Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of HAP800, HAP800-BSA, and HAP800-PEG
2.2. Optical Property Measurement
2.3. In Vitro Photothermal Conversion Efficiency
2.4. HT-29 Xenograft Mouse Model
2.5. In Vivo Biodistribution and Tumor Imaging
2.6. In Vivo Photothermal Therapeutic Efficacy
2.7. Histological Analysis
3. Results and Discussion
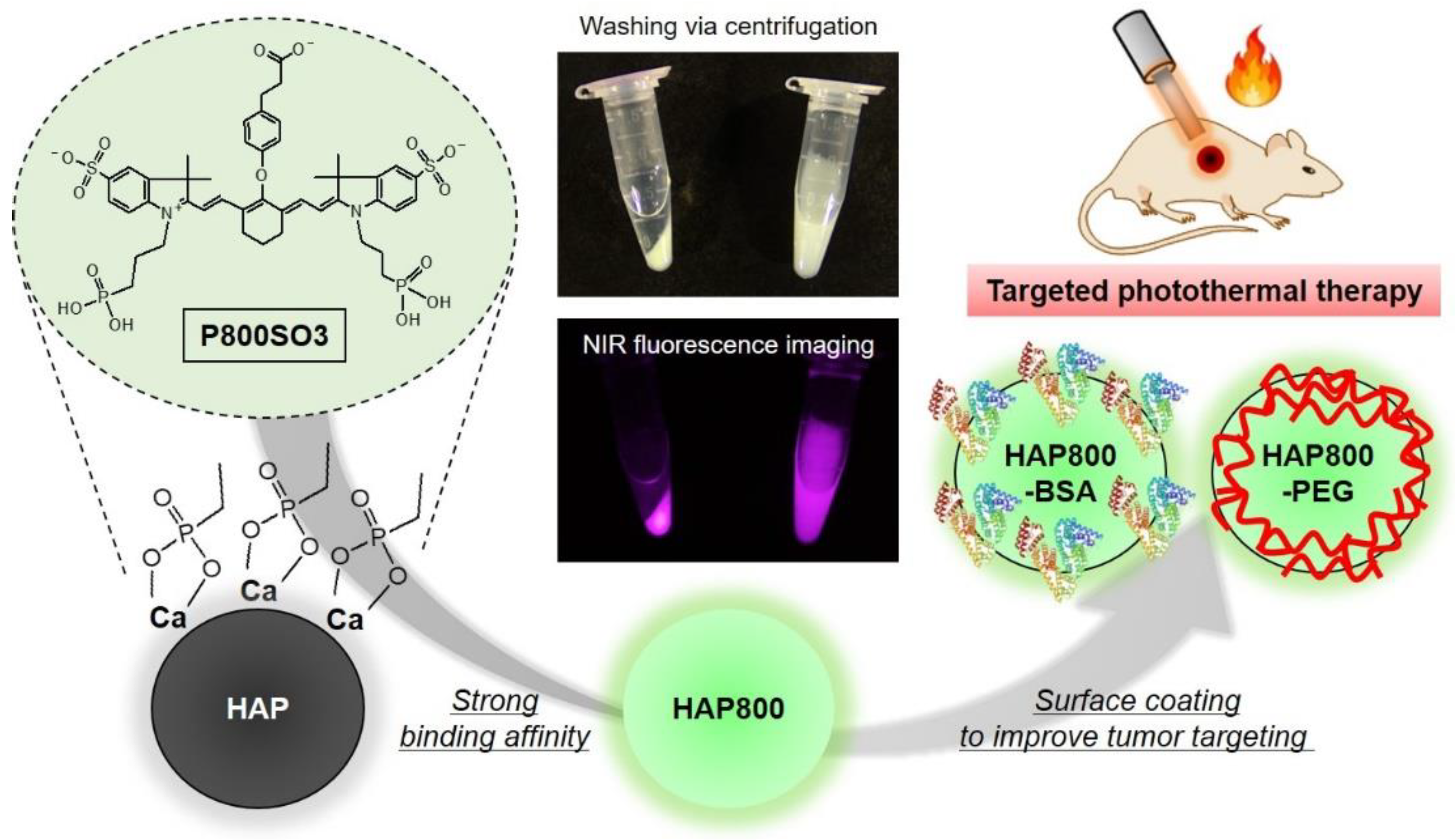
3.1. Preparation of NIR Fluorescent HAP Nanoparticles
3.2. Optical and Size Characterization of HAP Nanoparticles
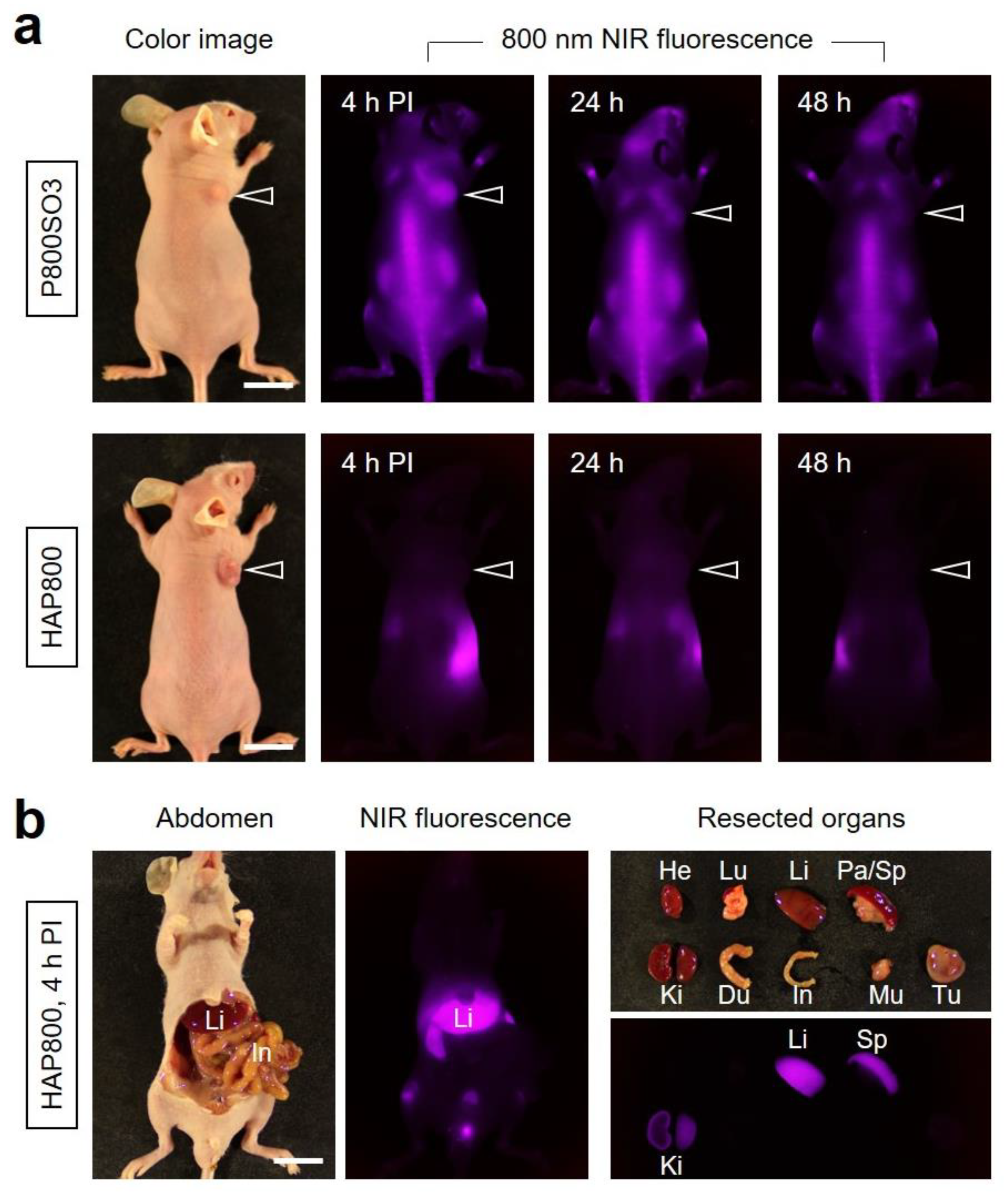
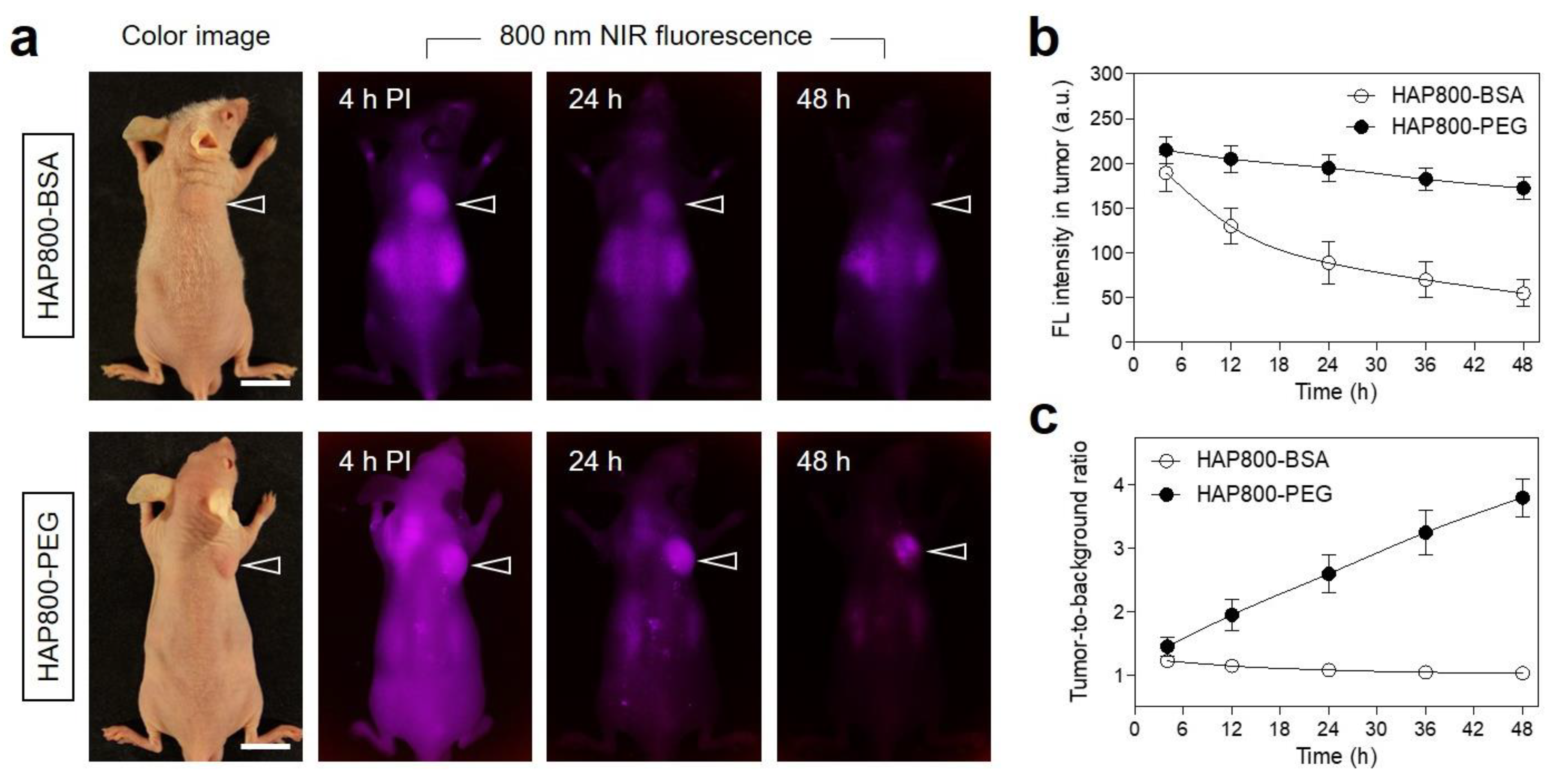
3.3. Time-Dependent In Vivo Tumor Imaging and Biodistribution
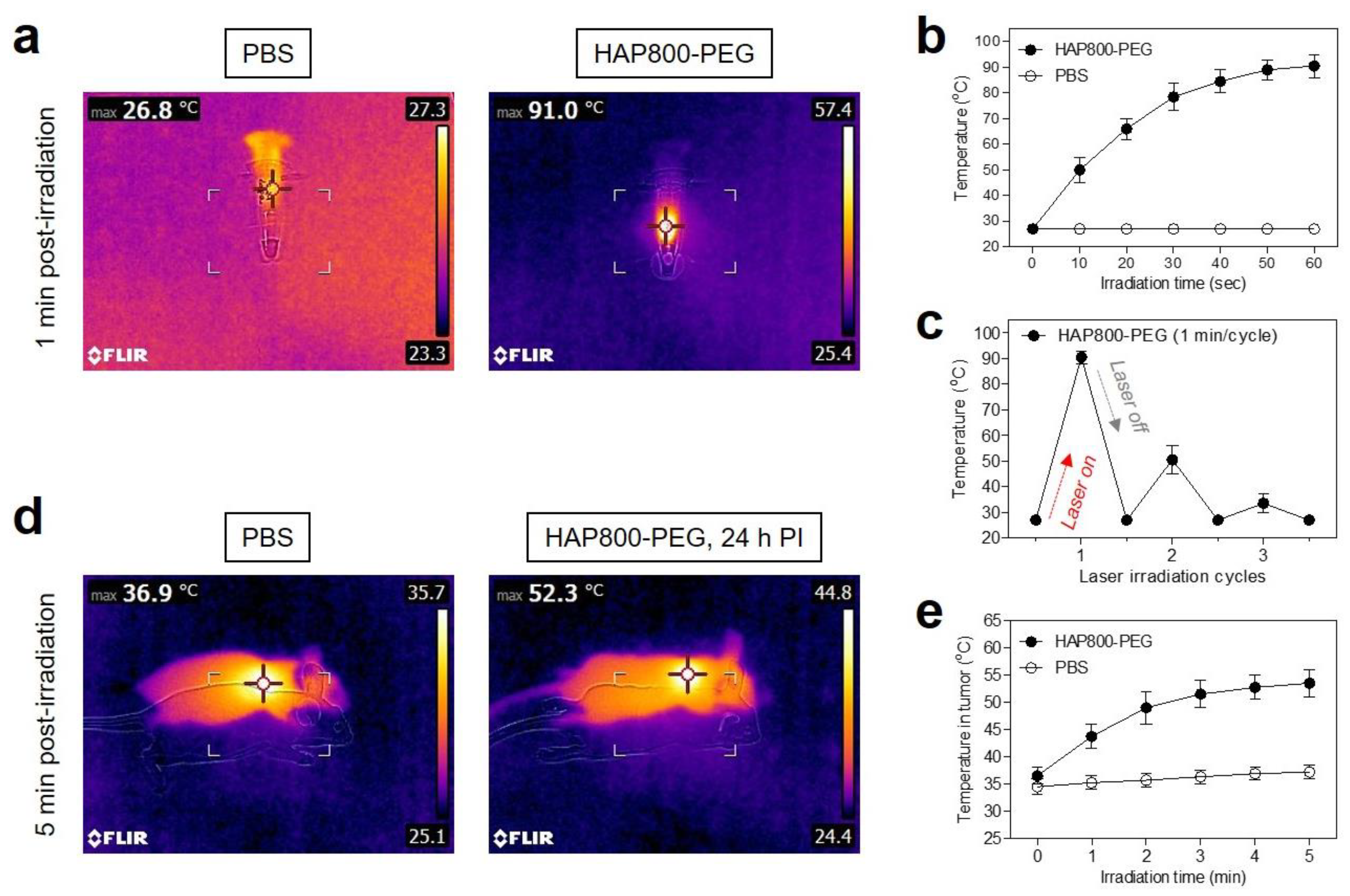
3.4. In Vitro and In Vivo Photothermal Effects
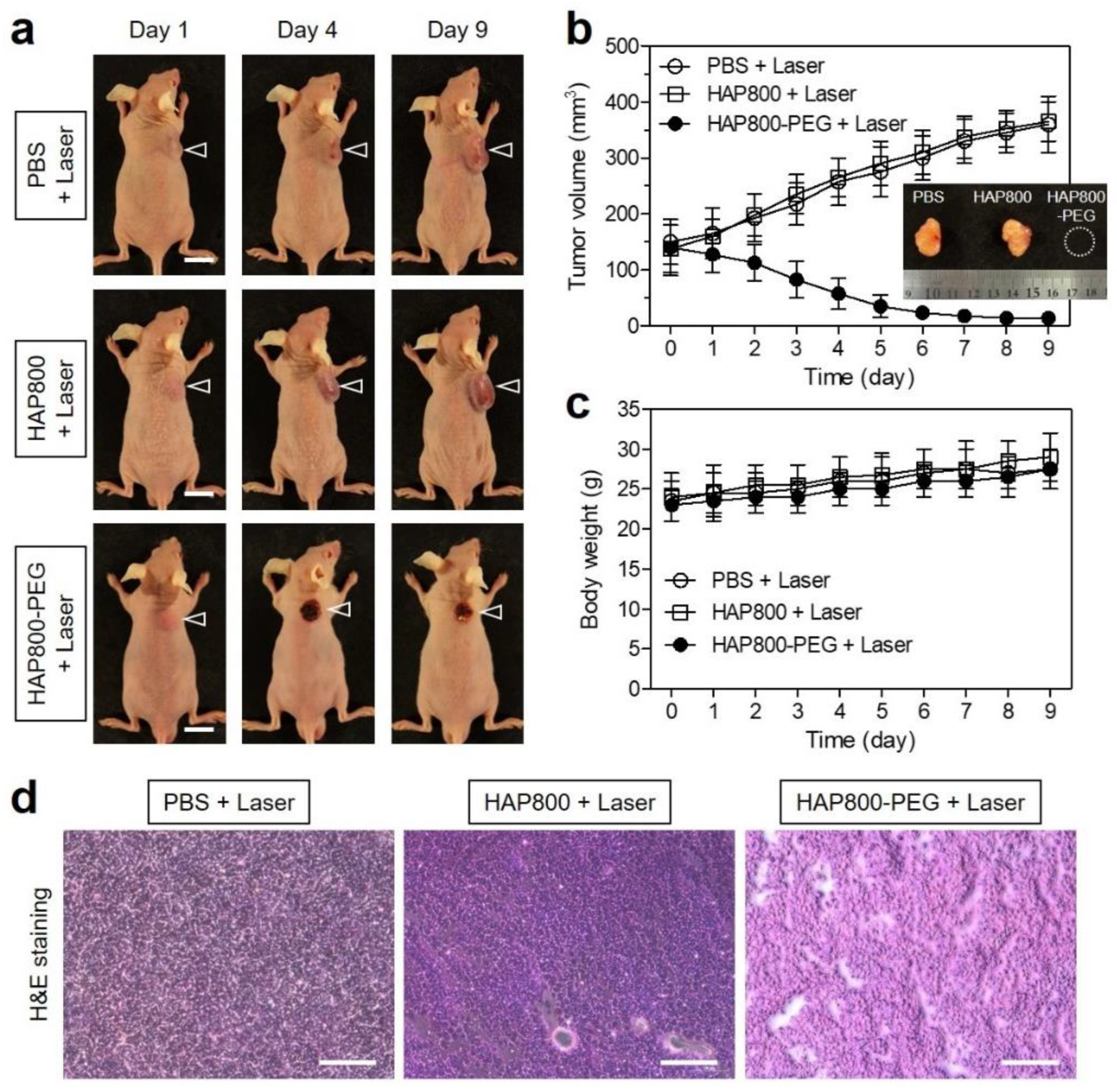
3.5. In Vivo Photothermal Therapeutic Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Yin, X.; Cheng, Y.; Feng, Y.; Stiles, W.R.; Park, S.H.; Kang, H.; Choi, H.S. Phototheranostics for multifunctional treatment of cancer with fluorescence imaging. Adv. Drug Deliv. Rev. 2022, 189, 114483. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, P.; Chen, P.; Pu, K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6, 746–765. [Google Scholar] [CrossRef]
- Locatelli, E.; Li, Y.; Monaco, I.; Guo, W.; Maturi, M.; Menichetti, L.; Armanetti, P.; Martin, R.C.; Franchini, M.C. A novel theranostic gold nanorods- and Adriamycin-loaded micelle for EpCAM targeting, laser ablation, and photoacoustic imaging of cancer stem cells in hepatocellular carcinoma. Int. J. Nanomed. 2019, 14, 1877–1892. [Google Scholar] [CrossRef] [PubMed]
- Melo-Diogo, D.D.; Lima-Sousa, R.; Alves, C.G.; Correia, I.J. Graphene family nanomaterials for application in cancer combination photothermal therapy. Biomater. Sci. 2019, 7, 3534–3551. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Sambri, L.; Tortorella, S.; Šturm, S.; Kostevšek, N.; Franchini, M.C. Synthesis of Ultrasmall Single-Crystal Gold-Silver Alloy Nanotriangles and Their Application in Photothermal Therapy. Nanomaterials 2021, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, H.M.; Song, G.; Li, Z.; Zhang, X.B. Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Zhang, T.; Zhu, Y.; Ni, Y.; Wang, C.; Raya, F.M.S.; Zou, L.; Wang, L.; Liang, G. A Self-Evaluating Photothermal Therapeutic Nanoparticle. ACS Nano 2020, 14, 9585–9593. [Google Scholar] [CrossRef]
- Park, M.H.; Jo, G.; Kim, E.J.; Hyun, H. Tumor-Targeted ZW800-1 Analog for Enhanced Tumor Imaging and Photothermal Therapy. Pharmaceutics 2021, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, K.; Chen, H.; Shi, Q.; Liu, H.; Zhong, X.; Qian, H.; Chen, X.; Cheng, L.; Wang, X. Liquid exfoliation of V8C7 nanodots as peroxidase-like nanozymes for photothermal-catalytic synergistic antibacterial treatment. Acta Biomater. 2022, 149, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dai, X.; Zhang, W.; Zhu, X.; Zha, Z.; Qian, H.; Cheng, L.; Wang, X. Liquid exfoliation of ultrasmall zirconium carbide nanodots as a noninflammatory photothermal agent in the treatment of glioma. Biomaterials 2023, 292, 121917. [Google Scholar] [CrossRef]
- Yin, M.; Chen, X.; Guo, Q.; Xiao, L.; Gao, P.; Zang, D.; Dong, J.; Zha, Z.; Dai, X.; Wang, X. Ultrasmall zirconium carbide nanodots for synergistic photothermal-radiotherapy of glioma. Nanoscale 2022, 14, 14935–14949. [Google Scholar] [CrossRef]
- Guo, Q.; Yin, M.; Fan, J.; Yang, Y.; Liu, T.; Qian, H.; Dai, X.; Wang, X. Peroxidase-mimicking TA-VOx nanobranches for enhanced photothermal/chemodynamic therapy of glioma by inhibiting the expression of HSP60. Mater. Des. 2022, 224, 111366. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yue, Q.; Xu, H.; Zhong, X.; Sun, L.; Li, G.; Gong, Y.; Yang, N.; Wang, Z.; et al. Liquid exfoliation of TiN nanodots as novel sonosensitizers for photothermal-enhanced sonodynamic therapy against cancer. Nano Today 2021, 39, 101170. [Google Scholar] [CrossRef]
- Jo, G.; Kim, E.J.; Hyun, H. Enhanced Tumor Accumulation of Low-Molecular-Weight Hyaluronic Acid/Chitosan Nanocomplexes for Photothermal Therapy. Pharmaceutics 2023, 15, 613. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef]
- Kim, D.; Jo, G.; Chae, Y.; Subramani, S.; Lee, B.Y.; Kim, E.J.; Ji, M.K.; Sim, U.; Hyun, H. Bioinspired Camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy. Nanoscale 2021, 13, 14426–14434. [Google Scholar] [CrossRef]
- Ren, X.; Yi, Z.; Sun, Z.; Ma, X.; Chen, G.; Chen, Z.; Li, X. Natural polysaccharide-incorporated hydroxyapatite as size-changeable, nuclear-targeted nanocarrier for efficient cancer therapy. Biomater. Sci. 2020, 8, 5390–5401. [Google Scholar] [CrossRef]
- Padilla, S.; Izquierdo-Barba, I.; Vallet-Regí, M. High Specific Surface Area in Nanometric Carbonated Hydroxyapatite. Chem. Mater. 2008, 20, 5942–5944. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, Y.; Zhang, Y.; Jia, C.; Wei, B.; Hu, T.; Tang, R.; Li, C. Glucose-Targeted Hydroxyapatite/Indocyanine Green Hybrid Nanoparticles for Collaborative Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37665–37679. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Ma, X.; Yuan, Y.; Liu, C.; Kohn, J.; Qian, J. Mitochondria-Targeted Hydroxyapatite Nanoparticles for Selective Growth Inhibition of Lung Cancer in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 25680–25690. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, S.; Ni, J.; Zhou, J.; Yao, J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials 2016, 94, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, C.; Gao, D.; Chen, S.; Zhu, Y.; Sun, J.; Luo, H.; Yu, K.; Fan, H.; Zhang, X. Antitumor Effect by Hydroxyapatite Nanospheres: Activation of Mitochondria-Dependent Apoptosis and Negative Regulation of Phosphatidylinositol-3-Kinase/Protein Kinase B Pathway. ACS Nano 2018, 12, 7838–7854. [Google Scholar] [CrossRef]
- Chang, L.; Huang, S.; Zhao, X.; Hu, Y.; Ren, X.; Mei, X.; Chen, Z. Preparation of ROS active and photothermal responsive hydroxyapatite nanoplatforms for anticancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 125, 112098. [Google Scholar] [CrossRef]
- Park, S.; Choi, J.; Doan, V.H.M. Biodegradable manganese-doped hydroxyapatite antitumor adjuvant as a promising photo-therapeutic for cancer treatment. Front. Mol. Biosci. 2022, 9, 1085458. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, M.; Wei, J.; Zhang, H.; Chen, Y. Surface modification of hydroxyapatite nanoparticles by poly(L-phenylalanine) via ROP of L-phenylalanine N-carboxyanhydride (Pha-NCA). Appl. Surf. Sci. 2012, 258, 2850–2855. [Google Scholar] [CrossRef]
- Hyun, H.; Wada, H.; Bao, K.; Gravier, J.; Yadav, Y.; Laramie, M.; Henary, M.; Frangioni, J.V.; Choi, H.S. Phosphonated near-infrared fluorophores for biomedical imaging of bone. Angew. Chem. Int. Ed. 2014, 53, 10668–10672. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xi, D.; Zhang, Z.; Long, S.; Chen, P.; Du, J.; Sun, W.; Fan, J.; Peng, X. Light-triggered dePEGylation with decreasing the diameter of hydroxyapatite nanocarriers for enhanced cellular uptake and tumor penetration. Nano Select 2021, 2, 1954–1961. [Google Scholar] [CrossRef]
- Lim, W.; Byun, J.Y.; Jo, G.; Kim, E.J.; Park, M.H.; Hyun, H. Molecular Tuning of IR-786 for Improved Tumor Imaging and Photothermal Therapy. Pharmaceutics 2022, 14, 676. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.S.; Jo, G.; Yang, D.H.; Koh, Y.S.; Hyun, H. Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy. Cancers 2019, 11, 1286. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Karbasi, S.; Pieles, U.; Daniels, A.U. Preparation, chemistry and physical properties of bone-derived hydroxyapatite particles having a negative zeta potential. Mater. Chem. Phys. 2012, 132, 446–452. [Google Scholar] [CrossRef]
- Lee, S.; Jo, G.; Jung, J.S.; Yang, D.H.; Hyun, H. Near-infra-red fluorescent chitosan oligosaccharide lactate for targeted cancer imaging and photothermal therapy. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1144–1152. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, G.; Park, Y.; Park, M.H.; Hyun, H. Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy. Pharmaceutics 2023, 15, 1374. https://doi.org/10.3390/pharmaceutics15051374
Jo G, Park Y, Park MH, Hyun H. Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy. Pharmaceutics. 2023; 15(5):1374. https://doi.org/10.3390/pharmaceutics15051374
Chicago/Turabian StyleJo, Gayoung, Yoonbin Park, Min Ho Park, and Hoon Hyun. 2023. "Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy" Pharmaceutics 15, no. 5: 1374. https://doi.org/10.3390/pharmaceutics15051374
APA StyleJo, G., Park, Y., Park, M. H., & Hyun, H. (2023). Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy. Pharmaceutics, 15(5), 1374. https://doi.org/10.3390/pharmaceutics15051374





