Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
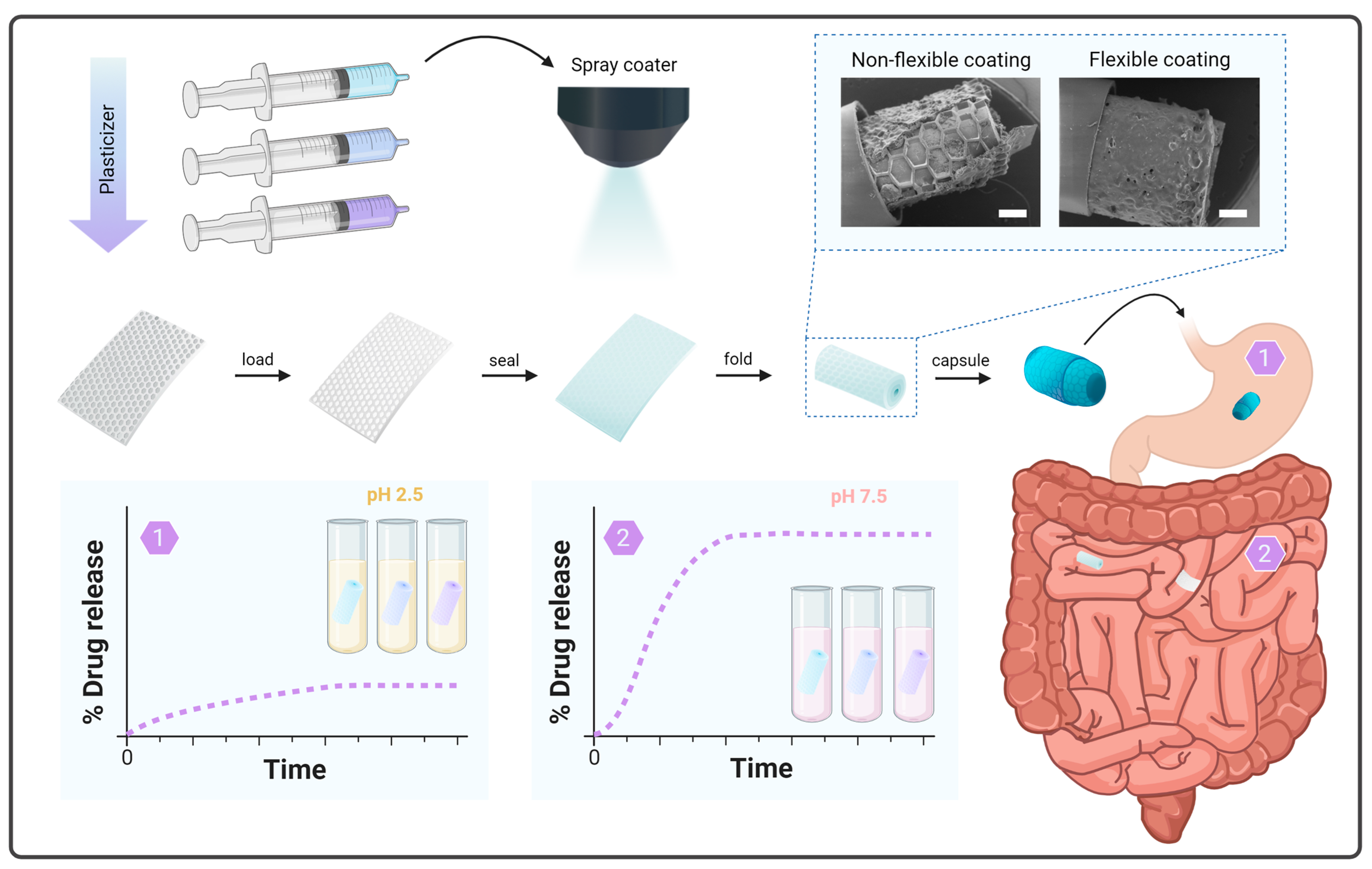
2.2. Fabrication of PDMS Foils
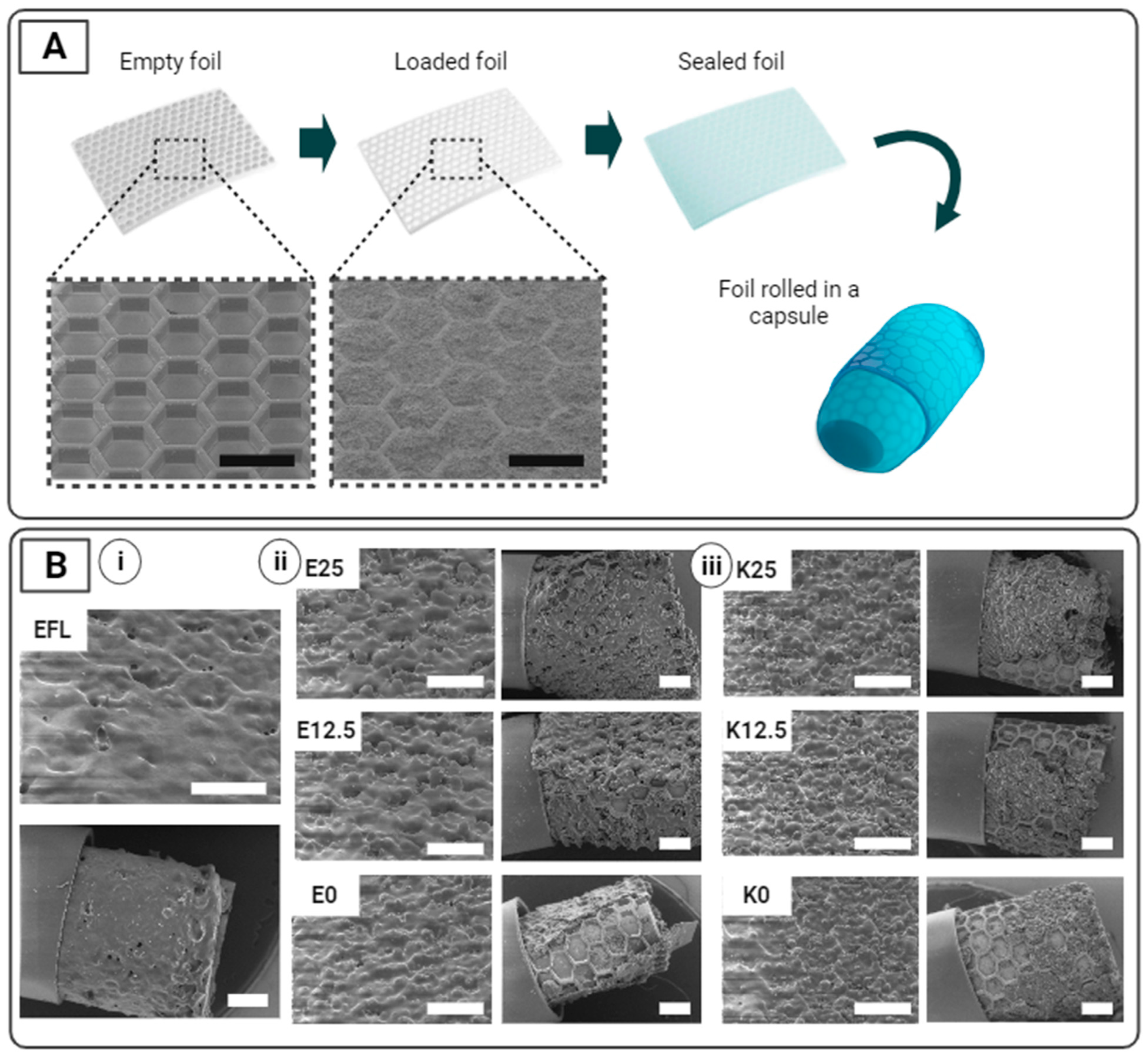
2.3. Preparation and Incorporation of SUFs into a Capsule
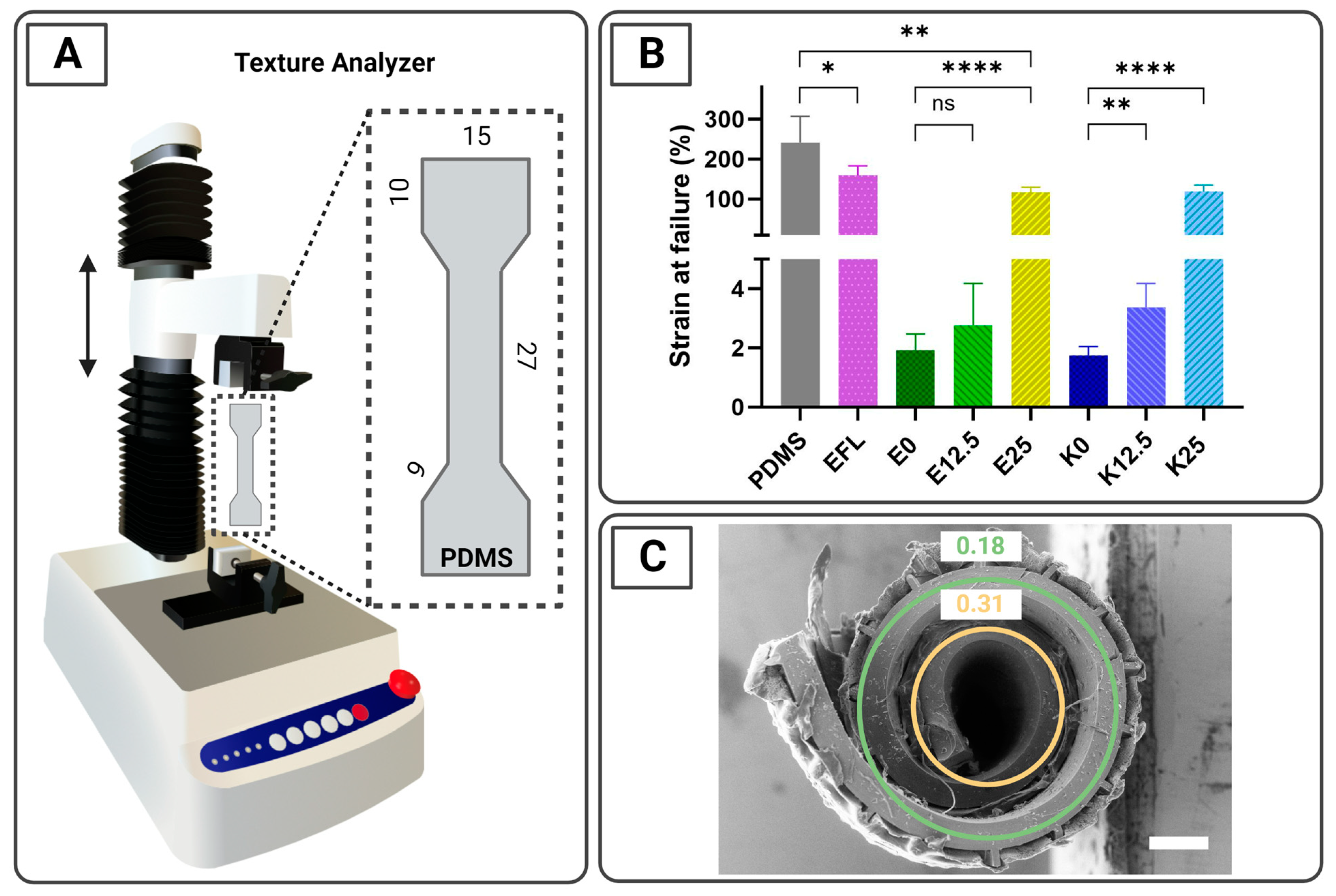
2.4. Tensile Characterization of the Coatings Using a Texture Analyzer
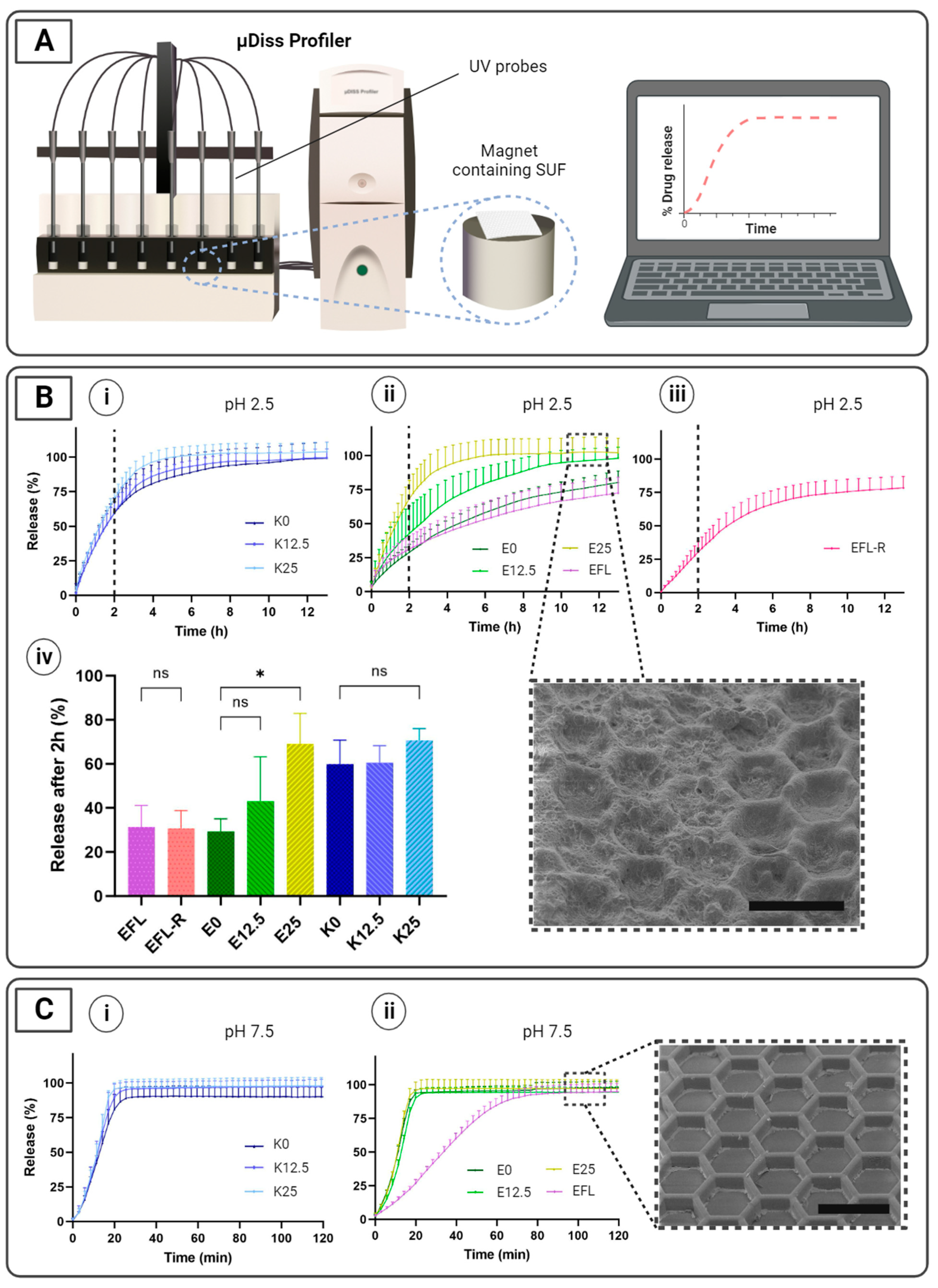
2.5. In Vitro Release Studies
2.6. Data Treatment
3. Results and Discussion
3.1. Preparation and Incorporation of SUFs into a Capsule
3.2. Tensile Characterization of the Coatings Using a Texture Analyzer
3.3. In Vitro Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Z.; Paunović, N.; Leroux, J.C. Physical Methods for Enhancing Drug Absorption from the Gastrointestinal Tract. Adv. Drug Deliv. Rev. 2021, 175, 113814. [Google Scholar] [CrossRef]
- Murakami, T. Absorption Sites of Orally Administered Drugs in the Small Intestine. Expert Opin. Drug Discov. 2017, 12, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kou, Y.; Zhang, X.; Cheng, H.; Chen, X.; Mao, S. Strategies and Industrial Perspectives to Improve Oral Absorption of Biological Macromolecules. Expert Opin. Drug Deliv. 2017, 15, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Toward Oral Delivery of Biopharmaceuticals: An Assessment of the Gastrointestinal Stability of 17 Peptide Drugs. Mol. Pharm. 2015, 12, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Han, L.; Xia, S.; Zhang, X.; Xue, P.; Kang, Y.; Ming, J.; Xu, Z. Microenvironment Responsive Pod-Structured Astaxanthin Nanocarrier for Ameliorating Inflammatory Bowel Disease. Chin. Chem. Lett. 2023, 109029. [Google Scholar] [CrossRef]
- Rump, A.; Kromrey, M.L.; Scheuch, E.; Jannin, V.; Rehenbrock, L.; Tzvetkov, M.V.; Weitschies, W.; Grimm, M. In Vivo Evaluation of a Gastro-Resistant HPMC-Based “Next Generation Enteric” Capsule. Pharmaceutics 2022, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Zhang, L.; Ma, Y.; Zhu, J.; Xiao, W. A Novel Electrostatic Dry Coating Process for Enteric Coating of Tablets with Eudragit® L100-55. Eur. J. Pharm. Biopharm. 2013, 83, 293–300. [Google Scholar] [CrossRef]
- Cui, C.; Sun, J.; Wang, X.; Yu, Z.; Shi, Y. Factors Contributing to Drug Release From Enteric-Coated Omeprazole Capsules: An In Vitro and In Vivo Pharmacokinetic Study and IVIVC Evaluation in Beagle Dogs. Dose-Response 2020, 18, 1559325820908980. [Google Scholar] [CrossRef]
- Amaral Silva, D.; Gomes Davanço, M.; Davies, N.M.; Krämer, J.; de Oliveira Carvalho, P.; Löbenberg, R. Physiologically Relevant Dissolution Conditions towards Improved In Vitro-In Vivo Relationship—A Case Study with Enteric Coated Pantoprazole Tablets. Int. J. Pharm. 2021, 605, 120857. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Thumma, S.; Chen, G.H.; Deng, W.B.; Repka, M.A.; Li, S.M. In Vitro and In Vivo Evaluation of Tegaserod Maleate PH-Dependent Tablets. Eur. J. Pharm. Biopharm. 2008, 69, 247–254. [Google Scholar] [CrossRef]
- Christfort, J.F.; Strindberg, S.; Al-khalili, S.; Bar-Shalom, D.; Boisen, A.; Nielsen, L.H.; Müllertz, A. In Vitro and In Vivo Comparison of Microcontainers and Microspheres for Oral Drug Delivery. Int. J. Pharm. 2021, 600, 120516. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Broekhuizen, C.A.N.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Transport of Octreotide and Evaluation of Mechanism of Opening the Paracellular Tight Junctions Using Superporous Hydrogel Polymers in Caco-2 Cell Monolayers. J. Pharm. Sci. 2004, 93, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Evaluation of Superporous Hydrogel (SPH) and SPH Composite in Porcine Intestine Ex-Vivo: Assessment of Drug Transport, Morphology Effect, and Mechanical Fixation to Intestinal Wall. Eur. J. Pharm. Biopharm. 2002, 53, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Verhoef, J.C.; Verheijden, J.H.M.; Rafiee-Tehrani, M.; Borchard, G.; Junginger, H.E. Peroral Absorption of Octreotide in Pigs Formulated in Delivery Systems on the Basis of Superporous Hydrogel Polymers. Pharm. Res. 2002, 19, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Setyaningsih, D.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Effects of Superporous Hydrogels on Paracellular Drug Permeability and Cytotoxicity Studies in Caco-2 Cell Monolayers. Int. J. Pharm. 2002, 241, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Verhoef, J.C.; Borchard, G.; Rafiee-Tehrani, M.; Verheijden, J.H.M.; Junginger, H.E. Intestinal Absorption of Human Insulin in Pigs Using Delivery Systems Based on Superporous Hydrogel Polymers. Int. J. Pharm. 2002, 247, 47–55. [Google Scholar] [CrossRef]
- Dorkoosh, F.A.; Stokkel, M.P.M.; Blok, D.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Feasibility Study on the Retention of Superporous Hydrogel Composite Polymer in the Intestinal Tract of Man Using Scintigraphy. J. Control. Release 2004, 99, 199–206. [Google Scholar] [CrossRef]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular Stomach Absorption of a Derivatized Glucagon-like Peptide-1 Receptor Agonist. Sci. Transl. Med. 2018, 10, eaar7047. [Google Scholar] [CrossRef]
- Maher, S.; Brayden, D.J. Formulation Strategies to Improve the Efficacy of Intestinal Permeation Enhancers. Adv. Drug Deliv. Rev. 2021, 177, 113925. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-salvador, E.; Soares, V.; Minahan, D.; Tian, R.Y.; Lu, X.; Dellal, D.; Gao, Y.; Kim, S.; Wainer, J.; et al. A Luminal Unfolding Microneedle Injector for Oral Delivery of Macromolecules. Nat. Med. 2019, 25, 1512–1518. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubálek, F.; Water, J.J.; et al. An Ingestible Self-Orienting System for Oral Delivery of Macromolecules. Science 2019, 363, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Abramson, A.; Frederiksen, M.R.; Vegge, A.; Jensen, B.; Poulsen, M.; Mouridsen, B.; Jespersen, M.O.; Kirk, R.K.; Windum, J.; Hubálek, F.; et al. Oral Delivery of Systemic Monoclonal Antibodies, Peptides and Small Molecules Using Gastric Auto-Injectors. Nat. Biotechnol. 2022, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.; Korupolu, R.; Syed, B.; Horlen, K.; Beraki, S.; Karamchedu, P.; Dhalla, A.K.; Ruffy, R.; Imran, M. Jejunal Wall Delivery of Insulin via an Ingestible Capsule in Anesthetized Swine—A Pharmacokinetic and Pharmacodynamic Study. Pharmacol. Res. Perspect. 2019, 7, e00522. [Google Scholar] [CrossRef] [PubMed]
- Home—Rani Therapeutics. Available online: https://www.ranitherapeutics.com/ (accessed on 26 December 2023).
- Dhalla, A.K.; Al-Shamsie, Z.; Beraki, S.; Dasari, A.; Fung, L.C.; Fusaro, L.; Garapaty, A.; Gutierrez, B.; Gratta, D.; Hashim, M.; et al. A Robotic Pill for Oral Delivery of Biotherapeutics: Safety, Tolerability, and Performance in Healthy Subjects. Drug Deliv. Transl. Res. 2022, 12, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.R.; Jepsen, M.L.; Nielsen, L.H.; Dufva, M.; Nielsen, H.M.; Rades, T.; Boisen, A.; Müllertz, A. Microcontainers for Oral Insulin Delivery—In Vitro Studies of Permeation Enhancement. Eur. J. Pharm. Biopharm. 2019, 143, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.R.; Thamdrup, L.H.E.; Kamguyan, K.; Nielsen, L.H.; Nielsen, H.M.; Boisen, A.; Rades, T.; Müllertz, A. Design of a Self-Unfolding Delivery Concept for Oral Administration of Macromolecules. J. Control. Release 2021, 329, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Pedersen, J.; Bech, R.; Kristian, A.; Alstrup, O.; Zhang, Z.; Koulianou, V.; Palmfeldt, J.; Vorup-jensen, T.; Thamdrup, E.; et al. A Self-Unfolding Proximity Enabling Device for Oral Delivery of Macromolecules. J. Control. Release 2023, 361, 40–52. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Auffret, A.D.; Humphrey, M.J.; Stevens, H.N.; Eccleston, G.M. In Vitro Drug Release Studies of Polymeric Freeze-Dried Wafers and Solvent-Cast Films Using Paracetamol as a Model Soluble Drug. Int. J. Pharm. 2009, 378, 66–72. [Google Scholar] [CrossRef]
- Obeidat, W.M.; Nokhodchi, A.; Alkhatib, H. Evaluation of Matrix Tablets Based on Eudragit® E100/Carbopol® 971P Combinations for Controlled Release and Improved Compaction Properties of Water Soluble Model Drug Paracetamol. AAPS PharmSciTech 2015, 16, 1169–1179. [Google Scholar] [CrossRef]
- Osman, Z.; Farah, Y.; Ali Hassan, H.; Elsayed, S. Comparative Physicochemical Evaluation of Starch Extracted from Pearl Millet Seeds Grown in Sudan as a Pharmaceutical Excipient against Maize and Potato Starch, Using Paracetamol as a Model Drug. Ann. Pharm. Françaises 2021, 79, 28–35. [Google Scholar] [CrossRef]
- Serrati, S.; Martinelli, C.; Palazzo, A.; Iacobazzi, R.M.; Perrone, M.; Ong, Q.K.; Luo, Z.; Bekdemir, A.; Pinto, G.; Cavalleri, O.; et al. Reproducibility Warning: The Curious Case of Polyethylene Glycol 6000 and Spheroid Cell Culture. PLoS ONE 2020, 15, e0224002. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.V.; Suhail, S.; Rose, J.; Vella, T.; Nair, L.S. Polymers and Composites for Orthopedic Applications. Mater. Devices Bone Disord. 2017, 8, 349–403. [Google Scholar] [CrossRef]
- Katz, S.; Lachman, N.; Hafif, N.; Rosh, L.; Pevzner, A.; Lybman, A.; Amitay-Rosen, T.; Nir, I.; Rotter, H. Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica. Polymers 2023, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic Surface Modification of Pdms for Droplet Microfluidics Using a Simple, Quick, and Robust Method via Pva Deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef] [PubMed]
- DiNunzio, J.; Forster, S.; Brown, C. Melt Extrusion: Pharmaceutical Applications. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2017; pp. 827–844. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Tran, P.H.; Tran, T.T. A Single Layer Film Coating for Colon-Targeted Oral Delivery. Int. J. Pharm. 2019, 25, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Marizza, P.; Keller, S.S.; Müllertz, A.; Boisen, A. Polymer-Filled Microcontainers for Oral Delivery Loaded Using Supercritical Impregnation. J. Control. Release 2013, 173, 1–9. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Melero, A.; Keller, S.S.; Jacobsen, J.; Garrigues, T.; Rades, T.; Müllertz, A.; Boisen, A. Polymeric Microcontainers Improve Oral Bioavailability of Furosemide. Int. J. Pharm. 2016, 504, 98–109. [Google Scholar] [CrossRef]
- Abid, Z.; Strindberg, S.; Javed, M.M.; Mazzoni, C.; Vaut, L.; Nielsen, L.H.; Gundlach, C.; Petersen, R.S.; Müllertz, A.; Boisen, A.; et al. Biodegradable Microcontainers-towards Real Life Applications of Microfabricated Systems for Oral Drug Delivery. Lab Chip 2019, 19, 2905–2914. [Google Scholar] [CrossRef]
- Christfort, J.F.; Strindberg, S.; Plum, J.; Hall-Andersen, J.; Janfelt, C.; Nielsen, L.H.; Müllertz, A. Developing a Predictive In Vitro Dissolution Model Based on Gastrointestinal Fluid Characterisation in Rats. Eur. J. Pharm. Biopharm. 2019, 142, 307–314. [Google Scholar] [CrossRef]
- Müller-Albers, J.; Guha, A.; Assmus, M. Use of an Advanced New Enteric Combination Polymer with Multiple Unit Pellet Systems and Other Multiparticulates. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/357688-Use-of-an-Advanced-New-Enteric-Combination-Polymer-with-Multiple-Unit-Pellet-Systems-and-other-Multiparticulates/ (accessed on 13 September 2023).
- Zhang, S.; Ge, C.; Liu, R. Mechanical Characterization of the Stress-Strain Behavior of the Polydimethylsiloxane (PDMS) Substate of Wearable Strain Sensors under Uniaxial Loading Conditions. Sens. Actuators A Phys. 2022, 341, 113580. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EU) No 231/2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012R0231 (accessed on 27 November 2023).
- Leonardi, D.; Barrera, M.G.; Lamas, M.C.; Salomón, C.J. Development of Prednisone: Polyethylene Glycol 6000 Fast-Release Tablets from Solid Dispersions: Solid-State Characterization, Dissolution Behavior, and Formulation Parameters. AAPS PharmSciTech 2007, 8, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, C.; Tentor, F.; Strindberg, S.A.; Nielsen, L.H.; Keller, S.S.; Alstrøm, T.S.; Gundlach, C.; Müllertz, A.; Marizza, P.; Boisen, A. From Concept to In Vivo Testing: Microcontainers for Oral Drug Delivery. J. Control. Release 2017, 268, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Granberg, R.A.; Rasmuson, Å.C. Solubility of Paracetamol in Pure Solvents. J. Chem. Eng. Data 1999, 44, 1391–1395. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milián-Guimerá, C.; De Vittorio, L.; McCabe, R.; Göncü, N.; Krishnan, S.; Thamdrup, L.H.E.; Boisen, A.; Ghavami, M. Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery. Pharmaceutics 2024, 16, 81. https://doi.org/10.3390/pharmaceutics16010081
Milián-Guimerá C, De Vittorio L, McCabe R, Göncü N, Krishnan S, Thamdrup LHE, Boisen A, Ghavami M. Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery. Pharmaceutics. 2024; 16(1):81. https://doi.org/10.3390/pharmaceutics16010081
Chicago/Turabian StyleMilián-Guimerá, Carmen, Laura De Vittorio, Reece McCabe, Nuray Göncü, Samvrta Krishnan, Lasse Højlund Eklund Thamdrup, Anja Boisen, and Mahdi Ghavami. 2024. "Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery" Pharmaceutics 16, no. 1: 81. https://doi.org/10.3390/pharmaceutics16010081
APA StyleMilián-Guimerá, C., De Vittorio, L., McCabe, R., Göncü, N., Krishnan, S., Thamdrup, L. H. E., Boisen, A., & Ghavami, M. (2024). Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery. Pharmaceutics, 16(1), 81. https://doi.org/10.3390/pharmaceutics16010081





