An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. HPLC Characterization of the Extracts
2.3. Effects of the Extracts on Ileum and Trachea Contractions
2.3.1. Experimental Animals
2.3.2. Isolation and Placement of Ileum and Trachea
2.3.3. Experimental Design with Ileum
2.3.4. Experimental Design with Rat Trachea
2.4. Molecular Docking Analysis
2.5. Evaluation of Antimicrobial Activity of the Extracts
2.6. Statistical Analysis
3. Results
3.1. Chemical Characterization of the Extracts
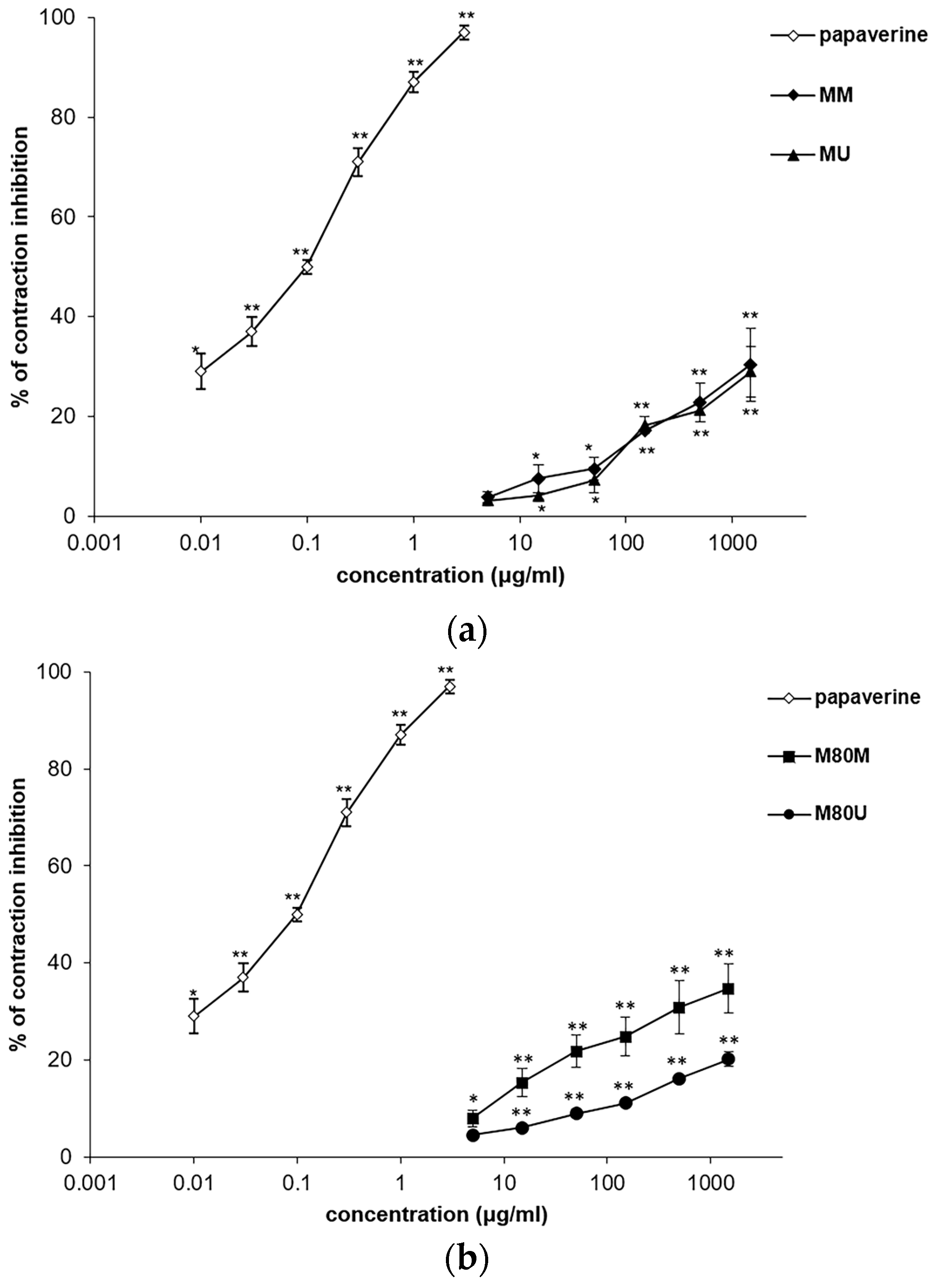
3.2. Spasmolytic Effects of the Extracts on Spontaneous Ileum Contractions
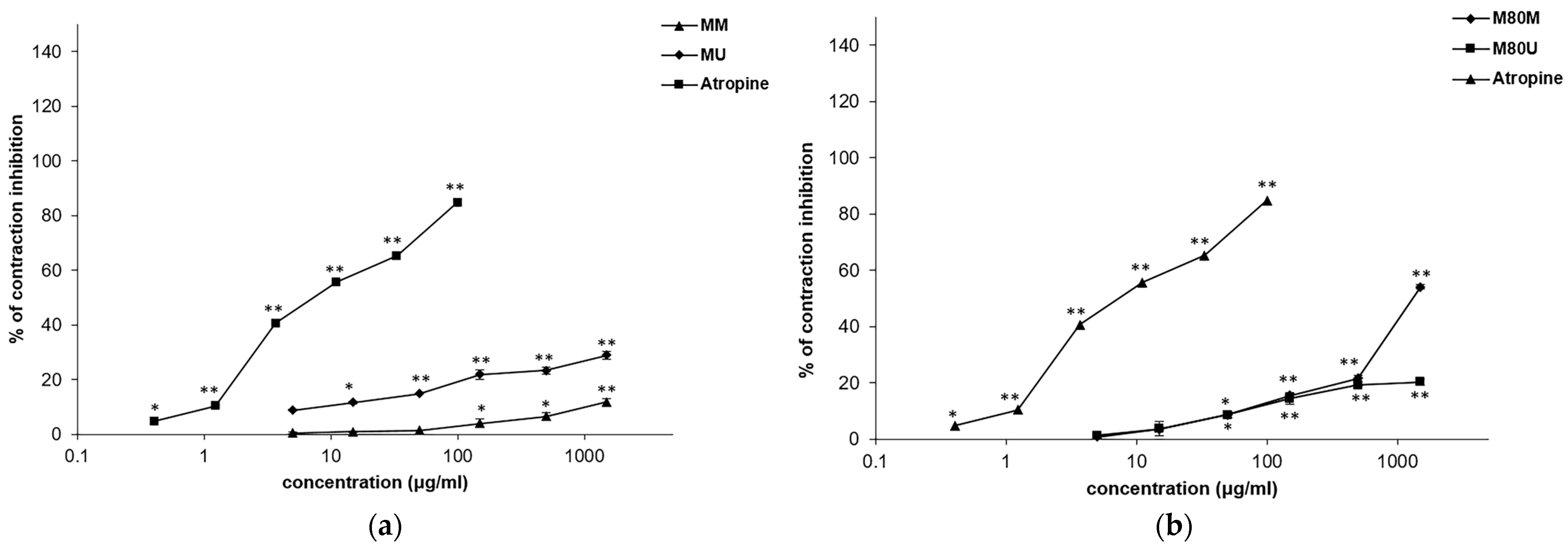
3.3. Spasmolytic Effects of the Extracts on KCl-Induced Ileum Contractions
3.4. Spasmolytic Effects of the Extracts on Acetylcholine-Induced Ileum Contractions
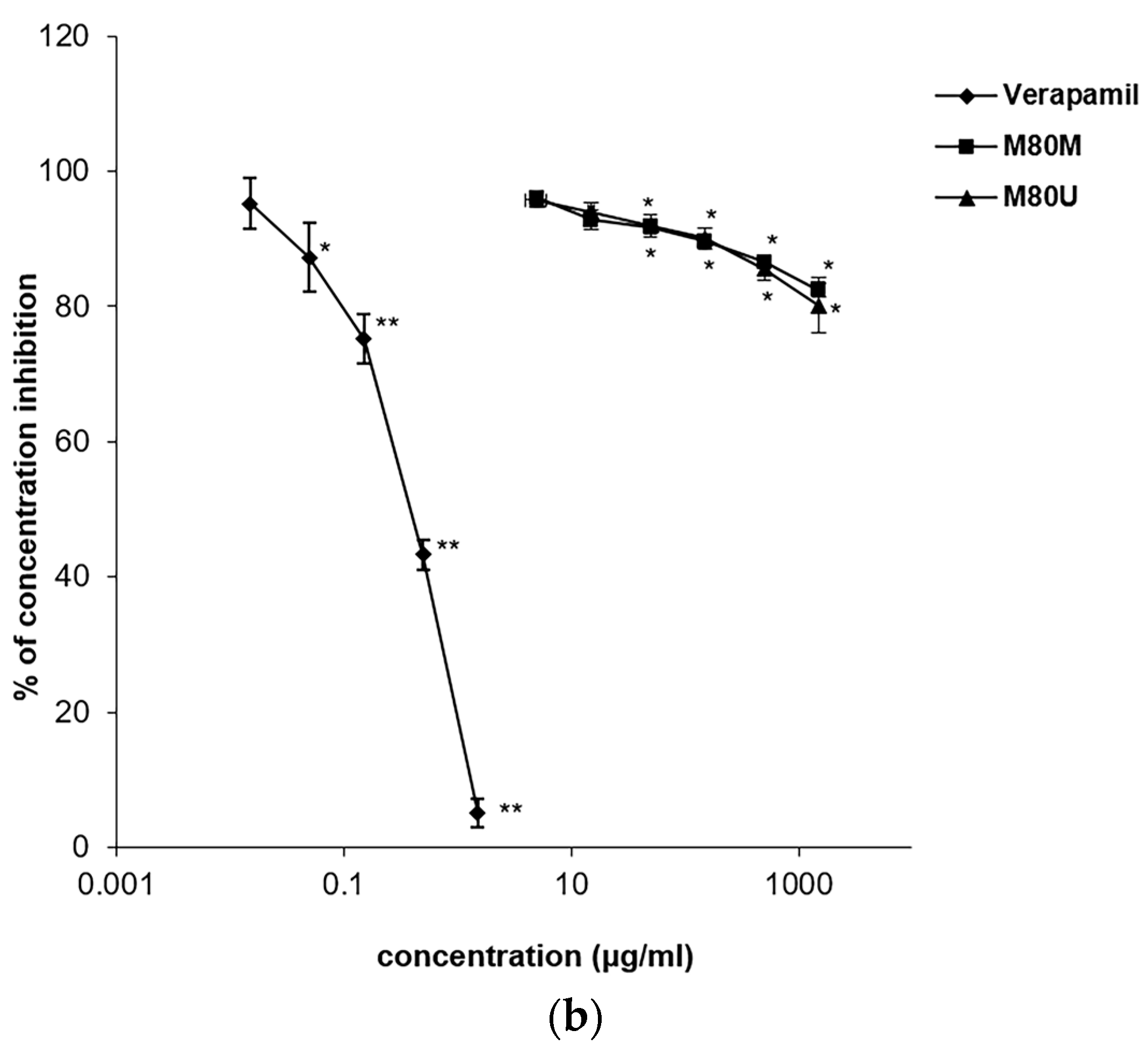
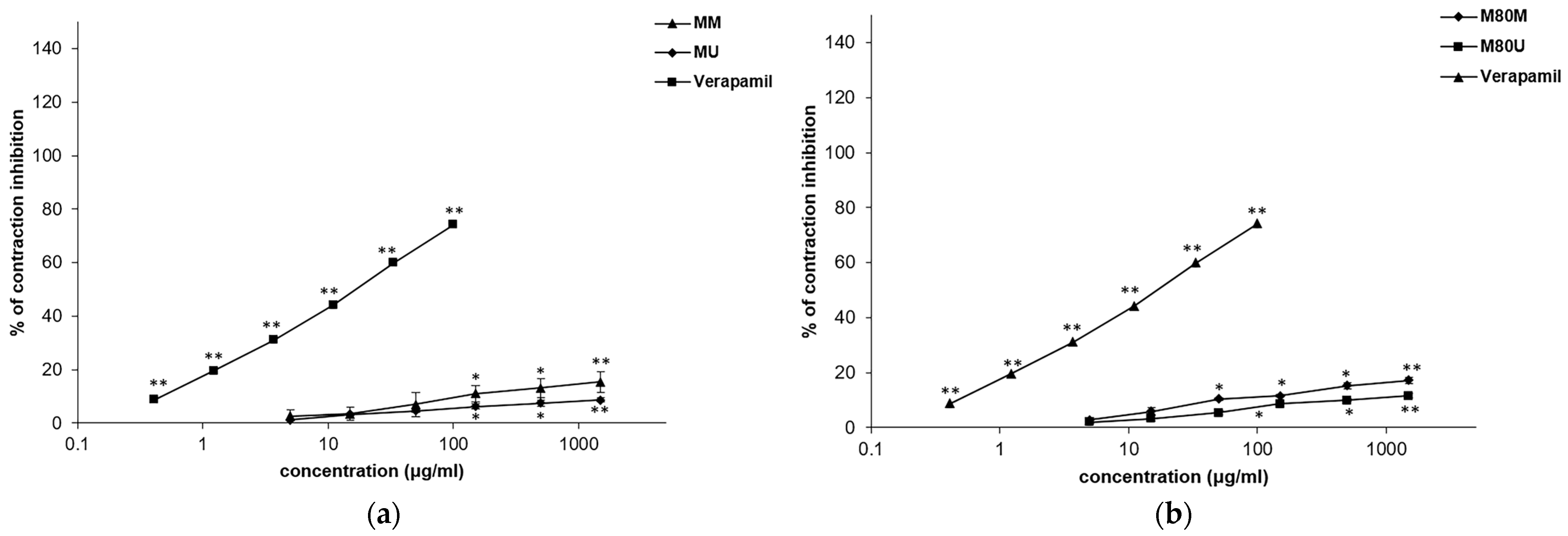
3.5. Spasmolytic Effects of the Extracts on KCl-Induced Tracheal Contractions
3.6. Spasmolytic Effects of the Extracts on Carbachol-Induced Tracheal Contractions
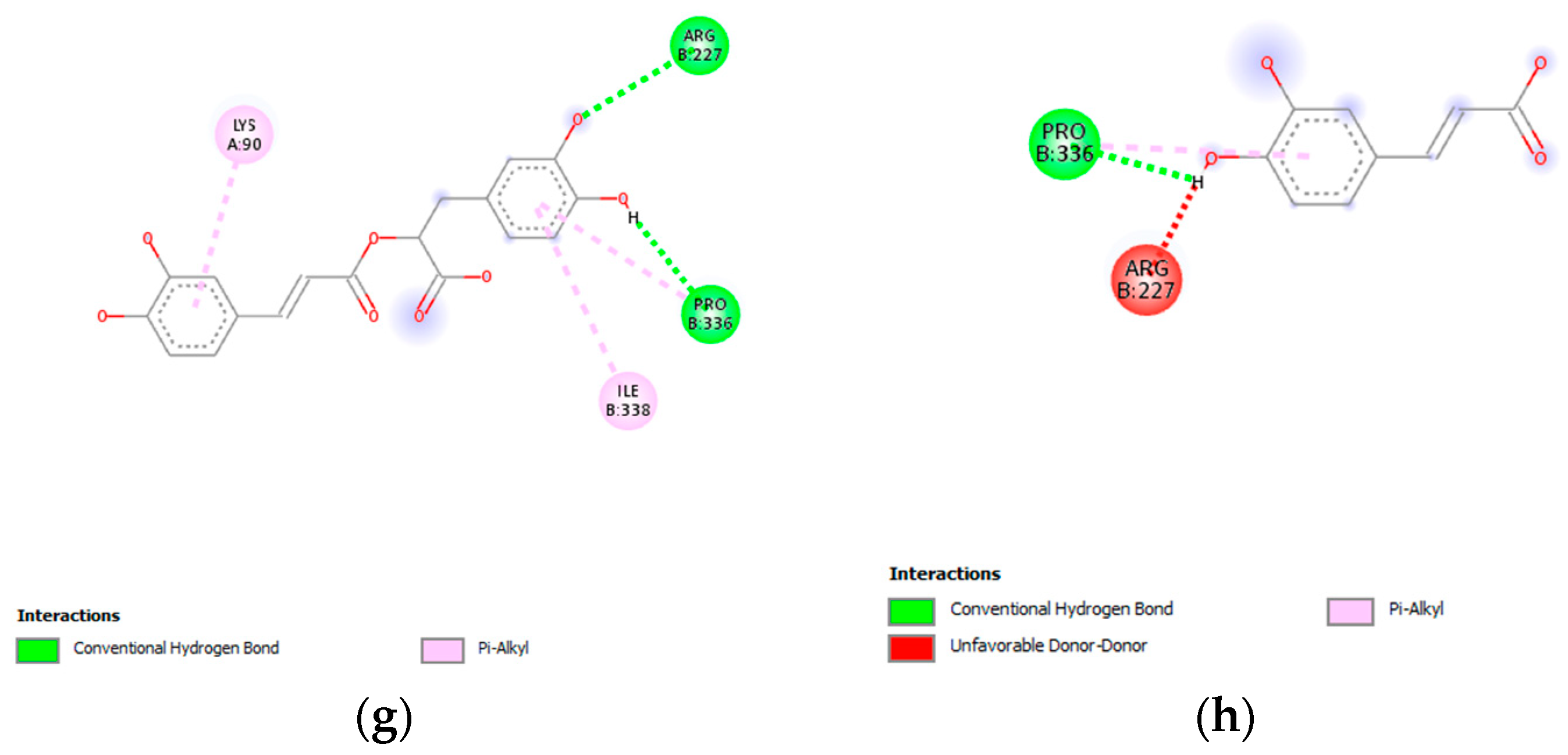
3.7. Molecular Docking Analysis
3.8. Antimicrobial Activity of the Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palhares, R.M.; Drummond, M.G.; Brasil, B.D.S.A.F.; Cosenza, G.P.; Brandão, M.D.G.L.; Oliveira, G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS ONE 2015, 10, e0127866. [Google Scholar] [CrossRef]
- Ahn, K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017, 50, 111–116. [Google Scholar] [CrossRef]
- Bone, K.; Mills, S.Y. Principles and Practice of Phytotherapy, Modern Herbal Medicine, 2nd ed.; Churchill Livingstone Elsevier: London, UK, 2013. [Google Scholar]
- Ben-Arye, E.; Dudai, N.; Eini, A.; Torem, M.; Schiff, E.; Rakover, Y. Treatment of upper respiratory tract infections in primary care: A randomized study using aromatic herbs. Evid. Based Complement. Altern. Med. 2011, 2011, 690346. [Google Scholar] [CrossRef]
- Engelbertz, J.; Lechtenberg, M.; Studt, L.; Hensel, A.; Verspohl, E.J. Bioassay-guided fractionation of a thymol-deprived hydrophilic thyme extract and its antispasmodic effect. J. Ethnopharmacol. 2012, 141, 848–853. [Google Scholar] [CrossRef]
- Cosio, B.G.; Rosado, J.R.; Rossi, F.F. Asthma: Epidemiology, pathophysiology, and risk factors. In Clinical Respiratory Medicine, 4th ed.; Spiro, S.G., Silvestri, G.A., Agusti, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Carović-Stanko, K.; Petek, M.; Martina, G.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Satović, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Baricevic, D.; Bartol, T. The biological/pharmacological activity of the Salvia genus. In Sage: The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Dweck, A.C. Folklore and cosmetic use of various Salvia species. In Sage: The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Koutsaviti, A.; Tzini, D.I.; Tzakou, O. Greek Salvia sclarea L. essential oils: Effect of hydrodistillation time, comparison of the aroma chemicals using hydrodistillation and HS-SPME techniques. Rec. Nat. Prod. 2016, 10, 800–805. [Google Scholar]
- Lawrence, B.M. Production of Clary Sage Oil and Sclareol in North America. In Proceedings of the 4emes Recontres Internacionales, Nyons, France, 5–7 December 1994; pp. 41–58. [Google Scholar]
- Leporatti, M.L.; Pavesi, A.; Posocco, E. Phytotherapy in the Valnerina Marche (Central Italy). J. Ethnopharmacol. 1985, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Hansel, R.; Keller, K.; Rimpler, H.; Schneider, G. Hagers Handbuch der Pharmazeutischen Praxis: Drogen P-Z Folgeband 2; Springer: Berlin, Germany, 1994. [Google Scholar]
- Raafat, K.; Habib, J. Phytochemical compositions and antidiabetic potentials of Salvia sclarea L. essential oils. J. Oleo Sci. 2018, 67, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.D.; Peana, A.T.; Satta, M. A study on anti-inflammatory and peripheral analgesic action of Salvia sclarea oil and its main components. J. Essent. Oil Res. 1997, 9, 199–204. [Google Scholar] [CrossRef]
- Zengin, G.; Senkardes, I.; Mollica, A.; Picot-Allain, C.M.N.; Bulut, G.; Dogan, A.; Mahomoodally, M.F. New insights into the in vitro biological effects, in silico docking and chemical profile of clary sage—Salvia sclarea L. Comput. Biol. Chem. 2018, 75, 111–119. [Google Scholar] [CrossRef]
- Kuzma, L.; Kalemba, D.; Rozalski, M.; Rozalska, B.; Wieckowska-Szakiel, M.; Krajewska, U.; Wysokińska, H. Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Randjelović, M.; Branković, S.; Miladinović, B.; Milutinović, M.; Živanović, S.; Mihajilov-Krstev, T.; Kitić, D. The benefits of Salvia sclarea L. ethanolic extracts on gastrointestinal and respiratory spasms. S. Afr. J. Bot. 2022, 150, 621–632. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Eriş, C.; Sönmez, U.; Kartal, M.; Kurucu, S.; Bozok-Johansson, C. Terpenoids from Salvia sclarea. Phytochemistry 1994, 36, 971–974. [Google Scholar] [CrossRef]
- Graves, N.S. Acute gastroenteritis. Prim. Care 2013, 40, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Lumb, A.B.; Horncastle, E. 29—Pulmonary physiology. In Pharmacology and Physiology for Anesthesia, 2nd ed.; Hugh, C., Hemmings, H.C., Egan, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar] [CrossRef]
- Pharmacopoea Jugoslavica, 4th ed.; Federal Institute of Public Health: Belgrade, Serbia, 1984.
- Kostić, M.; Kitić, D.; Petrović, M.B.; Jevtović-Stoimenov, T.; Jović, M.; Petrović, A.; Živanović, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Branković, S.; Šavikin, K.; Kostić, M.; Kitić, N.; Miladinović, B.; Kitić, D. Antispasmodic effects of black chokeberry (Aronia melanocarpa (Michx.) Elliott) extracts and juice and their potential use in gastrointestinal disorders. J. Berry Res. 2020, 10, 175–192. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 15th Informational Supplement, M100-S15; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2005. [Google Scholar]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Elez Garofulić, I.; Bosiljkov, T.; Ježek, D.; Brnčić, M. Comparison of conventional and ultrasound-assisted extraction techniques on mass fraction of phenolic compounds from sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- de Lima Silva, L.; Heldwein, C.G.; Reetz, L.G.B.; Zanella, R.; Pereira, A.M.S.; Heinzmann, B.M. Influence of extraction method on antibacterial activity of ethanolic extracts of Ocimum gratissimum L. J. Med. Plants Res. 2015, 9, 199–206. [Google Scholar] [CrossRef]
- Sruthi, D.R.; Indira, G. A comparative evaluation of maceration, soxhlation and ultra sound assisted extraction for the phytochemical screening of the leaves of Nephelium lappaceum. L. (Sapindaceae). J. Pharmacogn. Phytochem. 2016, 5, 386–389. [Google Scholar]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef]
- Mapel, D.W. Functional disorders of the gastrointestinal tract: Cost effectiveness review. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 913–931. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA/HMPC/277152/2015. European Union Herbal Monograph on Salvia officinalis L., Folium; European Medicines Agency: Amsterdam, The Netherlands, 2016.
- Gómez-Rivera, A.; González-Cortazar, M.; Gallegos-García, A.J.; Escobar-Ramos, A.; Flores-Franco, G.; Lobato-García, C.E. Spasmolytic, anti-inflammatory, and antioxidant activities of Salvia gesneriflora Lindley. Afr. J. Tradit. Complement. 2018, 15, 72–82. [Google Scholar] [CrossRef]
- Islam, A.; Amjad, A.; Maisa, A.Q.; Mohamed, A.E.; Maha, E.; Darwish, B. Spasmolytic effects of Salvia triloba leaf extract on smooth muscles of the duodenum in rats. Ethno Med. 2019, 13, 169–174. [Google Scholar] [CrossRef]
- Omar, G.; Dwikat, M.; Abdallah, L.; Ismaeil, S. Effect of ethanol extract from five species of Salvia on the spontaneous contractile activity of isolated rabbit ileum. Palest. Med. Pharm. J. 2019, 4, 41–47. [Google Scholar] [CrossRef]
- Todorov, S.; Philianos, S.; Petkov, V.; Harvala, C.; Zamfirova, R.; Olimpiou, H. Experimental pharmacological study of three species from genus Salvia. Acta Physiol. Pharmacol. Bulg. 1984, 10, 13–20. [Google Scholar]
- Karaki, H.; Weiss, G. Calcium release in smooth muscles. Life Sci. 1988, 42, 111–122. [Google Scholar] [CrossRef]
- Godfrain, T. Calcium entry blockade and excitation contraction coupling in the cardiovascular system (with an attempt of pharmacological classification). Acta Pharmacol. Toxicol. 1986, 58, 5–30. [Google Scholar] [CrossRef]
- Ehlert, F.J.; Sawyer, G.W.; Esqueda, E.E. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999, 64, 387–394. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, Y.; Wang, X.Q.; Tian, F.; Cao, H.; Wang, M.W.; Sun, Q.S. Effects of magnolol and honokiol derived from traditional Chinese herbal remedies on gastrointestinal movement. World J. Gastroenterol. 2005, 11, 4414–4418. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F. Scientific basis for medicinal use of Citrullus lanatus (Thunb.) in diarrhea and asthma: In vitro, in vivo and in silico studies. Phytomedicine 2022, 98, 153978. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Khayrani, A.C.; Irdiani, R.; Aditama, R.; Pratami, D.K.; Lischer, K.; Ansari, M.J.; Chinnathambi, A.; Alharbi, S.A.; Almoallim, H.S.; Sahlan, M. Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study. J. King Saud Univ. Sci. 2021, 33, 101297. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Hart, S. A preliminary study of the effect of essential oils on skeletal and smooth muscle in vitro. J. Ethnopharmacol. 1997, 58, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bazylko, A.; Zygmunt, M.; Sapa, J.; Strzelecka, H.; Filipek, B. Determination of spasmolytic and antispasmodic activities of thyme extracts and one of their major components, rosmarinic acid, in isolated rabbit ileum and isolated rat aorta. Acta Biol. Crac. Ser. Zool. 2009, 51, 49–54. [Google Scholar]
- Lemmens-Gruber, R.; Marchart, E.; Rawnduzi, P.; Engel, N.; Benedek, B.; Kopp, B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea-pig ilea. Arzneimittelforschung 2006, 56, 582–588. [Google Scholar] [CrossRef]
- Abdalla, S.; Zarga, A.; Sabri, S. Effects of the flavone luteolin, isolated from Colchicum richii, on guinea-pig isolated smooth muscle and heart and on blood pressure and blood flow. Phytother. Res. 1994, 8, 265–270. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghanadian, M.; Asghari, G.; Sekhavati, N. Antispasmodic activity of apigenin and luteolin, two components of Dracocephalum kotschyi extract, on rat ileum contractions. J. HerbMed Pharmacol. 2018, 7, 100–105. [Google Scholar] [CrossRef]
- Blažeković, B.; Stanić, G.; Vladimir-Knežević, S. Morphology, anatomy and phytochemistry of Thymus vulgaris L. and Thymus pulegioides L. Farm. Glas. 2006, 62, 121–130. [Google Scholar]
- Janbaz, K.H.; Hamid, I.; Qadir, M.I. Spasmolytic, bronchodilator and vasodilator activities of aqueous-methanolic extract of Ocimum basilicum. Int. J. Agric. Biol. 2014, 16, 321–327. [Google Scholar]
- Eftekhar, N.; Moghimi, A.; Boskabady, M.H. Prophylactic effect of rosmarinic acid on tracheal responsiveness, white blood cell count and oxidative stress markers in lung lavage of sensitized rats. Pharmacol. Rep. 2018, 70, 119–125. [Google Scholar] [CrossRef]
- Ko, W.C.; Shih, C.M.; Leu, I.J.; Chen, T.T.; Chang, J.P. Mechanisms of relaxant action of luteolin in isolated guinea pig trachea. Planta Med. 2005, 71, 406–411. [Google Scholar] [CrossRef]
- Chen, J.L.; Ko, W.C. Relaxation of isolated guinea-pig trachea by apigenin, a constituent of celery, via inhibition of phosphodiesterase. Eur. J. Pharmacol. 2017, 811, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Ozçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Soković, M. Antibacterial and antifungal activities of methanol extract and phenolic compounds from Diospyros virginiana L. Ind. Crops Prod. 2014, 59, 210–215. [Google Scholar] [CrossRef]
- Chiruvella, K.K.; Mohammed, A.; Dampuri, G.; Ghanta, R.G.; Raghavan, S.C. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O-glucoside isolated from callus cultures of Soymida febrifuga. Int. J. Biomed. Sci. 2007, 3, 269–278. [Google Scholar]
- Cottiglia, F.; Loy, G.; Garau, D.; Floris, C.; Caus, M.; Pompei, R.; Bonsignore, L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine 2001, 8, 302–305. [Google Scholar] [CrossRef]
- Alimpić, A.; Knežević, A.; Milutinović, M.; Stević, T.; Šavikin, K.; Stajić, M.; Marković, S.; Marin, P.D.; Matevski, V.; Duletić-Laušević, S. Biological activities and chemical composition of Salvia amplexicaulis Lam. extracts. Ind. Crops Prod. 2017, 105, 1–9. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef]
- Parker, D.; Prince, A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol. 2012, 34, 281–297. [Google Scholar] [CrossRef]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Microbe Profile: Listeria monocytogenes: A paradigm among intracellular bacterial pathogens. Microbiology 2019, 165, 719–721. [Google Scholar] [CrossRef]
- Chovanova, R.; Mikulašova, M.; Vaverkova, Š. In vitro antibacterial and antibiotic resistance modifying effect of bioactive plant extracts on methicillin-resistant Staphylococcus epidermidis. Int. J. Microbiol. 2013, 2013, 760969. [Google Scholar] [CrossRef]
- Gulcin, I.; Uguz, M.T.; Oktay, M.; Beydemir, S.; Kufrevioglu, O.I. Evaluation of the antioxidant and antimicrobial activities of clary sage (Salvia sclarea L.). Turk. J. Agric. For. 2004, 28, 25–33. [Google Scholar]
- Wilson, M.G.; Pandey, S. Pseudomonas aeruginosa; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557831/ (accessed on 12 January 2023).
- Tulukcu, E.; Sagdic, O.; Albayrak, S.; Ekici, L.; Yetim, H. Effect of collection time on biological activity of clary sage (Salvia sclarea). J. Appl. Bot. Food Qual. 2009, 83, 44–49. [Google Scholar]
- Kuzma, L.; Rozalski, M.; Walencka, E.; Rozalska, B.; Wysokinska, H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: Salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine 2007, 14, 31–35. [Google Scholar] [CrossRef]
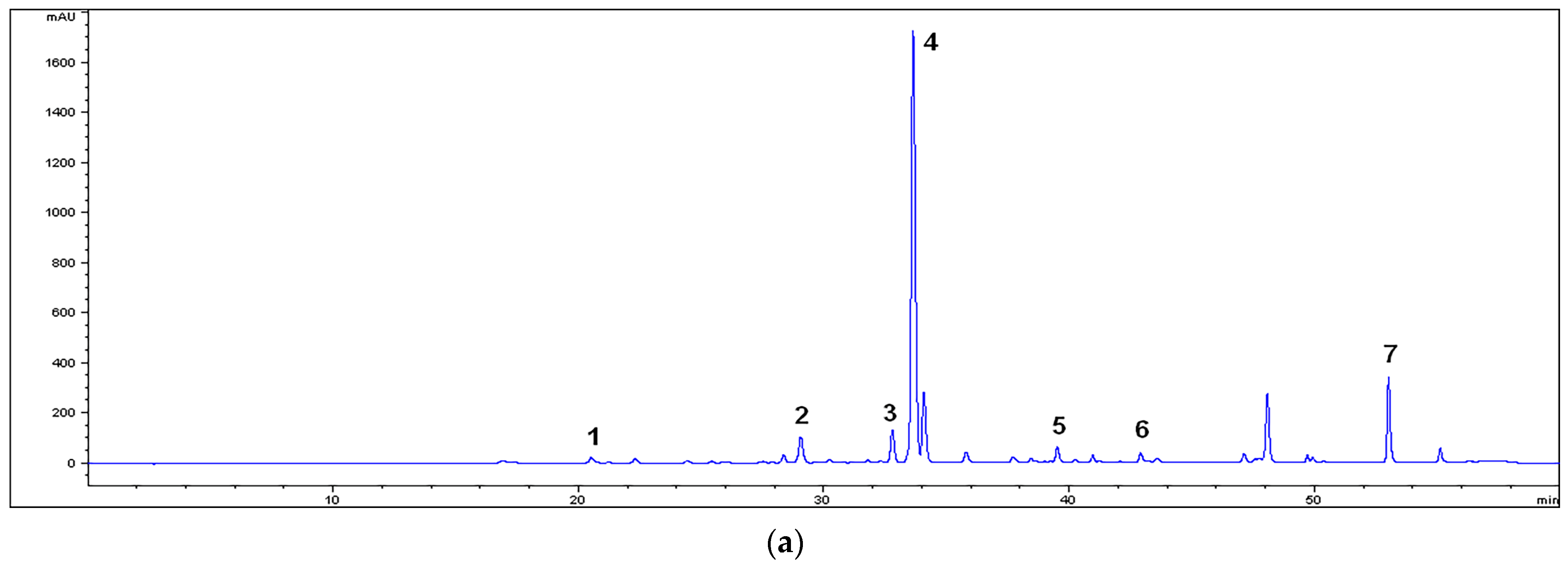
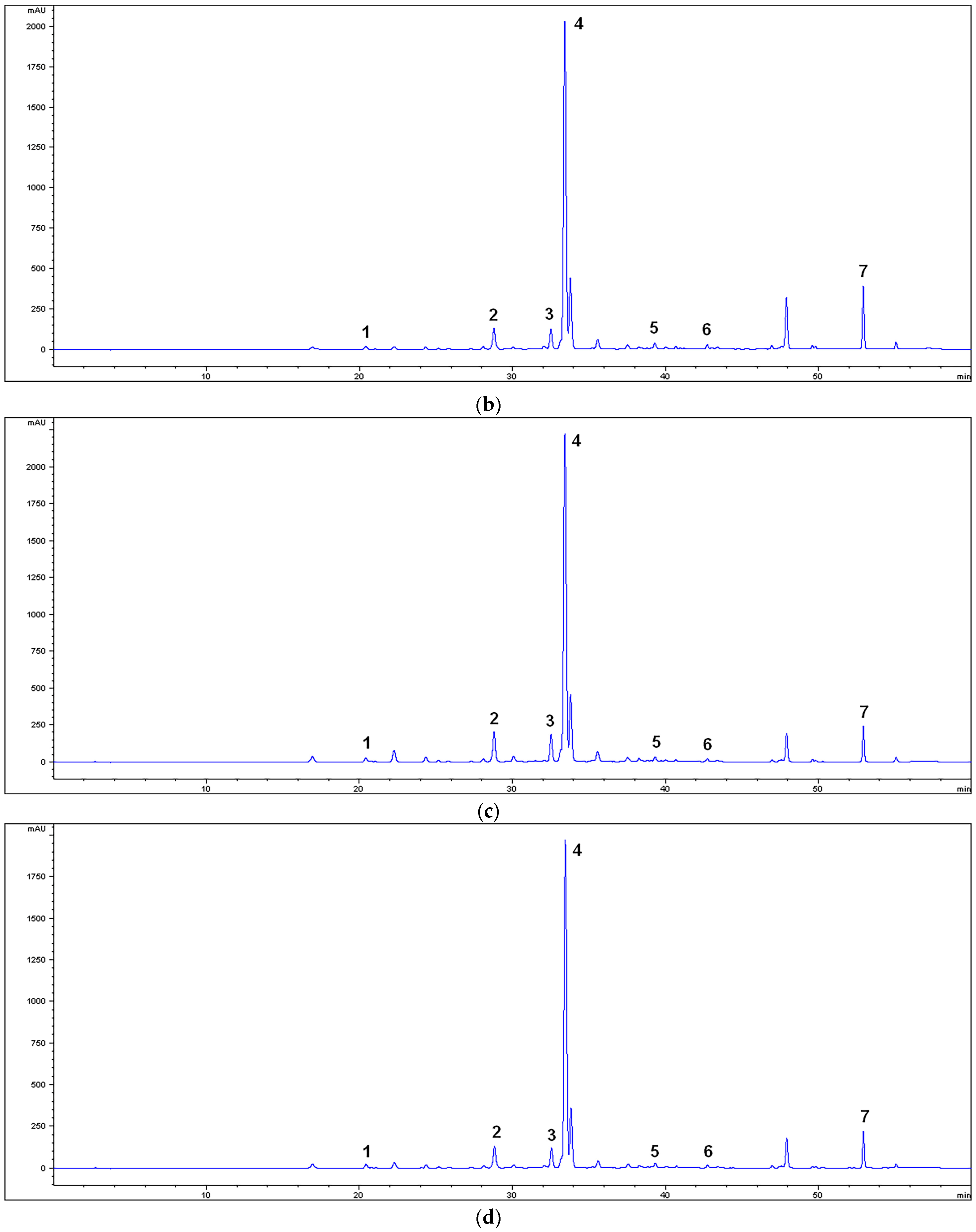



| Compounds | RT (min) | MM | MU | M80M | M80U |
|---|---|---|---|---|---|
| µg/mg | |||||
| caffeic acid | 20.5 | 0.79 ± 0.02 a | 0.63 ± 0.04 b | 0.97 ± 0.07 b | 0.81 ± 0.01 a |
| luteolin-7-O-glucoside | 29.0 | 5.18 ± 0.21 a | 5.65 ± 0.34 a | 8.84 ± 0.54 b | 5.61 ± 0.31 a |
| apigenin-7-O-glucoside | 32.5 | 5.63 ± 0.21 a | 4.56 ± 0.02 b | 7.00 ± 0.55 c | 4.45 ± 0.18 d |
| rosmarinic acid | 33.5 | 175.66 ± 2.02 a | 177.77 ± 1.89 a | 197.48 ± 2.00 b | 171.99 ± 1.88 c |
| luteolin | 39.3 | 1.45 ± 0.01 a | 1.13 ± 0.01 b | 0.96 ± 0.02 c | 0.80 ± 0.02 d |
| apigenin | 42.7 | 0.78 ± 0.01 a | 0.78 ± 0.00 a | 0.72 ± 0.00 b | 0.53 ± 0.00 c |
| salvigenin | 53.0 | 3.72 ± 0.09 a | 4.05 ± 0.02 b | 2.54 ± 0.04 c | 2.27 ± 0.01 d |
| EC50 | ||
|---|---|---|
| Spontaneous Contractions | KCl-Induced Contractions | |
| mg/mL | ||
| MM | 2.62 ± 0.24 a | 3.69 ± 0.30 a |
| MU | 2.69 ± 0.22 a | 4.90 ± 0.33 b |
| M80M | 2.44 ± 0.10 a | 5.76 ± 0.34 c |
| M80U | 4.59 ± 0.33 b | 4.63 ± 0.26 b |
| papaverine | 1.2 × 10−4 ± 0.1 × 10−4 c | / |
| verapamil | / | 6.3 × 10−4 ± 0.5 × 10−4 d |
| MM | MU | M80M | M80U | Atropine | |
|---|---|---|---|---|---|
| EC50 of Acetylcholine (nM) | |||||
| control | 0.29 ± 0.01 a | 0.03 ± 0.00 a | 0.01 ± 0.00 a | 0.17 ± 0.00 a | 0.10 ± 0.00 a |
| 0.5 mg/mL | 0.40 ± 0.01 b | 0.12 ± 0.00 b | 0.15 ± 0.00 b | 0.36 ± 0.00 b | / |
| 1.5 mg/mL | 18.21 ± 0.65 c | 0.47 ± 0.01 c | 7.25 ± 0.22 c | 62.15 ± 3.22 c | / |
| 140 nM | / | / | / | / | 18,261.96 ± 958.32 b |
| EC50 | ||
|---|---|---|
| KCl-Induced Contractions | Carbachol-Induced Contractions | |
| mg/mL | ||
| MM | 6.27 ± 0.16 a | 6.92 ± 0.04 a |
| MU | 15.38 ± 1.02 b | 3.26 ± 0.02 b |
| M80M | 6.03 ± 0.33 a | 1.36 ± 0.01 c |
| M80U | 9.02 ± 0.11 c | 4.26 ± 0.04 d |
| verapamil | 1.53 × 10−2 ± 8.00 × 10−5 d | / |
| atropine | / | 9.78 × 10−3 ± 0.00 f |
| Compounds | Binding Affinity (kcal/mol) | Hydrogen Bonds | Electrostatic/Hydrophobic Bonds |
|---|---|---|---|
| V | −6.6 | Conventional hydrogen bond: Arg65 (2.36) | π-Cation: Arg227 (4.23) Alkyl: Lys110 (4.12), Ala409 (4.50), Lys90 (4.13), Pro336 (4.38) π-Alkyl: Ala409 (5.06), Tyr402 (4.64), Tyr406 (5.00) |
| A | −6.7 | Conventional hydrogen bond: Asp91 (2.05), Glu111 (2.86), Ala405 (2.30) | π-Cation, π-donor hydrogen bond: Lys110 (2.92) π-Anion: Asp91 (4.07) π-Alkyl: Ala409 (5.37), Ala405 (5.31), Ala409 (4.06), Leu108 (5.34), Lys110 (4.22) |
| AG | −8.6 | Conventional hydrogen bond: Val109 (2.09), Glu381 (2.33), Arg65 (2.21) Carbon hydrogen bond: Ser382 (3.65), Ser330 (3.49) | π-Cation: Arg227 (4.24), Arg227 (4.26) π-Anion: Asp384 (3.85), Asp384 (3.84) π-π T-shaped: Phe92 (4.83) |
| L | −6.8 | Conventional hydrogen bond: Phe383 (2.49), Asp384 (2.55), Pro326 (2.47), Arg65 (2.65) | π-Alkyl: Pro336 (4.98) |
| LG | −8.6 | Conventional hydrogen bond: Pro326 (2.61), Val109 (1.82), Gln380 (2.45), Arg65 (2.52) Carbon hydrogen bond: Ser382 (3.56), Ala335 (3.64), Pro336 (3.67) | π-Cation: Arg227 (4.17), Arg227 (4.00) π-Anion: Asp384 (3.40), Asp384 (3.96) π-π T-shaped: Phe92 (4.82) |
| S | −6.9 | Conventional hydrogen Bbond: Pro336 (2.49), Arg65 (2.70), Arg65 (2.14) Carbon Hydrogen Bond: Ala335 (3.51) | Alkyl: Pro378 (4.61), Lys90 (3.85) π-Alkyl: Ala327 (5.26) |
| RA | −6.6 | Conventional hydrogen bond: Pro336 (2.72), Arg227 (2.74), Arg227 (2.95) | π-Alkyl: Pro336 (5.05), Ile338 (5.35), Lys90 (4.60) |
| CA | −5.8 | Conventional hydrogen bond: Pro336 (2.11) | π-Alkyl: Pro336 (4.89) |
| Extracts | MM | MU | M80M | M80U | S | |
|---|---|---|---|---|---|---|
| Bacterial Strain | MIC/MBC (mg/mL) | MIC/MBC (mg/mL) | MIC/MBC (mg/mL) | MIC/MBC (mg/mL) | MIC/MBC (μg/mL) | |
| Gram (+) | ATCC | Chlor. | ||||
| Staphylococcus aureus | 6538 | 12.5/>100 | 6.25/>100 | 12.5/100 | 25/>100 | 7.81/15.61 |
| Streptococcus pneumoniae | 6301 | 100/>100 | 100/>100 | 50/>100 | 100/100 | 0.06/0.12 |
| Streptococcus pyogenes | 19615 | >100/>100 | >100/>100 | 100/>100 | 100/>100 | 0.25/0.49 |
| Enterococcus faecalis | 9433 | >100/>100 | >100/>100 | 100/>100 | 100/>100 | 3.91/7.81 |
| Bacillus cereus | 11778 | 25.0/>100 | 12.5/>100 | 12.5/>100 | 50/>100 | 7.81/15.61 |
| Listeria monocytogenes | 15313 | 25.0/>100 | 25/>100 | 50/>100 | 50/>100 | 0.25/0.49 |
| Gram (−) | ATCC | Str. | ||||
| Pseudomonas aeruginosa | 9027 | 100/>100 | 50/>100 | 50/50 | 100/100 | 0.60/0.60 |
| Proteus mirabilis | 12453 | >100/>100 | >100/>100 | 100/100 | 100/>100 | 0.30/0.30 |
| Salmonella enteritidis | 13076 | 100/>100 | >100/>100 | 100/100 | 100/>100 | 0.30/0.30 |
| Escherichia coli | 8739 | 100/>100 | >100/>100 | 100/>100 | 100/>100 | 0.16/0.16 |
| Enterobacter aerogenes | 13048 | 100/>100 | 50/>100 | 100/>100 | 100/>100 | 0.60/0.60 |
| Klebsiella pneumoniae | 10031 | 100/>100 | 100/>100 | 100/>100 | 100/>100 | 0.30/0.30 |
| fungal strain | MIC/MFC (mg/mL) | MIC/MFC (mg/mL) | MIC/MFC (mg/mL) | MIC/MFC (mg/mL) | MIC/MFC (μg/mL) | |
| yeast | ATCC | Nys. | ||||
| Candida albicans | 24433 | 100/>100 | 100/>100 | 100/>100 | >100/>100 | 3.91/7.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randjelović, M.; Branković, S.; Jovanović, M.; Kitić, N.; Živanović, S.; Mihajilov-Krstev, T.; Miladinović, B.; Milutinović, M.; Kitić, D. An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics 2023, 15, 1376. https://doi.org/10.3390/pharmaceutics15051376
Randjelović M, Branković S, Jovanović M, Kitić N, Živanović S, Mihajilov-Krstev T, Miladinović B, Milutinović M, Kitić D. An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics. 2023; 15(5):1376. https://doi.org/10.3390/pharmaceutics15051376
Chicago/Turabian StyleRandjelović, Milica, Suzana Branković, Miloš Jovanović, Nemanja Kitić, Slavoljub Živanović, Tatjana Mihajilov-Krstev, Bojana Miladinović, Milica Milutinović, and Dušanka Kitić. 2023. "An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents" Pharmaceutics 15, no. 5: 1376. https://doi.org/10.3390/pharmaceutics15051376
APA StyleRandjelović, M., Branković, S., Jovanović, M., Kitić, N., Živanović, S., Mihajilov-Krstev, T., Miladinović, B., Milutinović, M., & Kitić, D. (2023). An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics, 15(5), 1376. https://doi.org/10.3390/pharmaceutics15051376








