Hyaluronic Acid-Mediated Phenolic Compound Nanodelivery for Cancer Therapy
Abstract
1. Introduction
1.1. Why Should Chemotherapy Be Improved?
1.2. Strategies for Innovating Anticancer Chemotherapy
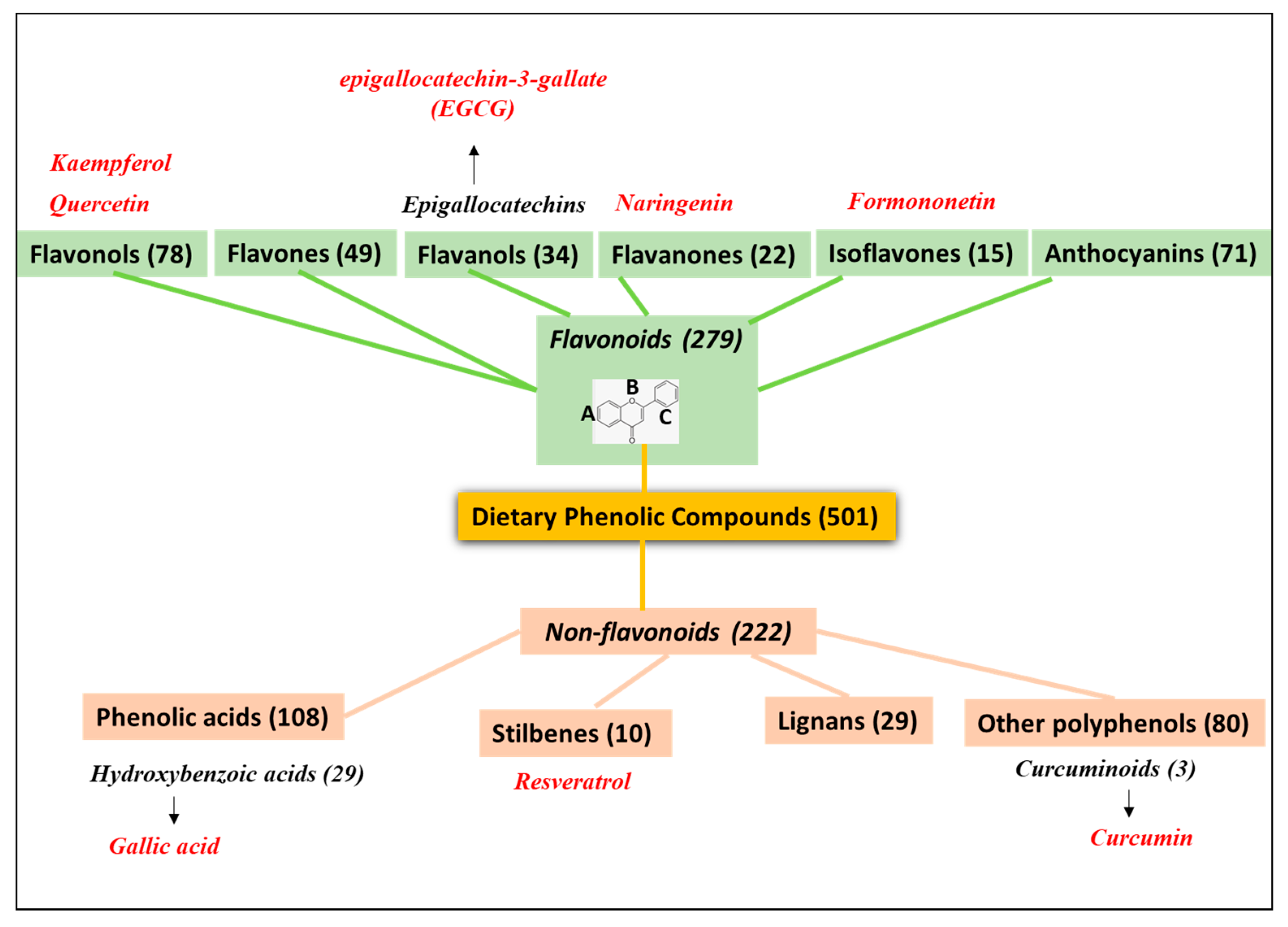
2. PheCs Included in Drug Nanodelivery Systems for Tumor Targeting
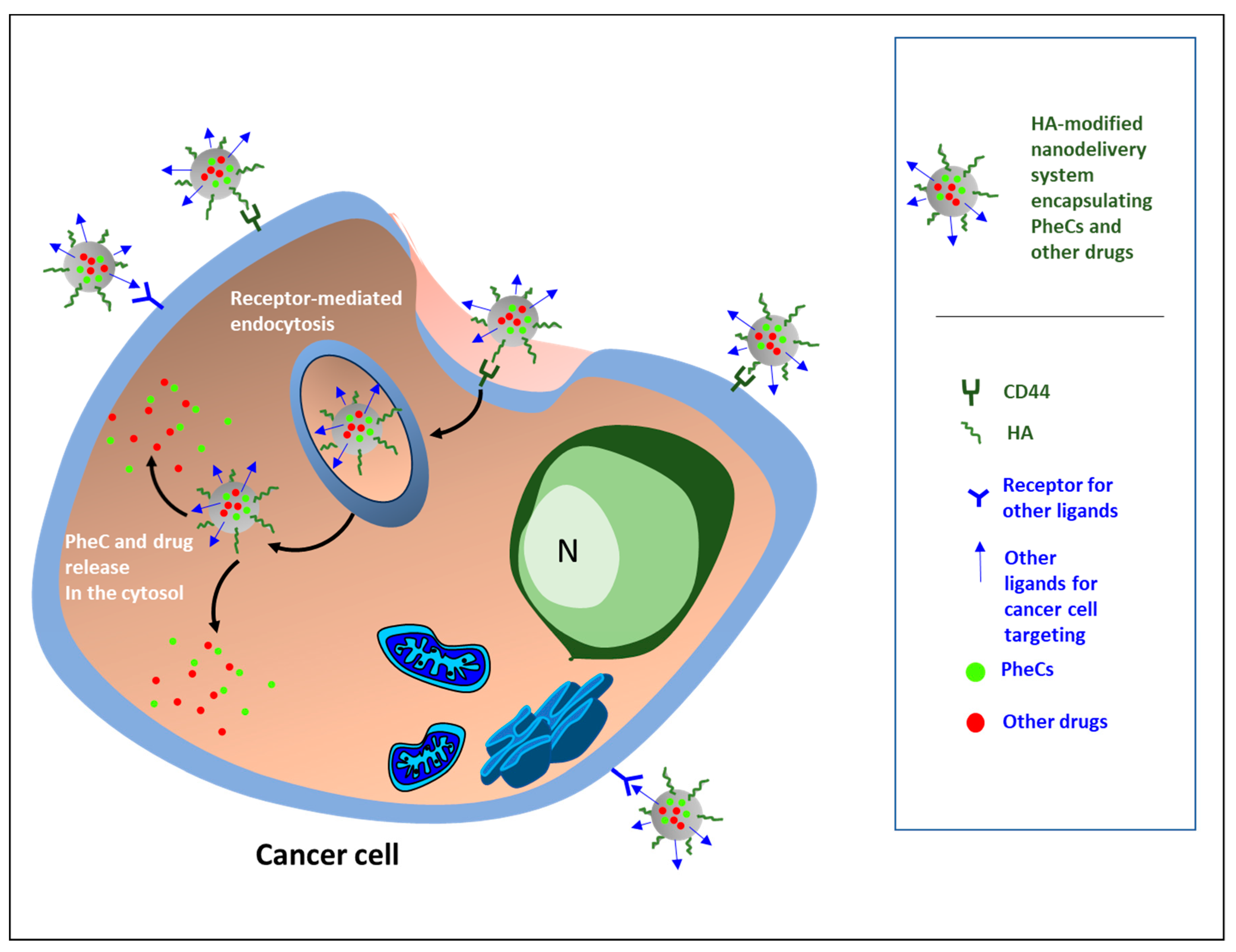
3. Hyaluronic Acid: An Efficient Carrier for the Specific Delivery of Antineoplastic Drugs and Natural Bioactive Products
4. Newly Developed HA-Based Nanocarrier Systems Loading PheCs and Their Effects against Cancer
4.1. HA-Based Nanocarrier Systems Loading Flavonoids
4.2. HA-Based Nanocarrier Systems Loading Non-Flavonoids
- (A)
- Polymeric micelles co-encapsulating icariin and CUR and based on pH-sensitive hydrazone bond, folic acid, and biotin-conjugated [67] (Table 2). This therapeutic strategy is interesting since it allows the codelivery of two PheCs known for their powerful anticancer activities (CUR and icariin, a prenyl-derivative of the flavonoid kaempferol). These PheCs were conjugated through a pH-sensitive hydrazone bond, which allows them to be easily released in the acidic tumor microenvironment. Moreover, to improve the recognition and internalization of the nanomicelles, besides HA, also folic acid and vitamin biotin were bound to their exterior. In fact, due to their high-grade proliferation, cancer cells need vitamins and overexpress receptors for them on their surface [124,125];
- (B)
- HA-functionalized mesoporous silica NPs (MSNPs) enclosing CUR alone [66] (Table 2). This approach was based on a series of favorable features of MSNPs, such as their chemical and mechanical stability, the possibility to easily functionalize their surface, their suitable endocytic behavior, as well as their biocompatibility;
- (C)
- HA- and riboflavin-coated transition metals-based nanoplatforms enclosing CUR [74] (Table 2). This interesting approach was aimed at increasing the anticancer effectiveness of transitional metals such as nickel, manganese, and iron that were used to synthesize photosensitizers for the photodynamic therapy of tumors. This effect was obtained through the inclusion of the CUR in the nanosystem to take advantage of the possible synergistic effect deriving from chemotherapy and photodynamic therapy simultaneously;
- (D)
- HA-decorated self-assembled nanomicelles consisting of a biocompatible amphiphilic polymer formed by styrene maleic anhydride (SMA) and TPGS, loading the hydrophobic molecule of difluorobenzylidene diferuloylmethane (CDF) (a stable cytotoxic CUR analog) [65] (Table 2). Biocompatibility, specific CD44 targeting, and TPGS activity against possible drug resistance represent the strength of this nanocarrier designed for TNBC that showed the high capability to be specifically accumulated in the tumor obtained by transplanting these BCa cells in nude mice and not in a series of normal tissues investigated. The authors, however, did not investigate their effect on tumor growth in vivo.
- (A)
- HA cross-linked zein nanogels [64]. Zein is a natural hydrophobic biopolymer that, similarly to HA, shows high biodegradability and biocompatibility [127]. Moreover, being easily extracted from corn, it also possesses the appreciable quality of being quite inexpensive, and for all these reasons, it has attracted considerable attention as an excellent nanocarrier for hydrophobic bioactive compounds [128]. However, this was the first time that a nanogel able to carry hydrophobic drugs was created with zein and HA, starting from the hypothesis that zein could be easily cross-linked with an anion such as HA.
- (B)
- An analogous HA–zein hydrogel NP [72] that, when compared with similar NPs where HA was substituted with other polysaccharides (arabic gum or pectin), was the most effective against CRC cells.
- (C)
- HA–lactoferrin–EGCG-containing composite NPs [75]. The main aim of the authors was to obtain an effective anticancer CUR-loading nanosystem that could possibly be used in food and supplements for both the prevention and the therapy of CRC. For this reason, they needed to produce edible NPs, and for this purpose they used natural safe products such as lactoferrin or EGCG that could increase the stability of loaded CUR through the gastrointestinal tract, allowing its higher bioavailability. Moreover, the choice of lactoferrin and EGCG for constructing CUR-loading NPs seems very appropriate since, besides ensuring safe delivery of this bioactive compound, they could synergistically reinforce the antineoplastic effect of CUR since they are known for exerting anticancer properties against CRC cells by themselves [129,130]. Furthermore, it is very interesting that the NP decoration with HA resulted in being critical for enhancing the NP anticancer properties, especially toward CRC cells characterized by a high degree of proliferation and malignancy, such as HT-29 and CT-26 CRC cells, which are known to show increased expression of CD44 receptor. On the contrary, the anticancer effect was not obtained toward CaCo-2 cells, which are CRC cells still able to differentiate, showing low levels of CD44 expression [75].
- (A)
- Liposomes decorated with HA and glycyrrhetinic acid and co-loading CUR and aprepitant [71]. This new therapeutic nanoplatform appears particularly appropriate for the therapy of HCC. In fact, it has become clear that HSCs represent important components of the tumor microenvironment, playing a crucial role in the development and progression of this kind of cancer [133]. The antiemetic drug aprepitant was found able to inhibit the activation of HSCs from their quiescent phenotype to the carcinoma-associated fibroblasts (CAFs) by blocking the neurokinin–receptor signal pathway activated by the neuropeptide SP, locally secreted by peripheral nerves [134]. Thus, the aprepitant-dependent inhibition of HSC activation could prevent the cross-talk between CAFs and HCC cells that have been involved in HCC development and progression [135]. Moreover, the co-targeting lysosomes through glycyrrhetinic acid and HA has the potential of making their delivery highly specific for this kind of cancer since, on the one hand, glycyrrhetinic acid receptors are overexpressed in HCC cells [136], and HA-specific CD44 receptors in activated HSCs [137].
- (B)
- Analogous HA and glycyrrhetinic acid-modified liposomes synthesized by the same group [73] but co-delivering CUR and berberin, an anticancer bioactive vegetal alkaloid [138], with the identical aim of combining anti-HCS and pro-apoptotic activities and inhibiting the cross-talk between the tumor and the activated HSCs. The authors’ hypothesis assumed that berberine could have the potential to inhibit the TGF-β-induced phenotypic switch of HSCs into the fibrogenic and carcinogenic myofibroblasts [139,140].
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Canceratlas. Available online: https://canceratlas.cancer.org/wp-content/uploads/2019/10/ACS_CA3_Book.pdf (accessed on 27 January 2023).
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Klassen, P.; Schiessel, D.L.; Baracos, V.E. Adverse effects of systemic cancer therapy on skeletal muscle: Myotoxicity comes out of the closet. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 210–218. [Google Scholar] [CrossRef]
- Michot, J.; Lazarovici, J.; Tieu, A.; Champiat, S.; Voisin, A.; Ebbo, M.; Godeau, B.; Michel, M.; Ribrag, V.; Lambotte, O. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur. J. Cancer 2019, 122, 72–90. [Google Scholar] [CrossRef]
- Nevins, S.; McLoughlin, C.D.; Oliveros, A.; Stein, J.B.; Rashid, M.A.; Hou, Y.; Jang, M.; Lee, K. Nanotechnology Approaches for Prevention and Treatment of Chemotherapy-Induced Neurotoxicity, Neuropathy, and Cardiomyopathy in Breast and Ovarian Cancer Survivors. Small 2023, e2300744. [Google Scholar] [CrossRef]
- Gupta, G.; Borglum, K.; Chen, H. Immunogenic Cell Death: A Step Ahead of Autophagy in Cancer Therapy. J. Cancer Immunol. 2021, 3, 47–59. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Huang, Z.; Guo, Y.; Li, Q. Triggering Immune System With Nanomaterials for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2022, 10, 878524. [Google Scholar] [CrossRef]
- Meng, Q.; Ding, B.; Ma, P.; Lin, J. Interrelation between Programmed Cell Death and Immunogenic Cell Death: Take Antitumor Nanodrug as an Example. Small Methods 2023, 7, e2201406. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Huang, N.; Yu, Y.; Qiao, J. Dual role for the unfolded protein response in the ovary: Adaption and apoptosis. Protein Cell 2017, 8, 14–24. [Google Scholar] [CrossRef]
- Jia, P.; Dai, C.; Cao, P.; Sun, D.; Ouyang, R.; Miao, Y. The role of reactive oxygen species in tumor treatment. RSC Adv. 2020, 10, 7740–7750. [Google Scholar] [CrossRef]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Teong, B.; Lin, C.-Y.; Chang, S.-J.; Niu, G.C.-C.; Yao, C.-H.; Chen, I.-F.; Kuo, S.-M. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J. Mater. Sci. Mater. Med. 2015, 26, 49. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Tu, M.; Wang, W.; Zhang, G.; Hammock, B.D. ω-3 Polyunsaturated Fatty Acids on Colonic Inflammation and Colon Cancer: Roles of Lipid-Metabolizing Enzymes Involved. Nutrients 2020, 12, 3301. [Google Scholar] [CrossRef]
- He, H.; Ma, Y.; Huang, H.; Huang, C.; Chen, Z.; Chen, D.; Gu, Y.; Wang, X.; Chen, J. A comprehensive understanding about the pharmacological effect of diallyl disulfide other than its anti-carcinogenic activities. Eur. J. Pharmacol. 2021, 893, 173803. [Google Scholar] [CrossRef]
- Wei, L.-Y.; Zhang, J.-K.; Zheng, L.; Chen, Y. The functional role of sulforaphane in intestinal inflammation: A review. Food Funct. 2022, 13, 514–529. [Google Scholar] [CrossRef]
- Sansone, C.; Bruno, A.; Piscitelli, C.; Baci, D.; Fontana, A.; Brunet, C.; Noonan, D.M.; Albini, A. Natural Compounds of Marine Origin as Inducers of Immunogenic Cell Death (ICD): Potential Role for Cancer Interception and Therapy. Cells 2021, 10, 231. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Shen, Y.-L. Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity. Molecules 2014, 19, 6694–6706. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Wang, P.-H.; Chen, S.-S.; Wen, C.-C.; Chen, Y.-H.; Yang, W.-C.; Yang, N.-S. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol. Immunother. 2012, 61, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Bin Muhsinah, A.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Gan, R.Y.; Chan, C.L.; Yang, Q.Q.; Li, H.B.; Zhang, D.; Ge, Y.Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Feng, H., Nemzer, B., DeVries, J.W., Eds.; Woodhead Publishing: Cambridge, UK; AACC International Press: Cambridge, UK, 2019; pp. 191–246. ISBN 9780128115251. [Google Scholar]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Phenol-Explorer. Available online: http://phenol-explorer.eu/compounds (accessed on 17 January 2023).
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, S.; Luo, F.; Tang, D.; Yang, T.; Yang, X.; Xie, Y. A Nanosized Codelivery System Based on Intracellular Stimuli-Triggered Dual-Drug Release for Multilevel Chemotherapy Amplification in Drug-Resistant Breast Cancer. Pharmaceutics 2022, 14, 422. [Google Scholar] [CrossRef]
- Naseer, F.; Ahmad, T.; Kousar, K.; Kakar, S.; Gul, R.; Anjum, S.; Shareef, U. Formulation for the Targeted Delivery of a Vaccine Strain of Oncolytic Measles Virus (OMV) in Hyaluronic Acid Coated Thiolated Chitosan as a Green Nanoformulation for the Treatment of Prostate Cancer: A Viro-Immunotherapeutic Approach. Int. J. Nanomed. 2023, 18, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Gennari, A.; Jain, S.A.; Rosso, F.; Iaffaioli, R.V.; Barbarisi, A.; Barbarisi, M.; Tirelli, N. Double-responsive hyaluronic acid-based prodrugs for efficient tumour targeting. Mater. Sci. Eng. C 2021, 131, 112475. [Google Scholar] [CrossRef]
- Qian, J.; Liu, S.; Yang, T.; Xiao, Y.; Sun, J.; Zhao, J.; Zhang, Z.; Xie, Y. Polyethyleneimine- -Tocopherol Hydrogen Succinate/Hyaluronic Acid-Quercetin (PEI-TOS/HA-QU) Core–Shell Micelles Delivering Paclitaxel for Combinatorial Treatment of MDR Breast Cancer. J. Biomed. Nanotechnol. 2021, 17, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Wang, Y.; Wan, J.; Yuan, P.; Chen, H.; Zhang, L. Facile preparation of hyaluronic acid-based quercetin nanoformulation for targeted tumor therapy. Int. J. Biol. Macromol. 2020, 147, 937–945. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. Combination Therapy of Doxorubicin and Quercetin on Multidrug-Resistant Breast Cancer and Their Sequential Delivery by Reduction-Sensitive Hyaluronic Acid-Based Conjugate/d-α-Tocopheryl Poly(ethylene glycol) 1000 Succinate Mixed Micelles. Mol. Pharm. 2020, 17, 1415–1427. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- Chen, M.-L.; Lai, C.-J.; Lin, Y.-N.; Huang, C.-M.; Lin, Y.-H. Multifunctional nanoparticles for targeting the tumor microenvironment to improve synergistic drug combinations and cancer treatment effects. J. Mater. Chem. B 2020, 8, 10416–10427. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.-P.; Zhu, J.-J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tsai, S.-C.; Ko, H.-Y.; Wu, C.-C.; Lin, Y.-H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Lai, C.-H.; Peng, S.-L.; Hsu, C.-Y.; Hsu, P.-H.; Chu, P.-Y.; Feng, C.-L.; Lin, Y.-H. Targeting Tumor Cells with Nanoparticles for Enhanced Co-Drug Delivery in Cancer Treatment. Pharmaceutics 2021, 13, 1327. [Google Scholar] [CrossRef]
- Bao, J.; Zhao, Y.; Xu, J.; Guo, Y. Design and construction of IR780- and EGCG-based and mitochondrial targeting nanoparticles and their application in tumor chemo-phototherapy. J. Mater. Chem. B 2021, 9, 9932–9945. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, Y.; Guo, J.; Tian, C.; Pan, W.; Wang, H.; Yan, J. Prostate Cancer Therapy Using Docetaxel and Formononetin Combination: Hyaluronic Acid and Epidermal Growth Factor Receptor Targeted Peptide Dual Ligands Modified Binary Nanoparticles to Facilitate the in vivo Anti-Tumor Activity. Drug Des. Dev. Ther. 2022, 16, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, G.; Zhou, H.; Li, W.; Sun, J.; Tao, T.; Li, J. A ROS-Responsive Self-Assembly Driven by Multiple Intermolecular Interaction Enhances Tumor-Targeted Chemotherapy. J. Pharm. Sci. 2021, 110, 1668–1675. [Google Scholar] [CrossRef]
- Chu, A.J. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Front. Biosci. 2022, 27, 134. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Nakamura, C.V.; Auzély-Velty, R. Boronate-ester crosslinked hyaluronic acid hydrogels for dihydrocaffeic acid delivery and fibroblasts protection against UVB irradiation. Carbohydr. Polym. 2020, 247, 116845. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, W.; Gao, C.; Zhou, Y.; Zhang, C.; Zhang, J.; Wang, R.; Wang, Y.; Wang, S. A polyphenol-assisted IL-10 mRNA delivery system for ulcerative colitis. Acta Pharm. Sin. B 2022, 12, 3367–3382. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Rangasami, V.K.; Sarlus, H.; Samal, J.R.; Evans, A.D.; Parihar, V.S.; Varghese, O.P.; Harris, R.A.; Oommen, O.P. Interpenetrating gallol functionalized tissue adhesive hyaluronic acid hydrogel polarizes macrophages to an immunosuppressive phenotype. Acta Biomater. 2022, 142, 36–48. [Google Scholar] [CrossRef]
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782. [Google Scholar] [CrossRef]
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Barucca, G.; Notarstefano, V.; Cantarini, M.; Armeni, T.; Mobbili, G. Encapsulation of a Neutral Molecule into a Cationic Clay Material: Structural Insight and Cytotoxicity of Resveratrol/Layered Double Hydroxide/BSA Nanocomposites. Nanomaterials 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Al-Jubori, A.A.; Sulaiman, G.M.; Tawfeeq, A.T.; Mohammed, H.A.; Khan, R.A.; Mohammed, S.A.A. Layer-by-Layer Nanoparticles of Tamoxifen and Resveratrol for Dual Drug Delivery System and Potential Triple-Negative Breast Cancer Treatment. Pharmaceutics 2021, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, X.; Tang, X.; Zhen, N.; Wang, Y.; Luo, Z.; Zhang, H.; Liu, J.; Zhou, D.; Huang, K. In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci. Nutr. 2021, 9, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.R.; Kim, H.E.; Ju, H.J.; Kim, J.H.; Choi, S.; Choi, H.S.; Kim, M.S. Injectable click-crosslinked hydrogel containing resveratrol to improve the therapeutic effect in triple negative breast cancer. Mater. Today Bio 2022, 16, 100386. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.-Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.-K.; Kim, Y.-C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sau, S.; Alsaab, H.O.; Iyer, A.K. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef]
- Liu, M.; Wang, B.; Guo, C.; Hou, X.; Cheng, Z.; Chen, D. Novel multifunctional triple folic acid, biotin and CD44 targeting pH-sensitive nano-actiniaes for breast cancer combinational therapy. Drug Deliv. 2019, 26, 1002–1016. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of dual-targeted nano-dandelion based on an oligomeric hyaluronic acid polymer targeting tumor-associated macrophages for combination therapy of non-small cell lung cancer. Drug Deliv. 2019, 26, 1265–1279. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual targeting curcumin loaded alendronate-hyaluronan- octadecanoic acid micelles for improving osteosarcoma therapy. Int. J. Nanomed. 2019, 14, 6425–6437. [Google Scholar] [CrossRef]
- Zhao, M.-D.; Li, J.-Q.; Chen, F.-Y.; Dong, W.; Wen, L.-J.; Fei, W.; Zhang, X.; Yang, P.-L.; Zhang, X.-M.; Zheng, C.-H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. GA&HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. Int. J. Nanomed. 2022, 17, 2559–2575. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.-W. Polysaccharide-Zein Composite Nanoparticles for Enhancing Cellular Uptake and Oral Bioavailability of Curcumin: Characterization, Anti-colorectal Cancer Effect, and Pharmacokinetics. Front. Nutr. 2022, 9, 846282. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, C.; Wang, H.; Wang, Q.; Sun, J.; Dong, J.; Yu, G.; Gao, Z.; Zhang, B.; Tian, G. Curcumin and berberine co-loaded liposomes for anti-hepatocellular carcinoma therapy by blocking the cross-talk between hepatic stellate cells and tumor cells. Front. Pharmacol. 2022, 13, 961788. [Google Scholar] [CrossRef]
- Zamani, M.; Aghajanzadeh, M.; Jashnani, S.; Shahangian, S.S.; Shirini, F. Hyaluronic acid coated spinel ferrite for combination of chemo and photodynamic therapy: Green synthesis, characterization, and in vitro and in vivo biocompatibility study. Int. J. Biol. Macromol. 2022, 219, 709–720. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhang, S.; Gu, Q.; McClements, D.J.; Chen, S.; Liu, X.; Liu, F. Lactoferrin-Based Ternary Composite Nanoparticles with Enhanced Dispersibility and Stability for Curcumin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 18166–18181. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Garduño, J.; León-Rodríguez, R.; Alemón-Medina, R.; Pérez-Guillé, B.E.; Soriano-Rosales, R.E.; González-Ortiz, A.; Chávez-Pacheco, J.L.; Solorio-López, E.; Fernandez-Pérez, P.; Rivera-Espinosa, L. Phytochemicals That Interfere With Drug Metabolism and Transport, Modifying Plasma Concentration in Humans and Animals. Dose-Response 2022, 20, 15593258221120485. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–Phenolic Networks as Versatile Coating Materials for Biomedical Applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef]
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-Based Particles for Theranostics. Theranostics 2019, 9, 3170–3190. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Chen, D.; Lian, S.; Sun, J.; Liu, Z.; Zhao, F.; Jiang, Y.; Gao, M.; Sun, K.; Liu, W.; Fu, F. Design of novel multifunctional targeting nano-carrier drug delivery system based on CD44 receptor and tumor microenvironment pH condition. Drug Deliv. 2016, 23, 798–803. [Google Scholar] [CrossRef]
- Labie, H.; Perro, A.; Lapeyre, V.; Goudeau, B.; Catargi, B.; Auzély, R.; Ravaine, V. Sealing hyaluronic acid microgels with oppositely-charged polypeptides: A simple strategy for packaging hydrophilic drugs with on-demand release. J. Colloid Interface Sci. 2019, 535, 16–27. [Google Scholar] [CrossRef]
- Johnson, P. CD44 and its Role in Inflammation and Inflammatory Diseases. Inflamm. Allergy-Drug Targets 2009, 8, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mesrati, M.H.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Ponomarev, A.; Gilazieva, Z.; Solovyeva, V.; Allegrucci, C.; Rizvanov, A. Intrinsic and Extrinsic Factors Impacting Cancer Stemness and Tumor Progression. Cancers 2022, 14, 970. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G. The role of CD44 in epithelial–mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar] [CrossRef]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 1–23. [Google Scholar] [CrossRef]
- de la Rosa, J.M.R.; Pingrajai, P.; Pelliccia, M.; Spadea, A.; Lallana, E.; Gennari, A.; Stratford, I.J.; Rocchia, W.; Tirella, A.; Tirelli, N. Binding and Internalization in Receptor-Targeted Carriers: The Complex Role of CD44 in the Uptake of Hyaluronic Acid-Based Nanoparticles (siRNA Delivery). Adv. Healthc. Mater. 2019, 8, e1901182. [Google Scholar] [CrossRef]
- Fu, C.-P.; Cai, X.-Y.; Chen, S.-L.; Yu, H.-W.; Fang, Y.; Feng, X.-C.; Zhang, L.-M.; Li, C.-Y. Hyaluronic Acid-Based Nanocarriers for Anticancer Drug Delivery. Polymers 2023, 15, 2317. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 760. [Google Scholar] [CrossRef]
- Asemi, Z.; Homayoonfal, M.; Aminianfar, A.; Yousefi, B. Application of nanoparticles for efficient delivery of quercetin in cancer cells. Curr. Med. Chem. 2023, 30. [Google Scholar] [CrossRef]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H.; Sheridan, C.; et al. CD44+/CD24-breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef]
- Ferrara, B.; Dugnani, E.; Sordi, V.; Pasquale, V.; Pellegrini, S.; Reni, M.; Balzano, G.; Piemonti, L. A Comprehensive Characterization of Stemness in Cell Lines and Primary Cells of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 10663. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Hristova-Avakumova, N.; Hadjimitova, V.; Traykov, T.; Petrov, P. Evaluation of physicochemical and antioxidant properties of nanosized copolymeric micelles loaded with kaempferol. Pharmacia 2020, 67, 49–54. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.N.H.; Choi, J.-W.; Jeong, H. Anti-cancer effects of polyphenolic compounds in epidermal growth factor receptor tyrosine kinase inhibitor-resistant non-small cell lung cancer. Pharmacogn. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef]
- Taherkhani, A.; Khodadadi, P.; Samie, L.; Azadian, Z.; Bayat, Z. Flavonoids as Strong Inhibitors of MAPK3: A Computational Drug Discovery Approach. Int. J. Anal. Chem. 2023, 2023, 8899240. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Greco, A.; Riva, F.; Dorati, R.; Conti, B.; Modena, T.; Genta, I. CD44-Targeted Carriers: The Role of Molecular Weight of Hyaluronic Acid in the Uptake of Hyaluronic Acid-Based Nanoparticles. Pharmaceuticals 2022, 15, 103. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: Antineoplastic effect in human ovarian cancer cells in vitro. Int. J. Pharm. 2021, 606, 120899. [Google Scholar] [CrossRef]
- Wolny, P.M.; Banerji, S.; Gounou, C.; Brisson, A.R.; Day, A.J.; Jackson, D.G.; Richter, R.P. Analysis of CD44-Hyaluronan Interactions in an Artificial Membrane System Insights into the Distinct Binding Properties of High and Low Molecular Weight Hyaluronan. J. Biol. Chem. 2010, 285, 30170–30180. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Talih, T.; Oz, B.; Halaclar, B.; Caglayan, K.; Sipahi, M. Comparison of lovastatin and hyaluronic acid/carboxymethyl cellulose on experimental created peritoneal adhesion model in rats. Int. J. Surg. 2014, 12, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, K.; Baut, E.; Scaltriti, M.; Jena, P.V.; et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra87. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wang, Z.; Chen, J.; Chen, X.; Li, J.; Li, Y.; Li, R.; Liu, X.; Song, B.; Cheong, K.-L.; et al. Physicochemical Characterization and Antitumor Activity of Fucoidan and Its Degraded Products from Sargassum hemiphyllum (Turner) C. Agardh. Molecules 2023, 28, 2610. [Google Scholar] [CrossRef]
- Du, G.; Jin, L.; Han, X.; Song, Z.; Zhang, H.; Liang, W. Naringenin: A Potential Immunomodulator for Inhibiting Lung Fibrosis and Metastasis. Cancer Res. 2009, 69, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Chang, Y.-M.; Lai, S.-C.; Chen, K.-M.; Wang, K.-C.; Chiu, T.-T.; Chang, F.-H.; Hsu, L.-S. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Gu, R.; Zhang, M.; Meng, H.; Xu, D.; Xie, Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed. Pharmacother. 2018, 105, 491–497. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Soltani, A.; Abadi, M.S.S.; Raeisi, M.; Kouhihabibidehkordi, G.; Eshaghi, F.; Mohreh, O. Apoptosis-inducing Plant-based phenolic compounds are effective on leukemia cell lines. Curr. Pharm. Des. 2023, 29, 1092–1104. [Google Scholar] [CrossRef]
- Franciosoa, A.; Mastromarino, P.; Masci, A.; D’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A Promising Therapeutic Advancement over Native Curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, N.; Gong, X.; Kawashima, Y.; Cui, F.; Sun, S. Tumor-targeting micelles based on folic acid and α-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery. Colloids Surf. B Biointerfaces 2019, 177, 11–18. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Biotin functionalized PEGylated poly(amidoamine) dendrimer conjugate for active targeting of paclitaxel in cancer. Int. J. Pharm. 2019, 557, 329–341. [Google Scholar] [CrossRef]
- He, Q.; Liu, C.; Wang, X.; Rong, K.; Zhu, M.; Duan, L.; Zheng, P.; Mi, Y. Exploring the mechanism of curcumin in the treatment of colon cancer based on network pharmacology and molecular docking. Front. Pharmacol. 2023, 14, 737. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Xu, X.; Liu, X.; Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: A review. Front. Nutr. 2022, 9, 999373. [Google Scholar] [CrossRef]
- Li, H.-Y.; Li, M.; Luo, C.-C.; Wang, J.-Q.; Zheng, N. Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef]
- Wei, R.; Wirkus, J.; Yang, Z.; Machuca, J.; Esparza, Y.; Mackenzie, G.G. EGCG sensitizes chemotherapeutic-induced cytotoxicity by targeting the ERK pathway in multiple cancer cell lines. Arch. Biochem. Biophys. 2020, 692, 108546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qu, J.; Fan, Y.; Zhang, R.; Wang, X. Curcumin Inhibits Invasion and Epithelial–Mesenchymal Transition in Hepatocellular Carcinoma Cells by Regulating TET1/Wnt/β-catenin Signal Axis. Bull. Exp. Biol. Med. 2022, 173, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Ren, S.; Chen, T.; Zhai, B.; Cheng, J.; Shi, X.; Song, L.; Fan, Y.; Guo, D. Investigation and experimental validation of curcumin-related mechanisms against hepatocellular carcinoma based on network pharmacology. J. Zhejiang Univ. B 2022, 23, 682–698. [Google Scholar] [CrossRef]
- Reyes, A.G.Q.; Sepulveda, S.A.L.; Martinez-Acuña, N.; Islas, J.F.; Gonzalez, P.D.; Torres, T.G.H.; Perez, J.R.; Treviño, E.N.G. Cancer Stem Cell and Hepatic Stellate Cells in Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2023, 22, 15330338231163677. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, 147276. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Li, Y.; Wang, X.; Lu, Q.; Wang, D.; Qi, C.; Li, C.; Li, Z.; Lian, B.; et al. Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and metastasis via targeting “substance P-hepatic stellate cells-hepatocellular carcinoma” axis. Biomaterials 2021, 276, 121003. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Z.-P.; Tian, G.-X.; Pan, R.-Y.; Xu, C.-M.; Zhang, B.; Wu, J.-L. Liver-targeted liposomes for codelivery of curcumin and combretastatin A4 phosphate: Preparation, characterization, and antitumor effects. Int. J. Nanomed. 2019, 14, 1789–1804. [Google Scholar] [CrossRef]
- Castilho-Fernandes, A.; De Almeida, D.C.; Fontes, A.M.; Melo, F.U.F.; Picanço-Castro, V.; Freitas, M.C.; Orellana, M.D.; Palma, P.V.; Hackett, P.B.; Friedman, S.L.; et al. Human hepatic stellate cell line (LX-2) exhibits characteristics of bone marrow-derived mesenchymal stem cells. Exp. Mol. Pathol. 2011, 91, 664–672. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Lee, D.Y.; White, E.S.; Cui, Z.; Larios, J.M.; Chacon, R.; Horowitz, J.C.; Day, R.M.; Thomas, P.E. Myofibroblast Differentiation by Transforming Growth Factor-beta1 Is Dependent on Cell Adhesion and Integrin Signaling via Focal Adhesion Kinase. J. Biol. Chem. 2003, 278, 12384–12389. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, J.; Pan, Y.; Shen, W. Berberine Suppressed the Progression of Human Glioma Cells by Inhibiting the TGF-β1/SMAD2/3 Signaling Pathway. Integr. Cancer Ther. 2022, 21, 15347354221130303. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Pharmacological effects of baicalin in lung diseases. Front. Pharmacol. 2023, 14, 1077. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sima, M.; Kopečková, P.; Wu, K.; Gao, S.; Liu, J.; Wang, D.; Miller, S.C.; Kopeček, J. Biodistribution and Pharmacokinetic Studies of Bone-Targeting N-(2-Hydroxypropyl)methacrylamide Copolymer−Alendronate Conjugates. Mol. Pharm. 2008, 5, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Majidi, A.; Na, R.; Dixon-Suen, S.; Jordan, S.J.; Webb, P.M. Common medications and survival in women with ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2020, 157, 678–685. [Google Scholar] [CrossRef]
| Preclinical Cancer Model | Nanoformulation | Antineoplastic Drug/Nutraceutic Enclosed in the Same Nanoformulation or Administered Separately | PheC (Flavonoid) in the Nanoformulation | Anticancer Effect(s) of Nanoformulation (or Combination of Formulations) Respect to the Drugs Alone | Ref. |
|---|---|---|---|---|---|
| In vitro: A549 lung carcinoma cells | Two molecular weight HA-modified kaempferol-loaded nanostructured lipid carriers (HA-KA-NLCs). Size: HA20-KA-NLC: 92.7 ± 4.6 nm HA130-KA-NLC: 97.6 ± 4.3 nm | None | Kaempferol (Flavonol) | In vitro: ↓ Cell viability; ↑ IC50 ↓ Cell proliferation; ↓ Cell migration rate ↑ Apoptosis | [34] |
| In vitro: MCF-7/ADR human breast cancer cells and 3D spheroids In vivo: MCF-7/ADR subcutaneously injected in nude mice breast. Drugs i.v. injected when the tumor reached 200 mm3. | Hybrid polymeric NPs for PTX and QU co-loading. Size:187.97 ± 1.66 nm | PTX | QU (Flavonol) | In vitro: ↓ IC50; Apoptosis In vivo: ↓ Tumor volume | [35] |
| In vivo: LNCaP human prostate cancer cells transplanted in the subcutaneous space of SCID mouse dorsa. Drugs i.v. injected in tumor-bearing mice after 14 days. | A boronated derivative of HA linked to QU | None | QU (Flavonol) | ↓ Tumor growth | [37] |
| In vitro: MDA-MB-231/MDR1 breast cancer cells In vivo: same cells transplanted in mice | Amphiphilic PEI-TOS/HA-QU core-shell micelles for the targeted co-delivery of PTX and QU. Size: 167.60 ± 8.185 nm | PTX | QU (Flavonol) | In vitro: ↓ IC50; ↑ Mitochondrial-dependent apoptosis In vivo: ↓ Tumor size | [38] |
| In vitro: 4T1 Breast cancer cell lines. In vivo: 4T1 Breast cancer cell lines injected subcutaneously into the right hind hip of Balb/c mice. Drugs i.v. injected every other day when the tumor reached 80–100 mm3 | HA acid-based QU nanoformulation. Size: 235.9 ± 3.2 nm | None | QU (Flavonol) | In vitro: ↓ Cell viability; ↑Apoptosis In vivo: ↓ Tumor volume | [39] |
| In vitro: MDA-MB-231/MDR1 breast cancer cells. In vivo: MDA-MB-231/MDR1 cells inoculated subcutaneously in the breast of nude mice. Drugs i.v. injected when the tumor reached 200 mm3. | HA-Based Conjugate/D α-TPGS Mixed Micelles loaded with: QU (average size: 329.83 nm) or DOX (average size: 201.2 nm) | DOX | QU (Flavonol) | In vitro: ↓ Cell viability; ↑Apoptosis; ↓ P-gp expression In vivo: ↓ Tumor volume | [40] |
| In vitro: Mia-PaCa-2 and PANC-1 pancreatic cancer cell lines | HA-decorated poly-ethylene oxide NPs loaded with: CD/QU (size: 135 ± 7 nm) or GMC (size: 175 ± 10 nm) | Gemcitabine | QU (Flavonol) | In vitro: ↓ Cell viability; ↑ Sensitivity of cancer cells to the anti-inflammatory effect of QU loaded in NP. | [41] |
| In vitro: Human prostate cancer PC3 cells In vivo: PC3 cells subcutaneously implanted in SCID mice. Drugs i.v. injected 6 times at 3 day intervals | TPGS-conjugated HA and fucoidan-based NPs. 205.48 ± 8.52 nm | DXT | EGCG (Flavanol) | In vitro: ↓ PC3 cell viability In vivo: ↓ tumor volume and tumor weight; ↑cell apoptosis (M30 protein) and ↓ cell proliferation (Ki67 ) in tumors | [42] |
| In vitro: MDA-MB-231/MDR1 breast cancer cells In vivo: MDA-MB-231/MDR1 bearing Balb/c nude mice. Drugs i.v. injected 6 times at 3 day intervals. | HA-coated EGCG, siRNA and protamine nanogel Average size: 80 nm | siRNA (for silencing CTGF, associated to drug resistance, promotion of cell proliferation, and migration) | EGCG (Flavanol) | In vitro:↓ MDA-MB-231/MDR1 cell viability and ↑apoptosis; ↓ expression of the drug resistance associated proteins cIAP1, Bcl-xL, and CTGF In vivo: ↓ Tumor volume | [43] |
| In vitro: Luc PC3 prostate cancer cells In vivo: Luc PC3 cells orthotopically injected in SCID mice. Drugs i.v. injected 6 times at 3 day intervals | HA, fucoidan, and poly(ethylene glycol)-gelatin NPs encapsulating EGCG and CU. Size: 197.73 ± 18.53 nm | CU | EGCG (Flavanol) | In vitro: ↓ PC3 cell viability; In vivo: ↓ Tumor cell proliferation (Ki-67) | [44] |
| In vitro: B16F10 mouse melanoma cells and DC2.4 mouse dendritic cell line. In vivo: B16F10 cells subcutaneously injected into the flank of C57BL/6J mice. Drugs i.v. injected every 3 days for four times. | Nanogels containing HA, cyclodextrin, and pH-sensitive ketone cross-linker DMAEP coloading ECGC and R848. Size: from 161.4 ± 2.86 to 170.17 ± 4.95 nm | Resiquimod (R848) (immune modulator) | EGCG (Flavanol) | In vitro: ↑ Maturation of dendritic cells and CTL stimulation. In vivo: ↑ PDL1 expression in tumors; ↓ CTL activation and infiltration into tumors; ↑ Treg suppressive effects | [45] |
| In vitro: Stable luciferase-expressing human MKN45 gastric cancer cells (Luc MKN45); In vivo: Luc MKN45 cells orthotopically inoculated in SCID mice. Drugs injected 5 times within 2 weeks | Fucoidan and TPGS-conjugated HA-based NPs. Size: 200–230 nm | DOX | EGCG (Flavanol) | In vitro: ↓ PC3 cell viability; arrest in G2/M cell cycle phase; ↑ apoptosis: In vivo: ↓ luminescence in luciferase-expressing gastric tumors; ↑cell apoptosis (M30 protein) and ↓cell proliferation (Ki67) in tumors | [46] |
| In vitro: A549 lung carcinoma cells. In vivo: A549 cells microinjected into the yolk of zebrafish embryos cultured for 48 h. Embryos treated with the drugs after 4 h. | Mitochondrial-targeting reduction-responsive nano drug delivery system (EGCG@THSI NPs). Size: about 173.2 nm | IR780-iodide (IR780) (photoactivator) | EGCG (Flavanol) | ↓ Cell proliferation (in vitro and in vivo) ↑ ROS production ↓ Cell invasion, metastasis and angiogenesis (in vivo) | [47] |
| In vitro: A549 lung carcinoma cells. In vivo: chemically-induced lung cancer in Wistar rats by 3 i.p. injections of urethane, every 2 days for a week. Drug orally administrated (50 mg/kg) at the start of the experiment or 15 days prior the beginning of the experiment. | HA-decorated caprolactone NPs. Size: 251.6 ± 3.22 nm | None | Naringenin (Flavanone) | In vitro: ↓ A549 cell viability; ↑ Cell-cycle arrest in G2-M phase In vivo: ↑ Animal survival ↓ Lung weight | [48] |
| In vitro: Human PC3 prostate adenocarcinoma cell line. In vivo: PC3 cell subcutaneously injected into the right flank of Balb/c nude mice. Drugs i.v. injected as the tumor reached 100 mm3. | GE11-modified nanoparticles (GE-NPs) loaded with DTX assembled with HA-decorated NPs (HANPs) encapsulating Formononetin Size: 189.5 ± 3.3 nm, | DTX | Formononetin (Isoflavon) | In vitro:↓ PC3 cell viability In vivo: ↓ Tumor volume ↑ Drug distribution in tumor | [49] |
| Preclinical Model | Nanoformulation (as Reported by the Authors) | Antineoplastic Drug/Nutraceutic enclosed in One Nanoformulation or Administered Separately | PheC (Non-Flavonoid) in the Nanoformulation | Anticancer Effect(s) of the Nanoformulation (or Combination of Formulations) Respect to the Enclosed Free Compounds | Ref. |
|---|---|---|---|---|---|
| In vitro: DOX-resistant HL-60 promyelocytic leukemia cells and DOX-resistant K562 chronic myeloid leukemia cells. In vivo: DOX-resistant HL-60 cells subcutaneously injected in Balb/c nude mice. Drugs i.v. injected every 3 days for 7 times. | Lipid-polymeric hybrid NPs consisting in: HA conjugated with PEG-DSPE co-loading DOX and GA. Size: 165.7 ± 4.6 nm | DOX | GA (Phenolic acid) | In vitro: ↓Cell viability ↓ IC50 (maximally at 2:1 DOX/GA ratio) In vivo: ↓ Tumor volume ↓ Decrease in body weight | [55] |
| In vitro: Human MCF-7 and CAL-51 breast cancer cell lines. | Liquid crystalline NPs (LCNPs) enclosing Tamoxifen plus RES and coated with multiple layers of chitosan and HA. Size: about 217 nm | TAM | RES (Stilbene) | In vitro: ↓ Cell viability *; ↑ Apoptosis * In vivo: No change in body weight during treatment | [61] |
| In vitro: 4T1 murine breast cancer cells | RES-loaded Zein-SHA NPs. Average size: about 152.13 nm | None | RES (Stilbene) | In vitro: ↓ Cell viability ↓ IC50 | [62] |
| In vitro: MDA-MB-231 triple-negative breast cancer cells In vivo: subcutaneous inoculation of MDA-MB-231 cells in the abdomen of nude mice. Drugs injected into the tumors as they reached a volume of about 80 mm3. | Injectable Res-Cx-HA hydrogel Size: Non reported | None | Resveratrol (RES) (Stilbene) | In vitro: ↓ Cell viability; In vivo: Tumor tissue inject with the Hydrogel: ↑ necrosis rate of tumor tissue; ↓ Angiogenesis | [63] |
| In vitro: CT26 colon cancer cell line; In vivo: CT26 cells xenografted in the flank of Balb/c nude mice. Drugs i.v. injected every day for 2 weeks. | HA-Zein-CUR NG Size: from 200–250 nm. | None | CUR (Curcuminoid) | In vitro: ↓ Cell viability; ↑ Apoptosis In vivo: ↓ Tumor volume and weight | [64] |
| In vitro: MDA-MB-231 and MDA-MB-468 breast cancer cells | CDF loaded in HA-SMA-TPGS nanomicelles. Average size: 129.4 nm | None | CDF (Curcuminoid) | In vitro: ↓ Cell viability; ↑ Apoptosis ↑ PTEN (pro-apoptotic) and ↓ NF-kB (tumorigenic) expression | [65] |
| In vitro: MDA-MB-231 breast cancer cell line. In vivo: Swiss albino mice injected with Erlich Ascites Carcinoma. Drugs i.v. injected after 10 days every 2 days for 2 weeks. | HA-tagged mesoporous silica NPs loaded with CUR. Average size: 161.3 nm | None | CUR (Curcuminoid) | In vitro: ↓ Cell viability; ↓ in-vitro cellular migration; ↑ Apoptosis; Cell cycle arrest at G2/M phase In vivo: ↓ Tumor volume and tumor mass | [66] |
| In vitro: MCF-7 cells breast cancer cells and breast cancer stem cells. In vivo: MCF-7 cells injected in nude mice. Drugs i.v. injected as tumors reached 400 mm3 | Icariin and CUR co-encapsulated in polymeric micelles based on pH-sensitive hydrazone bond, FA and biotin-conjugated HA. Size: 162.7 ± 5 nm. | Icariin | CUR (Curcuminoid) | In vitro: ↓ Cell viability; ↓ Invasion ability (Transwell assay) In vivo: ↓ Tumor volume | [67] |
| In vitro: A549 lung carcinoma cells and RAW264.7 cells. In vivo: A549 cells trasplanted in mice. Drugs i.v. injected as the tumor reached an appropriate size. | QU-dithiodipropionic acid-oligomeric HA-mannose-ferulic acid self-assembled and encapsulating CUR and Baicalin. Size: 121.0 ± 15 nm | Baicalin | CUR (Curcuminoid) | In vitro: ↓ Cell viability; In vivo: ↓ Tumor volume; ↓ Decrease in body weight | [68] |
| In vitro: MG-63 osteosarcoma cells In vivo: MG-63 cells injected into the right tibia of nude mice; at 12 days, drugs injected every 2 days for 20 days. | ALN-HA-C18 loading CUR. Size: 118 ± 3.6 nm | None | CUR (Curcuminoid) | In vitro: ↓ Cell viability; In vivo: ↓ Tumor volume; | [69] |
| In vitro: Human SKOV3 and SKOV3-TR30 ovarian cancer cells (multi-drug resistant); In vivo: SKOV3 cells subcutaneously xenografted. Drugs i.v. injected as tumor reached 200 mm3. | HA-coated PEI-SA copolymer co-encapsulating PTX and CUR. Average Size: 187.77 nm | PTX | CUR (Curcuminoid) | In vitro: ↓ IC50 (in both the cells) ↓ Cell invasiveness (of both cells) In vivo: ↓ Tumor volume | [70] |
| In vitro: LX-2 human hepatic stellate cells (HSCs) and SMMC-7721 human hepatocarcinoma cells (HCCs). In vivo: H22 mouse HCCs, mouse HSCs and SP subcutaneously injected into the right flank of mice. Drugs i.v. injected 7 times as tumors reached 150 mm3. | HA and glycyrrhetinic acid-modified liposomes co-delivering aprepitant and CUR. Size: 117.40 ± 0.62 | APR | CUR (Curcuminoid) | In vitro: ↓ Cell viability of LX-2 cells and of SP-treated LX-2 + HCCs co-culture; ↓ Cell migration in HCCs and in SP-treated LX-2 + HCCs co-culture In vivo: ↓ Tumor volume | [71] |
| In vitro: HCT116, HCT8, and HT29 colon cancer cells. | Zein-HA NPs enclosing CUR. Average size: about 300 nm | None | CUR (Curcuminoid) | In vitro: ↓ Cell viability of all cancer cell and IC50 with Zein-HA NPs (respect to CUR alone or to all other NPs) | [72] |
| In vitro: Human HCCs (BEL-7402), human-derived HSCs (LX-2), mouse HCCs (H22), and mouse HSCs (mHSCs); In vivo: H22+mHSCs injected in the flank of Balb/c mice. Drugs i.v. injected as tumors reached 200 mm3. | Glycyrrhetinic acid- and HA-modified liposomes co-delivering Berberine and CUR. Size: 159.39 ± 3.16 nm | Berberine | CUR (Curcuminoid) | In vitro: ↓ Cell viability of BEL-7402 cells and co-cultured BEL-7402+LX- cells; In vivo: ↓Tumor volume in H22+m-HSC tumor-bearing mice model | [73] |
| In vitro: Human breast adenocarcinoma cell line (MDA). | HA- and riboflavin-coated transition metals-based nanoplatforms enclosing CUR. Average size: about 70 nm | None | CUR (Curcuminoid) | In vitro: ↓ Cancer cell viability (vs. NPs not containing CUR, not vs. CUR alone) | [74] |
| In vitro: human HT-29 and mouse CT-26 colon cancer cells | Lactoferrin-EGCG-NP coated with HA and loading CUR. Average size: 144.7 nm | None | CUR (Curcuminoid) | ↓ Cancer cell viability ↑ Apoptosis | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Trombino, S.; Curcio, F.; Sole, R.; Cassano, R.; Calviello, G. Hyaluronic Acid-Mediated Phenolic Compound Nanodelivery for Cancer Therapy. Pharmaceutics 2023, 15, 1751. https://doi.org/10.3390/pharmaceutics15061751
Serini S, Trombino S, Curcio F, Sole R, Cassano R, Calviello G. Hyaluronic Acid-Mediated Phenolic Compound Nanodelivery for Cancer Therapy. Pharmaceutics. 2023; 15(6):1751. https://doi.org/10.3390/pharmaceutics15061751
Chicago/Turabian StyleSerini, Simona, Sonia Trombino, Federica Curcio, Roberta Sole, Roberta Cassano, and Gabriella Calviello. 2023. "Hyaluronic Acid-Mediated Phenolic Compound Nanodelivery for Cancer Therapy" Pharmaceutics 15, no. 6: 1751. https://doi.org/10.3390/pharmaceutics15061751
APA StyleSerini, S., Trombino, S., Curcio, F., Sole, R., Cassano, R., & Calviello, G. (2023). Hyaluronic Acid-Mediated Phenolic Compound Nanodelivery for Cancer Therapy. Pharmaceutics, 15(6), 1751. https://doi.org/10.3390/pharmaceutics15061751











