Respiratory Delivery of Lacticaseibacillus rhamnosus GG by Vibrating-Mesh and Jet Nebulisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing
2.2. pH and Osmolality Measurements
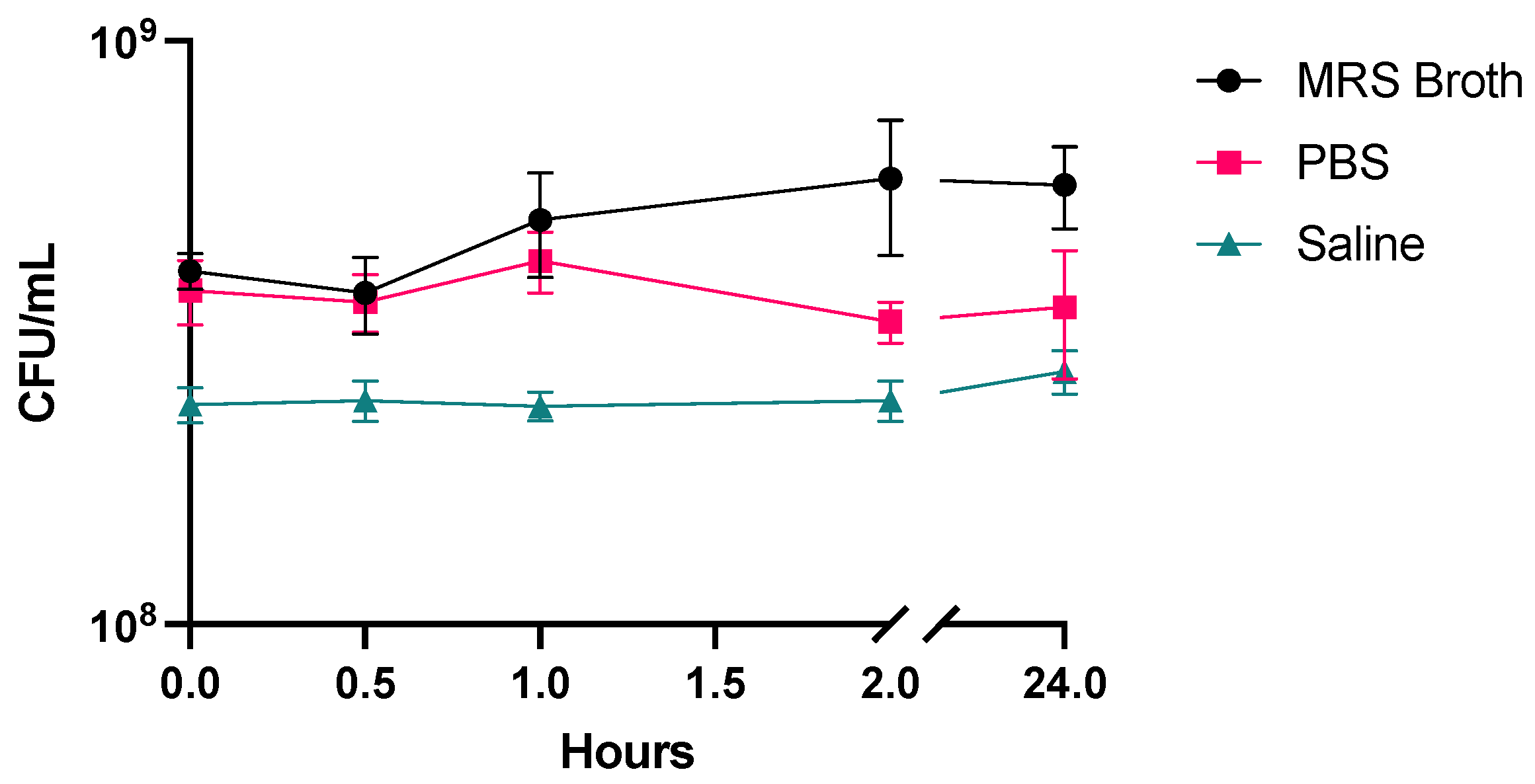
2.3. Stability of LGG
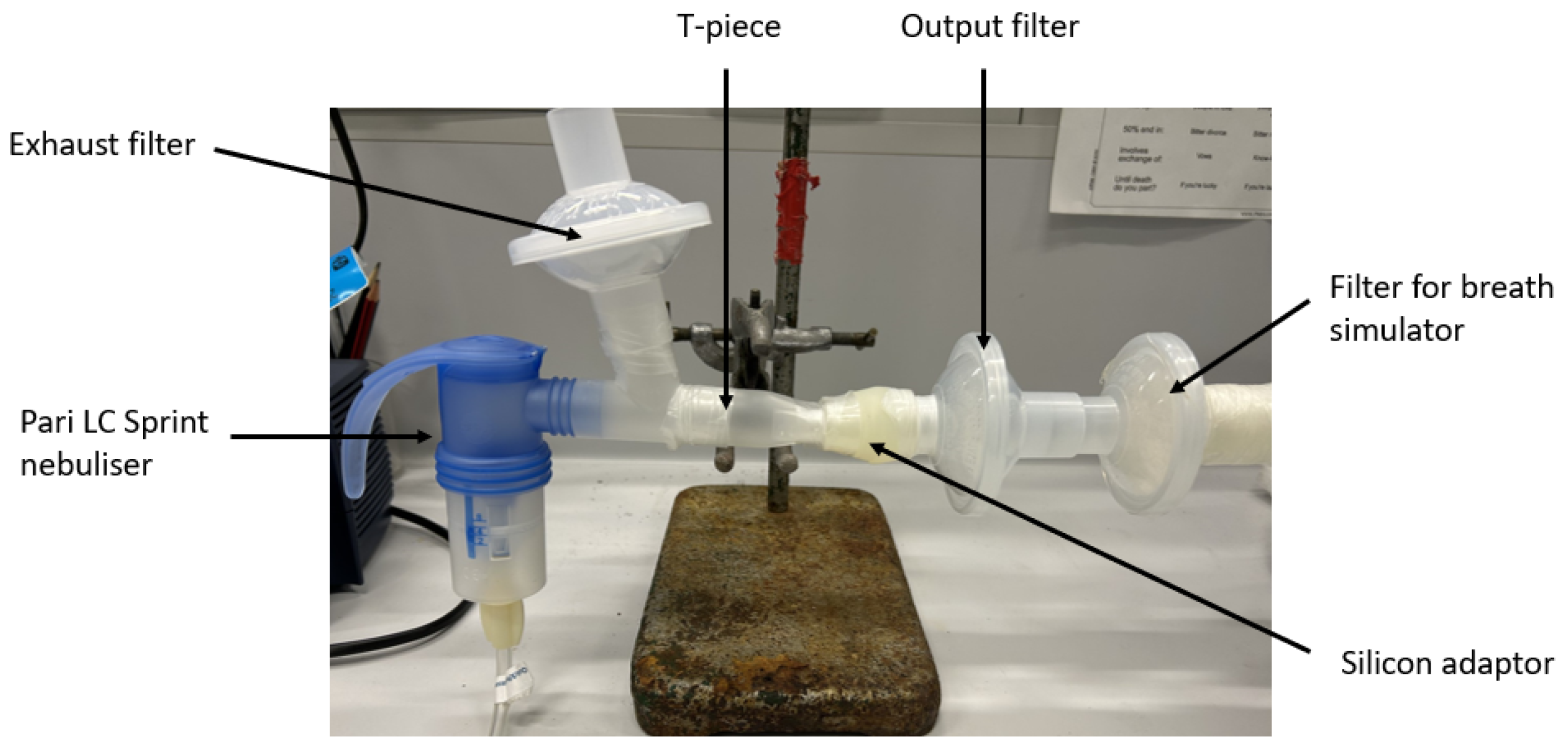
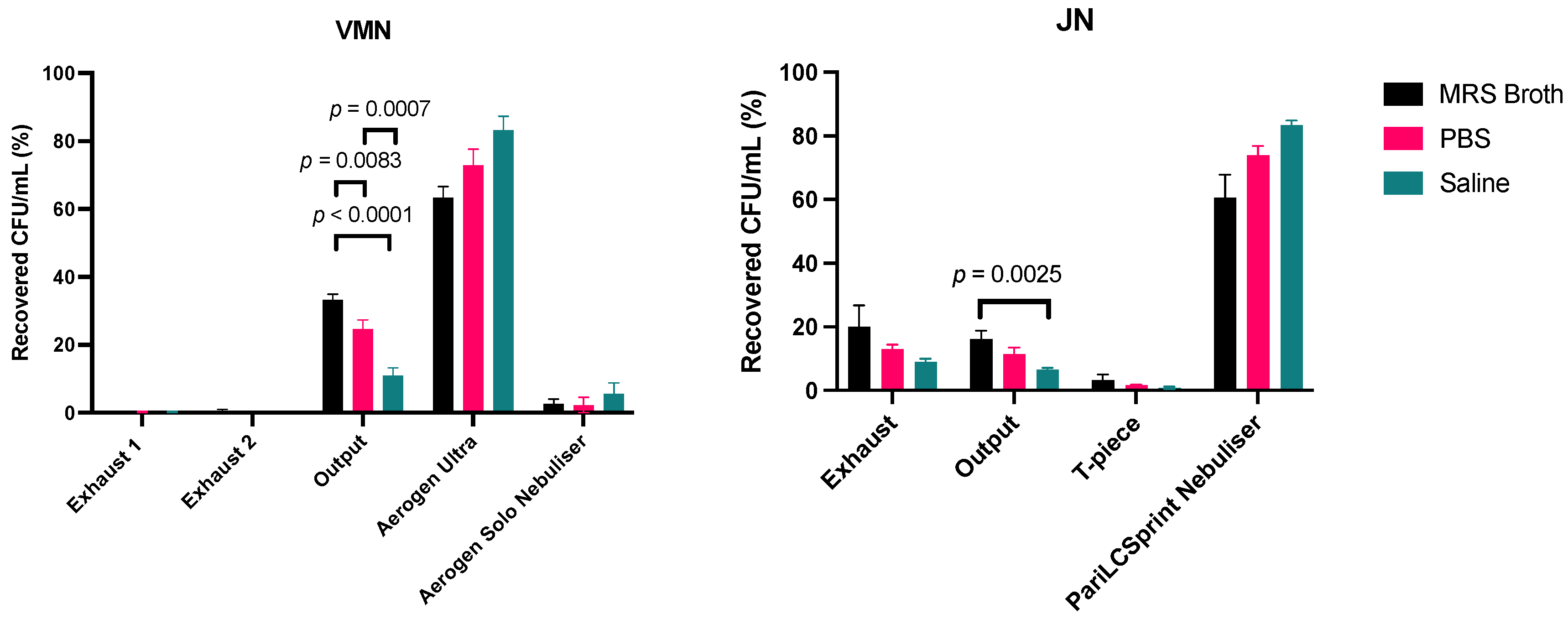
2.4. Dose Output
2.5. Timed Nebulisation
2.6. Laser Diffraction
2.7. Cascade Impaction
2.8. Real-Time Bacterial Imaging
2.9. Statistical Analysis
3. Results
3.1. pH, Osmolality, Refractive Indices Measurement
3.2. Stability
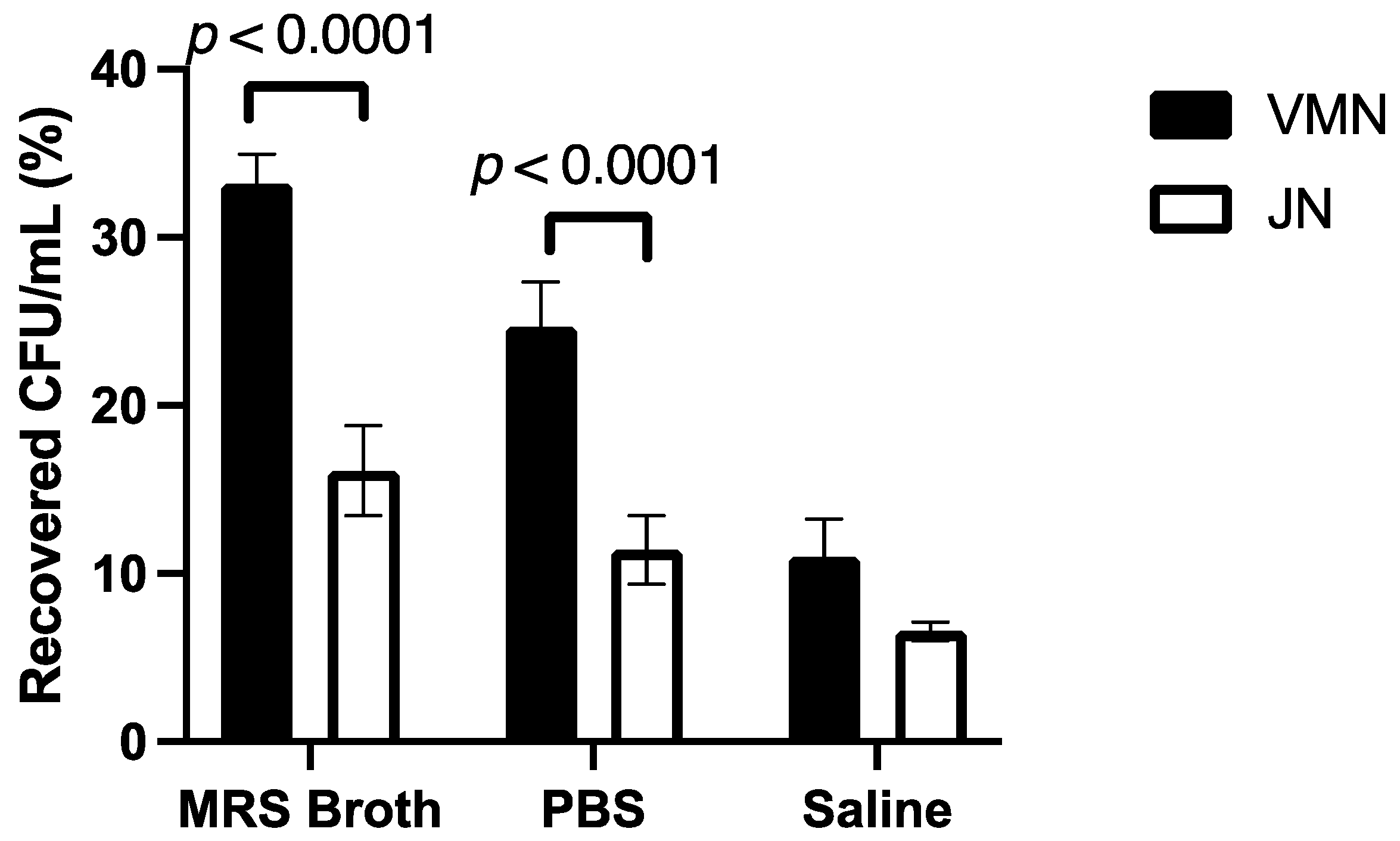
3.3. Dose Output
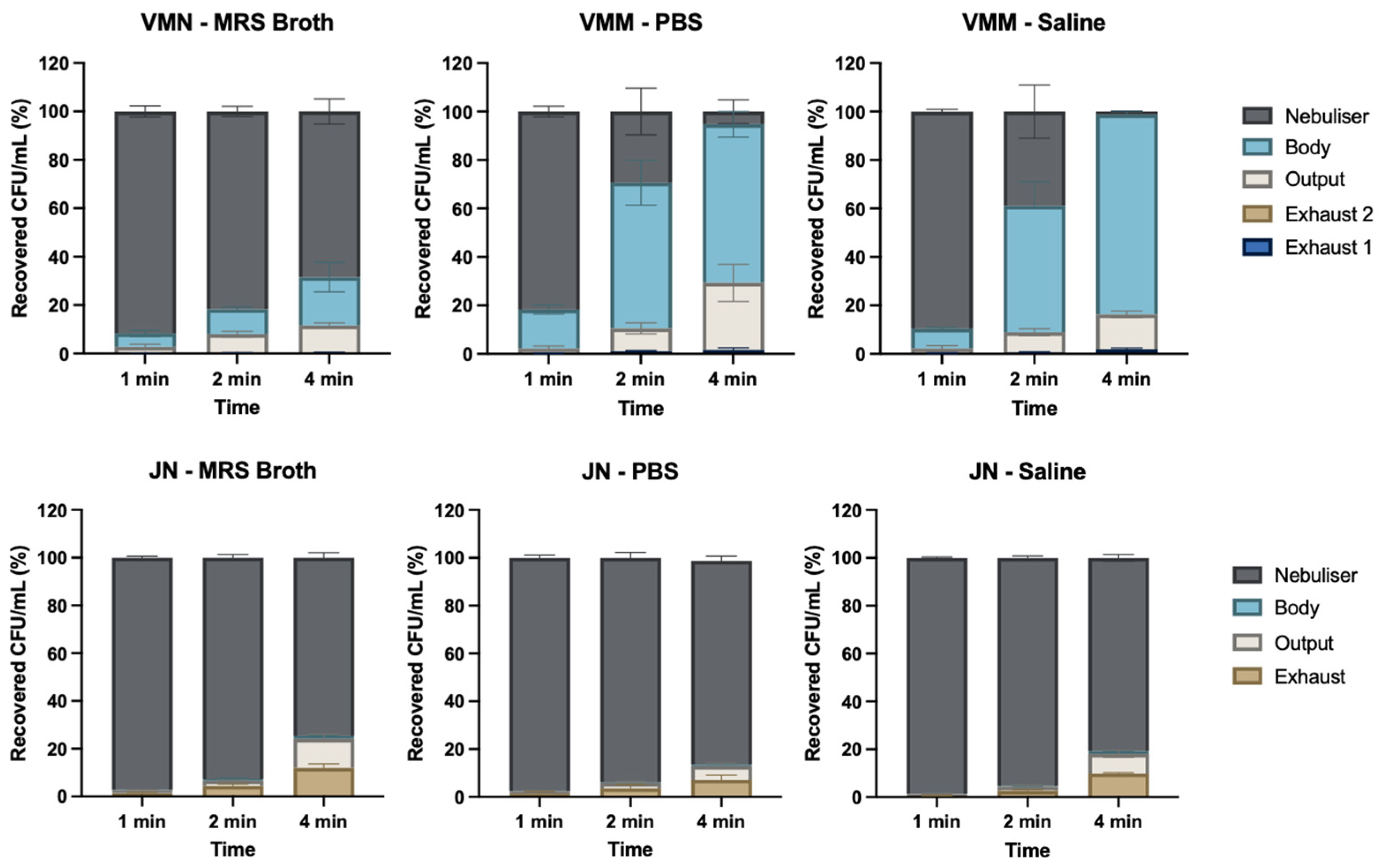
3.4. Timed Nebulisation
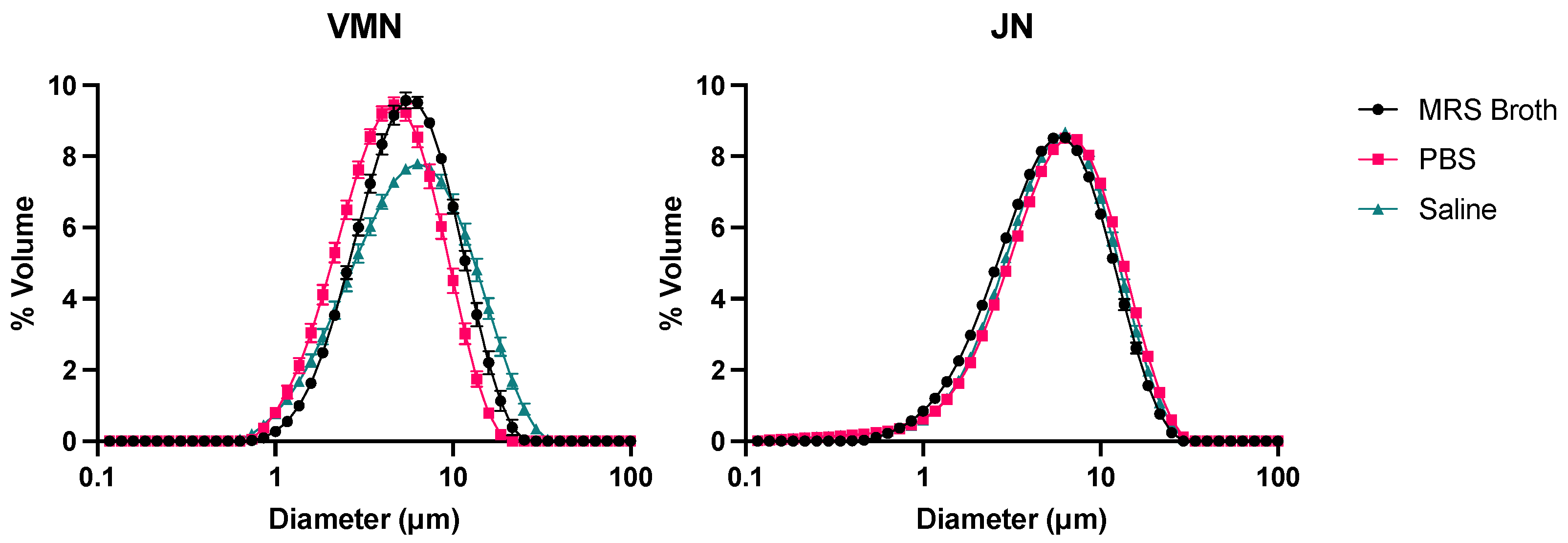
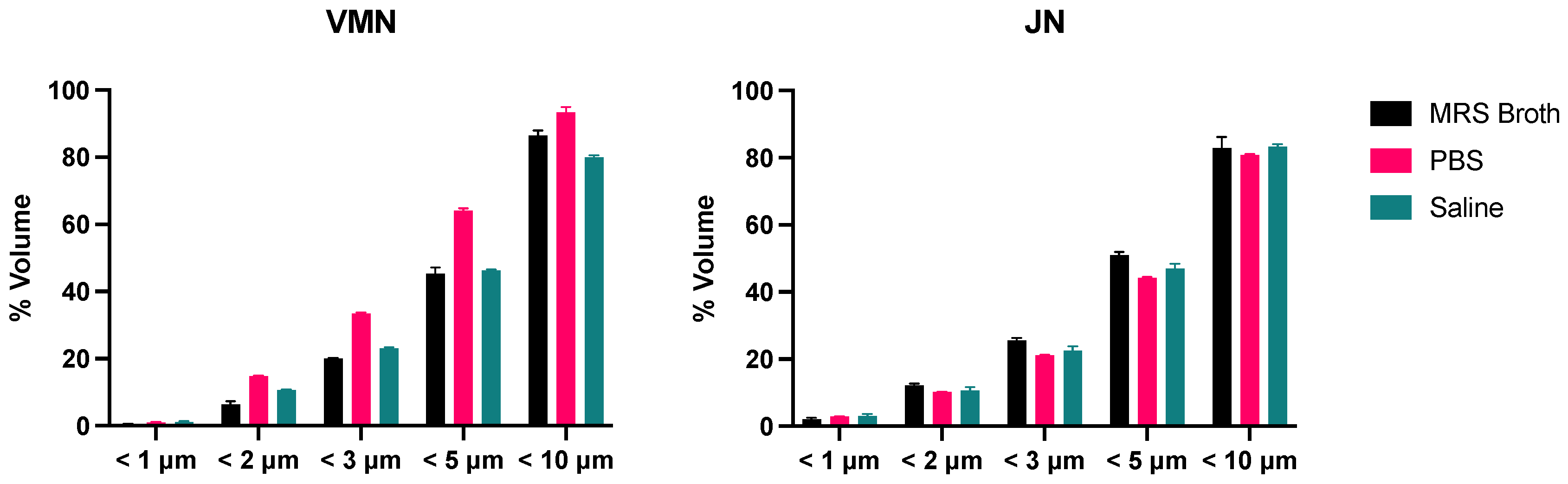
3.5. Laser Diffraction
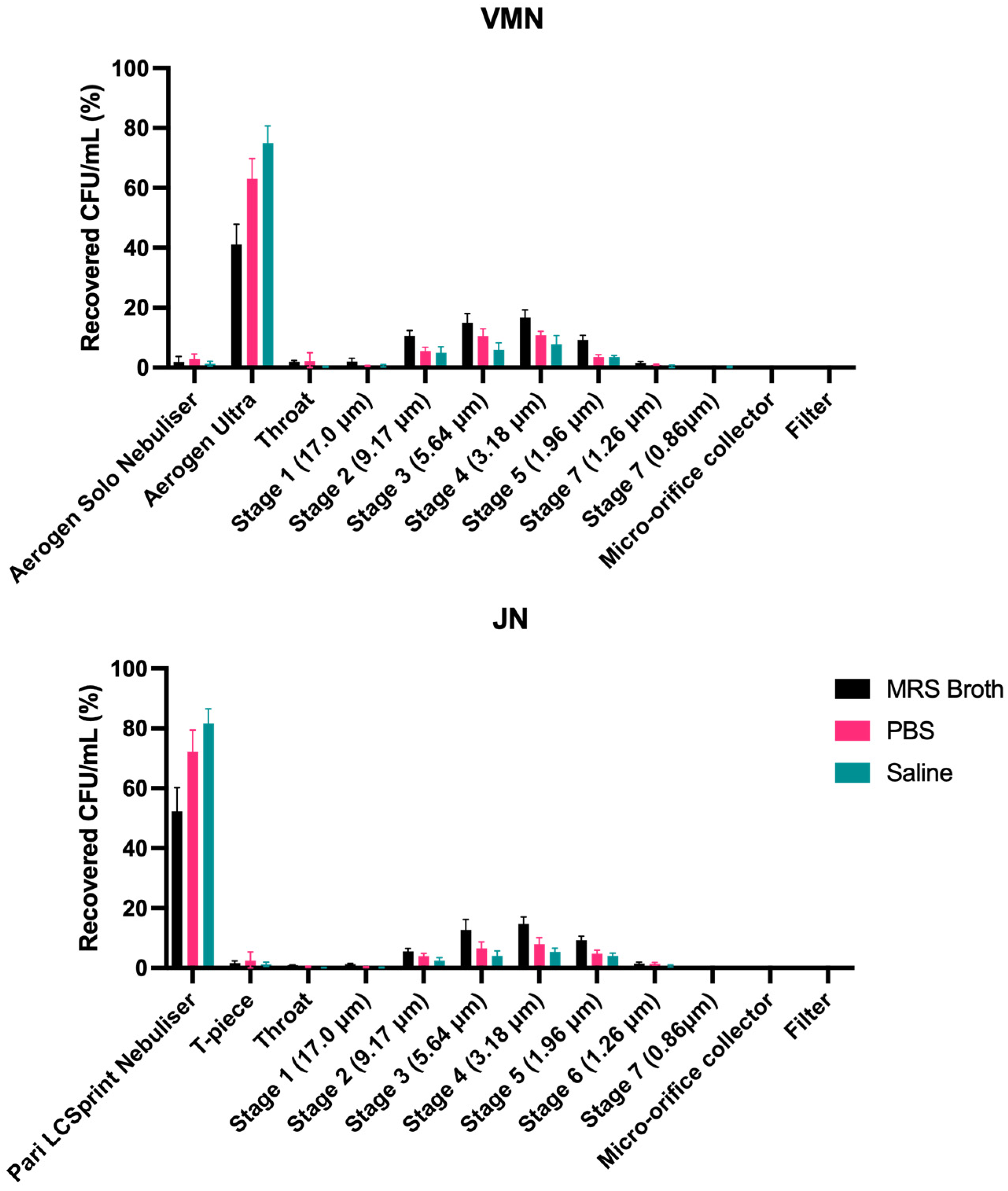
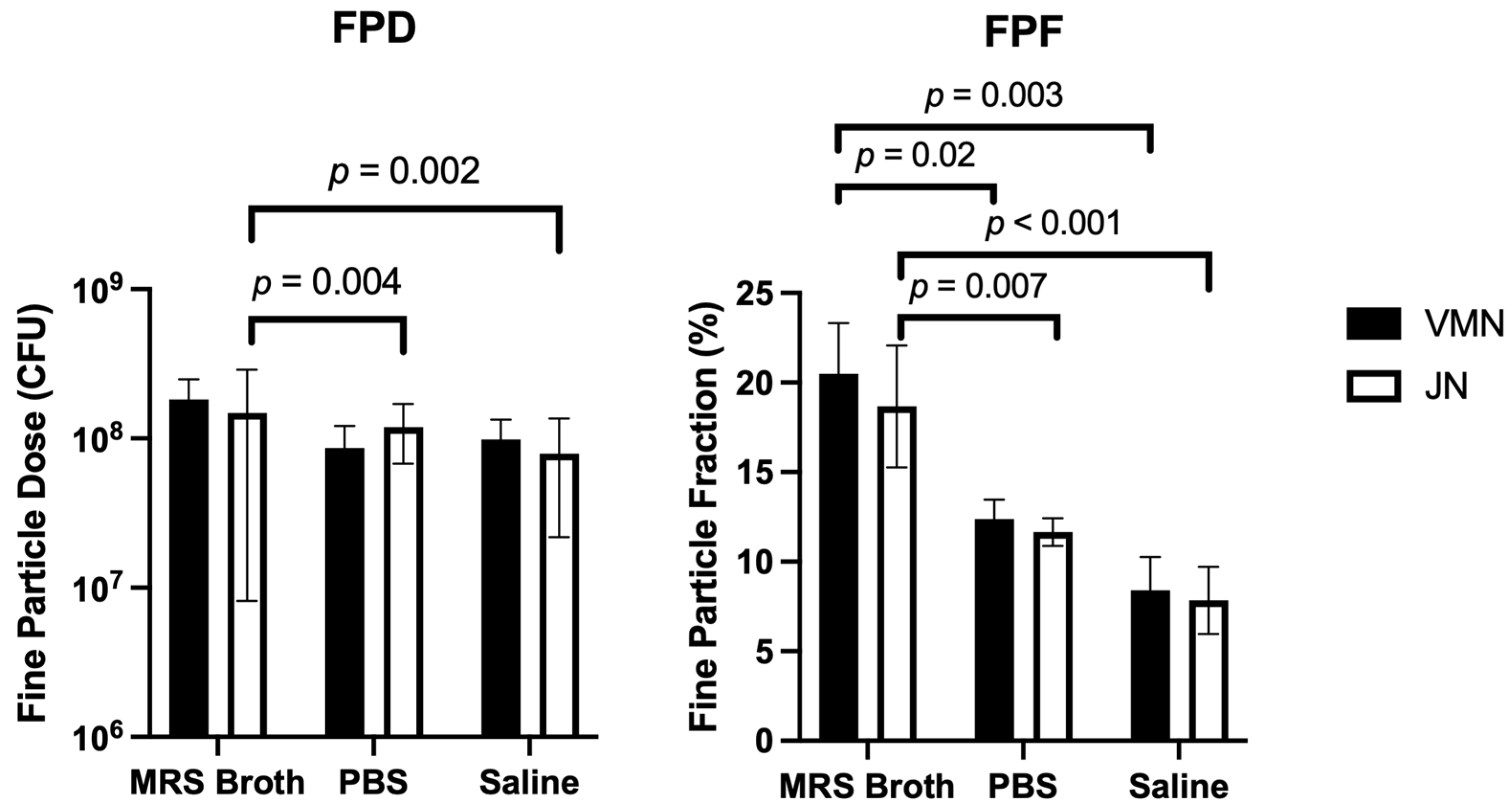
3.6. Cascade Impaction
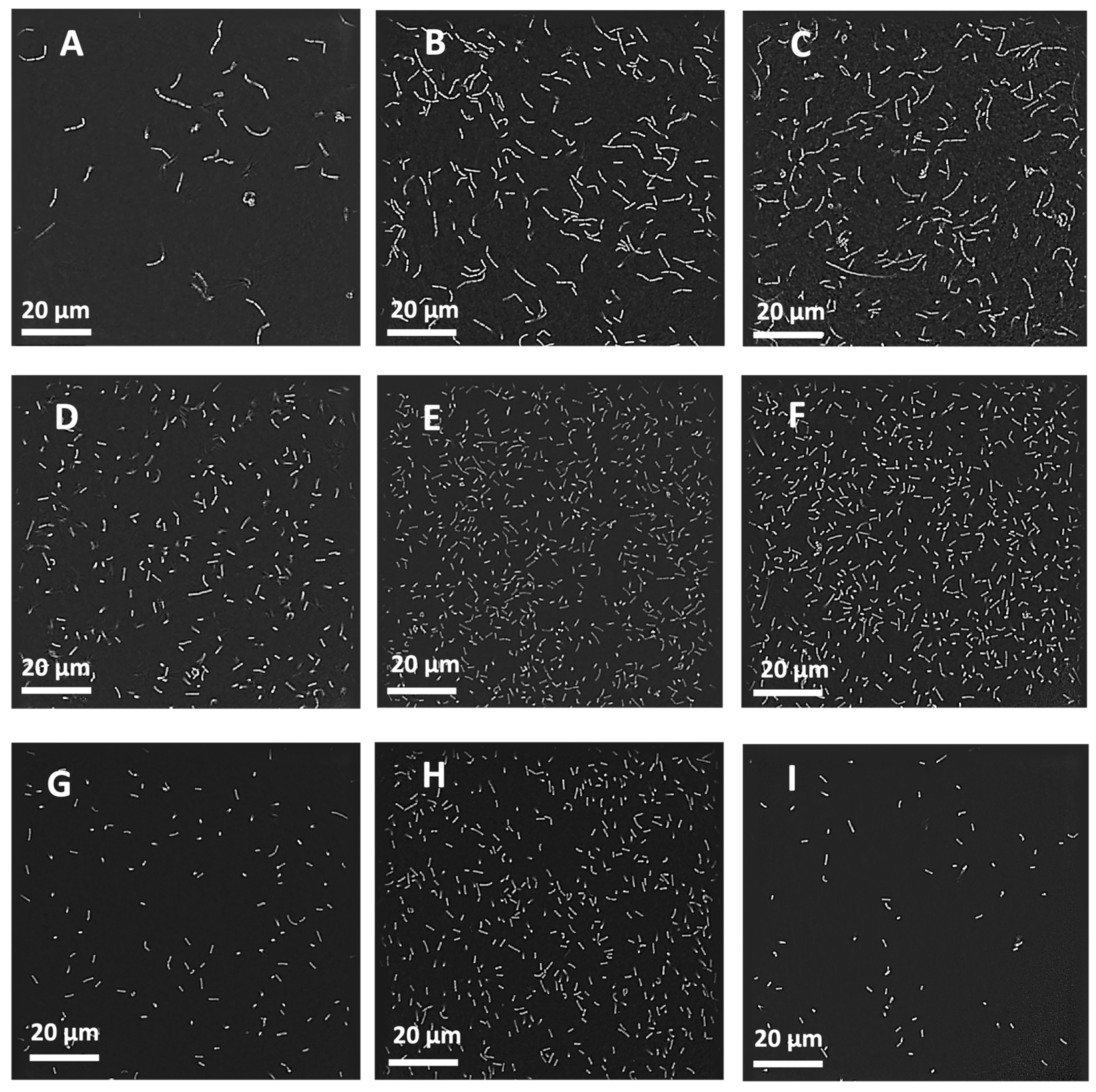
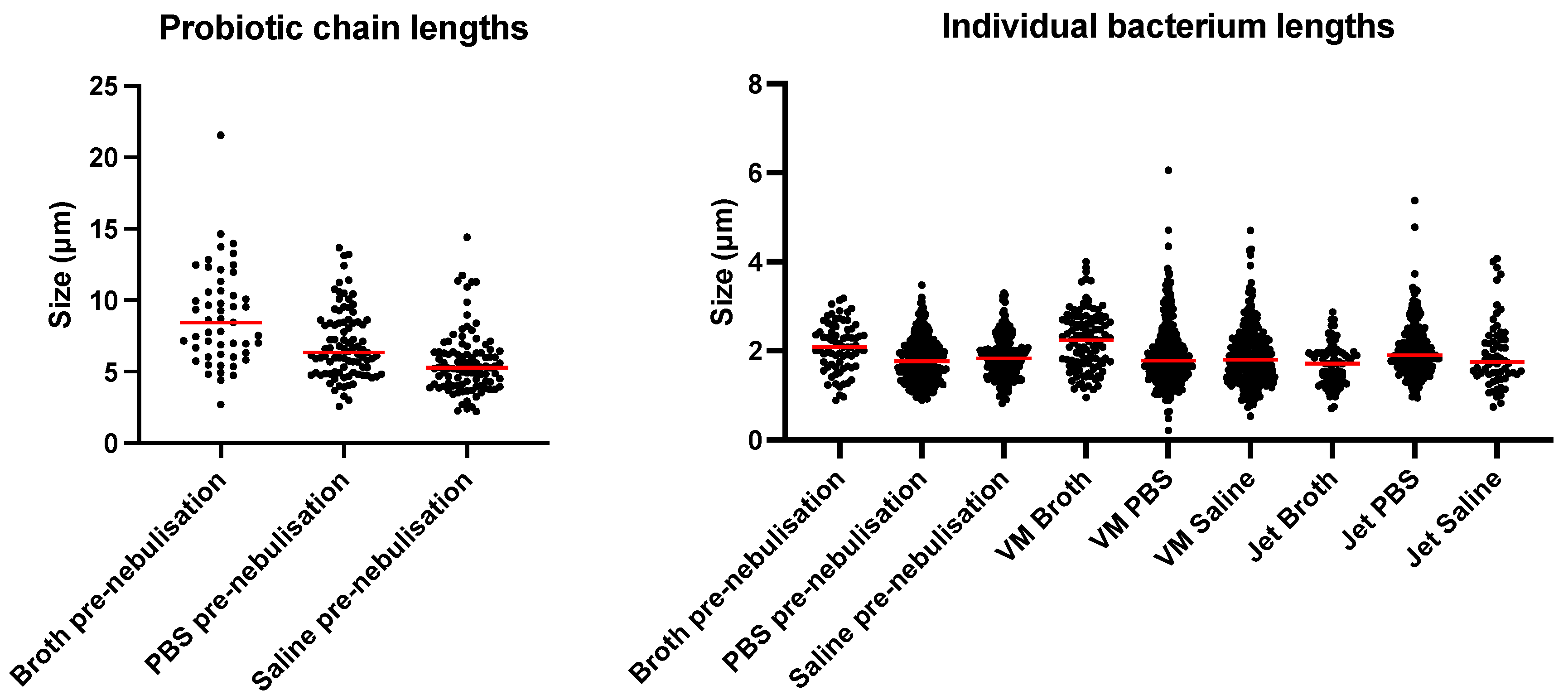
3.7. Real-Time Bacterial Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. eClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nair, H. Trends in the global burden of lower respiratory infections: The knowns and the unknowns. Lancet Infect. Dis. 2022, 22, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 5 November 2023).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Guitor, A.K.; Wright, G.D. Antimicrobial resistance and respiratory infections. Chest 2018, 154, 1202–1212. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Behzadi, M.; Leyva-Grado, V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. About Influenza Antiviral Medications. Available online: https://www.cdc.gov/flu/hcp/antivirals/index.html (accessed on 23 August 2023).
- Toussi, S.S.; Hammond, J.L.; Gerstenberger, B.S.; Anderson, A.S. Therapeutics for COVID-19. Nat. Microbiol. 2023, 8, 771–786. [Google Scholar]
- Esposito, S.; Amirthalingam, G.; Bassetti, M.; Blasi, F.; De Rosa, F.G.; Halasa, N.B.; Hung, I.; Osterhaus, A.; Tan, T.; Torres, J.P.; et al. Monoclonal antibodies for prophylaxis and therapy of respiratory syncytial virus, SARS-CoV-2, human immunodeficiency virus, rabies and bacterial infections: An update from the World Association of Infectious Diseases and Immunological Disorders and the Italian Society of Antinfective Therapy. Front. Immunol. 2023, 14, 1162342. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, R.; Mejias, A.; Ramilo, O. Monoclonal antibodies for prevention of respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2021, 40, S35–S39. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Pitkaranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, B.; Hao, Q. Probiotics for preventing acute upper respiratory tract infection. Cochrane Database Syst. Rev. 2022, 8, CD006895. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in treatment of viral respiratory infections and neuroinflammatory disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of probiotics, prebiotics and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Youn, H.; Lee, D.; Lee, Y.; Park, J.; Yuk, S.; Yang, S.; Lee, H.; Woo, S.; Kim, H.; Lee, J.; et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infections in mice. Antivir. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Ngo, V.; Kwon, Y.; Lee, Y.; Yoo, S.; Cho, Y.; Hong, S.; Hwang, H.; Ko, E.; Jung, Y.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innnate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, Y.; Ngo, V.; Cho, Y.; Ko, E.; Hong, S.; Kim, K.; Jang, J.; Oh, J.; Park, M.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.; Salva, S.; Kanmani, P.; Aguero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kybota, A.; Hiramatsu, M.; Yausi, H. Intranasal administraton of Lactobacillus rhamnosus GG protects mice from H1N1 infuenza virus infection by regulating respiratory immue responses. Lett. Apply Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef]
- Fangous, M.; Alexandre, Y.; Hymery, N.; Gouriou, S.; Arzur, D.; Le Blay, G.; Le Berre, R. Lactobacilli intra-tracheal administration protects from Pseudomonas aeruginosa pulmonary infection in mice-a proof of concept. Benef. Microbes 2019, 10, 893–900. [Google Scholar] [CrossRef]
- Fangous, M.; Gosset, P.; Galakhoff, N.; Gourious, S.; Guilloux, C.; Payan, C.; Vallet, S.; Hery-Arnaud, G.; Le Berre, R. Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice. BMC Microbiol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ibrahim, S.; Hayek, S. Recent application of probiotics in food and agricultural science. In Probiotics; Rigobelo, E., Ed.; Books on Demand: Norderstedt, Germay, 2012. [Google Scholar]
- Tran, D.; Tran, T.; Phung, T.; Bui, H.; Nguyen, P.; Vu, T.; Ngo, N.; Nguyen, M.; Nguyen, A.; Nguyen, A. Nasal-spraying Bacillus spores as an effective symptomatic treatment for children with acute respiratory syncytial virus infection. Sci. Rep. 2022, 12, 12402. [Google Scholar] [CrossRef]
- Endam, L.; Alromaih, S.; Gonzalez, E.; Madrenas, J.; Cousineau, B.; Renteria, A.; Desrosiers, M. Intranasal application of Lactococcus lactis w136 is safe in chronic rhinosinusitis patients with previous sinus surgery. Front. Cell Infect. Microbiol. 2020, 12, 440. [Google Scholar] [CrossRef]
- Tai, W.; Chow, M.; Chang, R.; Tang, P.; Gonda, I.; MacArthur, R.; Chan, H.; Kwok, P. Nebulised isotonic hydrochloroquine aerosols for potential treatment of COVID-19. Parmaceutics 2021, 13, 1260. [Google Scholar] [CrossRef]
- McGrath, J.; O’Sullivan, A.; Bennett, G.; O’Toole, C.; Joyce, M.; Byrne, M.A.; MacLoughlin, R. Investigation of the quality of exhaled aerosols released into the environment during nebulisation. Pharmaceutics 2019, 11, 75. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Barry, P.W. The science of nebulised drug delivery. Thorax 1997, 52, S31–S44. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; McConville, J.T. The function and performance of aqueous aerosol devices for inhalation therapy. J. Pharm. Pharmacol. 2016, 68, 556–578. [Google Scholar] [CrossRef] [PubMed]
- Sidler-Moix, A.; Di Paolo, E.R.; Dolci, U.; Berger-Gryllaki, M.; Cotting, J.; Pannatier, A. Physicochemical aspects and efficiency of albuterol nebulisation: Comparison of three aerosol types in an in vitro paediatric model. Respir. Care 2015, 60, 38–46. [Google Scholar] [CrossRef]
- Chang, K.H.; Moon, S.; Yoo, S.; Park, B.J.; Nam, K.C. Aerosol delivery of dornase alfa generated by jet and mesh nebulisers. Pharmaceutics 2020, 12, 721. [Google Scholar] [CrossRef]
- Carrigy, N.; Chang, R.; Leung, S.; Harrison, M.; Petrova, Z.; Pope, W.; Hatfull, G.; Britton, W.; Chan, H.; Sauvageau, D.; et al. Anti-tuberculosis bacteriophage D29 delivery with a vibrating mesh nebuliser, jet nebuliser, and soft mist inhaler. Pharm. Res. 2017, 34, 2084–2096. [Google Scholar] [CrossRef]
- Steckel, H.; Eskandar, F. Factors affecting aerosol performance during nebulisation with jet and ultrasonic nebulisers. Eur. J. Pharm. Sci. 2003, 19, 443–455. [Google Scholar] [CrossRef] [PubMed]
- McCallion, O.N.M.; Taylor, K.M.G.; Thomas, M.; Taylor, A.J. Nebulisation of fluids of different physicochemical properties with air-jet and ultrasonic nebulisers. Pharm. Res. 1995, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- McCallion, O.N.M.; Taylor, K.M.G.; Thomas, M.; Taylor, A.J. The influence of surface tension on aerosols produced by medical nebulisers. Int. J. Pharm. 1996, 129, 123–136. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yeom, E.; Lee, S. A microfluidic device for simultaneous measurement of viscosity and flow rate of blood in a complex fluidic network. Biomicrofluidics 2013, 7, 054111. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Elhissi, A.M.A.; Ding, Z.; Taylor, K.M.G. The influence of fluid physicochemical properties on vibrating-mesh nebulisation. Int. J. Pharm. 2007, 339, 103–111. [Google Scholar] [CrossRef]
- Najlah, M.; Vali, A.; Taylor, M.; Arafat, B.T.; Ahmed, W.; Phoenix, D.A.; Taylor, K.M.G.; Elhissi, A.M.A. A study of the effects of sodium halides on the performance of air-jet and vibrating-mesh nebulisers. Int. J. Pharm. 2013, 456, 520–527. [Google Scholar] [CrossRef]
- Byun, A.S.; Vitetta, L.; Chan, H.; Kwok, P. Respiratory Delivery of Probiotics to Improve Lung Health. In Respiratory Delivery of Biologics, Nucleic Acids, and Vaccines; Lam, J.K.W., Kwok, P., Eds.; AAPS Introductions in the Pharmaceutical Sciences; Springer: Cham, Switzerland, 2023; Volume 8. [Google Scholar]
- Glieca, S.; Quarta, E.; Bottari, B.; Bancalari, E.; Monica, S.; Scaltriti, E.; Tambassi, M.; Flammini, L.; Bertoni, S.; Buttini, F. Development of inhalation powders containing lactic acid bacteria with antimicrobial activity against Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2024, 63, 107001. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Cheow, W.S.; Pu, S.; Park, J.-W.; Hadinoto, K. Dry powder inhaler formulation of Lactobacillus rhamnosus GG targeting Pseudomonas aeruginosa infection in bronchiectasis maintenance therapy. Pharmaceutics 2024, 16, 980. [Google Scholar] [CrossRef]
- Astudillo, A.; Leung, S.; Kutter, E.; Morales, S.; Chan, H. Nebulisation effects on structural stability of bacteriophage PEV 44. Eur. J. Pharm. Biopharm. 2018, 125, 124–130. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Zhao, Q.; Fu, N.; Xiong, H.; Wu, W.; Chen, X. Spray drying of Lactobacillus rhamnosus GG with calcium-containing protectant for enhanced viability. Powder Technol. 2019, 358, 87–94. [Google Scholar] [CrossRef]
- Liao, C.; Shollenberger, L.M. Survivability and long-term preservation of bacteria in water and in phosphate buffered saline. Lett. Appl. Microbiol. 2003, 37, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Desager, K.N.; Van Bever, H.P.; Stevens, W.J. Osmolality and pH of anti-asthmatic drug solutions. Agents Actions 1990, 31, 225–228. [Google Scholar] [CrossRef]
- Law, S. Stability of preservative-free tobramycin in half-normal saline. Can. J. Hosp. Pharm. 2001, 54, 214–215. [Google Scholar]
- Markowiak-Kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.; Bidani, A.; Heming, T.A. Innate host defense of the lung: Effects of lung lining fluid pH. Lung 2004, 182, 297–317. [Google Scholar] [CrossRef]
- Respaud, R.; Marchand, D.; Parent, C.; Pelat, T.; Thullier, P.; Thournamille, J.; Viaud-Massuard, M.; Diot, P.; Si-Tahar, M.; Vecellio, L.; et al. Effect of formulation on the stability and aerosol performance of a nebulised antibody. mAbs 2014, 6, 1347–1355. [Google Scholar] [CrossRef]
- Gaston, B.; Kelly, R.; Urban, P.; Liu, L.; Henderson, E.M.; Doctor, A.; Teague, W.G.; Fitzpatrick, A.; Erzurum, S.; Hunt, J.F. Buffering airway acid decreases exhaled nitric oxide in asthma. J. Allergy Clin. Immunol. 2006, 118, 817–822. [Google Scholar] [CrossRef]
| Type of Nebuliser | MRS Broth | PBS | Saline |
|---|---|---|---|
| VMN | 9 min 1 s ± 28 s | 4 min 5 s ± 3 s | 4 min 3 s ± 13 s |
| JN | 8 min 0 s ± 16 s | 8 min 2 s ± 24 s | 7 min 40 s ± 23 s |
| VMN | JN | |||||
|---|---|---|---|---|---|---|
| Volumetric Diameter | MRS Broth | PBS | Saline | MRS Broth | PBS | Saline |
| D10 (μm) | 2.2 ± 0.0 | 1.7 ± 0.0 | 1.9 ± 0.0 | 1.8 ± 0.1 | 2.0 ± 0. | 1.9 ± 0.1 |
| D50 (μm) | 5.5 ± 0.1 | 4.0 ± 0.0 | 5.4 ± 0.0 | 4.9 ± 0.1 | 5.6 ± 0.0 | 5.3 ± 0.1 |
| D90 (μm) | 11.1 ± 0.5 | 8.8 ± 0.4 | 13.4 ± 0.2 | 11.3 ± 0.3 | 12.7 ± 0.1 | 12.0 ± 0.2 |
| Span | 1.7 ± 0.0 | 1.8 ± 0.1 | 2.1 ± 0.0 | 1.9 ± 0.0 | 1.9 ± 0.0 | 1.9 ± 0.0 |
| GSD | 1.8 ± 0.0 | 1.9 ± 0.2 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.1 |
| Suspension Media | MRS Broth | PBS | Saline | |||
|---|---|---|---|---|---|---|
| Fine Particle Parameters | FPD (CFU) | FPF (%) | FPD (CFU) | FPF (%) | FPD (CFU) | FPF (%) |
| VMN | 1.8 × 108 ± 6.7 × 107 | 20.5 ± 2.8 | 8.6 × 107 ± 3.5 × 107 | 12.4 ± 1.1 | 9.9 × 107 ± 3.5 × 107 | 8.4 ± 1.9 |
| JN | 1.5 × 108 ± 1.4 × 108 | 18.7 ± 3.4 | 1.9 × 108 ± 5.1 × 107 | 11.7 ± 0.8 | 7.9 × 107 ± 5.7 × 107 | 7.8 ± 1.9 |
| Lengths of L. rhamnosus GG Chains and Individual Bacterium (μm) | |||
|---|---|---|---|
| Suspension Media | Broth | PBS | Saline |
| Before nebulisation (chains) | 8.4 ± 3.4 | 6.9 ± 2.4 | 5.6 ± 2.2 |
| Before nebulisation (individual bacterium) | 2.8 ± 0.6 | 1.8 ± 0.5 | 1.9 ± 0.5 |
| After vibrating-mesh nebulisation | 1.7 ± 0.5 | 2.0 ± 0.6 | 1.9 ± 0.8 |
| After jet nebulisation | 2.2 ± 0.7 | 1.9 ± 0.8 | 1.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, A.S.; Vitetta, L.; Chan, H.-K.; Kwok, P.C.L. Respiratory Delivery of Lacticaseibacillus rhamnosus GG by Vibrating-Mesh and Jet Nebulisation. Pharmaceutics 2024, 16, 1326. https://doi.org/10.3390/pharmaceutics16101326
Byun AS, Vitetta L, Chan H-K, Kwok PCL. Respiratory Delivery of Lacticaseibacillus rhamnosus GG by Vibrating-Mesh and Jet Nebulisation. Pharmaceutics. 2024; 16(10):1326. https://doi.org/10.3390/pharmaceutics16101326
Chicago/Turabian StyleByun, Alex Seungyeon, Luis Vitetta, Hak-Kim Chan, and Philip Chi Lip Kwok. 2024. "Respiratory Delivery of Lacticaseibacillus rhamnosus GG by Vibrating-Mesh and Jet Nebulisation" Pharmaceutics 16, no. 10: 1326. https://doi.org/10.3390/pharmaceutics16101326
APA StyleByun, A. S., Vitetta, L., Chan, H.-K., & Kwok, P. C. L. (2024). Respiratory Delivery of Lacticaseibacillus rhamnosus GG by Vibrating-Mesh and Jet Nebulisation. Pharmaceutics, 16(10), 1326. https://doi.org/10.3390/pharmaceutics16101326






