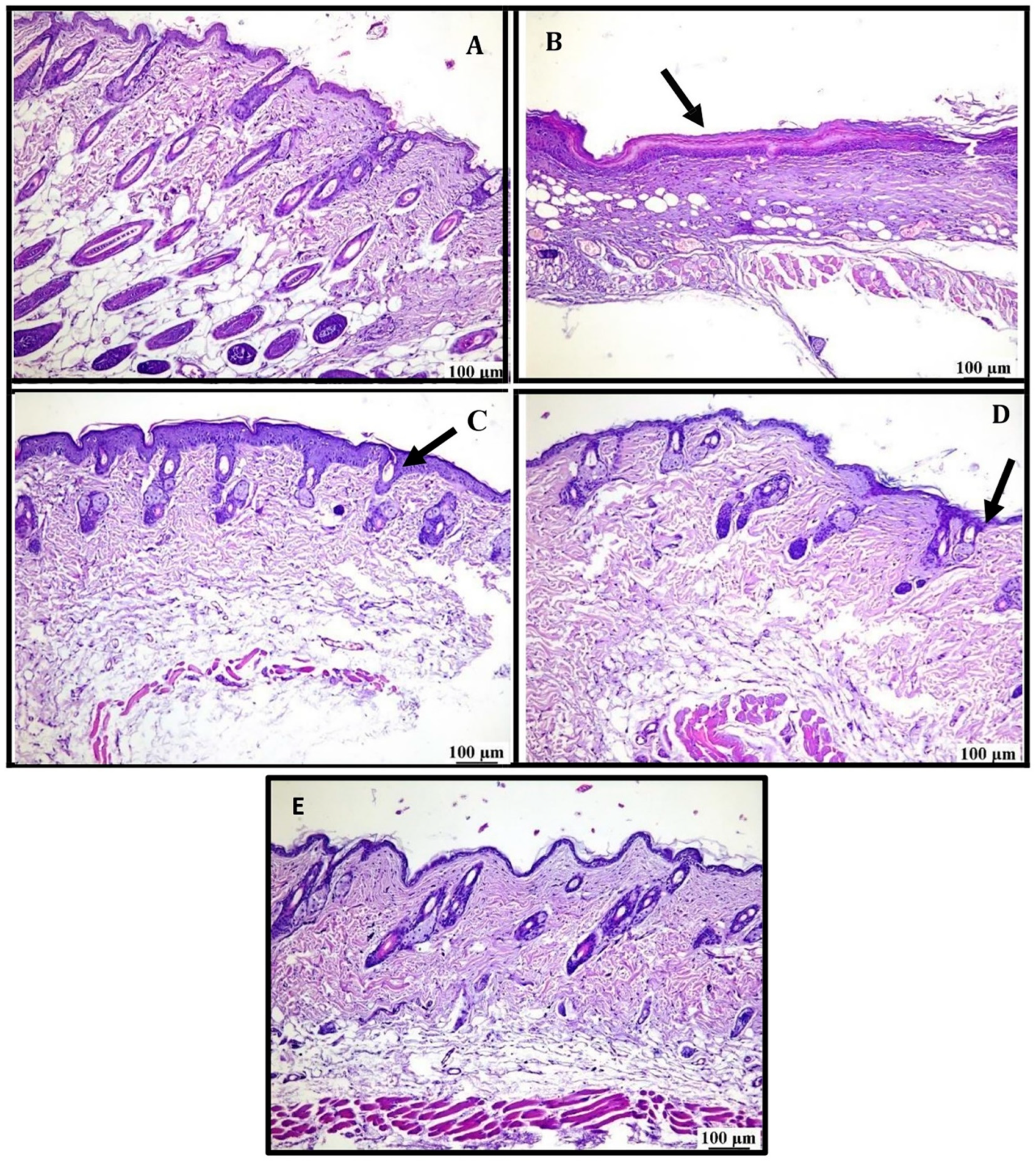
2.9.1. Skin Histopathology
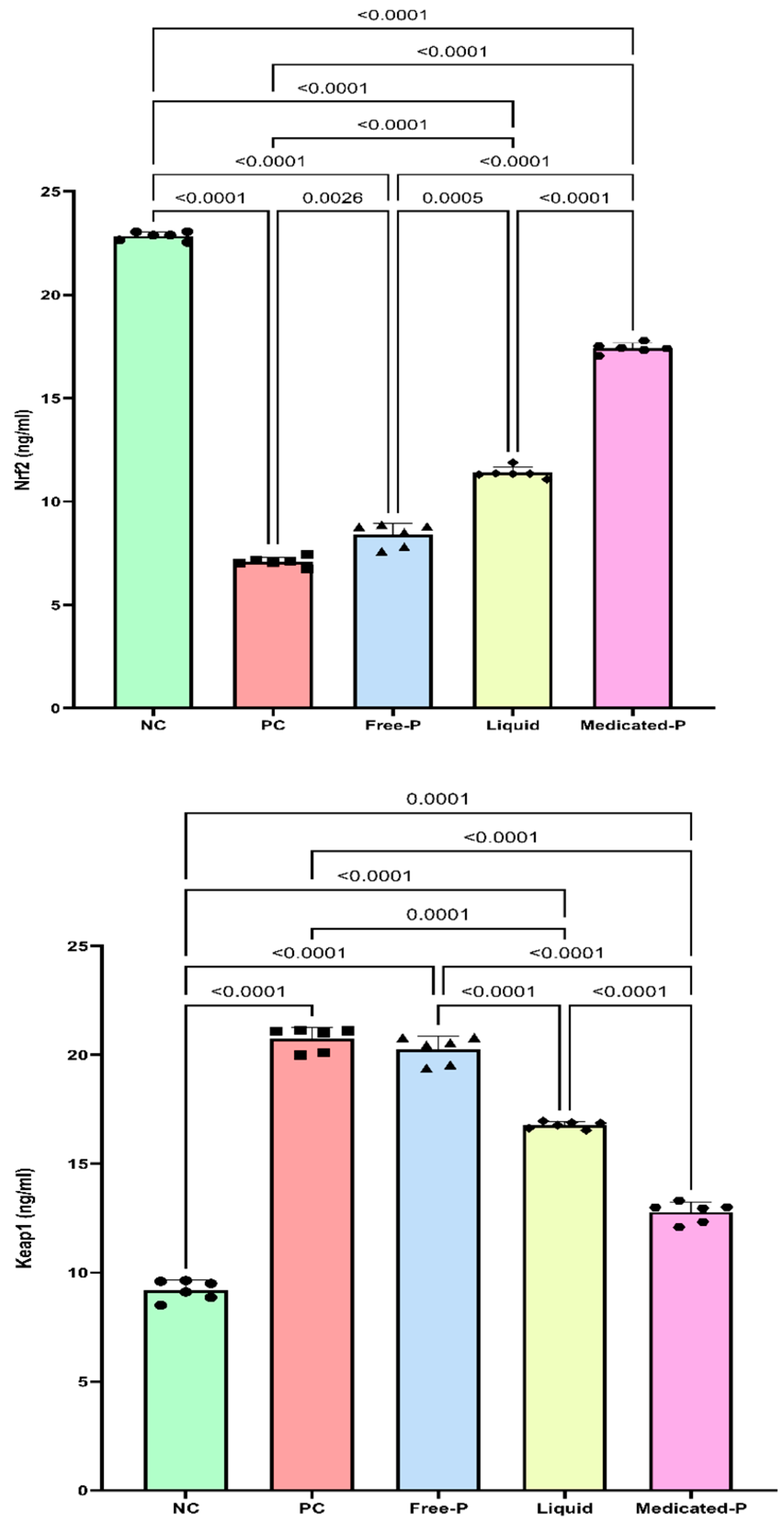
2.9.2. The Assessment of the Effect of the Medicated Nanofiber Patch on the Nrf2/Keap1 Signaling Pathway
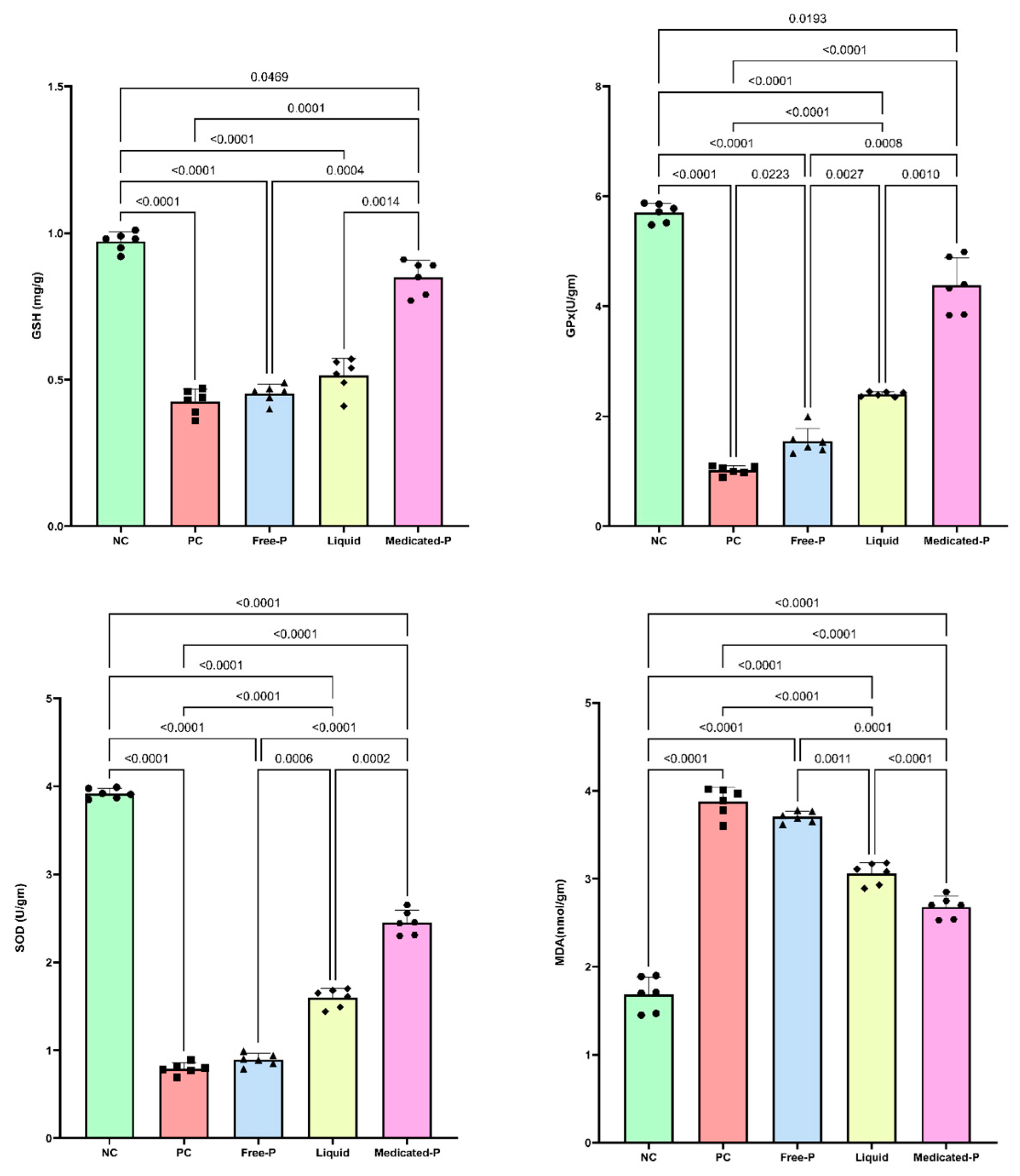
2.9.3. The Assessment of the Effect of the Medicated Nanofiber Patch on the Antioxidant Defenses in the Skin
2.9.4. Statistical Analysis
3. Results and Discussion
3.1. Callus Initiation
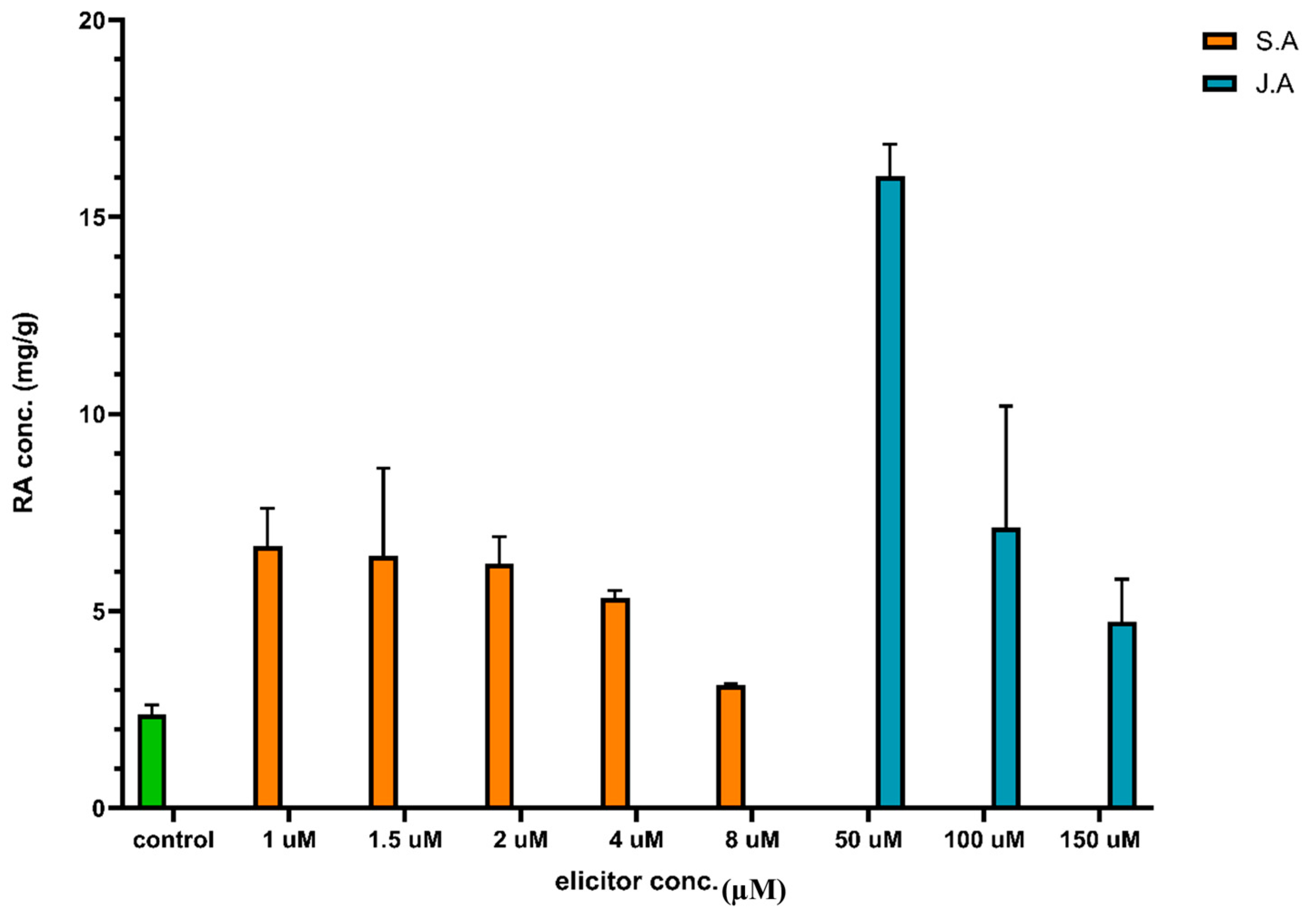
3.2. Eliciation Process with Jasmonic Acid (JA) and Salicylic Acid (SA)
3.3. Design Optimization for Eliciation Treatment
3.4. In Vitro Characterization of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
3.4.1. Drug Content
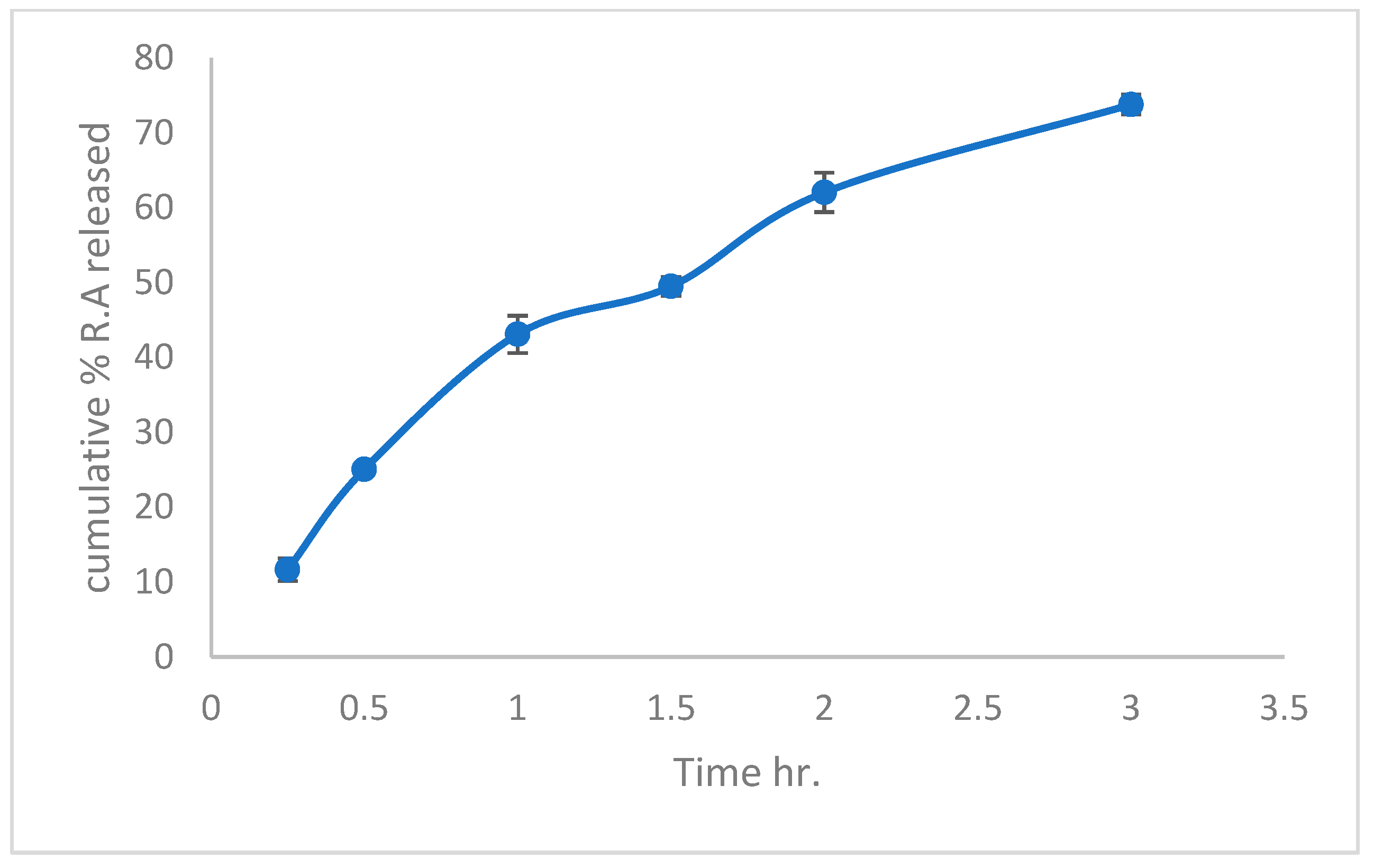
3.4.2. In Vitro Release of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
3.4.3. Swelling Degree of Extracted Rosmarinic Acid Electrospun Nanofiber Patch
3.4.4. Mechanical Strength of the Rosmarinic Acid-Loaded Nanofiber Patch
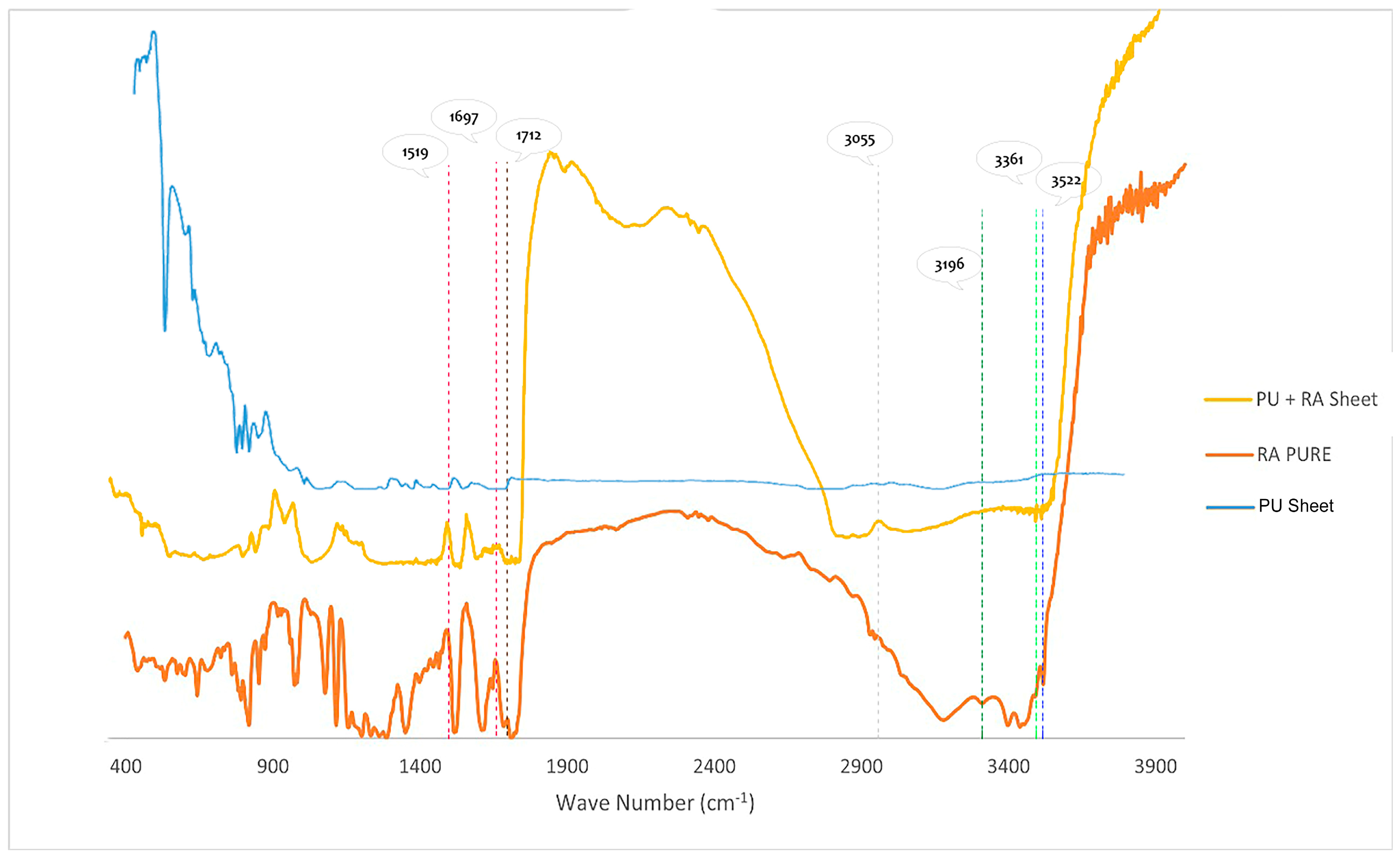
3.4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4.6. Impact of Storage on the Prepared Patch on Drug Content and In Vitro Release
3.5. Scanning Electron Microscope and Surface Roughness
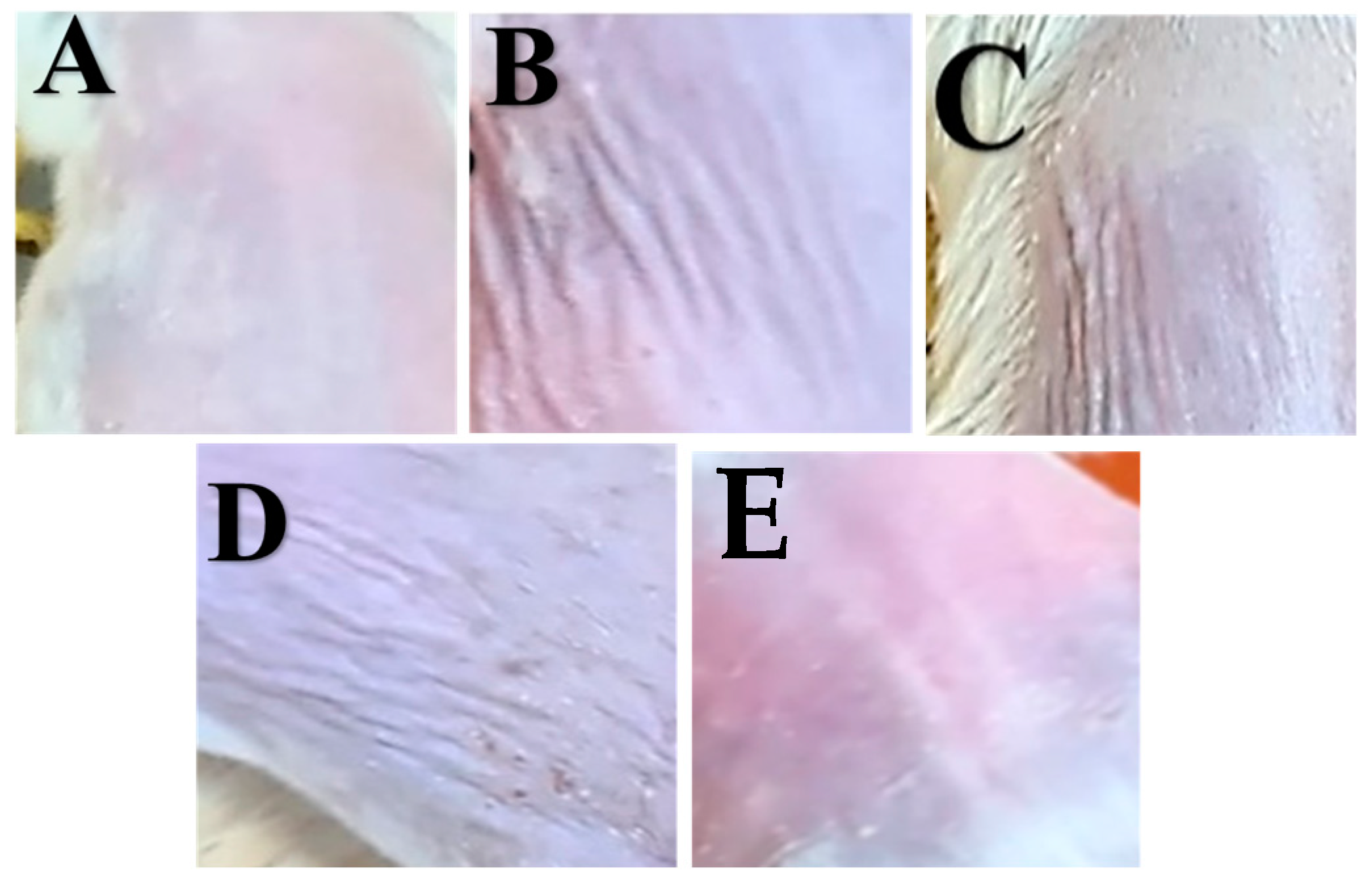
3.5.1. Physical Appearance and Skin Histopathology
3.5.2. The Medicated Nanofiber Patch Targets the Nrf2/Keap1 Signaling Pathway
3.5.3. Medicated Patch Had a Positive Impact on Cellular Oxidative Stress: Modulation of Glutathione, Glutathione Peroxidase, Superoxide Dismutase, and Malondialdehyde Levels
3.5.4. The Medicated Patch Subsides Skin Lipid Peroxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassen, S.I.; Diab, H.M.E.S.; Zayed, W.M.J.; Elhawatky, A.F. Comparison between Plasma gel (Filler) and Polydiaxone (PDO) Threads in Treating Fine and Medium Sized Infra-Orbital Wrinkles Using 3D Antera Camera. Egypt. J. Hosp. Med. 2023, 90, 2231–2241. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and functional changes and possible molecular mechanisms in aged skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Aioi, A. Trends in Immunotherapy. In Inflammaging in Skin and Intrinsic Underlying Factors; EnPress Publisher, LLC.: El Monte, CA, USA, 2021; Volume 5. [Google Scholar] [CrossRef]
- Fares, M.M.; Radaydeh, S.K. Novel mustard oil/aloe vera gel microemuslions as potential biomaterials. J. Mol. Liq. 2024, 397, 124077. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chandra, A. World Patent Information. An Insight into Patent Landscape Analysis of Plant Stem Cells; Elsevier: Amsterdam, The Netherlands, 2021; Volume 65. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Lobo, J.S.; Amaral, M.H. Biotechnology applied to cosmetics and aesthetic medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Jahangirian, H.; Azizi, S.; Rafiee-Moghaddam, R.; Baratvand, B.; Webster, T.J. Status of plant protein-based green scaffolds for regenerative medicine applications. Biomolecules 2019, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Meim, X.-D.; Cao, Y.-F.; Che, Y.-Y.; Li, J.; Shang, Z.-P.; Zhao, W.-J.; Qiao, Y.-J.; Zhang, J.-Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hu, Y.; Bai, X.; Liao, X. Rapid Screening of Chemical Components in Salvia miltiorrhiza with the Potential to Inhibit Skin Inflammation. Int. J. Mol. Sci. 2024, 25, 7369. [Google Scholar] [CrossRef]
- Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Impact of Heavy Metal Pollution in the Environment on the Metabolic Profile of Medicinal Plants and Their Therapeutic Potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef]
- Mu, X.; Yu, H.; Li, H.; Feng, L.; Ta, N.; Ling, L.; Bai, L.; A, R.; Borjigidai, A.; Pan, Y.; et al. Metabolomics analysis reveals the effects of Salvia miltiorrhiza Bunge extract on ameliorating acute myocardial ischemia in rats induced by isoproterenol. Heliyon 2024, 10, e30488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, S.; Zhu, J.; Zeng, G.; Lv, Z.; Zhang, M.; Yao, K.; Han, H. Rosmarinic acid-grafted gelatin nanogels for efficient diquafosol delivery in dry eye disease therapy. J. Control. Release 2024, 373, 306–318. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, J.; Yang, L.; Wang, D.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2016, 174, 1395–1425. [Google Scholar] [CrossRef]
- Khan, V.; Jha, A.; Princi; Seth, T.; Iqbal, N.; Umar, S. Exploring the role of jasmonic acid in boosting the production of secondary metabolites in medicinal plants: Pathway for future research. Ind. Crop. Prod. 2024, 220, 119227. [Google Scholar] [CrossRef]
- Song, L.; Zhao, T.; Luo, C.-L.; Li, J.-L.; Chen, S.-S.; Li, D.-D.; Liang, J.; Liu, H.-C.; Luo, F.-L.; Huang, M.-J.; et al. Establishment of a callus induction system of Saxifraga stolonifera Meerb. and its response to different elicitors. S. Afr. J. Bot. 2024, 171, 447–453. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Taibi, M.; Baraich, A.; Haddou, M.; Loukili, E.H.; Asehraou, A.; Mesnard, F.; Addi, M. Enhancing Secondary Metabolite Production in Pelargonium graveolens Hort. Cell Cultures: Eliciting Effects of Chitosan and Jasmonic Acid on Bioactive Compound Production. Horticulturae 2024, 10, 521. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2023, 5, 109–122. [Google Scholar] [CrossRef]
- Ko, J.; Kim, J.; Choi, S.; Kim, Y.; Park, S.; Kim, J.; Kim, H.; Lee, Y.; An, Y.; Hwang, N.S. Recent Advances in Nanotechnology-Mediated Noninvasive Transdermal and Topical Delivery of Proteins. Small Sci. 2024, 4, 2400175. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2023, 33, 355–376. [Google Scholar] [CrossRef]
- Salih, A.R.C.; Farooqi, H.M.U.; Amin, H.; Karn, P.R.; Meghani, N.; Nagendran, S. Hyaluronic acid: Comprehensive review of a multifunctional biopolymer. Futur. J. Pharm. Sci. 2024, 10, 63. [Google Scholar] [CrossRef]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Z.; Hu, H.; Kong, Z.; Chen, C.; Tian, Y.; Zhang, W.; Bin Ying, W.; Zhang, R.; Zhu, J. A polyurethane integrating self-healing, anti-aging and controlled degradation for durable and eco-friendly E-skin. Chem. Eng. J. 2021, 410, 128363. [Google Scholar] [CrossRef]
- Kim, E.; De Tollenaere, M.; Sennelier, B.; Lambert, C.; Durduret, A.; Kim, S.-Y.; Seo, H.-H.; Lee, J.-H.; Scandolera, A.; Reynaud, R.; et al. Analysis of Active Components and Transcriptome of Freesia refracta Callus Extract and Its Effects against Oxidative Stress and Wrinkles in Skin. Int. J. Mol. Sci. 2024, 25, 8150. [Google Scholar] [CrossRef]
- Lee, H.; Ye, S.; Kim, J.; Jun, S.-H.; Kang, N.-G. Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function. Curr. Issues Mol. Biol. 2024, 46, 5037–5051. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, H.; Wei, W.; Ning, R.; Chang, Y. International Immunopharmacology Advances in the study of ferroptosis and its relationship to autoimmune diseases. Int. Immunopharmacol. 2024, 140, 112819. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wu, C.-F.; Karioti, A.; Rohr, D.; Bilia, A.R.; Efferth, T. Production of rosmarinic acid and salvianolic acid B from callus culture of Salvia miltiorrhiza with cytotoxicity towards acute lymphoblastic leukemia cells. Food Chem. 2016, 201, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, T.; Wu, Y.; Zhou, Y.; Jiang, Y.; Zhang, L. Effect of elicitors on the metabolites in the suspension cell culture of Salvia miltiorrhiza Bunge. Physiol. Mol. Biol. Plants 2018, 25, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Aybastıer, O.; Işık, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; İnce, B.; Harmandar, M.; Topçu, G. A new rapid spectrophotometric method to determine the rosmarinic acid level in plant extracts. Food Chem. 2010, 123, 1352–1356. [Google Scholar] [CrossRef]
- Abdelrahman, R.; Eltorky, M.; El-Mokadem, H.E.; Hassan, H. Experimental research on the production of rosmarinic acid from different Ocimum basilicum In Vitro cultures. Middle East J. Appl. Sci. 2019, 9, 434–442. [Google Scholar] [CrossRef]
- Shah, A.A.; Yang, J.; Kumar, T.; Ayranci, C.; Zhang, X. Synthesis of transparent electrospun composite nanofiber membranes by asymmetric solvent evaporation process. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131264. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor activity of rosmarinic acid-loaded silk fibroin nanoparticles on hela and MCF-7 cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic acid-loaded electrospun nanofibers: In vitro release kinetic study and bioactivity assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef]
- Saragih, S.W.; Wirjosentono, B.; Eddiyanto Meliana, Y. Influence of crosslinking agent on the morphology, chemical, crystallinity and thermal properties of cellulose nanofiber using steam explosion. Case Stud. Therm Eng. 2020, 22, 100740. [Google Scholar] [CrossRef]
- Shaker, A.; Khedewy, A.T.; Hassan, M.A.; El-Baky, M.A.A. Thermo-mechanical characterization of electrospun polyurethane/carbon-nanotubes nanofibers: A comparative study. J. Sci. Rep. 2023, 13, 17368. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Şeker Karatoprak, G.; Degim, I.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Cesur, S.; Oktar, F.N.; Ekren, N.; Kilic, O.; Alkaya, D.B.; Seyhan, S.A.; Ege, Z.R.; Lin, C.-C.; Kuruca, S.E.; Erdemir, G.; et al. Preparation and characterization of electrospun polylactic acid/sodium alginate/orange oyster shell composite nanofiber for biomedical application. J. Aust. Ceram. Soc. 2020, 56, 533–543. [Google Scholar] [CrossRef]
- Sadeghi, S.M.; Vaezi, M.; Kazemzadeh, A.; Jamjah, R. 3D networks of TiO2 nanofibers fabricated by sol-gel/electrospinning/calcination combined method: Valuation of morphology and surface roughness parameters. Mater. Sci. Eng. B 2021, 271, 115254. [Google Scholar] [CrossRef]
- Bilkar, D.; Keshavamurthy, R.; Tambrallimath, V. Influence of carbon nanofiber reinforcement on mechanical properties of polymer composites developed by FDM. Mater. Today Proc. 2020, 46, 4559–4562. [Google Scholar] [CrossRef]
- Handbook of Bioethical Decisions; Springer: Berlin/Heidelberg, Germany, 2023; Volume 1, Available online: https://link.springer.com/chapter/10.1007/978-3-031-29451-8_5 (accessed on 6 November 2024).
- Savic, S.; Smiljic, S.; Lestarevic, S.; Ilic, A.; Mijovic, M.; Mandic, P.; Djerkovic, B. The structural characteristics of photoageing in mice caused by the effects of ultraviolet A radiation. Folia Morphol. 2020, 79, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Blythe, D. Theory Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone of ElSevier: Philadelphia, PA, USA, 2013; Chapter 18; pp. 386–431. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. Available online: http://www.ncbi.nlm.nih.gov/pubmed/13967893 (accessed on 6 November 2024). [PubMed]
- Ibrahim, M.M. Elicitor and Precursor Feeding as a Biotechnological tool for the Production of Bioactive Cardiac Glycosides in Cell Suspension Cultures of Digitalis lanata. Egypt. J. Chem. 2024, 67, 141–149. [Google Scholar] [CrossRef]
- Rastegarnejad, F.; Mirjalili, M.H.; Bakhtiar, Z. Enhanced production of tanshinone and phenolic compounds in hairy roots culture of Salvia miltiorrhiza Bunge by elicitation. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 156, 4. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Hassani, S.B.; Nohooji, M.G.; Riahi, H.; Shariatmadari, Z. Enhancement of Non-Enzymatic Antioxidant Compounds and Expression of Rosmarinic Acid Biosynthesis-Related Genes in Melissa officinalis Using Cyanobacteria. Iran. J. Sci. 2024, 48, 1099–1111. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, L.K.; Pérez-Bernal, J.E.; Santamaría-Torres, M.A.; Marquínez-Casas, X.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effect of methyl jasmonate and salicylic acid on the production of metabolites in cell suspensions cultures of Piper cumanense (Piperaceae). Biotechnol. Rep. 2020, 28, e00559. [Google Scholar] [CrossRef]
- Rasoli, F.; Gholipoor, M. Interactive effects of salicylic acid and jasmonic acid on secondary metabolite production in Echinacea purpurea. Int. J. Second. Metab. 2023, 10, 106–118. [Google Scholar] [CrossRef]
- Han, C.-F.; Liu, S.-T.; Yan, R.-R.; Li, J.; Chen, N.; Zhang, L.-L.; Jia, S.-R.; Han, P.-P. Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level. Foods 2023, 12, 915. [Google Scholar] [CrossRef]
- Woch, N.; Laha, S.; Gudipalli, P. Salicylic acid and jasmonic acid induced enhanced production of total phenolics, flavonoids, and antioxidant metabolism in callus cultures of Givotia moluccana (L.) Sreem. Vitr. Cell. Dev. Biol. Plant 2023, 59, 227–248. [Google Scholar] [CrossRef]
- Abdelmonem, R.; Eltahan, M.; El-Nabarawi, M. Development And Evaluation of Taste Masked Oro-Disintegrating Tablets of Itopride Hcl Using Different Co-Processed Excipients: Pharmacokinetics Study on Rabbits. Int. J. Appl. Pharm. 2022, 14, 69–79. [Google Scholar] [CrossRef]
- Zaid, A.N.; Al-Ramahi, R.J.; Abu Ghoush, A.; Qaddumi, A.; Abu Zaaror, Y. Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. Saudi Pharm. J. 2013, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Yu, D.-G.; Branford-White, C.; Zhu, L.-M. Sustained release of ethyl cellulose micro-particulate drug delivery systems prepared using electrospraying. J. Mater. Sci. 2011, 47, 1372–1377. [Google Scholar] [CrossRef]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog. Biomater. 2017, 7, 23–33. [Google Scholar] [CrossRef]
- Gencturk, A.; Kahraman, E.; Güngör, S.; Özhan, G.; Özsoy, Y.; Sarac, A.S. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: Characterization studies and In Vitro assays. Artif Cells Nanomed. Biotechnol. 2017, 45, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Deka, B.J.; Wong, P.W.; Sun, J.; An, A.K. Fabrication of robust green superhydrophobic hybrid nanofiber-nanosphere membrane for membrane distillation. Desalination 2021, 520, 115314. [Google Scholar] [CrossRef]
- Gavande, V.; Nagappan, S.; Lee, W.-K. Considering Electrospun Nanofibers as a Filler Network in Electrospun Nanofiber-Reinforced Composites to Predict the Tensile Strength and Young’s Modulus of Nanocomposites: A Modeling Study. Polymers 2022, 14, 5425. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Wei, S.; Huang, Y.; Yang, S.; Xue, Y.; Xuan, H.; Yuan, H. A Solvent System Involved Fabricating Electrospun Polyurethane Nanofibers for Biomedical Applications. Polymers 2020, 12, 3038. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2019, 146, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Kamble, R.N.; Mehtre, R.V.; Mehta, P.P.; Nangare, P.; Patil, S.S. Albendazole Electrospun Nanofiber Films: In-Vitro and Ex-Vivo Assessment. BioNanoScience 2019, 9, 625–636. [Google Scholar] [CrossRef]
- Fenner, J.; Clark, R.A. Anatomy, Physiology, Histology, and Immunohistochemistry of Human Skin. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes, J.H., IV, Eds.; Academic Press: Boston, MA, USA, 2016; Chapter 1; pp. 1–17. [Google Scholar] [CrossRef]
- Yehia, M.A.-H.; Fawzy, M.A.; Elbashar, Y.H.; Abdelmigid, B.A.; ElGhnam, S.M. Histomorphometry, immunohistochemistry, and fine structure of Rats skin exposed to Ultraviolet-A radiation. J. Opt. 2022, 51, 574–584. [Google Scholar] [CrossRef]
- Pinsky, M.A. Efficacy and Safety of an Anti-aging Technology for the Treatment of Facial Wrinkles and Skin Moisturization. J. Clin. Aesthetic Dermatol. 2017, 10, 27–35. [Google Scholar]
- Lu, M.; Ji, J.; Jiang, Z.; You, Q. The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef] [PubMed]
- Mrówka, M.; Lenża-Czempik, J.; Dawicka, A.; Skonieczna, M. Polyurethane-Based Nanocomposites for Regenerative Therapies of Cancer Skin Surgery with Low Inflammatory Potential to Healthy Fibroblasts and Keratinocytes In Vitro. ACS Omega 2023, 8, 37769–37780. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Dinkova-Kostova, A.T.; Cohen, G. NRF2 in dermatological disorders: Pharmacological activation for protection against cutaneous photodamage and photodermatosis. Free Radic Biol Med. 2022, 188, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, T.; Jin, J.; Li, Z.; Cheng, W.; Dai, X.; Yang, K.; Zhang, H.; Zhang, Z.; Zhang, H.; et al. Exosome-like nanovesicles derived from Phellinus linteus inhibit Mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J. Nanobiotechnol. 2022, 20, 455. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36271377 (accessed on 6 November 2024). [CrossRef]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef]
- Shah, N.; Sapra, R.; Kumari, M.; Sadhu, P.; Talele, C. Revolutionizing Drug Delivery: The Role of Nanofibers—A Review. J. Adv. Zool. 2024, 45, 512–520. [Google Scholar] [CrossRef]
- Parmar, G.; Chudasama, J.M.; Aundhia, C. Weaving the Future of Topical Medicine: A Journey with Electrospinning Nanofibre Scaffolds. Cur-Rent Nanosci. 2024, 20, 1875–6786. [Google Scholar] [CrossRef]
- Choi, S.-I.; Han, H.-S.; Kim, J.-M.; Park, G.; Jang, Y.-P.; Shin, Y.-K.; Ahn, H.-S.; Lee, S.-H.; Lee, K.-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef] [PubMed]





| Factors | Factor Type | Levels | |
|---|---|---|---|
| Low | High | ||
| X1: Conc. of elicitors X2: Type of elicitors | Numeric Categoric | 1 µM SA | 150 µM JA |
| Responses | Desirability constraints | ||
| Y1: Concentration of RA | Maximize | ||
| Treatments | Conc. of Elicitors (X1) (µM) | Type of Elicitors (X2) | Conc. of RA (mg/g) |
|---|---|---|---|
| T1 | 50 | JA | 16 ± 0.29 |
| T2 | 1 | SA | 6.65 ± 1.63 |
| T3 | 1.5 | SA | 6.4 ± 0.16 |
| T4 | 2 | SA | 6.21 ± 1.17 |
| T5 | 4 | SA | 5.32 ± 0.33 |
| T6 | 100 | JA | 7.13 ± 0.38 |
| T7 | 150 | JA | 4.74 ± 0.39 |
| T8 | 8 | SA | 3.114 ± 0.066 |
| Source | RA Conc. (mg/g) (Y1) |
|---|---|
| p value | <0.0001 |
| Model | Quadratic |
| X1 = A = Conc. of Elicitors | 0.0002 |
| X2 = B = Type of Elicitors | 0.0002 |
| Adequate precision R2 | 138.1224 0.9996 |
| Adjusted R2 | 0.9991 |
| Predicted R2 | −219.7502 |
| Significant factors | X1, X2 |
| Predicted value of the selected patch | 49.99 |
| Observed value of the selected patch | 50 ± 0.529 |
| The regression equation of the fitted model | +31.33 − 0.3.47 × A + 0.0012 × A2 |
| Composition | Ra (nm) | Rt (nm) | Rv (nm) | Rp (nm) |
|---|---|---|---|---|
| 10% PU polymer + Rosmarinic acid | 62.7 | 726.0 | 325.7 | 400.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmonem, R.; Bakr, A.; Badawy, I.; Abd El Maksoud, A.I.; Attia, R.T. Quality by Design Approach for the Formulation and Evaluation of Stem Cells Derived Rosmarinic Acid-Loaded Nanofibers as an Anti-Wrinkle Patch: In Vitro and In Vivo Characterizations. Pharmaceutics 2024, 16, 1598. https://doi.org/10.3390/pharmaceutics16121598
Abdelmonem R, Bakr A, Badawy I, Abd El Maksoud AI, Attia RT. Quality by Design Approach for the Formulation and Evaluation of Stem Cells Derived Rosmarinic Acid-Loaded Nanofibers as an Anti-Wrinkle Patch: In Vitro and In Vivo Characterizations. Pharmaceutics. 2024; 16(12):1598. https://doi.org/10.3390/pharmaceutics16121598
Chicago/Turabian StyleAbdelmonem, Rehab, Ahmed Bakr, Ingy Badawy, Ahmed Ibrahim Abd El Maksoud, and Reem T. Attia. 2024. "Quality by Design Approach for the Formulation and Evaluation of Stem Cells Derived Rosmarinic Acid-Loaded Nanofibers as an Anti-Wrinkle Patch: In Vitro and In Vivo Characterizations" Pharmaceutics 16, no. 12: 1598. https://doi.org/10.3390/pharmaceutics16121598
APA StyleAbdelmonem, R., Bakr, A., Badawy, I., Abd El Maksoud, A. I., & Attia, R. T. (2024). Quality by Design Approach for the Formulation and Evaluation of Stem Cells Derived Rosmarinic Acid-Loaded Nanofibers as an Anti-Wrinkle Patch: In Vitro and In Vivo Characterizations. Pharmaceutics, 16(12), 1598. https://doi.org/10.3390/pharmaceutics16121598






