The Effectiveness of a Bioactive Healing Abutment as a Local Drug Delivery System to Impact Peri-Implant Mucositis: A Prospective Case Series Study
Abstract
1. Introduction
2. Materials and Methods
2.1. General Statements
2.2. Participants
2.3. Case Qualification
2.4. Periodontal Examination
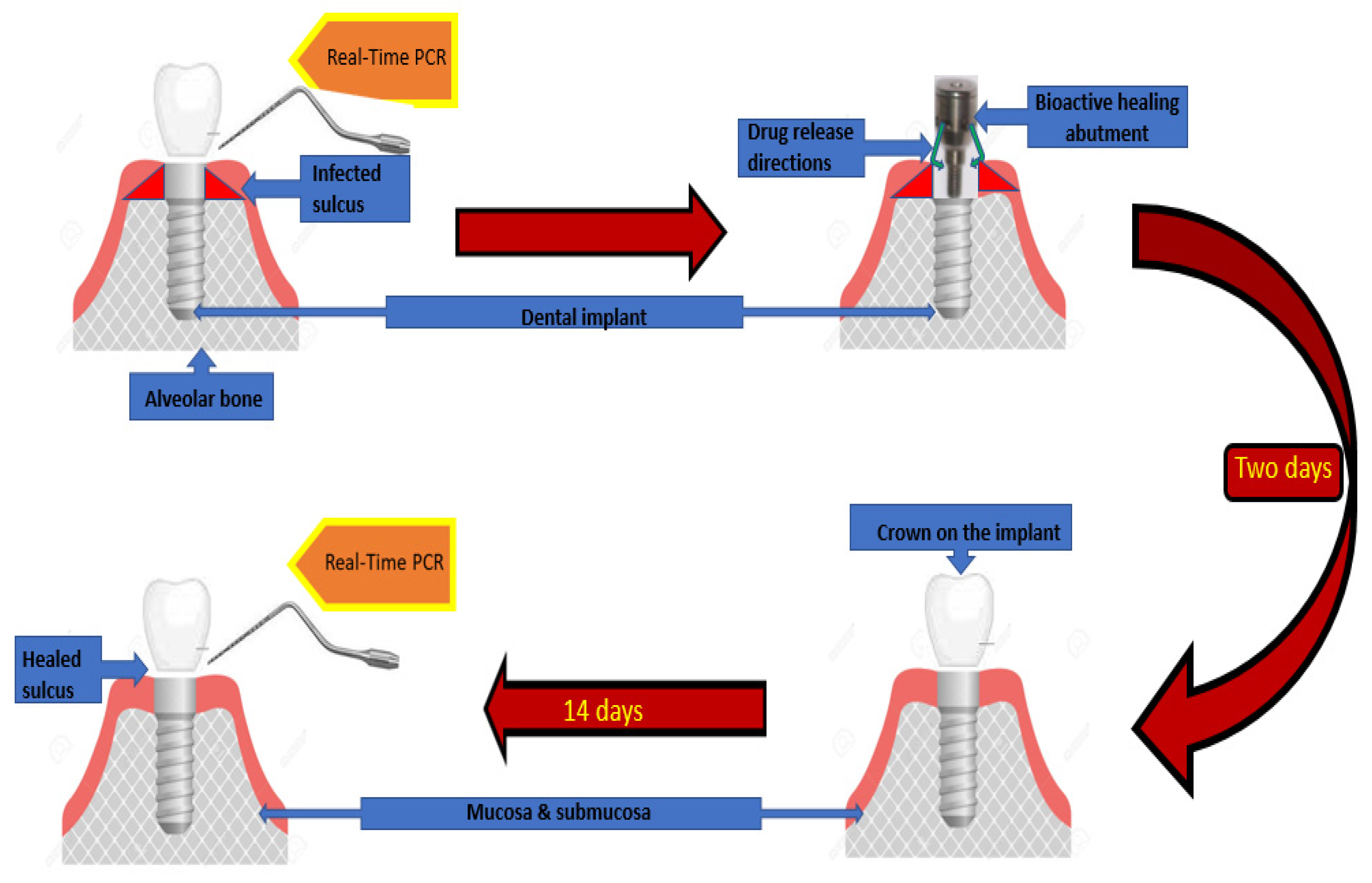
2.5. Study Design
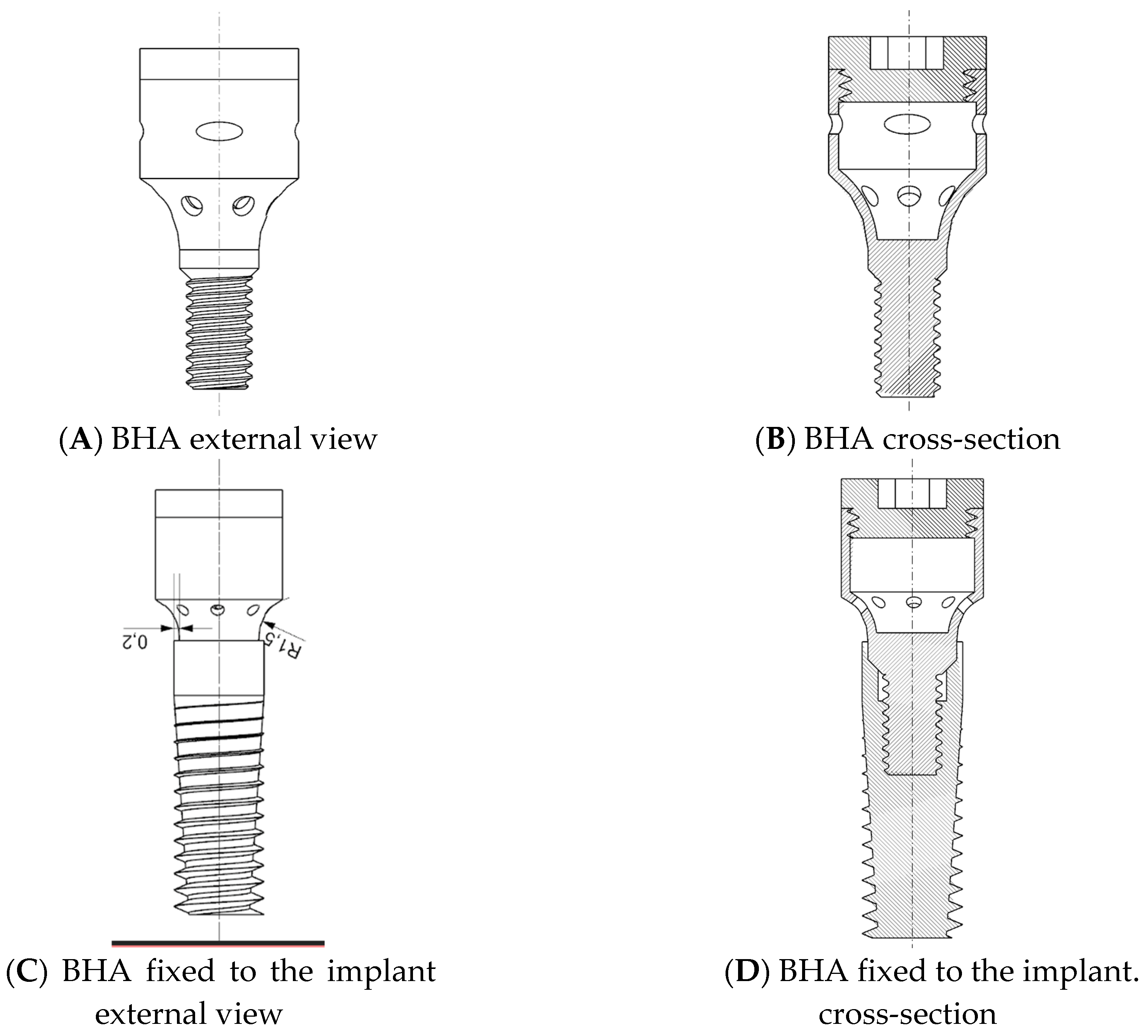
2.6. Preparation of the Insert for the Bioactive Healing Abutment
2.7. Statistical Analysis
3. Results
3.1. Bleeding Gums—Modified Sulcus Bleeding Index-mBI
3.2. Plaque Deposit—Modified Plaque Index-mPLI
3.3. Redness, Swelling and Exudation
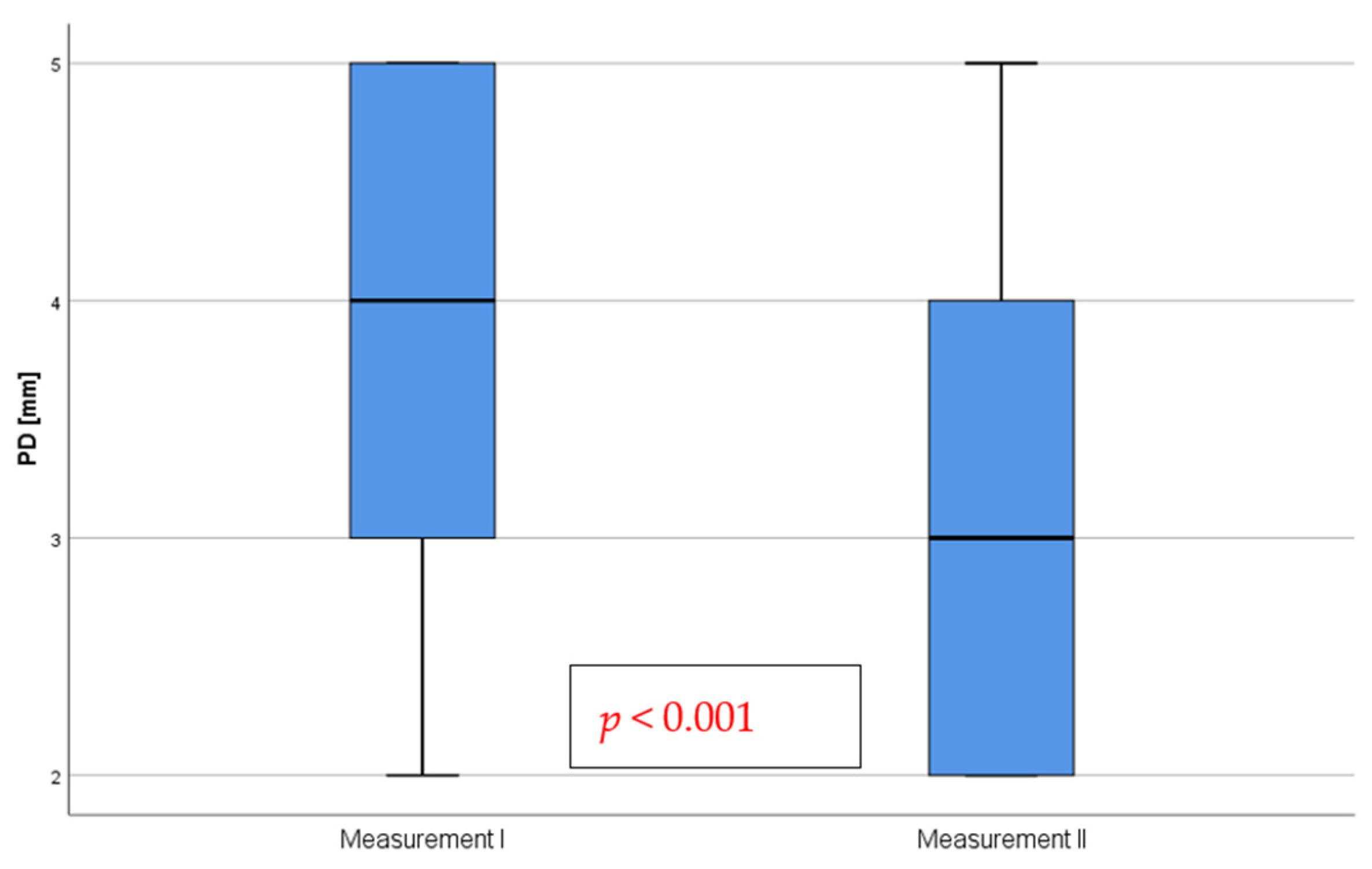
3.4. Depth of Pockets Formed by Implants
3.5. Occurrence of Bacterial Strains
3.6. Number of Bacteria Detected in Peri-Implant Soft Tissues
3.7. Number of Bacteria Detected in Peri-Implant Soft Tissues around Front and Posterior Implants
3.8. The Difference in the Number of Bacteria before and after Treatment
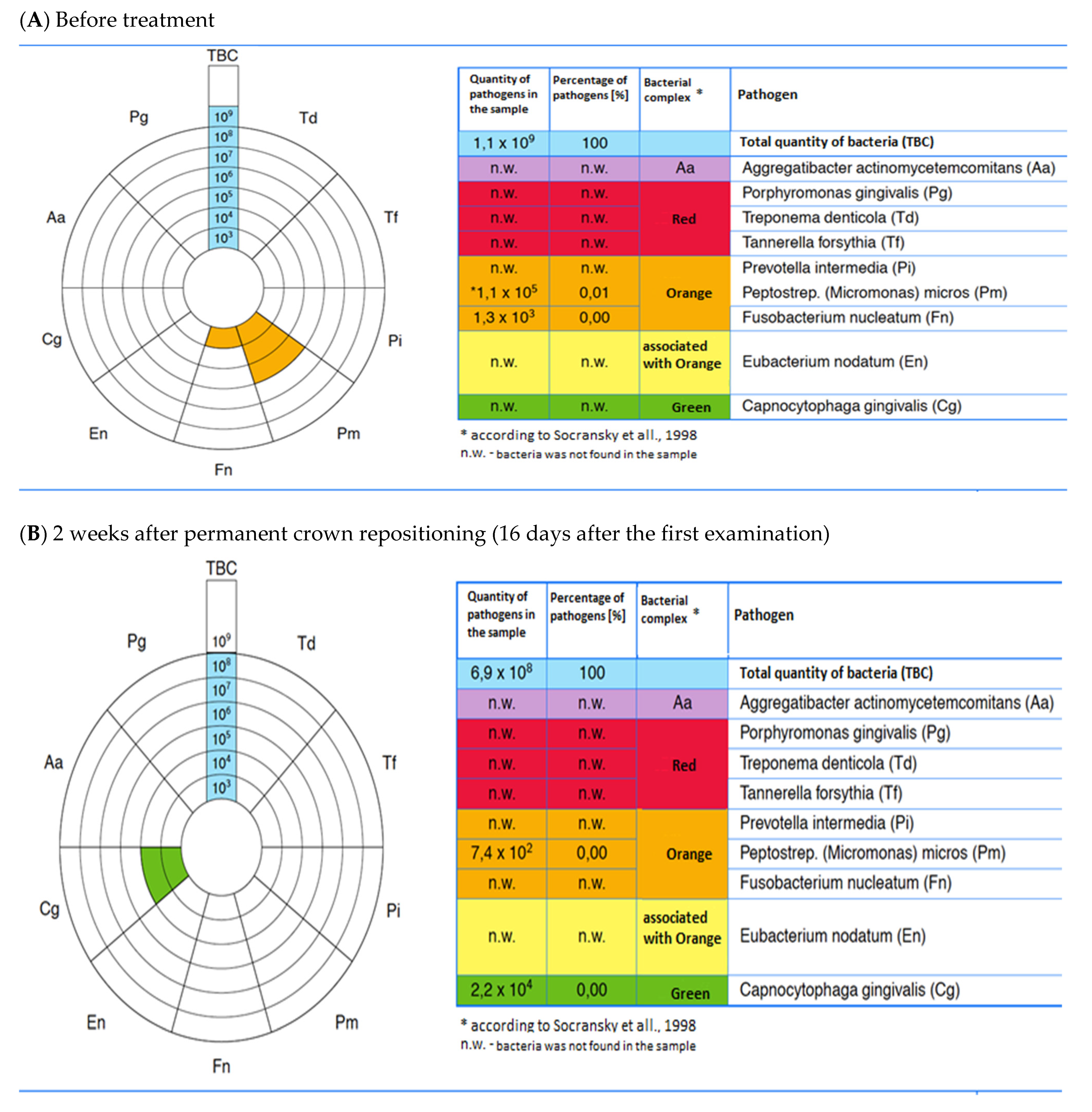
3.9. The Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guillaume, B. Dental implants: A review. Morphologie 2016, 100, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Wychowanski, P.; Woliński, J.; Morawiec, T.; Kownacki, P.; Starzynska, A.; Kosieradzki, M.; Fiedor, P. Preliminary Clinical Data and the Comparison of the Safety and Efficacy of Autogenous Bone Grafts Versus Xenograft Implantations in Vertical Bone Deficiencies Before Dental Implant Installation. Transplant. Proc. 2020, 52, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef]
- Wychowanski, P.; Wolinski, J.; Kacprzak, M.; Tomkiewicz, W.; Bartlomiej, I.; Szubinska-Lelonkiewicz, D.; Wojtowicz, A.; Nevins, M. Immediate Palatal Molar Implants: A Simple, Safe, Minimally Invasive Technique. Int. J. Periodontics Restor. Dent. 2017, 37, e297–e301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cruz, R.S.; Lemos, C.A.A.; Batista, V.E.S.; Oliveira, H.; Gomes, J.M.L.; Pellizzer, E.P.; Verri, F.R. Short implants versus longer implants with maxillary sinus lift. A systematic review and meta-analysis. Braz Oral Res 2018, 32, e86. [Google Scholar] [CrossRef]
- Di Murro, B.; Moretti, M.; De Smaele, E.; Letizia, C.; Lubrano, C.; Passarelli, P.C.; D’Addona, A.; Pompa, G.; Papi, P. Microbiological Profiles of Dental Implants in Metabolic Syndrome Patients: A Case-Control Study. Antibiotics 2021, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Spijkervet, F.; Raghoebar, G.M. The medically compromised patient: Are dental implants a feasible option? Oral Dis. 2018, 24, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Wychowanski, P.; Starzynska, A.; Woliński, J.; Kosieradzki, M.; Fiedor, P. New Surgical Technique Using Xenograft as a Microinvasive Method to Avoid Extensive Bone Reconstruction in Patients With Compromised General Health: Promising Surgical Methodology and First Clinical Results. Transplant. Proc. 2020, 52, 2244–2247. [Google Scholar] [CrossRef]
- Compton, S.M.; Clark, D.; Chan, S.; Kuc, I.; Wubie, B.A.; Levin, L. Dental Implants in the Elderly Population: A Long-Term Follow-up. Int. J. Oral Maxillofac. Implant. 2017, 32, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early dental implant failure: Risk factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef]
- Wychowański, P.; Starzyńska, A.; Jereczek-Fossa, B.A.; Iwanicka-Grzegorek, E.; Kosewski, P.; Adamska, P.; Woliński, J. The Effects of Smoking Cigarettes on Immediate Dental Implant Stability—A Prospective Case Series Study. Appl. Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Lang, N.P.; Kinane, D.F.; Lindhe, J.; Sanz, M.; Tonetti, M.S. Sixth European Workshop on Periodontology of the European Academy of Periodontology at the Charterhouse at Ittingen, Thurgau, Switzerland. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 1–2. [Google Scholar] [CrossRef]
- Butera, A.P.M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A.; Butera, A.B.A.; Pascadopoli, M. Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 286–291. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glockner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.-O.; Garcia-Sanchez, A.; Kewalramani, N.; Barone, A.; Martínez-González, J.-M.; Velasco-Ortega, E.; López-López, J.; Kaiser-Cifuentes, R.; Guerra, F.; Matos-Garrido, N.; et al. Consensus Report on Preventive Antibiotic Therapy in Dental Implant Procedures: Summary of Recommendations from the Spanish Society of Implants. Antibiotics 2022, 11, 655. [Google Scholar] [CrossRef]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants—Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar]
- Lopez, M.A.; Passarelli, P.C.; Godino, E.; Lombardo, N.; Altamura, F.R.; Speranza, A.; Lopez, A.; Papi, P.; Pompa, G.; D’Addona, A. The Treatment of Peri-Implant Diseases: A New Approach Using HYBENX® as a Decontaminant for Implant Surface and Oral Tissues. Antibiotics 2021, 10, 512. [Google Scholar] [CrossRef]
- Patil, C.; Agrawal, A.; Abullais, S.S.; Arora, S.; Khateeb, S.U.; Fadul, A. Elagib, M. Effectiveness of Different Chemotherapeutic Agents for Decontamination of Infected Dental Implant Surface: A Systematic Review. Antibiotics 2022, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Wychowanski, P.; Starzynska, A.; Adamska, P.; Slupecka-Ziemilska, M.; Sobocki, B.K.; Chmielewska, A.; Wysocki, B.; Alterio, D.; Marvaso, G.; Jereczek-Fossa, B.A.; et al. Methods of Topical Administration of Drugs and Biological Active Substances for Dental Implants-A Narrative Review. Antibiotics 2021, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Roca-Millan, E.; Estrugo-Devesa, A.; Merlos, A.; Jané-Salas, E.; Vinuesa, T.; López-López, J. Systemic Antibiotic Prophylaxis to Reduce Early Implant Failure: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.-O.; Peña-Cardelles, J.-F.; Kewalramani, N.; Mateos-Moreno, M.-V.; Jiménez-Guerra, Á.; Velasco-Ortega, E.; Uribarri, A.; Moreno-Muñoz, J.; Ortiz-García, I.; Núñez-Márquez, E.; et al. Preventive Antibiotic Therapy in the Placement of Immediate Implants: A Systematic Review. Antibiotics 2022, 11, 5. [Google Scholar] [CrossRef]
- Iwańczyk, B.; Wychowański, P.; Minkiewicz-Zochniak, A.; Strom, K.; Jarzynka, S.; Olędzka, G. Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study. Appl. Sci. 2020, 10, 5376. [Google Scholar] [CrossRef]
- Mombelli, A.; Samaranayake, L.P. Topical and systemic antibiotics in the management of periodontal diseases. Int. Dent. J. 2004, 54, 3–14. [Google Scholar] [CrossRef]
- Addy, L.D.; Martin, M.V. Clindamycin and dentistry. Br. Dent. J. 2005, 199, 23–26. [Google Scholar] [CrossRef]
- Vescovi, P.; Nammour, S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A critical review. Minerva Stomatol 2010, 59, 181–203, 204–213. [Google Scholar]
- Grimes, D.A.; Schulz, K.F. An overview of clinical research: The lay of the land. Lancet 2002, 359, 57–61. [Google Scholar] [CrossRef]
- Tonetti, M.; Palmer, R.; Working Group 2 of the VIII European Workshop on Periodontology*. Clinical research in implant dentistry: Study design, reporting and outcome measurements: Consensus report of Working Group 2 of the VIII European Workshop on Periodontology. J. Clin. Periodontol. 2012, 39, 73–80. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.C.; Schürch, E., Jr.; Lang, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.; Tonetti, M.S.; Cortellini, P.; Lang, N.P.; European Research Group on, P. Microbial colonization patterns predict the outcomes of surgical treatment of intrabony defects. J. Clin. Periodontol. 2006, 33, 62–68. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Szparecki, G.; Durlik, M.; Rydzewska, G.; Ziobrowski, I.; Gorska, R. The Q705K and F359L Single-Nucleotide Polymorphisms of NOD-Like Receptor Signaling Pathway: Association with Chronic Pancreatitis, Pancreatic Cancer, and Periodontitis. Arch Immunol. Ther. Exp. 2015, 63, 485–494. [Google Scholar] [CrossRef]
- Agina, O.A.; Cheah, K.T.; Sayuti, N.S.A.; Shaari, M.R.; Isa, N.M.M.; Ajat, M.; Zamri-Saad, M.; Mazlan, M.; Hamzah, H. High Granulocyte-Macrophage Colony Stimulating Factor to Interleukin 10 Ratio and Marked Antioxidant Enzyme Activities Predominate in Symptomatic Cattle Naturally Infected with Candidatus Mycoplasma haemobos, Theileria orientalis, Theileria sinensis and Trypanosoma evansi. Animals 2021, 11, 2235. [Google Scholar]
- Rubino, C.V.; Katz, B.G.; Langlois, K.; Wang, H.H.; Carrion, J.A.; Walker, S.G.; Collier, J.L.; Iacono, V.J.; Myneni, S.R. Evaluation of different materials used for sealing of implant abutment access channel and the peri-implant sulcus microbiota: A 6-month, randomized controlled trial. Clin. Oral. Implant. Res. 2021, 32, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennstrom, J.L.; Petzold, M.; Berglundh, T. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Cha, J.K.; Lee, J.S.; Kim, C.S. Surgical Therapy of Peri-Implantitis with Local Minocycline: A 6-Month Randomized Controlled Clinical Trial. J. Dent. Res. 2019, 98, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral Implant. Res. 2000, 11 (Suppl. 1), 146–155. [Google Scholar] [CrossRef] [PubMed]
- Cachovan, G.; Boger, R.H.; Giersdorf, I.; Hallier, O.; Streichert, T.; Haddad, M.; Platzer, U.; Schon, G.; Wegscheider, K.; Sobottka, I. Comparative efficacy and safety of moxifloxacin and clindamycin in the treatment of odontogenic abscesses and inflammatory infiltrates: A phase II, double-blind, randomized trial. Antimicrob. Agents Chemother. 2011, 55, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Lopez, M.A.; Guzman, I.C. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, e947–e951. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.P.M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]


| Measurement I mBI | ||||||
|---|---|---|---|---|---|---|
| Absent | Blood Point | Bloody Line | Profuse Bleeding | |||
| Measurement II mBI | absent | n | 0 | 7 | 6 | 1 |
| % | 0.00% | 17.10% | 14.60% | 2.40% | ||
| blood point | n | 0 | 5 | 10 | 10 | |
| % | 0.00% | 12.20% | 24.40% | 24.0% | ||
| bloody line | n | 0 | 0 | 0 | 2 | |
| % | 0.00% | 0.00% | 0.00% | 4.90% | ||
| Measurement I mPLI | ||||||
|---|---|---|---|---|---|---|
| Absent | Thin Layer of Plaque | Moderate Layer of Plaque | Abundant Plaque Deposits | |||
| Measurement II | absent | n | 3 | 5 | 6 | 4 |
| % | 7.30% | 12.20% | 14.60% | 9.80% | ||
| thin layer of plaque | n | 2 | 9 | 12 | 0 | |
| % | 4.90% | 22.00% | 29.30% | 0.00% | ||
| Measurement I | ||||||
|---|---|---|---|---|---|---|
| Redness | ||||||
| Absent | Present | |||||
| Measurement II | Redness | Absent | n | 0 | 41 | Z = −6.40 p < 0.001 r = 1 |
| % | 0% | 100% | ||||
| Present | n | 0 | 0 | |||
| % | 0% | 0% | ||||
| Swelling | ||||||
| Absent | Present | |||||
| Swelling | Absent | n | 1 | 36 | Z = −6.00 p < 0.001 r = 0.94 | |
| % | 2.4% | 87.8% | ||||
| Present | n | 0 | 4 | |||
| % | 0% | 9.8% | ||||
| Measurement I | ||||||
|---|---|---|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | ||||||
| Absent | Present | |||||
| Measurement II | Aa | Absent | n | 32 | 6 | Z= −2.45 p = 0.014 r = 0.38 |
| % | 78.00% | 14.60% | ||||
| Present | n | 0 | 3 | |||
| % | 0.00% | 7.30% | ||||
| Porphyromonas gingivalis | ||||||
| Absent | Present | |||||
| Pg | Absent | n | 25 | 8 | Z = −1.51 p = 0.132 | |
| % | 61.00% | 19.50% | ||||
| Present | n | 3 | 5 | |||
| % | 7.30% | 12.20% | ||||
| Treponema denticola | ||||||
| Absent | Present | |||||
| Td | Absent | n | 22 | 8 | Z= −2.83 p = 0.005 r = 0.44 | |
| % | 53.70% | 19.50% | ||||
| Present | n | 0 | 11 | |||
| % | 0.00% | 26.80% | ||||
| Tannerella forsythia | ||||||
| Absent | Present | |||||
| Tf | Absent | n | 14 | 20 | Z= −4.47 p < 0.001 r = 0.69 | |
| % | 34.10% | 48.80% | ||||
| Present | n | 0 | 7 | |||
| % | 0.00% | 17.10% | ||||
| Peptostrep. (Micromonas) micros | ||||||
| Absent | Present | |||||
| Pm | Absent | n | 1 | 17 | Z= −4.12 p < 0.001 r = 0.64 | |
| % | 2.40% | 41.50% | ||||
| Present | n | 0 | 23 | |||
| % | 0.00% | 56.10% | ||||
| Fusobacterium nucleatum | ||||||
| Absent | Present | |||||
| Fn | Absent | n | 4 | 15 | Z= −3.87 p < 0.001 r = 0.60 | |
| % | 9.80% | 36.60% | ||||
| Present | n | 0 | 22 | |||
| % | 0.00% | 53.70% | ||||
| Eubacterium nodatum | ||||||
| Absent | Present | |||||
| En | Absent | n | 36 | 3 | Z = −1.73 p = 0.083 r = 0.27 | |
| % | 87.80% | 7.30% | ||||
| Present | n | 0 | 2 | |||
| % | 0.00% | 4.90% | ||||
| Capnocytophaga gingivalis | ||||||
| Absent | Present | |||||
| Cg | Absent | n | 7 | 1 | Z = −1.00 p = 0.317 | |
| % | 17.10% | 2.40% | ||||
| Present | n | 3 | 30 | |||
| % | 7.30% | 73.20% | ||||
| Measurement I | Measurement II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Me | IQR | Min-Max | Me | IQR | Min-Max | Z | p | r | |
| Total number of bacteria | 560,000,000 | 962,000,000 | 7,298,300,000 | 9,300,000 | 62,825,000 | 1,099,810,000 | −4.61 | <0.001 | 0.72 |
| Aggregatibacter actinomycetemcomitans | 0 | 0 | 5300 | 0 | 0 | 210 | −5.48 | <0.001 | 0.86 |
| Porphyromonas gingivalis | 0 | 530 | 3,300,000 | 0 | 0 | 430 | −2.67 | 0.008 | 0.42 |
| Treponema denticola | 0 | 7850 | 132,000 | 0 | 25 | 23,000 | −3.03 | 0.002 | 0.47 |
| Tannerella forsythia | 450 | 3150 | 390,000 | 0 | 0 | 24,000 | −3.82 | <0.001 | 0.60 |
| Peptostrep. (Micromonas) micros | 19,000 | 183,625 | 930,000 | 140 | 390 | 24,000 | −4.54 | <0.001 | 0.71 |
| Fusobacterium nucleatum | 20,000 | 47,700 | 430,000 | 110 | 210 | 26,000 | −5.42 | <0.001 | 0.85 |
| Eubacterium nodatum | 0 | 0 | 7700 | 0 | 0 | 130 | −5.30 | <0.001 | 0.83 |
| Capnocytophaga gingivalis | 6800 | 169,810 | 590,000 | 720 | 1705 | 29,000 | −2.02 | 0.043 | 0.32 |
| Front Teeth (n = 12) | Side Teeth (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Me | IQR | Min-Max | Me | IQR | Min-Max | Z | p | r | |
| Measurement I | |||||||||
| Total numer of bacteria | 545,000,000 | 767,175,000 | 7,298,300,000 | 560,000,000 | 1,162 × 109 | 6,397,000,000 | −0.23 | 0.819 | 0.04 |
| Aggregatibacter actinomycetemcomitans | 0 | 0 | 2300 | 0 | 45 | 5300 | −0.63 | 0.527 | 0.10 |
| Porphyromonas gingivalis | 0 | 2607.5 | 3,300,000 | 0 | 460 | 2,700,000 | −0.26 | 0.795 | 0.04 |
| Treponema denticola | 1,500 | 14,500 | 95,000 | 0 | 5550 | 132,000 | −0.45 | 0.651 | 0.07 |
| Tannerella forsythia | 325 | 6375 | 390,000 | 590 | 11,900 | 330,000 | −0.54 | 0.588 | 0.08 |
| Peptostrep. (Micromonas) micros | 23,000 | 275,200 | 839,650 | 19,000 | 129,360 | 930,000 | −0.98 | 0.330 | 0.15 |
| Fusobacterium nucleatum | 46,500 | 36,975 | 430,000 | 6500 | 46,150 | 77,000 | −2.07 | 0.039 | 0.32 |
| Eubacterium nodatum | 0 | 0 | 7700 | 0 | 0 | 5700 | −0.61 | 0.545 | 0.09 |
| Capnocytophaga gingivalis | 27,000 | 465,100 | 509,180 | 1000 | 76,000 | 590,000 | −2.50 | 0.013 | 0.39 |
| Measurement II | |||||||||
| Total numer of bacteria | 18,000,000 | 116,547,500 | 1,099,810,000 | 6,300,000 | 50,465,000 | 689,770,000 | −0.92 | 0.359 | 0.14 |
| Aggregatibacter actinomycetemcomitans | 0 | 0 | 0 | 0 | 0 | 210 | −1.14 | 0.253 | 0.18 |
| Porphyromonas gingivalis | 0 | 362.5 | 430 | 0 | 0 | 310 | −1.74 | 0.082 | 0.27 |
| Treponema denticola | 0 | 0 | 30 | 0 | 45 | 23,000 | −1.18 | 0.240 | 0.18 |
| Tannerella forsythia | 0 | 0 | 290 | 0 | 0 | 24,000 | −0.04 | 0.965 | 0.01 |
| Peptostrep. (Micromonas) micros | 0 | 385 | 2100 | 200 | 430 | 24,000 | −0.94 | 0.346 | 0.15 |
| Fusobacterium nucleatum | 115 | 207.5 | 260 | 90 | 210 | 26,000 | −0.02 | 0.988 | 0.00 |
| Eubacterium nodatum | 0 | 0 | 130 | 0 | 0 | 100 | −0.69 | 0.490 | 0.11 |
| Capnocytophaga gingivalis | 670 | 2080 | 4300 | 720 | 1785 | 29,000 | −0.07 | 0.943 | 0.01 |
| Front Teeth (n = 12) | Side Teeth (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Me | IQR | Min-Max | Me | IQR | Min-Max | Z | p | r | |
| Total numer of bacteria | 474,615,000 | 729,090,000 | 6,210,900,000 | 420,700,000 | 1057 × 109 | 6,329,410,000 | −0.34 | 0.731 | 0.05 |
| Aggregatibacter actinomycetemcomitans | 0 | 0 | 2300 | 0 | 25 | 5,090 | −0.63 | 0.527 | 0.10 |
| Porphyromonas gingivalis | 0 | 2607.5 | 3,300,090 | 0 | 460 | 2,699,880 | −0.31 | 0.757 | 0.05 |
| Treponema denticola | 1485 | 14,500 | 95,000 | 0 | 3145 | 132,000 | −0.69 | 0.493 | 0.11 |
| Tannerella forsythia | 325 | 637.5 | 389,710 | 590 | 4400 | 329,790 | −0.54 | 0.588 | 0.08 |
| Peptostrep. (Micromonas) micros | 23,000 | 274,382.5 | 839,460 | 17,000 | 129,180 | 929,990 | −1.22 | 0.223 | 0.19 |
| Fusobacterium nucleatum | 46,305 | 3697.5 | 429,790 | 4610 | 45,995 | 76,790 | −2.14 | 0.033 | 0.33 |
| Eubacterium nodatum | 0 | 0 | 7570 | 0 | 0 | 5700 | −0.60 | 0.545 | 0.09 |
| Capnocytophaga gingivalis | 23,500 | 465,045 | 510,160 | 250 | 71,190 | 618,620 | −2.24 | 0.025 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wychowański, P.; Nowak, M.; Miskiewicz, A.; Morawiec, T.; Woliński, J.; Kucharski, Z.; Passarelli, P.C.; Bodnarenko, A.; Lopez, M.A. The Effectiveness of a Bioactive Healing Abutment as a Local Drug Delivery System to Impact Peri-Implant Mucositis: A Prospective Case Series Study. Pharmaceutics 2023, 15, 138. https://doi.org/10.3390/pharmaceutics15010138
Wychowański P, Nowak M, Miskiewicz A, Morawiec T, Woliński J, Kucharski Z, Passarelli PC, Bodnarenko A, Lopez MA. The Effectiveness of a Bioactive Healing Abutment as a Local Drug Delivery System to Impact Peri-Implant Mucositis: A Prospective Case Series Study. Pharmaceutics. 2023; 15(1):138. https://doi.org/10.3390/pharmaceutics15010138
Chicago/Turabian StyleWychowański, Piotr, Maciej Nowak, Andrzej Miskiewicz, Tadeusz Morawiec, Jarosław Woliński, Zbigniew Kucharski, Pier Carmine Passarelli, Alina Bodnarenko, and Michele Antonio Lopez. 2023. "The Effectiveness of a Bioactive Healing Abutment as a Local Drug Delivery System to Impact Peri-Implant Mucositis: A Prospective Case Series Study" Pharmaceutics 15, no. 1: 138. https://doi.org/10.3390/pharmaceutics15010138
APA StyleWychowański, P., Nowak, M., Miskiewicz, A., Morawiec, T., Woliński, J., Kucharski, Z., Passarelli, P. C., Bodnarenko, A., & Lopez, M. A. (2023). The Effectiveness of a Bioactive Healing Abutment as a Local Drug Delivery System to Impact Peri-Implant Mucositis: A Prospective Case Series Study. Pharmaceutics, 15(1), 138. https://doi.org/10.3390/pharmaceutics15010138






