Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms
Abstract
1. Antimicrobial Resistance
2. Biofilms: The Antibiotic-Resistant Home Self-Produced by Microorganisms
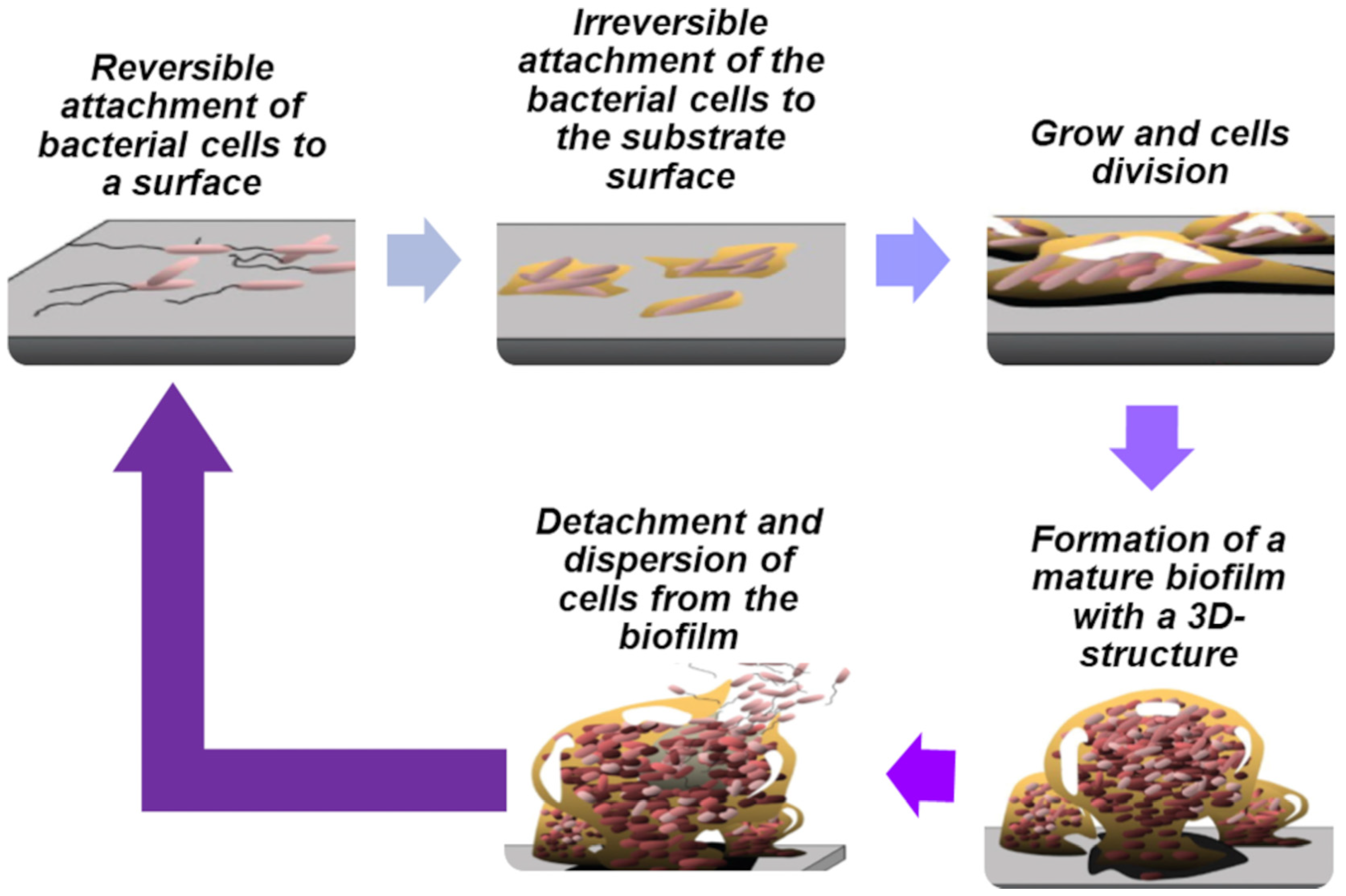
2.1. Biofilm Formation and Dispersal
2.1.1. Quorum Sensing (QS) in Bacterial Biofilms
2.1.2. Cyclic Dimeric Guanosine Monophosphate (c-di-GMP) in Bacterial Biofilms
3. Current and New Therapeutic Approaches against BF
3.1. Nitrogen-Based Quaternary Salts
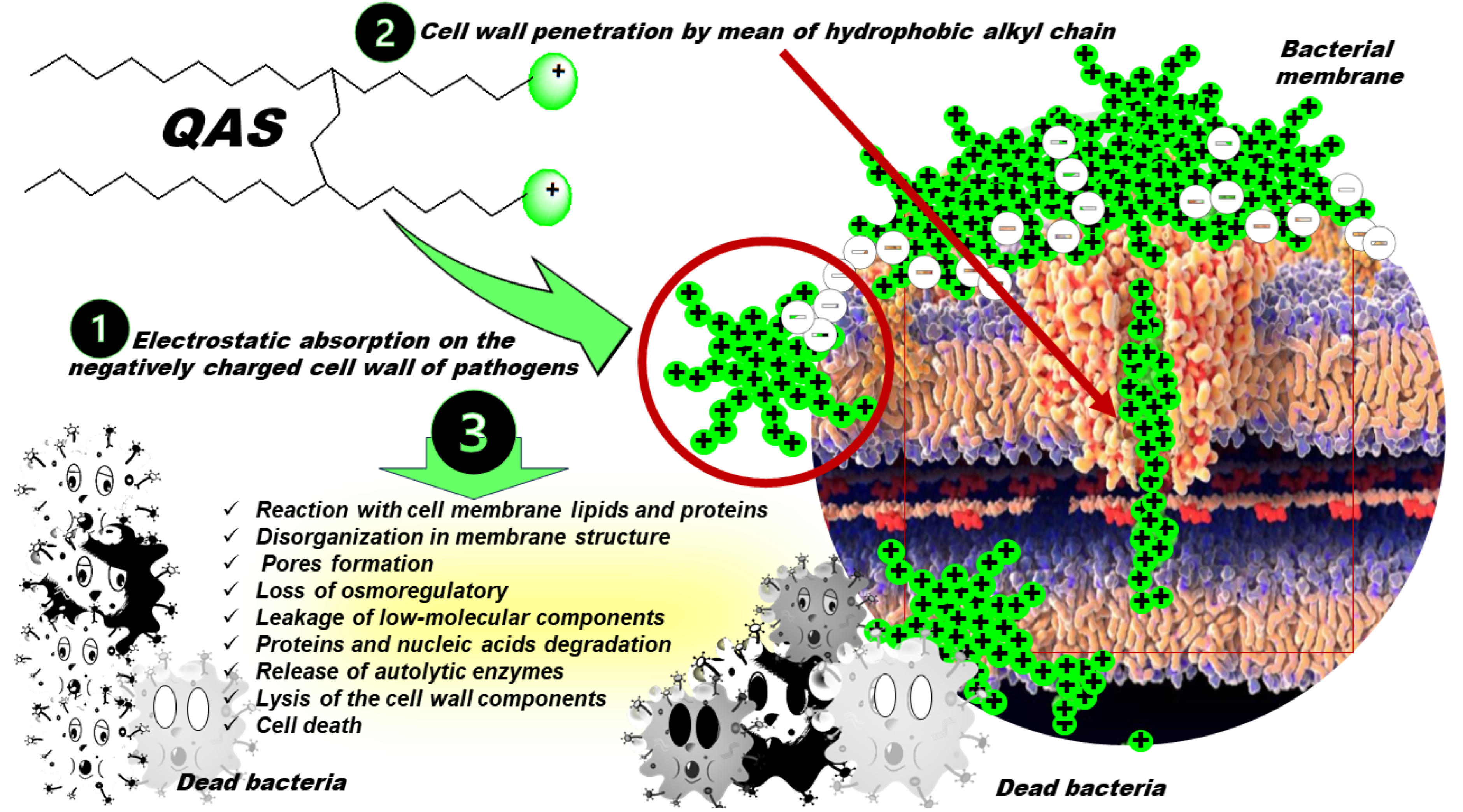
3.2. Quaternary Phosphonium Salts (QPS)
4. Synthetic Strategies Applied to Prepare the Aforementioned QPSs
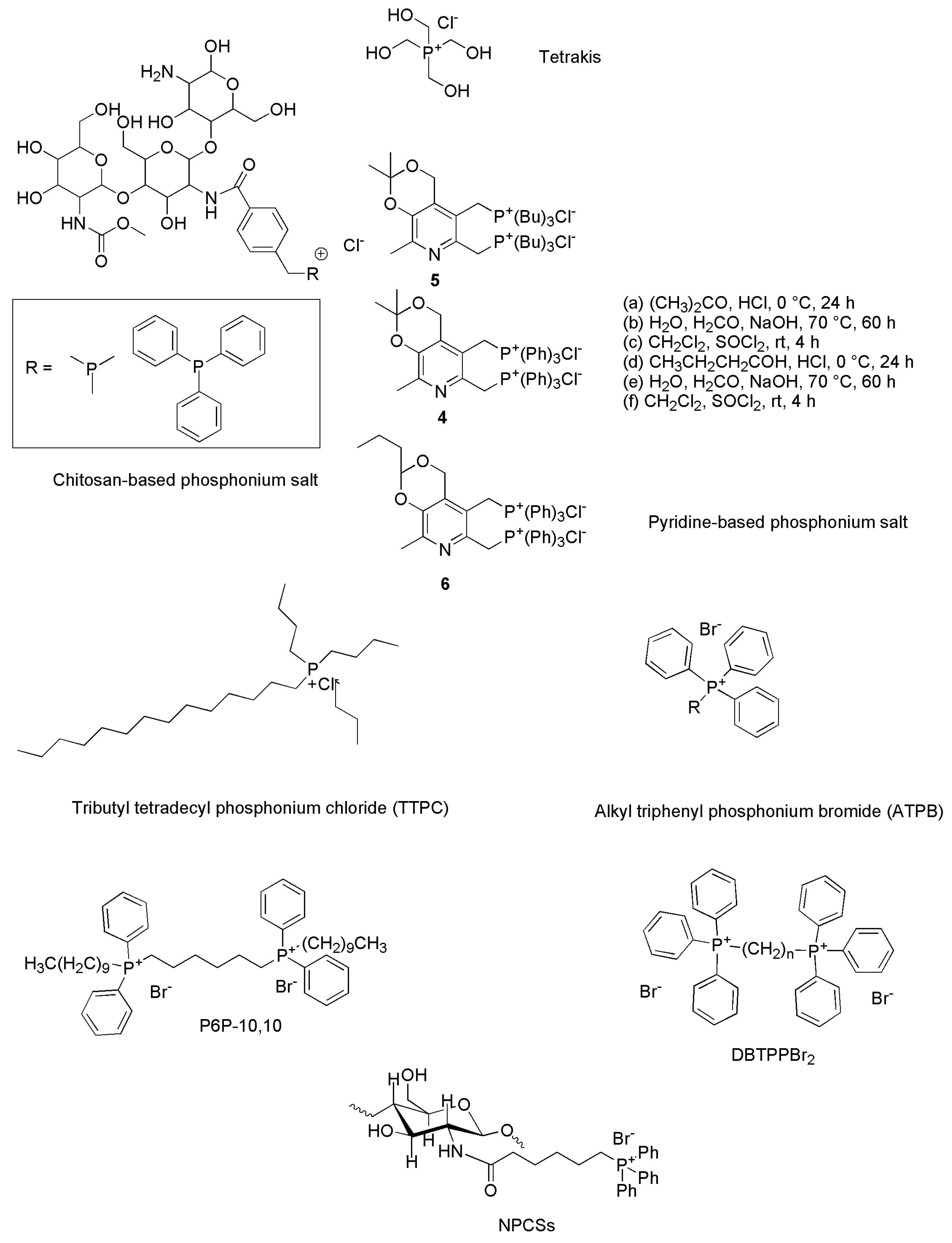
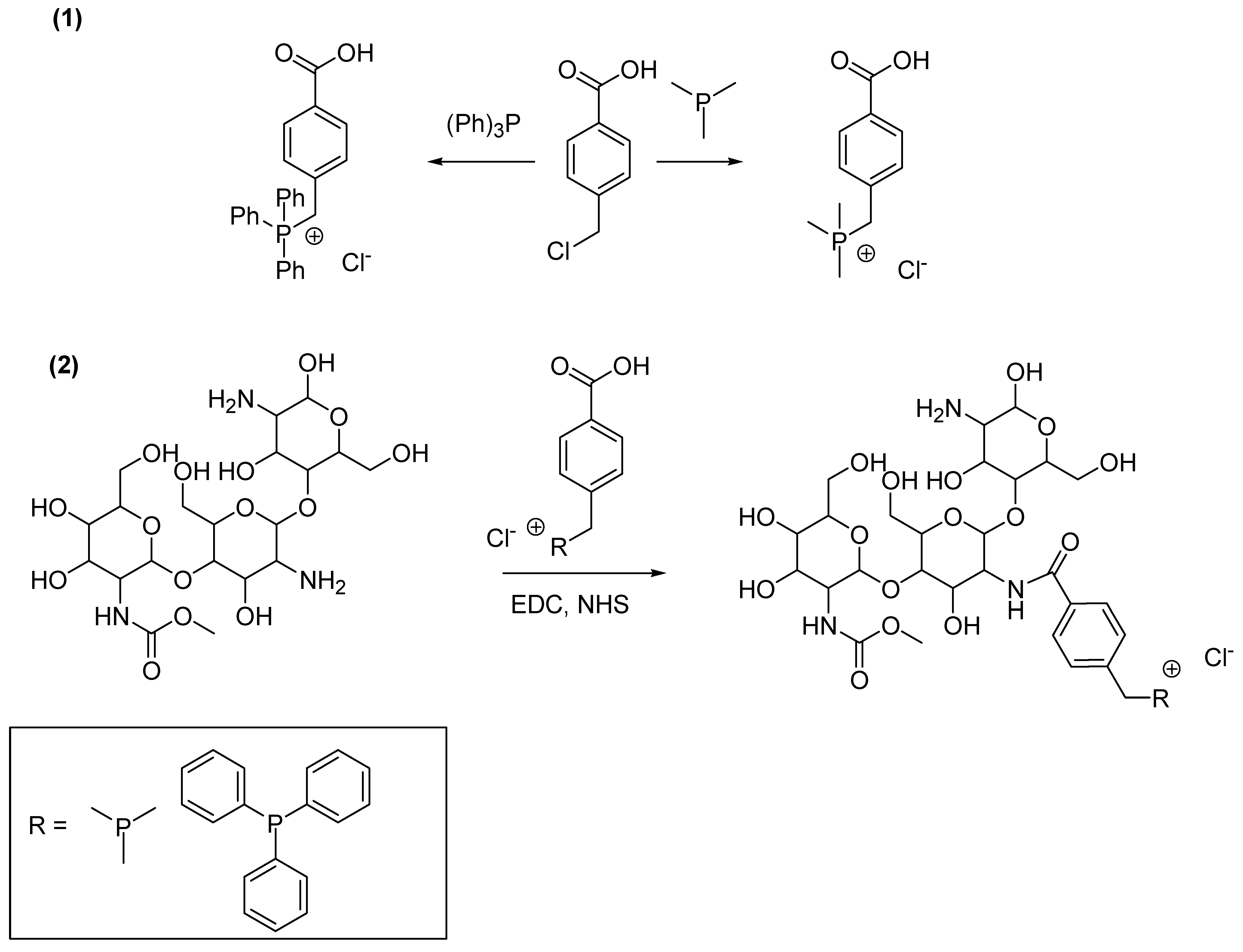
4.1. Synthesis of Chitosan-Based QPSs
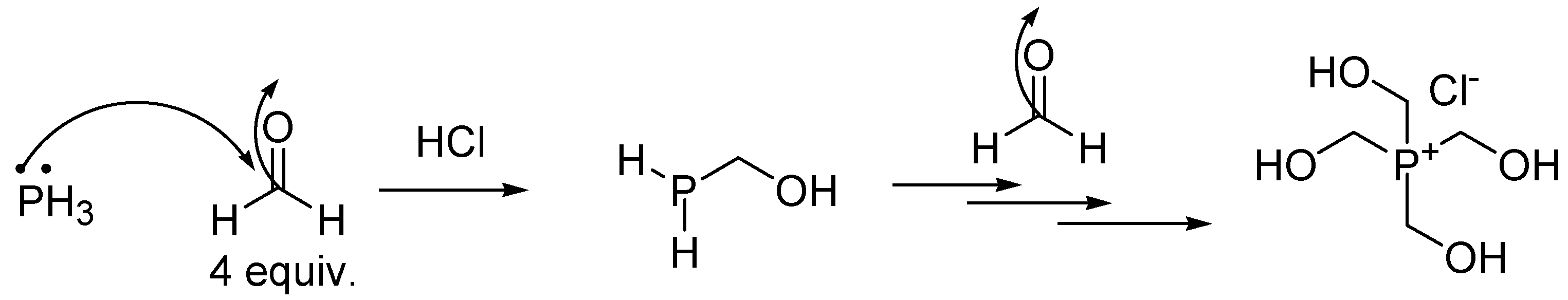
4.2. Synthesis of Tetrakis (Hydroxymethyl) Phosphonium Salt (THPS)
4.3. Synthesis of Bis-Phosphonium Salts of Pyridoxine
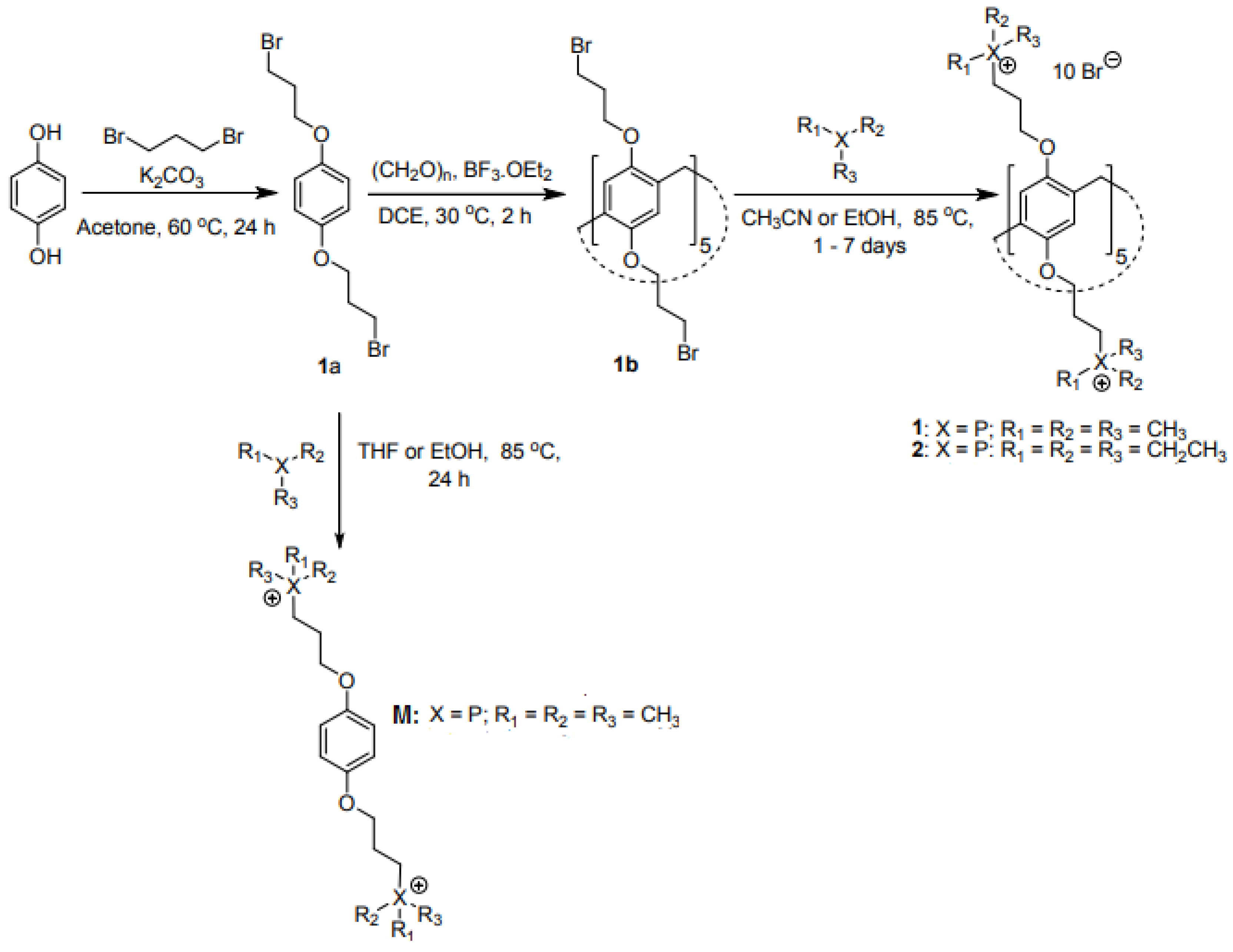
4.4. Synthesis of Pillar [5]Arene-Based QPSs
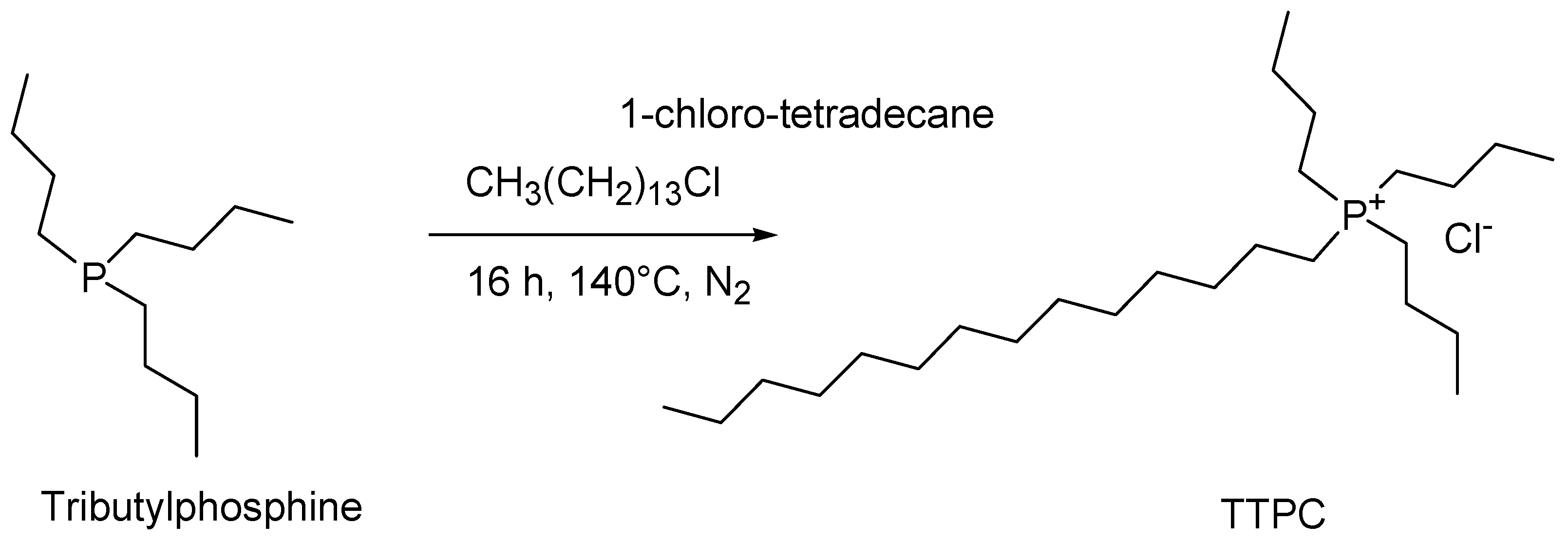
4.5. Synthesis of Tributyl Tetradecyl Phosphonium Chloride (TTPC)
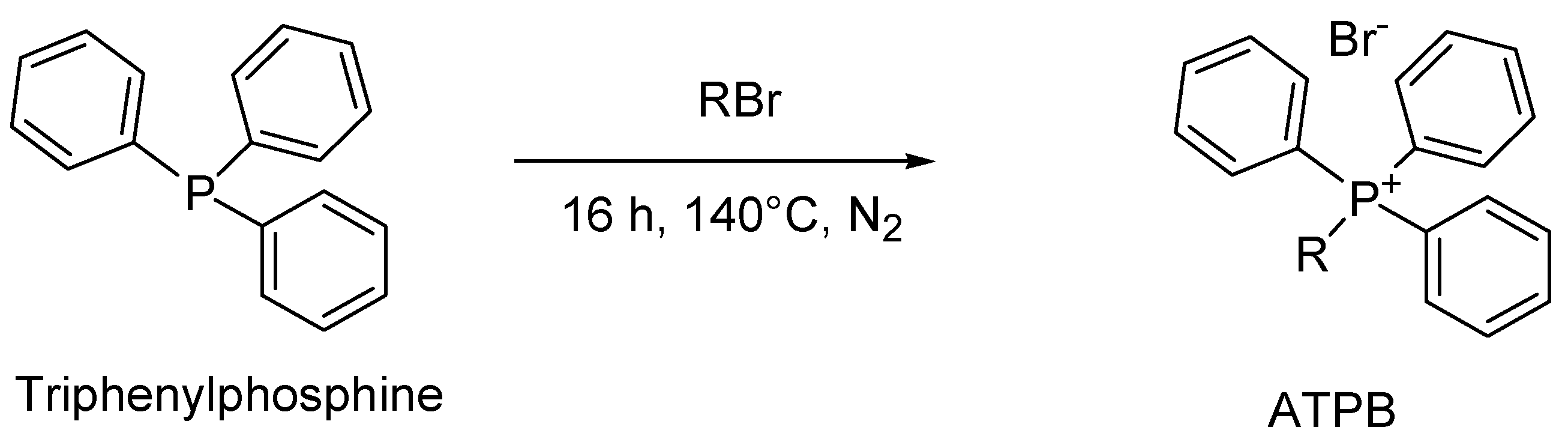
4.6. General Synthesis of Alkyl Triphenyl Phosphonium Bromide (ATPB)
4.7. Synthesis of Tetra Alkyl Tetraphenyl Bis-Phosphonium Compound (TATPBP, Namely P6P-10,10)
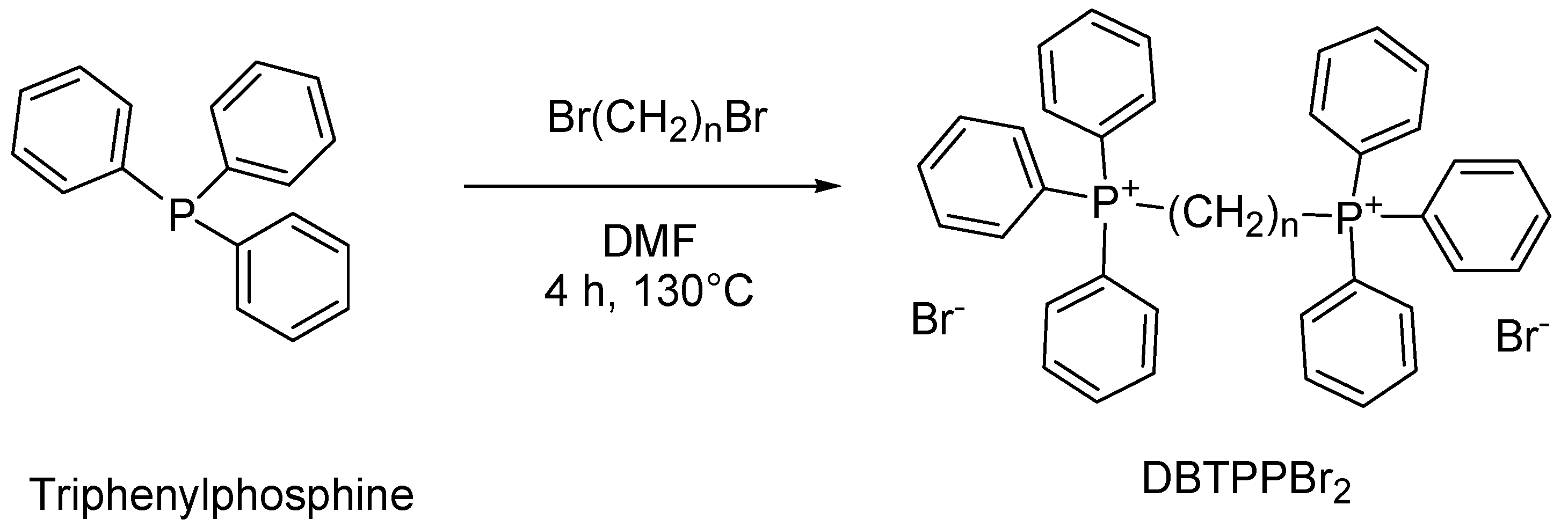
4.8. Synthesis of Alkyl Triphenyl Bis-Phosphonium Bromides
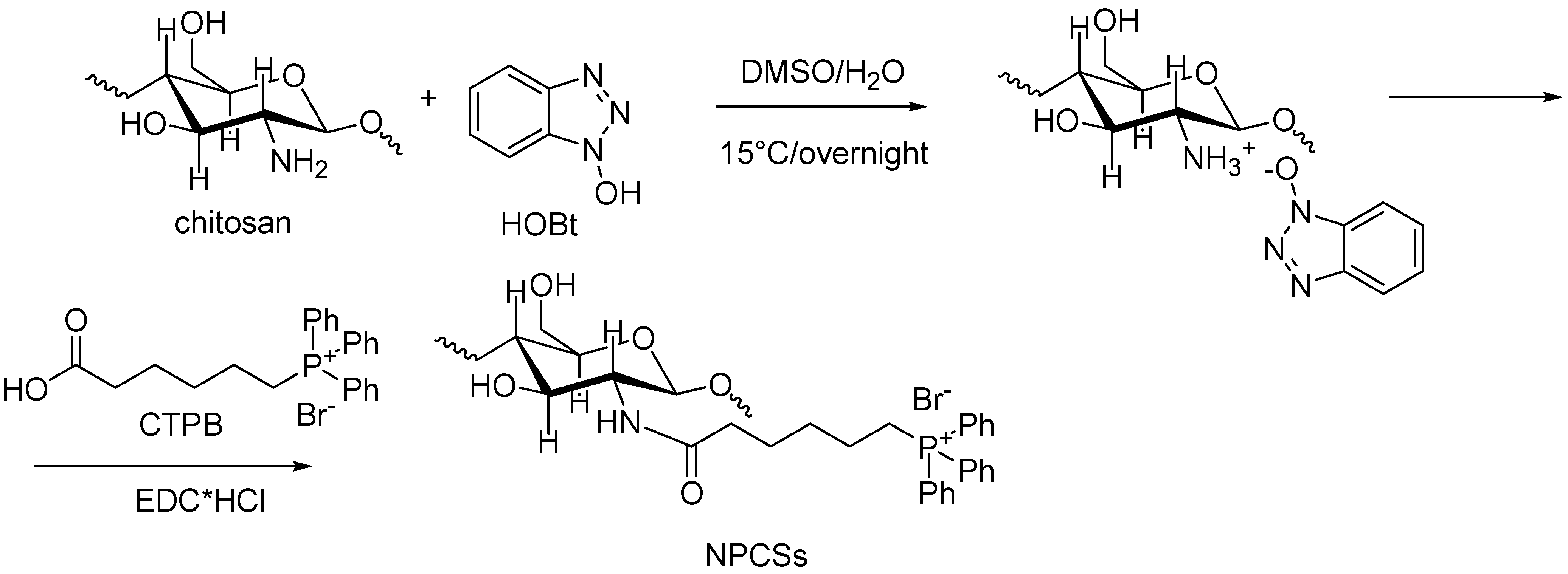
4.9. Synthesis of N-Phosphonium Chitosans (NPCSs) with Different Degrees of Substitution
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef] [PubMed]
- CDC. Center for Disease Control and Prevention. About Antimicrobial Resistance. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 23 November 2023).
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Jia, W.; Li, G.; Wang, W. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Hospital-Based Study in China. Int. J. Environ. Res. Public Health 2014, 11, 3424–3442. [Google Scholar] [CrossRef]
- Grassotti, T.T.; de Angelis, Z.D.; da Fontoura Xavier Costa, L.; de Araújo, A.J.G.; Pereira, R.I.; Soares, R.O.; Wagner, P.G.C.; Frazzon, J.; Frazzon, A.P.G. Antimicrobial Resistance Profiles in Enterococcus spp. Isolates from Fecal Samples of Wild and Captive Black Capuchin Monkeys (Sapajus nigritus) in South Brazil. Front. Microbiol. 2018, 9, 2366. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Galindo-Méndez, M. Antimicrobial Resistance in Escherichia coli. In E. Coli Infections-Importance of Early Diagnosis and Efficient Treatment; Louis, R., Ed.; IntechOpen: London, UK, 2020; p. 234. [Google Scholar] [CrossRef]
- Moya, C.; Maicas, S. Antimicrobial Resistance in Klebsiella pneumoniae Strains: Mechanisms and Outbreaks. Proceedings 2020, 66, 11. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic Resistance in Staphylococcus aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance Among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I.W., Shlaes, D., Drlica, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–38. ISBN 978-3-319-78538-7. [Google Scholar]
- Cuypers, W.L.; Jacobs, J.; Wong, V.; Klemm, E.J.; Deborggraeve, S.; Van Puyvelde, S. Fluoroquinolone Resistance in Salmonella: Insights by Whole-Genome Sequencing. Microb. Genom. 2018, 4, e000195. [Google Scholar] [CrossRef] [PubMed]
- Lampel, K.A. Chapter 7—Antimicrobial Resistance in Shigella Species. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 119–135. ISBN 978-0-12-801214-7. [Google Scholar]
- WHO. Multi-Drug Resistant Gonorrhoea. Available online: https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea (accessed on 21 November 2023).
- Li, H.; Yuan, J.; Duan, S.; Pang, Y. Resistance and Tolerance of Mycobacterium tuberculosis to Antimicrobial Agents—How M. Tuberculosis Can Escape Antibiotics. WIREs Mech. Dis. 2022, 14, e1573. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.; Stevens, D.A. Antifungal Drug Resistance. Clin. Infect. Dis. 2003, 36, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Rodero, L.; Mellado, E.; Rodriguez, A.C.; Salve, A.; Guelfand, L.; Cahn, P.; Cuenca-Estrella, M.; Davel, G.; Rodriguez-Tudela, J.L. G484S Amino Acid Substitution in Lanosterol 14-α Demethylase (ERG11) Is Related to Fluconazole Resistance in a Recurrent Cryptococcus Neoformans Clinical Isolate. Antimicrob. Agents Chemother. 2003, 47, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Arendrup, M.C. Acquired Antifungal Drug Resistance in Aspergillus fumigatus: Epidemiology and Detection. Med. Mycol. 2011, 49, S90–S95. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Buitrago, M.J.; Monzón, A.; Rodriguez-Tudela, J.L. Scopulariopsis brevicaulis, a Fungal Pathogen Resistant to Broad-Spectrum Antifungal Agents. Antimicrob. Agents Chemother. 2003, 47, 2339–2341. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral Drug Resistance of Human Cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef]
- Wutzler, P. Antiviral Therapy of Herpes Simplex and Varicella-Zoster Virus Infections. Intervirology 2008, 40, 343–356. [Google Scholar] [CrossRef]
- Cortez, K.J.; Maldarelli, F. Clinical Management of HIV Drug Resistance. Viruses 2011, 3, 347–378. [Google Scholar] [CrossRef]
- Hurt, A.C. The Epidemiology and Spread of Drug Resistant Human Influenza Viruses. Curr. Opin. Virol. 2014, 8, 22–29. [Google Scholar] [CrossRef]
- Suppiah, J.; Mohd Zain, R.; Haji Nawi, S.; Bahari, N.; Saat, Z. Drug-Resistance Associated Mutations in Polymerase (p) Gene of Hepatitis B Virus Isolated from Malaysian HBV Carriers. Hepat. Mon. 2014, 14, e13173. [Google Scholar] [CrossRef]
- Bloland, P.B. Drug Resistance in Malaria; A Background Document for the WHO Global Strategy for Containment of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Vanaerschot, M.; Dumetz, F.; Roy, S.; Ponte-Sucre, A.; Arevalo, J.; Dujardin, J.-C. Treatment Failure in Leishmaniasis: Drug-Resistance or Another (Epi-) Phenotype? Expert Rev. Anti-Infect. Ther. 2014, 12, 937–946. [Google Scholar] [CrossRef]
- Mohapatra, S. Drug Resistance in Leishmaniasis: Newer Developments. Trop. Parasitol. 2014, 4, 4–9. [Google Scholar] [CrossRef]
- Fallon, P.G.; Doenhoff, M.J. Drug-Resistant Schistosomiasis: Resistance to Praziquantel and Oxamniquine Induced in Schistosoma Mansoni in Mice Is Drug Specific. Am. J. Trop. Med. Hyg. 1994, 51, 83–88. [Google Scholar] [CrossRef]
- Qi, L.; Cui, J. A Schistosomiasis Model with Praziquantel Resistance. Discret. Dyn. Nat. Soc. 2013, 2013, 945767. [Google Scholar] [CrossRef]
- Bansal, D.; Malla, N.; Mahajan, R. Drug Resistance in Amoebiasis. Indian J. Med. Res. 2006, 123, 115–118. [Google Scholar]
- Muzny, C.A.; Schwebke, J.R. The Clinical Spectrum of Trichomonas Vaginalis Infection and Challenges to Management. Sex. Transm. Infect. 2013, 89, 423–425. [Google Scholar] [CrossRef]
- McFadden, D.C.; Tomavo, S.; Berry, E.A.; Boothroyd, J.C. Characterization of Cytochrome b from Toxoplasma Gondii and Qo Domain Mutations as a Mechanism of Atovaquone-Resistance. Mol. Biochem. Parasitol. 2000, 108, 1–12. [Google Scholar] [CrossRef]
- Nagamune, K.; Moreno, S.N.; Sibley, L.D. Artemisinin-Resistant Mutants of Toxoplasma gondii Have Altered Calcium Homeostasis. Antimicrob. Agents Chemother. 2007, 51, 3816–3823. [Google Scholar] [CrossRef]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Sauvage, V.; Velard, F.; Schmid, A.; Villena, I. Sulfadiazine Resistance in Toxoplasma gondii: No Involvement of Overexpression or Polymorphisms in Genes of Therapeutic Targets and ABC Transporters. Parasite 2013, 20, 19. [Google Scholar] [CrossRef]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef]
- Osmanov, A.; Farooq, Z.; Richardson, M.D.; Denning, D.W. The antiseptic Miramistin: A review of its comparative in vitro and clinical activity. FEMS Microbiol. Rev. 2020, 44, 399–417. [Google Scholar] [CrossRef]
- Kampf, G. Didecyldimethylammonium Chloride. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Kampf, G., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 371–394. [Google Scholar]
- Mao, X.; Auer David, L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Assadian, O. Octenidine dihydrochloride: Chemical characteristics and antimicrobial properties. J. Wound Care 2016, 25, S3–S6. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Michaud, M.E.; Allen, R.A.; Morrison-Lewis, K.R.; Sanchez, C.A.; Minbiole, K.P.C.; Post, S.J.; Wuest, W.M. Quaternary Phosphonium Compound Unveiled as a Potent Disinfectant against Highly Resistant Acinetobacter baumannii Clinical Isolates. ACS Infect. Dis. 2022, 8, 2307–2314. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Santos, A.L.S.; dos Galdino, A.C.M.; Mello, T.P.; de Ramos, L.D.S.; Branquinha, M.H.; Bolognese, A.M.; Columbano Neto, J.; Roudbary, M. What Are the Advantages of Living in a Community? A Microbial Biofilm Perspective! Memórias Do Inst. Oswaldo Cruz 2018, 113, e180212. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Dincer, S.; Özdenefe, M.S.; Arkut, A. Bacterial Biofilms; IntechOpen: Rijeka, Yugoslavia, 2020; ISBN 978-1-78985-900-3. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- de Vos, W.M. Microbial Biofilms and the Human Intestinal Microbiome. NPJ Biofilms Microbiomes 2015, 1, 15005. [Google Scholar] [CrossRef]
- Carthey, A.J.R.; Blumstein, D.T.; Gallagher, R.V.; Tetu, S.G.; Gillings, M.R. Conserving the Holobiont. Funct. Ecol. 2020, 34, 764–776. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Percival, S.L.; Kite, P. Intravascular Catheters and Biofilm Control. J. Vasc. Access 2007, 8, 69–80. [Google Scholar] [CrossRef]
- Safdar, N.; Mermel, L.A.; Maki, D.G. The epidemiology of catheter-related infection in the critically ill. In Catheter-Related Infections in the Critically Ill; O’Grady, N., Pittet, D., Eds.; Kluwer: New York, NY, USA, 2004; pp. 1–23. [Google Scholar]
- Donlan, R.; Murga, R.; Carson, L. Growing biofilms in intravenous fluids. In Biofilms, the Good, the Bad, and the Ugly; Wimpenny, J., Gilbert, P., Walker, J., Brading, M., Bayston, R., Eds.; Biofilm Club: Powys, UK, 1999; pp. 23–29. [Google Scholar]
- Chow, K.M.; Li, P.K.-T.; Cho, Y.; Abu-Alfa, A.; Bavanandan, S.; Brown, E.A.; Cullis, B.; Edwards, D.; Ethier, I.; Hurst, H.; et al. ISPD Catheter-Related Infection Recommendations: 2023 Update. Perit. Dial. Int. 2023, 43, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.M.D.; Romanelli, A.M. An Overview of Prosthetic Joint Infection (PJI) Definition and Diagnosis. 13 July 2021. Available online: https://health.ucdavis.edu/blog/lab-best-practice/an-overview-of-prosthetic-joint-infection-pji-definition-and-diagnosis/2021/07#:~:text=Pathogens%20involved%20in%20Prosthetic%20joint%20infection%201%20Early,after%20surgery%29%20Staphylococcus%20aureus%20Gram-negative%20bacilli%20Beta-hemolytic%20streptococci (accessed on 21 November 2023).
- Palmeri, N.O.; Kramer, D.B.; Karchmer, A.W.; Zimetbaum, P.J. A Review of Cardiac Implantable Electronic Device Infections for the Practicing Electrophysiologist. JACC Clin. Electrophysiol. 2021, 7, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Kokare, C.R.; Chakraborty, S.; Khopade, A.; Mahadik, K.R. Biofilm: Importance and Applications. Indian J. Biotechnol. 2009, 8, 159–168. [Google Scholar]
- Stickler, D.J. Bacterial Biofilms and the Encrustation of Urethral Catheters. Biofouling 1996, 9, 293–305. [Google Scholar] [CrossRef]
- Pentland, D.R.; Stevens, S.; Williams, L.; Baker, M.; McCall, C.; Makarovaite, V.; Balfour, A.; Mühlschlegel, F.A.; Gourlay, C.W. Precision Antifungal Treatment Significantly Extends Voice Prosthesis Lifespan in Patients Following Total Laryngectomy. Front. Microbiol. 2020, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Kokare, C.R.; Kadam, S.S.; Mahadik, K.R.; Chopade, B.A. Studies on bioemulsifier production from marine Streptomyces sp. S1. Ind. J. Biotechnol. 2007, 6, 78–84. [Google Scholar]
- Lamont, R.J.; Jenkinson, H.F. Life Below the Gum Line: Pathogenic Mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998, 62, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Overman, P.R. Biofilm: A New View of Plaque. J. Contemp. Dent. Pract. 2000, 1, 18–29. [Google Scholar] [CrossRef]
- Nasser, A.; Azimi, T.; Ostadmohammadi, S.; Ostadmohammadi, S. A comprehensive review of bacterial osteomyelitis with emphasis on Staphylococcus aureus. Microb. Pathog. 2020, 148, 104431. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The Biofilm-Associated Bacterial Infections Unrelated to Indwelling Devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef]
- Arunasri, K.; Mohan, S.V. Chapter 2.3—Biofilms: Microbial Life on the Electrode Surface. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–313. ISBN 978-0-444-64052-9. [Google Scholar]
- Jamal, M.; Tasneem, U.; Hussain, T.; Andleeb, S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. Res. Rev. J. Microbiol. Biotechnol. 2015, 4, 59. [Google Scholar]
- Monroe, D. Looking for Chinks in the Armor of Bacterial Biofilms. PLoS Biol. 2007, 5, e307. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N.H. A Fatty Acid Messenger Is Responsible for Inducing Dispersion in Microbial Biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.A.; Koh, K.S.; Queck, S.Y.; Labbate, M.; Lam, K.W.; Kjelleberg, S. Biofilm Formation and Sloughing in Serratia marcescens Are Controlled by Quorum Sensing and Nutrient Cues. J. Bacteriol. 2005, 187, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.; Sandulescu, O. Communication Is the Key: Biofilms, Quorum Sensing, Formation and Prevention. Discoveries 2019, 7, e10. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.C.; Miyashiro, T. Quorum Sensing in the Squid-Vibrio Symbiosis. Int. J. Mol. Sci. 2013, 14, 16386–16401. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Li, Z.; Nair, S.K. Quorum Sensing: How Bacteria Can Coordinate Activity and Synchronize Their Response to External Signals? Protein Sci. 2012, 21, 1403–1417. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. BioMed Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef]
- Schirmer, T. C-Di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation. J. Mol. Biol. 2016, 428, 3683–3701. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Mi, D.; Hou, Y.; Lin, M.; Xie, Q.; Niu, X.; Chen, Y.; He, C.; Tao, J.; Li, C. Systematic Analysis of the Roles of C-Di-GMP Signaling in Xanthomonas oryzae Pv. Oryzae Virulence. FEMS Microbiol. Lett. 2022, 369, fnac068. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Filloux, A. Biofilms and Cyclic Di-GMP (c-Di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting Bacterial Cooperation Is an Evolutionarily Robust Anti-Biofilm Strategy. Nat. Commun. 2020, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Mataraci, E.; Dosler, S. In Vitro Activities of Antibiotics and Antimicrobial Cationic Peptides Alone and in Combination against Methicillin-Resistant Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef]
- Dosler, S.; Mataraci, E. In Vitro Pharmacokinetics of Antimicrobial Cationic Peptides Alone and in Combination with Antibiotics against Methicillin Resistant Staphylococcus aureus Biofilms. Peptides 2013, 49, 53–58. [Google Scholar] [CrossRef]
- Dosler, S.; Karaaslan, E. Inhibition and Destruction of Pseudomonas aeruginosa Biofilms by Antibiotics and Antimicrobial Peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef]
- Field, D.; O’ Connor, R.; Cotter, P.D.; Ross, R.P.; Hill, C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus biofilms. Front. Microbiol. 2016, 7, 508. [Google Scholar] [CrossRef]
- Obłąk, E.; Gamian, A. Biologiczna aktywność czwartorzędowych soli amoniowych (CSA) * The biological activity of quaternary ammonium salts (QASs). Postepy Hig. Med. Dosw. 2010, 64, 201–211. [Google Scholar]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.C.; Addison, R.M.; Rubino, J.R.; Leese, K.E.; Dulaney, P.D.; Newell, M.S.; Wilkins, J.; Gaber, D.J.; Wineinger, T.; Criger, D.A. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J. Appl. Microbiol. 2003, 95, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Mbithi, J.N.; Springthorpe, V.S.; Sattar, S.A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl. Environ. Microbiol. 1990, 56, 3601–3604. [Google Scholar] [CrossRef] [PubMed]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? Chem. Med. Chem. 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Bragg, R.; Jansen, A.; Coetzee, M.; van der Westhuizen, W.; Boucher, C. Bacterial resistance to quaternary ammonium compounds (QAC) disinfectants. In Infectious Diseases and Nanomedicine II; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014; Volume 808, pp. 1–13. [Google Scholar]
- Peng, Z.-X.; Tu, B.; Shen, Y.; Du, L.; Wang, L.; Guo, S.-R.; Tang, T.-T. Quaternized Chitosan Inhibits icaA Transcription and Biofilm Formation by Staphylococcus on a Titanium Surface. Antimicrob. Agents Chemother. 2011, 55, 860–866. [Google Scholar] [CrossRef]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The Use of Quaternised Chitosan-Loaded PMMA to Inhibit Biofilm Formation and Downregulate the Virulence-Associated Gene Expression of Antibiotic-Resistant Staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef]
- Song, J.; Kong, H.; Jang, J. Bacterial Adhesion Inhibition of the Quaternary Ammonium Functionalized Silica Nanoparticles. Colloids Surf. B Biointerfaces 2011, 82, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Hamood, A.N.; de Souza, A.; Schultz, G.; Liesenfeld, B.; Mehta, D.; Reid, T.W. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kocak, H.S.; Bulut, O.; Yilmaz, M.D. A Dicationic BODIPY-Based Fluorescent Bactericide to Combat Infectious Diseases and to Eradicate Bacterial Biofilms. ACS Appl. BioMaterials 2023, 6, 1604–1610. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ibrahim, A.S.; Balhaddad, A.A.; Weir, M.D.; Lin, N.J.; Tay, F.R.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. A Novel Dental Sealant Containing Dimethylaminohexadecyl Methacrylate Suppresses the Cariogenic Pathogenicity of Streptococcus Mutans Biofilms. Int. J. Mol. Sci. 2019, 20, 3491. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.-K.; Nagendrababu, V.; Fawzy, A.S. A Quaternary Ammonium Silane Antimicrobial Triggers Bacterial Membrane and Biofilm Destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef]
- Balbinot, G.S.; Marcon, N.; Sauro, S.; Luxan, S.A.; Collares, F.M. Alkyl Trimethyl Ammonium Bromide for the Formulation of Antibacterial Orthodontic Resins. Clin. Oral Investig. 2022, 26, 7011–7019. [Google Scholar] [CrossRef]
- Phuangkaew, T.; Booranabunyat, N.; Kiatkamjornwong, S.; Thanyasrisung, P.; Hoven, V.P. Amphiphilic Quaternized Chitosan: Synthesis, Characterization, and Anti-Cariogenic Biofilm Property. Carbohydr. Polym. 2022, 277, 118882. [Google Scholar] [CrossRef]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-Biofilm Surfaces from Mixed Dopamine-Modified Polymer Brushes: Synergistic Role of Cationic and Zwitterionic Chains to Resist: Staphyloccocus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Zhou, X.; Han, Q.; Zhou, W.; Liang, J.; Xu, H.H.K.; Ren, B.; Peng, X.; Weir, M.D.; et al. Anti-Caries Effect of Resin Infiltrant Modified by Quaternary Ammonium Monomers. J. Dent. 2020, 97, 103355. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Cruz, C.D.; Morawska, M.; Ciura, K.; Gilbert-Girard, S.; Mazur, L.; Mäkkylä, H.; Ilina, P.; Savijoki, K.; Fallarero, A.; et al. Antibacterial and Antibiofilm Activity of Permanently Ionized Quaternary Ammonium Fluoroquinolones. European J. Med. Chem. 2023, 254, 115373. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Bapat, R.A.; Sidhu, P.; Ilyas, M.S.; Khan, A.S.; Mak, K.-K.; Pichika, M.R.; Nagendrababu, V.; Peters, O.A. Antibacterial and Antibiofilm Efficacy of K21-E in Root Canal Disinfection. Dent. Mater. 2021, 37, 1511–1528. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Esin, S.; Benedetti, A.; Maisetta, G.; Fabiano, A.; Zambito, Y.; Batoni, G. Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives. Int. J. Mol. Sci. 2019, 20, 6297. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, Ester-Free Monomers: Polymerization Kinetics, Mechanical Properties, Biocompatibility and Anti-Biofilm Activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rita Pereira, A.; Gomes, I.B.; Simões, M. Choline-Based Ionic Liquids for Planktonic and Biofilm Growth Control of Bacillus cereus and Pseudomonas fluorescens. J. Mol. Liq. 2022, 346, 117077. [Google Scholar] [CrossRef]
- Gaspar, C.; Rolo, J.; Cerca, N.; Palmeira de Oliveira, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Dequalinium Chloride Effectively Disrupts Bacterial Vaginosis (BV) Gardnerella Spp. Biofilms. Pathogens 2021, 10, 261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zheng, X.; Li, Z.; Wang, M.; Luo, K.; Zhang, C.; Xia, X.; Wang, Y.; Shi, C. Didecyldimethylammonium Bromide: Application to Control Biofilms of Staphylococcus aureus and Pseudomonas aeruginosa Alone and in Combination with Slightly Acidic Electrolyzed Water. Food Res. Int. 2022, 157, 111236. [Google Scholar] [CrossRef]
- Yu, W.; Ren, C.; Zhang, N.; Cao, L.; Weir, M.D.; Yang, K.; Xu, H.H.K.; Bai, Y. Dual Function of Anti-Biofilm and Modulating Biofilm Equilibrium of Orthodontic Cement Containing Quaternary Ammonium Salt. Dent. Mater. J. 2023, 42, 149–157. [Google Scholar] [CrossRef]
- Moshynets, O.V.; Baranovskyi, T.P.; Iungin, O.S.; Kysil, N.P.; Metelytsia, L.O.; Pokholenko, I.; Potochilova, V.V.; Potters, G.; Rudnieva, K.L.; Rymar, S.Y.; et al. eDNA Inactivation and Biofilm Inhibition by the Polymeric Biocide Polyhexamethylene Guanidine Hydrochloride (PHMG-Cl). Int. J. Mol. Sci. 2022, 23, 731. [Google Scholar] [CrossRef]
- Daood, U.; Burrow, M.F.; Yiu, C.K.Y. Effect of a Novel Quaternary Ammonium Silane Cavity Disinfectant on Cariogenic Biofilm Formation. Clin. Oral Investig. 2020, 24, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cheng, L.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Li, Q.; Hannig, M.; Rupf, S. Effects of Water Aging on the Mechanical and Anti-Biofilm Properties of Glass-Ionomer Cement Containing Dimethylaminododecyl Methacrylate. Dent. Mater. 2019, 35, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Song, H.; Loh, J.L.C.; She, J.; Deng, L.; Liu, B. Grafting Antibiofilm Polymer Hydrogel Film onto Catheter by SARA SI-ATRP. J. Biomater. Sci. Polym. Ed. 2018, 29, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Yasir, M.; Yee, E.; Willcox, M.; Black, D.S.C.; Kumar, N. Guanidine Functionalized Anthranilamides as Effective Antibacterials with Biofilm Disruption Activity. Org. Biomol. Chem. 2018, 16, 5871–5888. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Weir, M.D.; Passos, V.F.; Rolim, J.P.M.; Lynch, C.D.; Rodrigues, L.K.A.; Xu, H.H.K. Human in Situ Study of the Effect of Bis(2-Methacryloyloxyethyl) Dimethylammonium Bromide Immobilized in Dental Composite on Controlling Mature Cariogenic Biofilm. Int. J. Mol. Sci. 2018, 19, 3443. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.; Detusheva, E.; Fursova, N.; Ostashevskaya, I.; Vereshchagin, A. Microbiological Evaluation of Novel Bis-Quaternary Ammonium Compounds: Clinical Strains, Biofilms, and Resistance Study. Pharmaceuticals 2022, 15, 514. [Google Scholar] [CrossRef]
- Soares, N.S.; Hollanda, L.M.; Elias, C.M.V.; Azevedo, M.M.F.; Santos, F.E.P.; Lobo, A.O.; Marciano, F.R. Modified PCL/PEG/GelMA Electrospun Blends Reduced Biofilm Formation. Mater. Lett. 2022, 320, 132315. [Google Scholar] [CrossRef]
- Ran, H.-H.; Cheng, X.; Bao, Y.-W.; Hua, X.-W.; Gao, G.; Zhang, X.; Jiang, Y.-W.; Zhu, Y.-X.; Wu, F.-G. Multifunctional Quaternized Carbon Dots with Enhanced Biofilm Penetration and Eradication Efficiencies. J. Mater. Chem. B 2019, 7, 5104–5114. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, J.; Tian, Q.; Shen, C.; Zhang, L.; Yang, K.; Shang, L. One Step Synthesis of Positively Charged Gold Nanoclusters as Effective Antimicrobial Nanoagents against Multidrug-Resistant Bacteria and Biofilms. J. Colloid Interface Sci. 2020, 569, 235–243. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.-X.; Lu, P.; Zhu, B.; Wu, F.-G. One-Step Synthesis of Quaternized Silica Nanoparticles with Bacterial Adhesion and Aggregation Properties for Effective Antibacterial and Antibiofilm Treatments. J. Mater. Chem. B 2022, 10, 3073–3082. [Google Scholar] [CrossRef]
- Xia, L.; Tian, J.; Yue, T.; Cao, H.; Chu, J.; Cai, H.; Zhang, W. Pillar [5]Arene-Based Acid-Triggered Supramolecular Porphyrin Photosensitizer for Combating Bacterial Infections and Biofilm Dispersion. Adv. Healthc. Mater. 2022, 11, 2102015. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Guo, Q.; Shen, X. Polyethyleneimine and Quaternized Ammonium Polyethyleneimine: The Versatile Materials for Combating Bacteria and Biofilms. J. Biomater. Sci. Polym. Ed. 2019, 30, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Kuppusamy, R.; Yasir, M.; Hassan, M.M.; Sara, M.; Ho, J.; Willcox, M.D.P.; Black, D.S.; Kumar, N. Polyphenylglyoxamide-based Amphiphilic Small Molecular Peptidomimetics as Antibacterial Agents with Anti-biofilm Activity. Int. J. Mol. Sci. 2021, 22, 7344. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Ibrahim, M.S.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Pronounced Effect of Antibacterial Bioactive Dental Composite on Microcosm Biofilms Derived from Patients with Root Carious Lesions. Front. Mater. 2020, 7, 583861. [Google Scholar] [CrossRef]
- He, D.; Yu, Y.; Liu, F.; Yao, Y.; Li, P.; Chen, J.; Ning, N.; Zhang, S. Quaternary Ammonium Salt-Based Cross-Linked Micelle Templated Synthesis of Highly Active Silver Nanocomposite for Synergistic Anti-Biofilm Application. Chem. Eng. J. 2020, 382, 122976. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Yu, L.; Wang, Y.-R.; Yuan, X.; Tang, Y.-F.; Chen, W.; Zeng, L.-Z. Quaternary Ammonium Salt-Based Cross-Linked Micelle with Copper Nanoparticles for Treatment of Sulfate Reducing Bacteria Biofilm. React. Funct. Polym. 2022, 180, 105405. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhou, X.; Zou, Y.; Li, M.; Peng, X.; Ren, B.; Xu, H.H.K.; Weir, M.D.; Cheng, L.; et al. Short-Time Antibacterial Effects of Dimethylaminododecyl Methacrylate on Oral Multispecies Biofilm in Vitro. BioMed Res. Int. 2019, 2019, 6393470. [Google Scholar] [CrossRef]
- Hernando-Gozalo, M.; Aguilera-Correa, J.J.; Rescalvo-Casas, C.; Seijas-Pereda, L.; García-Bertolín, C.; de la Mata, F.J.; Sánchez-Nieves, J.; Cuadros, J.; Pérez-Tanoira, R. Study of the Antimicrobial Activity of Cationic Carbosilane Dendrimers against Clinical Strains of Multidrug-Resistant Bacteria and Their Biofilms. Front. Cell. Infect. Microbiol. 2023, 13, 3991. [Google Scholar] [CrossRef]
- Gao, S.; Sun, Y.; Lu, Z.; Jiang, N.; Yao, H. Synergistic Antibacterial and Biofilm Eradication Activity of Quaternary-Ammonium Compound with Copper Ion. J. Inorg. Biochem. 2023, 243, 112190. [Google Scholar] [CrossRef]
- Ali, I.; Burki, S.; El-Haj, B.M.; Parveen, S.; Nadeem, H.Ş.; Nadeem, S.; Shah, M.R. Synthesis and Characterization of Pyridine-Based Organic Salts: Their Antibacterial, Antibiofilm and Wound Healing Activities. Bioorganic Chem. 2020, 100, 103937. [Google Scholar] [CrossRef]
- Garipov, M.R.; Sabirova, A.E.; Pavelyev, R.S.; Shtyrlin, N.V.; Lisovskaya, S.A.; Bondar, O.V.; Laikov, A.V.; Romanova, J.G.; Bogachev, M.I.; Kayumov, A.R.; et al. Targeting Pathogenic Fungi, Bacteria and Fungal-Bacterial Biofilms by Newly Synthesized Quaternary Ammonium Derivative of Pyridoxine and Terbinafine with Dual Action Profile. Bioorganic Chem. 2020, 104, 104306. [Google Scholar] [CrossRef] [PubMed]
- Hympanova, M.; Terlep, S.; Markova, A.; Prchal, L.; Dogsa, I.; Pulkrabkova, L.; Benkova, M.; Marek, J.; Stopar, D. The Antibacterial Effects of New N-Alkylpyridinium Salts on Planktonic and Biofilm Bacteria. Front. Microbiol. 2020, 11, 573951. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.A.; Seferyan, M.A.; Valeev, A.B.; Saverina, E.A.; Detusheva, E.V.; Vereshchagin, A.N. The Antimicrobial and Antibiofilm Potential of New Water-Soluble Tris-Quaternary Ammonium Compounds. Int. J. Mol. Sci. 2023, 24, 10512. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, Enzymatic and Protein Profiles of Pseudomonas aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules 2018, 23, 1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xin, M.; Li, M.; Liu, W.; Mao, Y. Effect of the Structure of Chitosan Quaternary Phosphonium Salt and Chitosan Quaternary Ammonium Salt on the Antibacterial and Antibiofilm Activity. Int. J. Biol. Macromol. 2023, 242, 124877. [Google Scholar] [CrossRef]
- Keasler, V.; Bennett, B.; McGinley, H. Analysis of Bacterial Kill Versus Corrosion from Use of Common Oilfield Biocides. In Proceedings of the 2010 8th International Pipeline Conference, Calgary, AB, Canada, 27 September–1 October 2010; ASME: New York, NY, USA; Volume 1, pp. 935–944. [Google Scholar] [CrossRef]
- Kramer, J.F.; Arpita, S.; Strba, S.F. Comparative Performance of Biocides Versus Corrosion Causing Biofilms. In NACE CORROSION; NACE: San Antonio, TX, USA, 2010. [Google Scholar]
- Kayumov, A.R.; Nureeva, A.A.; Trizna, E.Y.; Gazizova, G.R.; Bogachev, M.I.; Shtyrlin, N.V.; Pugachev, M.V.; Sapozhnikov, S.V.; Shtyrlin, Y.G. New Derivatives of Pyridoxine Exhibit High Antibacterial Activity against Biofilm-Embedded Staphylococcus Cells. BioMed Res. Int. 2015, 2015, 890968. [Google Scholar] [CrossRef]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic Pillararenes Potently Inhibit Biofilm Formation without Affecting Bacterial Growth and Viability. J. Am. Chem. Soc. 2016, 138, 754–757. [Google Scholar] [CrossRef]
- Joseph, R.; Kaizerman, D.; Herzog, I.M.; Hadar, M.; Feldman, M.; Fridman, M.; Cohen, Y. Phosphonium Pillar [5]Arenes as a New Class of Efficient Biofilm Inhibitors: Importance of Charge Cooperativity and the Pillar Platform. Chem. Commun. 2016, 52, 10656–10659. [Google Scholar] [CrossRef]
- Das, S.; Paul, A.; Bera, D.; Dey, A.; Roy, A.; Dutta, A.; Ganguly, D. Design, Development and Mechanistic Insights into the Enhanced Antibacterial Activity of Mono and Bis-Phosphonium Fluoresceinate Ionic Liquids. Mater. Today Commun. 2021, 28, 102672. [Google Scholar] [CrossRef]
- Cieniecka-Rosłonkiewicz, A.; Pernak, J.; Kubis-Feder, J.; Ramani, A.; Robertson, A.J.; Seddon, K.R. Synthesis, Anti-Microbial Activities and Anti-Electrostatic Properties of Phosphonium-Based Ionic Liquids. Green Chem. 2005, 7, 855–862. [Google Scholar] [CrossRef]
- Shive, P.N.; Diehl, J.F. Reduction of Hematite to Magnetite under Natural and Laboratory Conditions. J. Geomagn. Geoelectr. 1977, 29, 345–354. [Google Scholar] [CrossRef]
- Atefi, F.; Garcia, M.T.; Singer, R.D.; Scammells, P.J. Phosphonium Ionic Liquids: Design, Synthesis and Evaluation of Biodegradability. Green Chem. 2009, 11, 1595–1604. [Google Scholar] [CrossRef]
- Metelytsia, L.O.; Hodyna, D.M.; Semenyuta, I.V.; Kovalishyn, V.V.; Rogalsky, S.P.; Derevianko, K.Y.; Brovarets, V.S.; Tetko, I.V. Theoretical and Experimental Studies of Phosphonium Ionic Liquids as Potential Antibacterials of MDR Acinetobacter baumannii. Antibiotics 2022, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Hemp, S.T.; Zhang, M.; Allen, M.H.; Cheng, S.; Moore, R.B.; Long, T.E. Comparing Ammonium and Phosphonium Polymerized Ionic Liquids: Thermal Analysis, Conductivity, and Morphology. Macromol. Chem. Phys. 2013, 214, 2099–2107. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Synthesis and Antimicrobial Activity of Dimethyl- and Trimethyl-Substituted Phosphonium Salts with Alkyl Chains of Various Lengths. Antimicrob. Agents Chemother. 1994, 38, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Hamada, N.; Kanazawa, A.; Endo, T. Study on Antibacterial Dental Resin Using Tri-n-Butyl(4-Vinylbenzyl)Phosphonium Chloride. Dent. Mater. J. 2011, 30, 960–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric Phosphonium Salts as a Novel Class of Cationic Biocides. V. Synthesis and Antibacterial Activity of Polyesters Releasing Phosphonium Biocides. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 3003–3011. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yang, M.-S.; Liang, M. The Synthesis, Characterization and Antibacterial Activity of Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)s Modified with Ammonium and Phosphonium Salts. React. Funct. Polym. 2010, 70, 944–950. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S.V. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorganic Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Hatakeyama, E.S.; Ju, H.; Gabriel, C.J.; Lohr, J.L.; Bara, J.E.; Noble, R.D.; Freeman, B.D.; Gin, D.L. New Protein-Resistant Coatings for Water Filtration Membranes Based on Quaternary Ammonium and Phosphonium Polymers. J. Membr. Sci. 2009, 330, 104–116. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, Y.; He, L.; Yao, J.; Suresh, A.K.; Bellare, J.; Sridhar, T.; Wang, H. Evaluation of Quaternary Phosphonium-Based Polymer Membranes for Desalination Application. Desalination 2012, 292, 119–123. [Google Scholar] [CrossRef]
- Brunel, F.; Lautard, C.; Giorgio, C.D.; Garzino, F.; Raimundo, J.-M.; Bolla, J.M.; Camplo, M. Antibacterial Activities of Mono-, Di- and Tri-Substituted Triphenylamine-Based Phosphonium Ionic Liquids. Bioorganic Med. Chem. Lett. 2018, 28, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Taladriz, A.; Healy, A.R.; Pérez, E.J.F.; García, V.H.; Martínez, C.H.R.; Alkhaldi, A.A.M.; Eze, A.A.; Kaiser, M.; Koning, H.P.; de Chana, A.; et al. Synthesis and Structure-Activity Analysis of New Phosphonium Salts with Potent Activity against African Trypanosomes. J. Med. Chem. 2012, 55, 2606–2622. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ortega, J.R.; Reuther, P.; Rivas, L.; Dardonville, C. New Benzophenone-Derived Bisphosphonium Salts as Leishmanicidal Leads Targeting Mitochondria through Inhibition of Respiratory Complex II. J. Med. Chem. 2010, 53, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Park, H.-D. Tributyl Tetradecyl Phosphonium Chloride for Biofouling Control in Reverse Osmosis Processes. Desalination 2015, 372, 39–46. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Babarro, J.M.F.; Lahoz, F.; Sansón, M.; Martín, V.S.; Norte, M.; Fernández, J.J. From Broad-Spectrum Biocides to Quorum Sensing Disruptors and Mussel Repellents: Antifouling Profile of Alkyl Triphenylphosphonium Salts. PLoS ONE 2015, 10, e0123652. [Google Scholar] [CrossRef]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small Molecule Control of Bacterial Biofilms. Org. Biomol. Chem. 2012, 10, 7457–7474. [Google Scholar] [CrossRef]
- Shi, L.W.; Zhuang, Q.Q.; Wang, T.Q.; Jiang, X.D.; Liu, Y.; Deng, J.W.; Sun, H.H.; Li, Y.; Li, H.H.; Liu, T.B.; et al. Synthetic Antibacterial Quaternary Phosphorus Salts Promote Methicillin-Resistant Staphylococcus aureus-Infected Wound Healing. Int. J. Nanomed. 2023, 18, 1145–1158. [Google Scholar] [CrossRef]
- Guo, A.; Wang, F.; Lin, W.; Xu, X.; Tang, T.; Shen, Y.; Guo, S. Evaluation of Antibacterial Activity of N-Phosphonium Chitosan as a Novel Polymeric Antibacterial Agent. Int. J. Biol. Macromol. 2014, 67, 163–171. [Google Scholar] [CrossRef]
- Pinnock, T.; Voordouw, J.; Voordouw, G. Use of Carbon Steel Ball Bearings to Determine the Effect of Biocides and Corrosion Inhibitors on Microbiologically Influenced Corrosion under Flow Conditions. Appl. Microbiol. Biotechnol. 2018, 102, 5741–5751. [Google Scholar] [CrossRef]
- Okoro, C.C. The Biocidal Efficacy of Tetrakis-hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on Oil Pipeline PigRuns Liquid Biofilms. Pet. Sci. Technol. 2015, 33, 1366–1372. [Google Scholar] [CrossRef]
- Svara, J.; Weferling, N.; Hofmann, T. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Pugachev, M.V.; Shtyrlin, N.; Sysoeva, L.P.; Nikitina, E.V.; Abdullin, T.I.; Iksanova, A.G.; Ilaeva, A.A.; Musin, R.Z.; Berdnikov, E.A.; Shtyrlin, Y.G. Synthesis and Antibacterial Activity of Novel Phosphonium Salts on the Basis of Pyridoxine. Bioorganic Med. Chem. 2013, 21, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Pugachev, M.V.; Shtyrlin, N.V.; Sapozhnikov, S.V.; Sysoeva, L.P.; Iksanova, A.G.; Nikitina, E.V.; Musin, R.Z.; Lodochnikova, O.A.; Berdnikov, E.A.; Shtyrlin, Y.G. Bis-Phosphonium Salts of Pyridoxine: The Relationship between Structure and Antibacterial Activity. Bioorganic Med. Chem. 2013, 21, 7330–7342. [Google Scholar] [CrossRef] [PubMed]
- Dharaskar, S.; Sillanpaa, M. Synthesis, Characterization, and Application of Trihexyl(Tetradecyl)Phosphonium Chloride as Promising Solvent for Extractive Desulfurization of Liquid Fuel. Chem. Eng. Res. Des. 2018, 133, 388–397. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Hermecz, I.; Odinets, I.L. Quaternary Phosphonium Salt and 1,3-Dialkylimidazolium Hexafluorophosphate Ionic Liquids as Green Chemical Tools in Organic Syntheses. Curr. Org. Chem. 2011, 15, 3824–3848. [Google Scholar] [CrossRef]
- Sommers, K.J.; Michaud, M.E.; Hogue, C.E.; Scharnow, A.M.; Amoo, L.E.; Petersen, A.A.; Carden, R.G.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Phosphonium Compounds: An Examination of Non-Nitrogenous Cationic Amphiphiles That Evade Disinfectant Resistance. ACS Infect. Dis. 2022, 8, 387–397. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Guo, S.; Peng, Z.; Tang, T. Novel Water Soluble Phosphonium Chitosan Derivatives: Synthesis, Characterization and Cytotoxicity Studies. Int. J. Biol. Macromol. 2011, 48, 375–380. [Google Scholar] [CrossRef]
- Qian, C.; Xu, X.; Shen, Y.; Li, Y.; Guo, S. Synthesis and Preliminary Cellular Evaluation of Phosphonium Chitosan Derivatives as Novel Non-Viral Vector. Carbohydr. Polym. 2013, 97, 676–683. [Google Scholar] [CrossRef]




| Device-associated | Type of Device | Typical Bacterial Species | Infection | Ref. |
| Contact lenses | E. coli P. aeruginosa S. aureus S. epidermidis Candida spp. Serratia spp. Proteus spp. | Keratitis | [56,57,58] | |
| Central venous catheters | P. aeruginosa Enterobacteriaceae Klebsiella spp. | Bacteremia | [57,58,59,60] | |
| Mechanical heart valves | Streptococci S. aureus S. epidermidis Bacillus spp. Enterococci Candida spp. | PVE | [56,57,58] | |
| Peritoneal dialysis catheters | Staphylococci P. aeruginosa E. coli | CESI TI PI | [61] | |
| Prosthetic joints | S. aureus CN Staphylococci Propionibacterium spp. Enterococcus spp. Gram-negative bacilli Beta-hemolytic streptococci | PPI | [62] | |
| Pacemakers | S. aureus CN S. aureus E. coli K. pneumoniae P. aeruginosa A. baumannii | Endocarditis | [63] | |
| Urinary catheters | E. coli Enterococcus faecalis S. epidermidis P. aeruginosa Proteus mirabilis K. pneumoniae | UTI | [57,58,64,65] | |
| Voice prostheses | Streptococcus mitis Streptococcus sobrinus S. aureus P. aeruginosa | TTI | [66] | |
| Non-device-associated | N.E. | P. aerobicus Fusobacterium nucleatum | Periodontitis | [67,68,69] |
| Different Bacteria Fungi | Osteomyelitis | [70] | ||
| Haemophilus influenzae S. pneumoniae | Otitis media | [71] | ||
| Fusobacterium Streptococcus Veillonella Actinomyces Prevotella Porphyromonas Neisseria Eubacteria Treponema Lactobacterium Haemophilus Eikenella Capnocytophaga Peptostreptococcus Leptotrichia Propionibacterium Staphylococcus | Dental plaque | |||
| Uropathogenic E. coli (UPEC) K. pneumoniae E. faecalis | UTI | |||
| P. aeruginosa | Cystic fibrosis | |||
| Viridans group Streptococci Staphylococcus spp. | Infective endocarditis | |||
| Group A Streptococci | Tonsillitis | |||
| Group A Streptococci | Necrotizing fasciitis | |||
| Gram-negative bacilli | Infection kidney stone |
| Mechanism of Action | Type of Compound | Target Pathogens | Effects on BFs | Contration | [Ref.] Year |
|---|---|---|---|---|---|
| Electrostatic interactions (EI) Membrane depolarization (MD) | C12 di-cationic BODIPY-based fluorescent amphiphiles | S. epidermidis 1 S. epidermidis 2 | Prevention of BF formation Destruction of mature BF | 16 µg/mL | [108] 2023 |
| EI Membrane disruption (MDI) | DMAHDM | Streptococcus mutans | ⇓ Colony-forming unit counts ⇓ Metabolic activity ⇓ Exopolysaccharide synthesis ⇓ Overall acid production ⇓ Tolerance to oxygen stress (OS) | 5 wt% # | [109] 2019 |
| EI, MDI | QASI | S. mutans Lactobacillus acidophilus | Sortase-A protein conformational change ⇓ Carbohydrate intensities Absence of bacterial colonies ⇓ DAPI staining ⇑ Fatty acid compositions | 1–2 wt% # | [110] 2020 |
| MDI Cell lysis | ATAB | S. mutans | ⇓ Viability of BF ⇓ Viability planktonic bacteria | 5 wt% # 10 wt% # | [111] 2022 |
| EI Cell wall disintegration | Amphiphilic quaternized chitosan | S. mutans | 29% residual BF | 1100 µg/mL | [112] 2022 |
| Mixed antifouling + bactericidal actions | PSBMA/PQA4C-10%, PSBMA/PQA4C-30% PSBMA/PQA8C-10% | S. aureus | Prevention of bacterial attachment and BF formation | N.A. | [113] 2019 |
| Contact inhibition | DMADDM | S. mutans | ⇓ Amount of BF ⇓ Metabolic activity ⇓ Lactic acid production ⇓ EPS ⇓ Viable S. mutans in BF | 2.5–10 wt% # | [114] 2020 |
| MDI Bind to DNA gyrase Bind to topoisomerase IV | QA fluoroquinolones | S. aureus | ⇓Total biomass of P. aeruginosa ATCC 15442 BF | 5–10 µM | [115] 2023 |
| P. aeruginosa | 25 µM | ||||
| MDI | QASI (K21) | E. faecalis | Destruction of BF | 0.5 wt% # | [116] 2021 |
| Interference with adhesion Interference with QS | QAL, QAH | S. epidermidis P. aeruginosa | 50% ⇓ in BF formation | 0.037–0.15 mg/mL | [117] 2019 |
| Contact-kill | C14-QAAM C14-QAMAM | S. mutans | ⇓ 99% in BF formation No BF adhesion | 10 wt% # | [118] 2019 |
| MDI | [Ch] [Gly], [Ch] [Ala] | Bacillus cereus Pseudomonas fluorescens. | 56–83% removal of biomass | 70–100 mg/mL | [119] 2022 |
| ⇓ 1.3–2 log CFU/cm2 culturable population | |||||
| Multiple mechanisms | Dequalinium chloride (DQC) | Bacterial vaginosis Gardnerella spp. | ⇓ 80% BF biomass ⇓ 80% metabolic activity | 25.64 µg/mL | [120] 2021 |
| MD | DDAB * | S. aureus | Total removal of BF | 32 µg/mL | [121] 2022 |
| P. aeruginosa | Disruption of BF structure ⇓ BF polysaccharides, proteins, and phospholipids | 600 µg/mL | |||
| MD | DMAHDM | S. mutans S. sanguinis | Suppression of cariogenic species in BF Modulation of BF equilibrium from cariogenic state to non-cariogenic state | 3% wt# | [122] 2023 |
| Formation of eDNA–PHMG-Cl complexes | PHMG-Cl | P. aeruginosa ATCC S. aureus ATCC | Blockage of BF development | 0.1% and 0.5% | [123] 2022 |
| Interference with the ion transport Membrane lysis | QASI | S. mutans L. acidophilus Actinomyces naeslundii S. sanguis | Significant decrease in BF growth | 2% | [124] 2020 |
| EI, MDI, Cytoplasmic leakage | DMADDM | N.R. | ⇓ BF viability ⇓ BF formation | 1.1–2.2 wt% # | [125] 2023 |
| N.R. | DADMAC-based coatings | Methicillin-resistant S. aureus (MRSA) | ⇓ 99% adhesion and BF grow | N.R. | [126] 2018 |
| Vancomycin-resistant Enterococcus spp. (VRE) | ⇓ 94% adhesion and BF grow | ||||
| N.R. | Guanidinium anthranilamide compounds | S. aureus | ⇓ 83–92%established BF | 62.4–64 µM | [127] 2018 |
| MDI | QADM | S. mutans | (1–3 days) Significant inhibition of BF formation | 10 wt% # | [128] 2022 |
| N.R. | Pyridine-based QASs | E. coli | BF eradication (MBEC) | 16 mg/L | [129] 2018 |
| S. aureus | 8–16 mg/L | ||||
| EI, MDI | GTMAC-modified PCL:PEG:GelMA | E. coli | Significant ⇓ in CFU | N.R. | [130] 2022 |
| S. aureus | |||||
| S. epidermidis | |||||
| EI, membrane infiltration MDI | Metal-free quaternized carbon dots | S. aureus | ⇓ BF viability ⇓ BF formation | 1000 µg/mL | [131] 2019 |
| MDI DNA damage ROS generation | MUTAB-based AuNCs | B.subtilis E. faecalis S. pneumoniae E. coli P. aeruginosa C. albicans | No live bacteria in BF of E. coli | 200 µg/mL | [132] 2020 |
| Hydrophobic and EI MDI ROS production DNA damage | Si-QAC/TEOS NPs | S. aureus | Eradication of mature BF Inhibition of BF formation | 100 µg/mL | [133] 2022 |
| Disrupt the charge balance of bacterial membranes ⇑ Membrane permeability ⇑ ROS by irradiation at 660 nm | CP5/TFPP-QA | E. coli MRSA | 70–80% BF dispersion | 100 µg/mL | [134] 2022 |
| N.R. | QA polyethyleneimine PEI1200-C6 | E. coli P. aeruginosa B. amyloliquefaciens S. aureus | >80% BF dispersion | 1–8 mg/mL | [135] 2019 |
| MD MDI | phenylglyoxamide-based QA iodide | S. aureus | 70% inhibition of BF formation 44% BF disruption | 16 µM 32 μM | [136] 2021 |
| E. coli | 28% BF disruption | 64 μM | |||
| Contact Killing | DMAHDM | Streptococci S. mutans Lactobacilli | ⇓ 80% metabolic activity ⇓ 95% lactic acid production | 3–5 wt% # | [137] 2020 |
| Acidic release of AgNPs MDI by QAS DNA damage | Ag@QAS@CM | S. aureus | ⇓ 80% BF mass | 6.25 μg/mL (Ag) | [138] 2020 |
| EPS penetration ability Acidic release of CuNPs MDI by QAS DNA damage | Cu@QAS@CM | Sulfate-reducing bacteria (SRB) | ⇓ 75% BF mass | 250 μM | [139] 2022 |
| EI, MDI, cytoplasmic leakage | DMADDM | S. mutans S. sanguinis S. gordonii | Significant ⇓ of multispecies BFs Significant ⇓ in metabolic activity of multispecies BFs | 40–200 μg/mL | [140] 2019 |
| Displacement of divalent cations from membrane Membrane destabilization Lysis | QA carbosilane dendrimers and dendron | S. aureus | BF inhibition | 16–64 μg/mL MBIC | [141] 2023 |
| BF eradication | 32–64 μg/mL MBEC | ||||
| MRSA | BF inhibition | 16–64 μg/mL MBIC | |||
| BF eradication | 16–64 μg/mL MBEC | ||||
| MDI ROS generation | Cu2+/PDDA | S. aureus | ⇓ EPS Kill cells in BF | 144 μg/mL PDDA 26.5 μM Cu2+ | [142] 2023 |
| EI, MDI, cytoplasmic leakage | N-alkylated pyridine-based QAS | S.aureus E. coli | 52–95% inhibition of BF formation | 50–250 μg/mL | [143] 2020 |
| MDI Pyridoxal-dependent enzyme targeting | Pyridoxine-based QAS of terbinafine (KFU127) | S. aureus (SA) C. albicans (CA) E. coli (EC) S. epidermidis (SE) | Full destruction of cells in BF | 400 μg/mL (CA) 400–800 μg/mL (CA+SA) | [144] 2020 |
| ⇓ >3 LogCFU/cm2 | 128 μg/mL (SA) 128 μg/mL (SE) 256 μg/mL (EC) | ||||
| EI, MDI, PAI | N-Alkyl pyridinium QAS | E. faecalis | 85–100% killed cells in BF No BF removal | 250 µM (60′) | [145] 2020 |
| 65–95% killed cells in BF 15–20% BF removal | 250 µM 1′ + 10 s PAI | ||||
| EI, MDI, EPS permeation | Pyridine-based tris-QASs | E. coli | MBIC MBEC | 8–32 μg/mL 16–32 μg/mL | [146] 2023 |
| K. pneumoniae | MBIC MBEC | 16–64 μg/mL 64–500 μg/mL | |||
| S. aureus | MBIC MBEC | 8 μg/mL 16 μg/mL | |||
| P. aeruginosa | MBIC MBEC | 64 μg/mL 250–500 μg/mL | |||
| A. baumannii | MBIC MBEC | 32 μg/mL 250 μg/mL | |||
| C. albicans | MBIC MBEC | 4–16 μg/mL 8–32 μg/mL | |||
| EI, MDI, cell lysis | DTAB vs. C6 | P. aeruginosa | Full eradication of BF (C6, 4 h) | 0.29 mM | [147] 2018 |
| EI, MDI, cytoplasmic leakage | TMACS | E. coli | BF inhibition rate 57.6% | 156 µg/mL | [148] 2023 |
| BF removal rate 41.6% | 2.5 mg/mL | ||||
| S. aureus | BF inhibition rate 58.5% | 20 µg/mL | |||
| BF removal rate 59.01% | 2.5 mg/mL | ||||
| EI, MDI | Glut/ADBAC | P. aeruginosa | 5 log killing of sessile cells | 1000 ppm (2 h) 100 ppm (24 h) | [149] 2010 |
| CDA | 100 ppm (2 h) 10 ppm (24 h) | ||||
| Glut/ADBAC | 30% (24 h)–35% (2 h) biomass residual | 100 ppm | |||
| 45% (24 h)–40% (2 h) biomass residual | 1000 ppm | ||||
| CDA | 30% (24 h)–30% (2 h) biomass residual | 100 ppm | |||
| 20% (24 h)–25% (2 h) biomass residual | 1000 ppm | ||||
| N.R. | Barquat® MB-50 Ucarcide® 42 | P. aeruginosa | N.R. | N.R. | [150] 2010 |
| EI, MDI EPS penetration ability due to the C18 chain | Pyridoxine-based QASs | S. aureus S. epidermidis | QAS (1–3) killed BF-detached Staphylococci cells | 16–32 µg/mL | [151] 2015 |
| S. aureus | MBCadh (QAS 3) | 32 µg/mL | |||
| S. epidermidis | MBCadh (QAS 3) | 16 µg/mL | |||
| EI by well-accessible positive charges Host–guest properties of pillar[n]arenes | Pillar[n]arene QASs and imidalonium salts | S. aureus E. faecalis S. epidermidis S. mutans | Inhibition of BF formation (MBIC50) No biocidal, no hemolytic | 0.4–8.8 µM | [152] 2016 |
| Pillar [5]arene QASs | S. aureus E. faecalis | Inhibition of BF formation (MBIC50) No biocidal, no hemolytic | 2–4 µg/mL | [153] 2016 |
| Mechanism of Action | Type of Compound | Target Pathogens | Effects on BF | Concentration | [Ref.] Year |
|---|---|---|---|---|---|
| EI, MDI, cytoplasmic leakage | TMPCS TPPCS | E. coli | BF inhibition rate 33.9% (TMPCS), 56.6% (TPPCS) | 156 µg/mL | [148] 2023 |
| BF removal rate 46.4% (TMPCS), 48.9% (TPPCS) | 2.5 mg/mL | ||||
| S. aureus | BF inhibition rate 53.8% (TMPCS), 62.2% (TPPCS) | 20 µg/mL | |||
| BF removal rate 60.4% (TMPCS), 69.9% V | 2.5 mg/mL | ||||
| Disrupts disulfide bonds on the cell surface | THPS | P. aeruginosa | 5 log killing of sessile cells | 100 ppm (2 h) 100 ppm (24 h) | [149] 2010 |
| 35% (24 h)–45% (2 h) biomass residual | 100 ppm | ||||
| 40% (24 h)–40% (2 h) biomass residual | 1000 ppm | ||||
| N.R. | Bellacide® 350 Tolcide® PS75 | P. aeruginosa | N.R. | N.R. | [150] 2010 |
| EI, MDI Partial diffusion in EPS | Pyridoxine-based QPSs | S. aureus S. epidermidis | QPS (6) killed detached S. epidermidis cells 68–77% cells located in BF killed | 32 µg/mL 8 µg/mL | [151] 2015 |
| EI by well-accessible positive charges on QPSs Host–guest properties of pillar[n]arenes | Pillar [5]arene QPS | S. aureus E. faecalis | Inhibition of BF formation (MBIC50) No biocidal, no hemolytic | 2–4 µg/mL | [153] 2016 |
| N.R. | TTPC | S. aureus | ⇓ BF (OD545 < 1) | 20 µg/mL | [170] 2015 |
| P. aeruginosa | ⇓ BF (OD545 < 1) | 40 µg/mL | |||
| ⇓ Biofilm thickness (73.9%) and volume (73.8%) | 40 µg/mL | ||||
| QS disruption | ATPB | Chromobacterium violaceum | Inhibition of BF formation (violacein inhibition) | 52.9–142.2 μM * | [171] 2015 |
| Vibrio harveyi. | Inhibition of BF formation (bioluminescence inhibition) | 128.6–348.5 μM * | |||
| EI, MDI Cell lysis, cytoplasmic leakage | TATPBP (P6P-10,10) | EDR A. baumannii | BF eradication (MBEC) | MBEC 32–63 µM | [45] 2022 |
| EI, MDI, MD ROS production | (1,2-DBTPP)Br2 (1,4-DBTPP)Br2 (1,6-DBTPP)Br2 | S. aureus | 100% inhibition of BF formation | 64–128 µg/mL ** | [173] 2023 |
| 80% ⇓ metabolic activity | 32–64 µg/mL ** | ||||
| MRSA | 100% inhibition of BF formation | 128–256 µg/mL ** | |||
| 80% ⇓ metabolic activity | 64–128 µg/mL ** | ||||
| EI with cell surface Cell wall penetration | NCPS | S. aureus | Significant ⇓ of BF formation | 64–128 µg/mL | [174] 2014 |
| E. coli | 64–256 µg/mL | ||||
| CTPBs | S. aureus E. coli | Not active | MIC > 1600 µg/mL | ||
| N.R. | THPS | Desulfovibrio Desulfomicrobium Desulfocurvus | ⇓ Microbiologically influenced corrosion | 17% | [175] 2018 |
| N.R. | THPS | SRB APB | Significant ability to penetrate BF 100% inhibition of SRB >85% inhibition of APB | 0.6% | [176] 2015 |
| Antioxidant effects Hydroxyl radical scavenging | ATBPB, ATPB | MDR A. baumannii | N.R. | 6.25–25.0 μM | [158] 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S. Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms. Pharmaceutics 2024, 16, 80. https://doi.org/10.3390/pharmaceutics16010080
Alfei S. Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms. Pharmaceutics. 2024; 16(1):80. https://doi.org/10.3390/pharmaceutics16010080
Chicago/Turabian StyleAlfei, Silvana. 2024. "Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms" Pharmaceutics 16, no. 1: 80. https://doi.org/10.3390/pharmaceutics16010080
APA StyleAlfei, S. (2024). Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms. Pharmaceutics, 16(1), 80. https://doi.org/10.3390/pharmaceutics16010080






