How to Completely Squeeze a Fungus—Advanced Genome Mining Tools for Novel Bioactive Substances
Abstract
1. Introduction
1.1. Fungal Secondary Metabolites as Biopharmaceuticals of Tomorrow
1.2. Genomic Organization and Evolution of BGCs
2. Deciphering the Genomic Potential of Fungal Genomes for Secondary Metabolites
BGC Prediction Methods
3. Activation of Microbial BGCs via Environmental Triggers
3.1. Mimicking Natural Habitats and Stress Conditions
3.2. Co-Cultivation
3.3. Conclusions of Initial Assessment
4. BGC Activation Using Molecular Biology Tools: The Way to Fully Exploit the Genome for Novel Bioactive Substances
4.1. Overexpression of a Pathway Specific Transcription Factor or Whole BGC
4.2. Heterologous Expression
4.2.1. Fungal Artificial Chromosomes (FAC-MS) [283,284]
4.2.2. CoIN [286]
4.2.3. Single Promoter BGC Expression [296]
4.2.4. Hex [285]
5. Chromatin: An Important Layer of (BGC) Gene Regulation
5.1. Heterochromatin
5.1.1. Constitutive Heterochromatin via H3K9 Methylation and Clr4/HP1
5.1.2. Facultative Heterochromatin via H3K27me3 and Polycomb Repressive Complex II (PRC2)
5.2. Euchromatin
5.2.1. Methylation of Histone 3 Lysin 4
5.2.2. H4K12 Acetylation
5.2.3. H3K9 Acetylation/H3K14 Acetylation
5.2.4. H3K27 Acetylation
5.3. The Histone Code of Actively Transcribed Genes
5.4. Finding New Bioactive Compounds by Breaking the Chromatin Silencing Machinery
6. CRISPR/Cas: New Opportunities in Molecular Biology: Targeted Gene Deletion, Activation and Epigenetic Editing in Filamentous Fungi
6.1. Principle of CRISPR-Cas Technology
6.2. Differences between CRISPR/Cas Variants
6.3. Gene Editing via CRISPR/Cas
6.4. Gene and BGC Activation via CRISPR/dCAS Variants
6.4.1. Transcriptional Regulation
6.4.2. Targeted Chromatin Remodelling Using CRISPR/Cas
6.5. Application Relevant Information for CRISPR/Cas-Methods
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A Review of the Microbial Production of Bioactive Natural Products and Biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef]
- Olsen, A. The Antibiotic Revolution. Kuhn’s Philos. Sci. 2013, 17. Available online: https://digitalcommons.providence.edu/kuhn_2013/6 (accessed on 28 July 2022).
- Dhillion, S.S.; Svarstad, H.; Amundsen, C.; Bugge, H.C. Bioprospecting: Effects on Environment and Development. AMBIO A J. Hum. Environ. 2002, 31, 491–493. [Google Scholar] [CrossRef]
- Laupacis, A.; Keown, P.A.; Ulan, R.A.; McKenzie, N.; Stiller, C.R. Cyclosporin A: A powerful immunosuppressant. Can. Med. Assoc. J. 1982, 126, 1041–1046. [Google Scholar]
- Moore, R.N.; Bigam, G.; Chan, J.K.; Hogg, A.M.; Nakashima, T.T.; Vederas, J.C. Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen, and oxygen atoms by carbon-13 NMR and mass spectrometry. J. Am. Chem. Soc. 1985, 107, 3694–3701. [Google Scholar] [CrossRef]
- Goldman, G.H.; Osmani, S.A. The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods; CRC press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Nakkeeran, S.; Rajamanickam, S.; Karthikeyan, M.; Mahendra, K.; Renukadevi, P.; Johnson, I. 11—Antimicrobial secondary metabolites from Trichoderma spp. as next generation fungicides. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 257–282. [Google Scholar]
- Savita; Sharma, A. Fungi as Biological Control Agents. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 395–411. [Google Scholar]
- Segaran, G.; Sathiavelu, M. Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 2019, 21, 101284. [Google Scholar] [CrossRef]
- Shi, T.Q.; Peng, H.; Zeng, S.Y.; Ji, R.Y.; Shi, K.; Huang, H.; Ji, X.J. Microbial production of plant hormones: Opportunities and challenges. Bioengineered 2017, 8, 124–128. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal Secondary Metabolites Produced by the Fungal Endophytes: Chemical Diversity and Potential Use in the Development of Biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary Metabolites with Agricultural Antagonistic Potentials from Beauveria felina, a Marine-Derived Entomopathogenic Fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Bioactive Products from Fungi. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–87. [Google Scholar]
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, T.-J.; Lu, X.-X.; Ma, B.-L.; Ren, A.; Shi, L.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Membrane fluidity is involved in the regulation of heat stress induced secondary metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and Their Biosynthesis in Fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Wong, H.J.; Mohamad-Fauzi, N.; Rizman-Idid, M.; Convey, P.; Alias, S.A. Protective mechanisms and responses of micro-fungi towards ultraviolet-induced cellular damage. Polar Sci. 2019, 20, 19–34. [Google Scholar] [CrossRef]
- Batey, S.F.D.; Greco, C.; Hutchings, M.I.; Wilkinson, B. Chemical warfare between fungus-growing ants and their pathogens. Curr. Opin. Chem. Biol. 2020, 59, 172–181. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T.; Holb, I.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Schrettl, M.; Haas, H. Iron homeostasis—Achilles’ heel of Aspergillus fumigatus? Curr. Opin. Microbiol. 2011, 14, 400–405. [Google Scholar] [CrossRef]
- Haas, H. How to trigger a fungal weapon. eLife 2015, 4, e10504. [Google Scholar] [CrossRef]
- Robey, M.T.; Caesar, L.K.; Drott, M.T.; Keller, N.P.; Kelleher, N.L. An interpreted atlas of biosynthetic gene clusters from 1,000 fungal genomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2020230118. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Heo, Y.M.; Oh, S.-Y.; Kim, K.; Han, S.-I.; Kwon, S.L.; Yoo, Y.; Kim, D.; Khim, J.S.; Kang, S.; Lee, H.; et al. Comparative Genomics and Transcriptomics Depict Marine Algicolous Arthrinium Species as Endosymbionts That Help Regulate Oxidative Stress in Brown Algae. Front. Mar. Sci. 2021, 8, 753222. [Google Scholar] [CrossRef]
- Martín, J.F. Transport systems, intracellular traffic of intermediates and secretion of β-lactam antibiotics in fungi. Fungal Biol. Biotechnol. 2020, 7, 6. [Google Scholar] [CrossRef]
- Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [Google Scholar] [CrossRef]
- Westphal, K.R.; Muurmann, A.T.; Paulsen, I.E.; Nørgaard, K.T.H.; Overgaard, M.L.; Dall, S.M.; Aalborg, T.; Wimmer, R.; Sørensen, J.L.; Sondergaard, T.E. Who Needs Neighbors? PKS8 Is a Stand-Alone Gene in Fusarium graminearum Responsible for Production of Gibepyrones and Prolipyrone B. Molecules 2018, 23, 2232. [Google Scholar] [CrossRef]
- Terabayashi, Y.; Sano, M.; Yamane, N.; Marui, J.; Tamano, K.; Sagara, J.; Dohmoto, M.; Oda, K.; Ohshima, E.; Tachibana, K.; et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 953–961. [Google Scholar] [CrossRef]
- Wiemann, P.; Guo, C.J.; Palmer, J.M.; Sekonyela, R.; Wang, C.C.; Keller, N.P. Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. USA 2013, 110, 17065–17070. [Google Scholar] [CrossRef]
- Dai, G.; Shen, Q.; Zhang, Y.; Bian, X. Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk. J. Fungi 2022, 8, 320. [Google Scholar] [CrossRef]
- Gluck-Thaler, E.; Haridas, S.; Binder, M.; Grigoriev, I.V.; Crous, P.W.; Spatafora, J.W.; Bushley, K.; Slot, J.C. The Architecture of Metabolism Maximizes Biosynthetic Diversity in the Largest Class of Fungi. Mol. Biol. Evol. 2020, 37, 2838–2856. [Google Scholar] [CrossRef]
- Doroghazi, J.R.; Albright, J.C.; Goering, A.W.; Ju, K.S.; Haines, R.R.; Tchalukov, K.A.; Labeda, D.P.; Kelleher, N.L.; Metcalf, W.W. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat. Chem. Biol. 2014, 10, 963–968. [Google Scholar] [CrossRef]
- Lind, A.L.; Wisecaver, J.H.; Lameiras, C.; Wiemann, P.; Palmer, J.M.; Keller, N.P.; Rodrigues, F.; Goldman, G.H.; Rokas, A. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLOS Biol. 2017, 15, e2003583. [Google Scholar] [CrossRef]
- Fedorova, N.D.; Khaldi, N.; Joardar, V.S.; Maiti, R.; Amedeo, P.; Anderson, M.J.; Crabtree, J.; Silva, J.C.; Badger, J.H.; Albarraq, A.; et al. Genomic Islands in the Pathogenic Filamentous Fungus Aspergillus fumigatus. PLOS Genet. 2008, 4, e1000046. [Google Scholar] [CrossRef]
- Andersen, M.R.; Salazar, M.P.; Schaap, P.J.; van de Vondervoort, P.J.I.; Culley, D.; Thykaer, J.; Frisvad, J.C.; Nielsen, K.F.; Albang, R.; Albermann, K.; et al. Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res. 2011, 21, 885–897. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Salichos, L.; Slot, J.C.; Rinker, D.C.; McGary, K.L.; King, J.G.; Klich, M.A.; Tabb, D.L.; McDonald, W.H.; Rokas, A. The Evolutionary Imprint of Domestication on Genome Variation and Function of the Filamentous Fungus Aspergillus oryzae. Curr. Biol. 2012, 22, 1403–1409. [Google Scholar] [CrossRef]
- Rokas, A.; Wisecaver, J.H.; Lind, A.L. The birth, evolution and death of metabolic gene clusters in fungi. Nat. Rev. Microbiol. 2018, 16, 731–744. [Google Scholar] [CrossRef]
- Cheng, K.; Rong, X.; Pinto-Tomás, A.A.; Fernández-Villalobos, M.; Murillo-Cruz, C.; Huang, Y.; Voordouw, G. Population Genetic Analysis of Streptomyces albidoflavus Reveals Habitat Barriers to Homologous Recombination in the Diversification of Streptomycetes. Appl. Environ. Microbiol. 2015, 81, 966–975. [Google Scholar] [CrossRef]
- Dal Grande, F.; Jamilloux, V.; Choisne, N.; Calchera, A.; Rolshausen, G.; Petersen, M.; Schulz, M.; Nilsson, M.A.; Schmitt, I. Transposable Elements in the Genome of the Lichen-Forming Fungus Umbilicaria pustulata and Their Distribution in Different Climate Zones along Elevation. Biology 2022, 11, 24. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef]
- Takeda, I.; Umemura, M.; Koike, H.; Asai, K.; Machida, M. Motif-independent prediction of a secondary metabolism gene cluster using comparative genomics: Application to sequenced genomes of Aspergillus and ten other filamentous fungal species. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2014, 21, 447–457. [Google Scholar] [CrossRef]
- Brown, C.A.; Murray, A.W.; Verstrepen, K.J. Rapid expansion and functional divergence of subtelomeric gene families in yeasts. Curr. Biol. 2010, 20, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Graham-Taylor, C.; Kamphuis, L.G.; Derbyshire, M.C. A detailed in silico analysis of secondary metabolite biosynthesis clusters in the genome of the broad host range plant pathogenic fungus Sclerotinia sclerotiorum. BMC Genom. 2020, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Rehmeyer, C.; Li, W.; Kusaba, M.; Kim, Y.-S.; Brown, D.; Staben, C.; Dean, R.; Farman, M. Organization of chromosome ends in the rice blast fungus, Magnaporthe oryzae. Nucleic Acids Res. 2006, 34, 4685–4701. [Google Scholar] [CrossRef] [PubMed]
- Farman, M.L. Telomeres in the rice blast fungus Magnaporthe oryzae: The world of the end as we know it. FEMS Microbiol. Lett. 2007, 273, 125–132. [Google Scholar] [CrossRef][Green Version]
- McDonagh, A.; Fedorova, N.D.; Crabtree, J.; Yu, Y.; Kim, S.; Chen, D.; Loss, O.; Cairns, T.; Goldman, G.; Armstrong-James, D.; et al. Sub-Telomere Directed Gene Expression during Initiation of Invasive Aspergillosis. PLOS Pathog. 2008, 4, e1000154. [Google Scholar] [CrossRef]
- Cairns, T.; Meyer, V. In silico prediction and characterization of secondary metabolite biosynthetic gene clusters in the wheat pathogen Zymoseptoria tritici. BMC Genom. 2017, 18, 631. [Google Scholar] [CrossRef]
- Morris, J.J.; Lenski, R.E.; Zinser, E.R. The Black Queen Hypothesis: Evolution of dependencies through adaptive gene loss. mBio 2012, 3. [Google Scholar] [CrossRef]
- Burkhardt, I.; Siemon, T.; Henrot, M.; Studt, L.; Rosler, S.; Tudzynski, B.; Christmann, M.; Dickschat, J.S. Mechanistic Characterisation of Two Sesquiterpene Cyclases from the Plant Pathogenic Fungus Fusarium fujikuroi. Angew. Chem. Int. Ed. Engl. 2016, 55, 8748–8751. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.E.; Thomma, B.P.H.J.; Seidl, M.F. Transposable Elements Contribute to Genome Dynamics and Gene Expression Variation in the Fungal Plant Pathogen Verticillium dahliae. Genome Biol. Evol. 2021, 13, evab135. [Google Scholar] [CrossRef] [PubMed]
- Valero-Jiménez, C.A.; Steentjes, M.B.F.; Slot, J.C.; Shi-Kunne, X.; Scholten, O.E.; van Kan, J.A.L. Dynamics in Secondary Metabolite Gene Clusters in Otherwise Highly Syntenic and Stable Genomes in the Fungal Genus Botrytis. Genome Biol. Evol. 2020, 12, 2491–2507. [Google Scholar] [CrossRef]
- Porquier, A.; Morgant, G.; Moraga, J.; Dalmais, B.; Luyten, I.; Simon, A.; Pradier, J.-M.; Amselem, J.; Collado, I.G.; Viaud, M. The botrydial biosynthetic gene cluster of Botrytis cinerea displays a bipartite genomic structure and is positively regulated by the putative Zn(II)2Cys6 transcription factor BcBot6. Fungal Genet. Biol. 2016, 96, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Mead, M.E.; Steenwyk, J.L.; Raja, H.A.; Oberlies, N.H. Biosynthetic gene clusters and the evolution of fungal chemodiversity. Nat. Prod. Rep. 2020, 37, 868–878. [Google Scholar] [CrossRef]
- Slot, J.C.; Rokas, A. Horizontal Transfer of a Large and Highly Toxic Secondary Metabolic Gene Cluster between Fungi. Curr. Biol. 2011, 21, 134–139. [Google Scholar] [CrossRef]
- Campbell, M.A.; Rokas, A.; Slot, J.C. Horizontal transfer and death of a fungal secondary metabolic gene cluster. Genome Biol. Evol. 2012, 4, 289–293. [Google Scholar] [CrossRef]
- Dhillon, B.; Feau, N.; Aerts, A.L.; Beauseigle, S.; Bernier, L.; Copeland, A.; Foster, A.; Gill, N.; Henrissat, B.; Herath, P.; et al. Horizontal gene transfer and gene dosage drives adaptation to wood colonization in a tree pathogen. Proc. Natl. Acad. Sci. USA 2015, 112, 3451–3456. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, X.; Köllner, T.G.; Rinkel, J.; Fu, J.; Labbé, J.; Xiong, W.; Dickschat, J.S.; Gershenzon, J.; Chen, F. Terpene Synthase Genes Originated from Bacteria through Horizontal Gene Transfer Contribute to Terpenoid Diversity in Fungi. Sci. Rep. 2019, 9, 9223. [Google Scholar] [CrossRef]
- Proctor, R.H.; Van Hove, F.; Susca, A.; Stea, G.; Busman, M.; van der Lee, T.; Waalwijk, C.; Moretti, A.; Ward, T.J. Birth, death and horizontal transfer of the fumonisin biosynthetic gene cluster during the evolutionary diversification of Fusarium. Mol. Microbiol. 2013, 90, 290–306. [Google Scholar] [CrossRef]
- Reynolds, H.T.; Vijayakumar, V.; Gluck-Thaler, E.; Korotkin, H.B.; Matheny, P.B.; Slot, J.C. Horizontal gene cluster transfer increased hallucinogenic mushroom diversity. Evol. Lett. 2018, 2, 88–101. [Google Scholar] [CrossRef]
- Schumacher, J.; Gautier, A.; Morgant, G.; Studt, L.; Ducrot, P.-H.; Le Pêcheur, P.; Azeddine, S.; Fillinger, S.; Leroux, P.; Tudzynski, B.; et al. A Functional Bikaverin Biosynthesis Gene Cluster in Rare Strains of Botrytis cinerea Is Positively Controlled by VELVET. PLoS ONE 2013, 8, e53729. [Google Scholar] [CrossRef]
- Kohli, G.S.; John, U.; Van Dolah, F.M.; Murray, S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016, 10, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Min, B.; Grigoriev, I.V.; Choi, I.-G. FunGAP: Fungal Genome Annotation Pipeline using evidence-based gene model evaluation. Bioinformatics 2017, 33, 2936–2937. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Kim, H.U.; Medema, M.H.; Weber, T. Recent development of antiSMASH and other computational approaches to mine secondary metabolite biosynthetic gene clusters. Brief Bioinform 2019, 20, 1103–1113. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Kopp, J.F.; Martínez-Guerrero, C.; Yáñez-Guerra, L.A.; Selem-Mojica, N.; Ramos-Aboites, H.; Feldmann, J.; Barona-Gómez, F. Phylogenomic Analysis of Natural Products Biosynthetic Gene Clusters Allows Discovery of Arseno-Organic Metabolites in Model Streptomycetes. Genome Biol. Evol. 2016, 8, 1906–1916. [Google Scholar] [CrossRef]
- Vignolle, G.A.; Schaffer, D.; Zehetner, L.; Mach, R.L.; Mach-Aigner, A.R.; Derntl, C. FunOrder: A robust and semi-automated method for the identification of essential biosynthetic genes through computational molecular co-evolution. PLOS Comput. Biol. 2021, 17, e1009372. [Google Scholar] [CrossRef]
- Walker, A.S.; Clardy, J. A Machine Learning Bioinformatics Method to Predict Biological Activity from Biosynthetic Gene Clusters. J. Chem. Inf. Modeling 2021, 61, 2560–2571. [Google Scholar] [CrossRef]
- Qiang, B.; Lai, J.; Jin, H.; Zhang, L.; Liu, Z. Target Prediction Model for Natural Products using Transfer Learning. Int. J. Mol. Sci. 2021, 22, 4632. [Google Scholar] [CrossRef]
- Moumbock, A.F.A.; Li, J.; Mishra, P.; Gao, M.; Günther, S. Current computational methods for predicting protein interactions of natural products. Comput. Struct. Biotechnol. J. 2019, 17, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.-F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic mapping of fungal secondary metabolite clusters. Fungal Genet. Biol. 2010, 47, 736–741. [Google Scholar] [CrossRef]
- Sélem-Mojica, N.; Aguilar, C.; Gutiérrez-García, K.; Martínez-Guerrero, C.E.; Barona-Gómez, F. EvoMining reveals the origin and fate of natural product biosynthetic enzymes. Microb. Genom. 2019, 5, e000260. [Google Scholar] [CrossRef]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Suarez Duran, H.G.; de los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. antiSMASH 4.0—improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef]
- Mungan, M.D.; Alanjary, M.; Blin, K.; Weber, T.; Medema, M.H.; Ziemert, N. ARTS 2.0: Feature updates and expansion of the Antibiotic Resistant Target Seeker for comparative genome mining. Nucleic Acids Res. 2020, 48, W546–W552. [Google Scholar] [CrossRef]
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.; Philmus, B.; Ziemert, N. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucleic Acids Res. 2017, 45, W42–W48. [Google Scholar] [CrossRef]
- Kjærbølling, I.; Vesth, T.; Andersen, M.R.; Sessions, P.F.D. Resistance Gene-Directed Genome Mining of 50 Aspergillus Species. mSystems 2019, 4, e00085-19. [Google Scholar] [CrossRef]
- Umemura, M.; Koike, H.; Nagano, N.; Ishii, T.; Kawano, J.; Yamane, N.; Kozone, I.; Horimoto, K.; Shin-ya, K.; Asai, K.; et al. MIDDAS-M: Motif-Independent De Novo Detection of Secondary Metabolite Gene Clusters through the Integration of Genome Sequencing and Transcriptome Data. PLoS ONE 2014, 8, e84028. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Ahamed, A.; Aslam, R.; Alako, B.T.F.; Burgin, J.; Buso, N.; Courtot, M.; Fan, J.; Gupta, D.; Haseeb, M.; et al. The European Nucleotide Archive in 2020. Nucleic Acids Res. 2021, 49, D82–D85. [Google Scholar] [CrossRef] [PubMed]
- Kanz, C.; Aldebert, P.; Althorpe, N.; Baker, W.; Baldwin, A.; Bates, K.; Browne, P.; van den Broek, A.; Castro, M.; Cochrane, G.; et al. The EMBL Nucleotide Sequence Database. Nucleic Acids Res. 2005, 33, D29–D33. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2012, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The Natural Products Atlas 2.0: A database of microbially-derived natural products. Nucleic Acids Res. 2021, 50, D1317–D1323. [Google Scholar] [CrossRef]
- Haft, D.H.; Selengut, J.D.; Richter, R.A.; Harkins, D.; Basu, M.K.; Beck, E. TIGRFAMs and Genome Properties in 2013. Nucleic Acids Res. 2012, 41, D387–D395. [Google Scholar] [CrossRef]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.-R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLOS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Almeida, H.; Palys, S.; Tsang, A.; Diallo, A.B. TOUCAN: A framework for fungal biosynthetic gene cluster discovery. NAR Genom. Bioinform. 2020, 2, lqaa098. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kautsar, S.A.; Medema, M.H.; Weber, T. The antiSMASH database version 3: Increased taxonomic coverage and new query features for modular enzymes. Nucleic Acids Res. 2020, 49, D639–D643. [Google Scholar] [CrossRef] [PubMed]
- Flissi, A.; Ricart, E.; Campart, C.; Chevalier, M.; Dufresne, Y.; Michalik, J.; Jacques, P.; Flahaut, C.; Lisacek, F.; Leclère, V.; et al. Norine: Update of the nonribosomal peptide resource. Nucleic Acids Res. 2019, 48, D465–D469. [Google Scholar] [CrossRef]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; van der Hooft, J.J.J.; van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2019, 48, D454–D458. [Google Scholar] [CrossRef]
- Palaniappan, K.; Chen, I.-M.A.; Chu, K.; Ratner, A.; Seshadri, R.; Kyrpides, N.C.; Ivanova, N.N.; Mouncey, N.J. IMG-ABC v.5.0: An update to the IMG/Atlas of Biosynthetic Gene Clusters Knowledgebase. Nucleic Acids Res. 2019, 48, D422–D430. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Prihoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Durcak, J.; Wurst, M.; Kotowski, J.; Chang, D.; et al. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 2019, 47, e110. [Google Scholar] [CrossRef]
- Agrawal, P.; Khater, S.; Gupta, M.; Sain, N.; Mohanty, D. RiPPMiner: A bioinformatics resource for deciphering chemical structures of RiPPs based on prediction of cleavage and cross-links. Nucleic Acids Res. 2017, 45, W80–W88. [Google Scholar] [CrossRef]
- Khater, S.; Gupta, M.; Agrawal, P.; Sain, N.; Prava, J.; Gupta, P.; Grover, M.; Kumar, N.; Mohanty, D. SBSPKSv2: Structure-based sequence analysis of polyketide synthases and non-ribosomal peptide synthetases. Nucleic Acids Res. 2017, 45, W72–W79. [Google Scholar] [CrossRef]
- Anand, S.; Prasad, M.V.R.; Yadav, G.; Kumar, N.; Shehara, J.; Ansari, M.Z.; Mohanty, D. SBSPKS: Structure based sequence analysis of polyketide synthases. Nucleic Acids Res. 2010, 38, W487–W496. [Google Scholar] [CrossRef]
- Zierep, P.F.; Padilla, N.; Yonchev, D.G.; Telukunta, K.K.; Klementz, D.; Günther, S. SeMPI: A genome-based secondary metabolite prediction and identification web server. Nucleic Acids Res. 2017, 45, W64–W71. [Google Scholar] [CrossRef] [PubMed]
- Wolf, T.; Shelest, V.; Nath, N.; Shelest, E. CASSIS and SMIPS: Promoter-based prediction of secondary metabolite gene clusters in eukaryotic genomes. Bioinformatics 2015, 32, 1138–1143. [Google Scholar] [CrossRef]
- Vesth, T.C.; Brandl, J.; Andersen, M.R. FunGeneClusterS: Predicting fungal gene clusters from genome and transcriptome data. Synth. Syst. Biotechnol. 2016, 1, 122–129. [Google Scholar] [CrossRef]
- Andersen, M.R.; Nielsen, J.B.; Klitgaard, A.; Petersen, L.M.; Zachariasen, M.; Hansen, T.J.; Blicher, L.H.; Gotfredsen, C.H.; Larsen, T.O.; Nielsen, K.F.; et al. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. USA 2013, 110, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Ravel, J. Chapter 8 Methods for In Silico Prediction of Microbial Polyketide and Nonribosomal Peptide Biosynthetic Pathways from DNA Sequence Data. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2009; Volume 458, pp. 181–217. [Google Scholar]
- Johnston, C.W.; Skinnider, M.A.; Wyatt, M.A.; Li, X.; Ranieri, M.R.M.; Yang, L.; Zechel, D.L.; Ma, B.; Magarvey, N.A. An automated Genomes-to-Natural Products platform (GNP) for the discovery of modular natural products. Nat. Commun. 2015, 6, 8421. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, Y.; Noé, L.; Leclère, V.; Pupin, M. Smiles2Monomers: A link between chemical and biological structures for polymers. J. Cheminform. 2015, 7, 62. [Google Scholar] [CrossRef]
- Conway, K.R.; Boddy, C.N. ClusterMine360: A database of microbial PKS/NRPS biosynthesis. Nucleic Acids Res. 2012, 41, D402–D407. [Google Scholar] [CrossRef]
- Röttig, M.; Medema, M.H.; Blin, K.; Weber, T.; Rausch, C.; Kohlbacher, O. NRPSpredictor2—A web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res. 2011, 39, W362–W367. [Google Scholar] [CrossRef]
- Weber, T.; Rausch, C.; Lopez, P.; Hoof, I.; Gaykova, V.; Huson, D.H.; Wohlleben, W. CLUSEAN: A computer-based framework for the automated analysis of bacterial secondary metabolite biosynthetic gene clusters. J. Biotechnol. 2009, 140, 13–17. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.; Rangel, D.E.; Fernandes, É.K.; Flint, S.D.; Roberts, D.W. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Heo, S.; Nah, G.; Chun, H.S. Transcriptomic responses of Aspergillus flavus to temperature and oxidative stresses during aflatoxin production. Sci. Rep. 2021, 11, 2803. [Google Scholar] [CrossRef]
- Gressler, M.; Meyer, F.; Heine, D.; Hortschansky, P.; Hertweck, C.; Brock, M. Phytotoxin production in Aspergillus terreus is regulated by independent environmental signals. eLife 2015, 4, e07861. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.S.; Pal, A.; Srinivasan, S.; Somvanshi, P.R.; Venkatesh, K.V. Global Transcriptional Regulators Fine-Tune the Translational and Metabolic Efficiency for Optimal Growth of Escherichia coli. mSystems 2021, 6, e00001-21. [Google Scholar] [CrossRef]
- Lind, A.L.; Wisecaver, J.H.; Smith, T.D.; Feng, X.; Calvo, A.M.; Rokas, A. Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus Aspergillus. PLOS Genet. 2015, 11, e1005096. [Google Scholar] [CrossRef]
- Fasoyin, O.E.; Wang, B.; Qiu, M.; Han, X.; Chung, K.R.; Wang, S. Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 2018, 115, 41–51. [Google Scholar] [CrossRef]
- Leiter, É.; Emri, T.; Pákozdi, K.; Hornok, L.; Pócsi, I. The impact of bZIP Atf1ortholog global regulators in fungi. Appl. Microbiol. Biotechnol. 2021, 105, 5769–5783. [Google Scholar] [CrossRef]
- Pfannmüller, A.; Boysen, J.M.; Tudzynski, B. Nitrate Assimilation in Fusarium fujikuroi Is Controlled by Multiple Levels of Regulation. Front. Microbiol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Wiemann, P.; Sieber, C.M.K.; von Bargen, K.W.; Studt, L.; Niehaus, E.-M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the Cryptic Genome: Genome-wide Analyses of the Rice Pathogen Fusarium fujikuroi Reveal Complex Regulation of Secondary Metabolism and Novel Metabolites. PLOS Pathog. 2013, 9, e1003475. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [PubMed]
- Pfannmüller, A.; Leufken, J.; Studt, L.; Michielse, C.B.; Sieber, C.M.K.; Güldener, U.; Hawat, S.; Hippler, M.; Fufezan, C.; Tudzynski, B. Comparative transcriptome and proteome analysis reveals a global impact of the nitrogen regulators AreA and AreB on secondary metabolism in Fusarium fujikuroi. PLoS ONE 2017, 12, e0176194. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Sarkar, A.; Schroeckh, V.; Dahse, H.-M.; Roth, M.; Brakhage, A.A.; Horn, U.; Hertweck, C. Two Induced Fungal Polyketide Pathways Converge into Antiproliferative Spiroanthrones. ChemBioChem 2011, 12, 1836–1839. [Google Scholar] [CrossRef]
- Giese, H.; Sondergaard, T.E.; Sørensen, J.L. The AreA transcription factor in Fusarium graminearum regulates the use of some nonpreferred nitrogen sources and secondary metabolite production. Fungal Biol. 2013, 117, 814–821. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.; Liu, G. Disruption of the nitrogen regulatory gene AcareA in Acremonium chrysogenum leads to reduction of cephalosporin production and repression of nitrogen metabolism. Fungal Genet. Biol. 2013, 61, 69–79. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Schäfer, K.; Hera, C.; Di Pietro, A. Combinatorial function of velvet and AreA in transcriptional regulation of nitrate utilization and secondary metabolism. Fungal Genet. Biol. 2014, 62, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Akhberdi, O.; Zhang, Q.; Wang, D.; Wang, H.; Hao, X.; Liu, Y.; Wei, D.; Zhu, X. Distinct Roles of Velvet Complex in the Development, Stress Tolerance, and Secondary Metabolism in Pestalotiopsis microspora, a Taxol Producer. Genes 2018, 9, 164. [Google Scholar] [CrossRef]
- Bayram, Ö.S.; Dettmann, A.; Karahoda, B.; Moloney, N.M.; Ormsby, T.; McGowan, J.; Cea-Sánchez, S.; Miralles-Durán, A.; Brancini, G.T.P.; Luque, E.M.; et al. Control of Development, Secondary Metabolism and Light-Dependent Carotenoid Biosynthesis by the Velvet Complex of Neurospora crassa. Genetics 2019, 212, 691–710. [Google Scholar] [CrossRef]
- Lan, N.; Yue, Q.; An, Z.; Bills, G.F. Apc.LaeA and Apc.VeA of the velvet complex govern secondary metabolism and morphological development in the echinocandin-producing fungus Aspergillus pachycristatus. J. Ind. Microbiol. Biotechnol. 2020, 47, 155–168. [Google Scholar] [CrossRef]
- Monroy, A.A.; Stappler, E.; Schuster, A.; Sulyok, M.; Schmoll, M. A CRE1—Regulated cluster is responsible for light dependent production of dihydrotrichotetronin in Trichoderma reesei. PLoS ONE 2017, 12, e0182530. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wang, Y.; Liu, F.; Li, E.; Ma, J.; Yang, B.; Zhang, C.; Li, L.; Liu, Y. Requirement of LaeA, VeA, and VelB on Asexual Development, Ochratoxin A Biosynthesis, and Fungal Virulence in Aspergillus ochraceus. Front. Microbiol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Amare, M.G.; Keller, N.P. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 2014, 66, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F. Key role of LaeA and velvet complex proteins on expression of β-lactam and PR-toxin genes in Penicillium chrysogenum: Cross-talk regulation of secondary metabolite pathways. J. Ind. Microbiol. Biotechnol. 2017, 44, 525–535. [Google Scholar] [CrossRef]
- Sarikaya-Bayram, Ö.; Palmer, J.M.; Keller, N.; Braus, G.H.; Bayram, Ö. One Juliet and four Romeos: VeA and its methyltransferases. Front. Microbiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Bok, J.W.; Keller, N.P.; Humpf, H.-U.; Tudzynski, B. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef]
- Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, Z.; Wu, Y.; Xu, L.; Du, H.; Wang, N.; Huang, L. LaeA Controls Virulence and Secondary Metabolism in Apple Canker Pathogen Valsa mali. Front. Microbiol. 2020, 11, 581203. [Google Scholar] [CrossRef]
- Kumar, D.; Barad, S.; Chen, Y.; Luo, X.; Tannous, J.; Dubey, A.; Glam Matana, N.; Tian, S.; Li, B.; Keller, N.; et al. LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol. Plant Pathol. 2017, 18, 1150–1163. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, M.; Li, L.; Si, J.; Song, B.; Zhou, C.; Yu, M.; Wang, X.; Zhang, Y.; Ding, G.; et al. Overexpression of the Global Regulator LaeA in Chaetomium globosum Leads to the Biosynthesis of Chaetoglobosin Z. J. Nat. Prod. 2016, 79, 2487–2494. [Google Scholar] [CrossRef]
- Butchko, R.A.E.; Brown, D.W.; Busman, M.; Tudzynski, B.; Wiemann, P. Lae1 regulates expression of multiple secondary metabolite gene clusters in Fusarium verticillioides. Fungal Genet. Biol. 2012, 49, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.M.; Rindermann, L.; Janevska, S.; Munsterkotter, M.; Guldener, U.; Tudzynski, B. Analysis of the global regulator Lae1 uncovers a connection between Lae1 and the histone acetyltransferase HAT1 in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2018, 102, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Barda, O.; Maor, U.; Sadhasivam, S.; Bi, Y.; Zakin, V.; Prusky, D.; Sionov, E. The pH-Responsive Transcription Factor PacC Governs Pathogenicity and Ochratoxin A Biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Luo, Q.; Yang, L.; He, X.; Wu, J.; Kachanuban, K.; Wilaipun, P.; Zhu, W.; Wang, Y. Bioactive Metabolites from Acid-Tolerant Fungi in a Thai Mangrove Sediment. Front. Microbiol. 2021, 11, 60995. [Google Scholar] [CrossRef]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH Regulation of Sterigmatocystin and Aflatoxin Biosynthesis in Aspergillus spp. Phytopathology 1997, 87, 643–648. [Google Scholar] [CrossRef]
- Shah, A.J.; Tilburn, J.; Adlard, M.W.; Arst, H.N., Jr. pH regulation of penicillin production in Aspergillus nidulans. FEMS Microbiol. Lett. 1991, 61, 209–212. [Google Scholar] [CrossRef]
- Trushina, N.; Levin, M.; Mukherjee, P.K.; Horwitz, B.A. PacC and pH–dependent transcriptome of the mycotrophic fungus Trichoderma virens. BMC Genom. 2013, 14, 138. [Google Scholar] [CrossRef]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Tian, S. Function of pH-dependent transcription factor PacC in regulating development, pathogenicity, and mycotoxin biosynthesis of phytopathogenic fungi. FEBS J. 2022, 289, 1723–1730. [Google Scholar] [CrossRef]
- Merhej, J.; Richard-Forget, F.; Barreau, C. The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet. Biol. 2011, 48, 275–284. [Google Scholar] [CrossRef]
- Tilburn, J.; Sarkar, S.; Widdick, D.A.; Espeso, E.A.; Orejas, M.; Mungroo, J.; Peñalva, M.A.; Arst, H.N., Jr. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. Embo j. 1995, 14, 779–790. [Google Scholar] [CrossRef]
- Lind, A.L.; Smith, T.D.; Saterlee, T.; Calvo, A.M.; Rokas, A. Regulation of Secondary Metabolism by the Velvet Complex Is Temperature-Responsive in Aspergillus. G3 2016, 6, 4023–4033. [Google Scholar] [CrossRef]
- Yogabaanu, U.; Weber, J.-F.F.; Convey, P.; Rizman-Idid, M.; Alias, S.A. Antimicrobial properties and the influence of temperature on secondary metabolite production in cold environment soil fungi. Polar Sci. 2017, 14, 60–67. [Google Scholar] [CrossRef]
- Overy, D.; Correa, H.; Roullier, C.; Chi, W.-C.; Pang, K.-L.; Rateb, M.; Ebel, R.; Shang, Z.; Capon, R.; Bills, G.; et al. Does Osmotic Stress Affect Natural Product Expression in Fungi? Mar. Drugs 2017, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, C.; Qian, X.; Huang, Y.; Zheng, Z.; Shen, Y. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol. Sin. 2011, 30, 118. [Google Scholar] [CrossRef]
- Sarri, E.; Termentzi, A.; Abraham, E.M.; Papadopoulos, G.K.; Baira, E.; Machera, K.; Loukas, V.; Komaitis, F.; Tani, E. Salinity Stress Alters the Secondary Metabolic Profile of M. sativa, M. arborea and Their Hybrid (Alborea). Int. J. Mol. Sci. 2021, 22, 4882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Sun, K.; Zhu, W. Effects of High Salt Stress on Secondary Metabolite Production in the Marine-Derived Fungus Spicaria elegans. Mar. Drugs 2011, 9, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Fil, T.P.; Pallini, H.F.; Amaral, L.D.S.; Silva, J.V.D.; Bidóia, D.L.; Peron, F.; Garcia, F.P.; Nakamura, C.V.; Rodrigues-Filho, E. Copper and Manganese Cations Alter Secondary Metabolism in the Fungus Penicillium brasilianum. J. Braz. Chem. Soc. 2016, 27, 1444–1451. [Google Scholar] [CrossRef]
- Scott, R.E.; Jones, A.; Lam, K.S.; Gaucher, G.M. Manganese and antibiotic biosynthesis. I. A specific manganese requirement for patulin production in Penicillium urticae. Can. J. Microbiol. 1986, 32, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Lechner, B.E.; Baccile, J.A.; Velk, T.A.; Yin, W.B.; Bok, J.W.; Pakala, S.; Losada, L.; Nierman, W.C.; Schroeder, F.C.; et al. Perturbations in small molecule synthesis uncovers an iron-responsive secondary metabolite network in Aspergillus fumigatus. Front. Microbiol. 2014, 5, 530. [Google Scholar] [CrossRef] [PubMed]
- Raffa, N.; Won, T.H.; Sukowaty, A.; Candor, K.; Cui, C.; Halder, S.; Dai, M.; Landero-Figueroa, J.A.; Schroeder, F.C.; Keller, N.P. Dual-purpose isocyanides produced by Aspergillus fumigatus contribute to cellular copper sufficiency and exhibit antimicrobial activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015224118. [Google Scholar] [CrossRef]
- Gressler, M.; Zaehle, C.; Scherlach, K.; Hertweck, C.; Brock, M. Multifactorial induction of an orphan PKS-NRPS gene cluster in Aspergillus terreus. Chem. Biol. 2011, 18, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villafán, B.; Cruz-Bautista, R.; Manzo-Ruiz, M.; Passari, A.K.; Villarreal-Gómez, K.; Rodríguez-Sanoja, R.; Sánchez, S. Carbon catabolite regulation of secondary metabolite formation, an old but not well-established regulatory system. Microb. Biotechnol. 2022, 15, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Schrettl, M.; Kragl, C.; Müller, D.; Illmer, P.; Haas, H. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef]
- Hong, S.Y.; Roze, L.V.; Linz, J.E. Oxidative stress-related transcription factors in the regulation of secondary metabolism. Toxins 2013, 5, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Chanda, A.; Wee, J.; Awad, D.; Linz, J.E. Stress-related transcription factor AtfB integrates secondary metabolism with oxidative stress response in aspergilli. J. Biol. Chem. 2011, 286, 35137–35148. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, G.; Geng, L.; Lu, X.; Yang, K.; Yuan, J.; Nie, X.; Zhuang, Z.; Wang, S. The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus. Toxins 2016, 8, 202. [Google Scholar] [CrossRef]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Punelli, F.; Camera, E.; Fabbri, C.; Picardo, M.; Fanelli, C.; Fabbri, A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: A role for the ApyapA gene. Eukaryot. Cell 2008, 7, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Gazzetti, K.; Punelli, F.; Scarpari, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl. Microbiol. Biotechnol. 2012, 95, 1293–1304. [Google Scholar] [CrossRef]
- Oakley, C.E.; Ahuja, M.; Sun, W.W.; Entwistle, R.; Akashi, T.; Yaegashi, J.; Guo, C.J.; Cerqueira, G.C.; Russo Wortman, J.; Wang, C.C.; et al. Discovery of McrA, a master regulator of Aspergillus secondary metabolism. Mol. Microbiol. 2017, 103, 347–365. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Schumacher, J.; Burkhardt, I.; Rabe, P.; Münsterkötter, M.; Güldener, U.; Sieber, C.M.K.; Dickschat, J.S.; Tudzynski, B. The GATA-Type Transcription Factor Csm1 Regulates Conidiation and Secondary Metabolism in Fusarium fujikuroi. Front. Microbiol. 2017, 8, 1175. [Google Scholar] [CrossRef]
- Kim, H.Y.; Heo, D.Y.; Park, H.M.; Singh, D.; Lee, C.H. Metabolomic and Transcriptomic Comparison of Solid-State and Submerged Fermentation of Penicillium expansum KACC 40815. PLoS ONE 2016, 11, e0149012. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Lee, S.; Singh, D.; Lee, N.R.; Lee, D.Y.; Lee, C.H. Comprehensive Secondary Metabolite Profiling Toward Delineating the Solid and Submerged-State Fermentation of Aspergillus oryzae KCCM 12698. Front. Microbiol. 2018, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Thammajaruk, N.; Sriubolmas, N.; Israngkul, D.; Meevootisom, V.; Wiyakrutta, S. Optimization of culture conditions for mycoepoxydiene production by Phomopsis sp. Hant25. J. Ind. Microbiol. Biotechnol. 2011, 38, 679–685. [Google Scholar] [CrossRef]
- Bigelis, R.; He, H.; Yang, H.Y.; Chang, L.-P.; Greenstein, M. Production of fungal antibiotics using polymeric solid supports in solid-state and liquid fermentation. J. Ind. Microbiol. Biotechnol. 2006, 33, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Nahlik, K.; Dumkow, M.; Bayram, O.; Helmstaedt, K.; Busch, S.; Valerius, O.; Gerke, J.; Hoppert, M.; Schwier, E.; Opitz, L.; et al. The COP9 signalosome mediates transcriptional and metabolic response to hormones, oxidative stress protection and cell wall rearrangement during fungal development. Mol. Microbiol. 2010, 78, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Gerke, J.; Bayram, O.; Feussner, K.; Landesfeind, M.; Shelest, E.; Feussner, I.; Braus, G.H. Breaking the silence: Protein stabilization uncovers silenced biosynthetic gene clusters in the fungus Aspergillus nidulans. Appl. Environ. Microbiol. 2012, 78, 8234–8244. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, E.; Chiang, Y.M.; Oakley, C.E.; Davidson, A.D.; Wang, C.C.; Oakley, B.R. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl. Environ. Microbiol. 2008, 74, 7607–7612. [Google Scholar] [CrossRef]
- Berger, H.; Bacher, M.; Labuda, R.; Eppel, I.M.; Bayer, F.; Sulyok, M.; Gasparotto, E.; Zehetbauer, F.; Doppler, M.; Gratzl, H.; et al. Polaramycin B, and not physical interaction, is the signal that rewires fungal metabolism in the Streptomyces–Aspergillus interaction. Environ. Microbiol. 2022. [Google Scholar] [CrossRef]
- Gu, Y.; Ding, P.; Liang, Z.; Song, Y.; Liu, Y.; Chen, G.; Li, J.L. Activated production of silent metabolites from marine-derived fungus Penicillium citrinum. Fitoterapia 2018, 127, 207–211. [Google Scholar] [CrossRef]
- Gila, B.C.; Antal, K.; Birko, Z.; Keseru, J.S.; Pocsi, I.; Emri, T. Strategies Shaping the Transcription of Carbohydrate-Active Enzyme Genes in Aspergillus nidulans. J. Fungi 2022, 8, 79. [Google Scholar] [CrossRef]
- Li, T.; Jiang, G.; Qu, H.; Wang, Y.; Xiong, Y.; Jian, Q.; Wu, Y.; Duan, X.; Zhu, X.; Hu, W.; et al. Comparative Transcriptome Analysis of Penicillium citrinum Cultured with Different Carbon Sources Identifies Genes Involved in Citrinin Biosynthesis. Toxins 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.M.; Sayed, A.M.; AboulMagd, A.M.; Hassan, H.M.; El Amir, D.; Abouzid, S.F.; El-Gendy, A.O.; Rateb, M.E.; Abdelmohsen, U.R.; Alhadrami, H.; et al. Metabolomic profiling, biological evaluation of Aspergillus awamori, the river Nile-derived fungus using epigenetic and OSMAC approaches. RSC Adv. 2021, 11, 6709–6719. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Hoffmeister, D.; Maggio-Hall, L.A.; Murillo, R.; Glasner, J.D.; Keller, N.P. Genomic mining for Aspergillus natural products. Chem. Biol. 2006, 13, 31–37. [Google Scholar] [CrossRef]
- Fountain, J.C.; Bajaj, P.; Pandey, M.; Nayak, S.N.; Yang, L.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Zhuang, W.; Scully, B.T.; et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016, 6, 38747. [Google Scholar] [CrossRef]
- Joffe, A.Z.; Lisker, N. Effects of light, temperature, and pH value on aflatoxin production in vitro. Appl. Microbiol. 1969, 18, 517–518. [Google Scholar] [CrossRef]
- Hagiwara, D.; Sakai, K.; Suzuki, S.; Umemura, M.; Nogawa, T.; Kato, N.; Osada, H.; Watanabe, A.; Kawamoto, S.; Gonoi, T.; et al. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS ONE 2017, 12, e0177050. [Google Scholar] [CrossRef] [PubMed]
- Berthier, E.; Lim, F.Y.; Deng, Q.; Guo, C.J.; Kontoyiannis, D.P.; Wang, C.C.; Rindy, J.; Beebe, D.J.; Huttenlocher, A.; Keller, N.P. Low-volume toolbox for the discovery of immunosuppressive fungal secondary metabolites. PLoS Pathog 2013, 9, e1003289. [Google Scholar] [CrossRef]
- Jyoti, S.; Singh, D.P. Production of Secondary Metabolites from Two Penicillium Strains Adapted to Different Temperature Conditions: A Study on DifferentialResponse of Fungal Strains to Temperature Stress. Cell. Mol. Biol. 2016. [Google Scholar] [CrossRef]
- Yaegashi, J.; Praseuth, M.B.; Tyan, S.W.; Sanchez, J.F.; Entwistle, R.; Chiang, Y.M.; Oakley, B.R.; Wang, C.C. Molecular genetic characterization of the biosynthesis cluster of a prenylated isoindolinone alkaloid aspernidine A in Aspergillus nidulans. Org. Lett. 2013, 15, 2862–2865. [Google Scholar] [CrossRef]
- Michielse, C.B.; Pfannmuller, A.; Macios, M.; Rengers, P.; Dzikowska, A.; Tudzynski, B. The interplay between the GATA transcription factors AreA, the global nitrogen regulator and AreB in Fusarium fujikuroi. Mol. Microbiol. 2014, 91, 472–493. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.M.; von Bargen, K.W.; Espino, J.J.; Pfannmuller, A.; Humpf, H.U.; Tudzynski, B. Characterization of the fusaric acid gene cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Son, H.; Lee, Y.-W. Effects of light on secondary metabolism and fungal development of Fusarium graminearum. J. Appl. Microbiol. 2014, 116, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, B.; Homann, V.; Feng, B.; Marzluf, G.A. Isolation, characterization and disruption of the areA nitrogen regulatory gene of Gibberella fujikuroi. Mol. Genet. Genom. 1999, 261, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mihlan, M.; Homann, V.; Liu, T.W.; Tudzynski, B. AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol. Microbiol. 2003, 47, 975–991. [Google Scholar] [CrossRef]
- Nazari, L.; Manstretta, V.; Rossi, V. A non-linear model for temperature-dependent sporulation and T-2 and HT-2 production of Fusarium langsethiae and Fusarium sporotrichioides. Fungal Biol. 2016, 120, 562–571. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, J.; Singh, D.K.; Mishra, A.; Verma, S.K.; Gond, S.K.; Kumar, A.; Singh, N.; Kharwar, R.N. Induction of Cryptic and Bioactive Metabolites through Natural Dietary Components in an Endophytic Fungus Colletotrichum gloeosporioides (Penz.) Sacc. Front. Microbiol. 2017, 8, 1126. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, T.; Wei, H.; Zhang, G.; Wang, H.; Gu, Q. Spicochalasin A and New Aspochalasins from the Marine-Derived Fungus Spicaria elegans. Eur. J. Org. Chem. 2009, 2009, 3045–3051. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Dyshlovoy, S.A.; von Amsberg, G.; Yurchenko, E.A.; Afiyatullov, S.S. Unique prostate cancer-toxic polyketides from marine sediment-derived fungus Isaria felina. J. Antibiot. 2017, 70, 856–858. [Google Scholar] [CrossRef]
- Pruss, S.; Fetzner, R.; Seither, K.; Herr, A.; Pfeiffer, E.; Metzler, M.; Lawrence, C.B.; Fischer, R. Role of the Alternaria alternata blue-light receptor LreA (white-collar 1) in spore formation and secondary metabolism. Appl. Environ. Microbiol. 2014, 80, 2582–2591. [Google Scholar] [CrossRef]
- Bazafkan, H.; Beier, S.; Stappler, E.; Böhmdorfer, S.; Oberlerchner, J.T.; Sulyok, M.; Schmoll, M. SUB1 has photoreceptor dependent and independent functions in sexual development and secondary metabolism in Trichoderma reesei. Mol. Microbiol. 2017, 106, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Li, X.-M.; Li, C.-S.; Wang, B.-G. Diverse Secondary Metabolites Produced by Marine-Derived Fungus Nigrospora sp. MA75 on Various Culture Media. Chem. Biodivers. 2012, 9, 1338–1348. [Google Scholar] [CrossRef]
- Siless, G.E.; Gallardo, G.L.; Rodriguez, M.A.; Rincon, Y.A.; Godeas, A.M.; Cabrera, G.M. Metabolites from the Dark Septate Endophyte Drechslera sp. Evaluation by LC/MS and Principal Component Analysis of Culture Extracts with Histone Deacetylase Inhibitors. Chem. Biodivers. 2018, 15, e1800133. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Bacha, N.; Ahmad, B.; Bakht, J.; Lutfullah, G.; Ali, J. Synthesis of secondary metabolites by Cladosporium resinae (NRL-6437) under different growth media and chemical inducers and their pharmaceutical activity. Pak. J. Pharm. Sci. 2017, 30, 1617–1624. [Google Scholar] [PubMed]
- Morishita, Y.; Okazaki, Y.; Luo, Y.Y.; Nunoki, J.; Taniguchi, T.; Oshima, Y.; Asai, T. Use of plant hormones to activate silent polyketide biosynthetic pathways in Arthrinium sacchari, a fungus isolated from a spider. Org. Biomol. Chem. 2019, 17, 780–784. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Leow, H.Y.; Lee, D.C.W.; Karisnan, K.; Song, A.A.L.; Mai, C.W.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Co-Culture Systems for the Production of Secondary Metabolites: Current and Future Prospects. Open Biotechnol. J. 2019, 13, 18–26. [Google Scholar] [CrossRef]
- Bertrand, S.; Schumpp, O.; Bohni, N.; Bujard, A.; Azzollini, A.; Monod, M.; Gindro, K.; Wolfender, J.-L. Detection of metabolite induction in fungal co-cultures on solid media by high-throughput differential ultra-high pressure liquid chromatography–time-of-flight mass spectrometry fingerprinting. J. Chromatogr. A 2013, 1292, 219–228. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Xu, C.; Sun, X.; Jin, M.; Zhang, X. A Novel Benzoquinone Compound Isolated from Deep-Sea Hydrothermal Vent Triggers Apoptosis of Tumor Cells. Mar. Drugs 2017, 15, 200. [Google Scholar] [CrossRef] [PubMed]
- Mandelare, P.E.; Adpressa, D.A.; Kaweesa, E.N.; Zakharov, L.N.; Loesgen, S. Coculture of Two Developmental Stages of a Marine-Derived Aspergillus alliaceus Results in the Production of the Cytotoxic Bianthrone Allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, W.; Ma, Z. Induced production of cytochalasans in co-culture of marine fungus Aspergillus flavipes and actinomycete Streptomyces sp. Nat. Prod. Res. 2016, 30, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual Induction of New Microbial Secondary Metabolites by Fungal Bacterial Co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Stroe, M.C.; Netzker, T.; Scherlach, K.; Kruger, T.; Hertweck, C.; Valiante, V.; Brakhage, A.A. Targeted induction of a silent fungal gene cluster encoding the bacteria-specific germination inhibitor fumigermin. Elife 2020, 9, e52541. [Google Scholar] [CrossRef]
- Gerke, J.; Köhler, A.M.; Wennrich, J.-P.; Große, V.; Shao, L.; Heinrich, A.K.; Bode, H.B.; Chen, W.; Surup, F.; Braus, G.H. Biosynthesis of Antibacterial Iron-Chelating Tropolones in Aspergillus nidulans as Response to Glycopeptide-Producing Streptomycetes. Front. Fungal Biol. 2022, 2, 777474. [Google Scholar] [CrossRef]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.G.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC approaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef]
- Moree, W.J.; Yang, J.Y.; Zhao, X.; Liu, W.-T.; Aparicio, M.; Atencio, L.; Ballesteros, J.; Sánchez, J.; Gavilán, R.G.; Gutiérrez, M.; et al. Imaging Mass Spectrometry of a Coral Microbe Interaction with Fungi. J. Chem. Ecol. 2013, 39, 1045–1054. [Google Scholar] [CrossRef]
- Oh, D.-C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced Production of Emericellamides A and B from the Marine-Derived Fungus Emericella sp. in Competing Co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef]
- Shang, Z.; Salim, A.A.; Capon, R.J. Chaunopyran A: Co-Cultivation of Marine Mollusk-Derived Fungi Activates a Rare Class of 2-Alkenyl-Tetrahydropyran. J. Nat. Prod. 2017, 80, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhu, L.-P.; Xu, X.-Y.; Tan, L.-L.; Sadilek, M.; Fan, H.; Hu, B.; Shen, X.-T.; Yang, J.; Qiao, B.; et al. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 2016, 6, 33237. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorganic Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic Polyketides from the Co-Cultivation of Marine-Derived Penicillium sp. WC-29-5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, W.; Wang, Q.; Ma, Z.; Xu, X.; Zhao, X.; Chen, Z. Induction of cryptic bioactive 2,5-diketopiperazines in fungus Penicillium sp. DT-F29 by microbial co-culture. Tetrahedron 2017, 73, 907–914. [Google Scholar] [CrossRef]
- Chen, H.; Aktas, N.; Konuklugil, B.; Mándi, A.; Daletos, G.; Lin, W.; Dai, H.; Kurtán, T.; Proksch, P. A new fusarielin analogue from Penicillium sp. isolated from the Mediterranean sponge Ircinia oros. Tetrahedron Lett. 2015, 56, 5317–5320. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Inducing Secondary Metabolite Production by Combined Culture of Talaromyces aculeatus and Penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef]
- Khalil, Z.G.; Cruz-Morales, P.; Licona-Cassani, C.; Marcellin, E.; Capon, R.J. Inter-Kingdom beach warfare: Microbial chemical communication activates natural chemical defences. ISME J. 2019, 13, 147–158. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat. Commun. 2020, 11, 6058. [Google Scholar] [CrossRef]
- Flaherty, J.E.; Payne, G.A. Overexpression of aflR Leads to Upregulation of Pathway Gene Transcription and Increased Aflatoxin Production in Aspergillus flavus. Appl. Environ. Microbiol. 1997, 63, 3995–4000. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Szewczyk, E.; Davidson, A.D.; Keller, N.; Oakley, B.R.; Wang, C.C. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. J. Am. Chem. Soc. 2009, 131, 2965–2970. [Google Scholar] [CrossRef] [PubMed]
- Janevska, S.; Arndt, B.; Baumann, L.; Apken, L.H.; Mauriz Marques, L.M.; Humpf, H.U.; Tudzynski, B. Establishment of the Inducible Tet-On System for the Activation of the Silent Trichosetin Gene Cluster in Fusarium fujikuroi. Toxins 2017, 9, 126. [Google Scholar] [CrossRef]
- Inglis, D.O.; Binkley, J.; Skrzypek, M.S.; Arnaud, M.B.; Cerqueira, G.C.; Shah, P.; Wymore, F.; Wortman, J.R.; Sherlock, G. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Ehrlich, K.C. Genome-wide analysis of the Zn(II)2Cys6 zinc cluster-encoding gene family in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2013, 97, 4289–4300. [Google Scholar] [CrossRef]
- Kwon, M.J.; Steiniger, C.; Cairns, T.C.; Wisecaver, J.H.; Lind, A.L.; Pohl, C.; Regner, C.; Rokas, A.; Meyer, V.; Goldman, G.H. Beyond the Biosynthetic Gene Cluster Paradigm: Genome-Wide Coexpression Networks Connect Clustered and Unclustered Transcription Factors to Secondary Metabolic Pathways. Microbiol. Spectr. 2021, 9, e00898-21. [Google Scholar] [CrossRef]
- Ahuja, M.; Chiang, Y.-M.; Chang, S.-L.; Praseuth, M.B.; Entwistle, R.; Sanchez, J.F.; Lo, H.-C.; Yeh, H.-H.; Oakley, B.R.; Wang, C.C.C. Illuminating the Diversity of Aromatic Polyketide Synthases in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 8212–8221. [Google Scholar] [CrossRef]
- Shimizu, K.; Hicks, J.K.; Huang, T.-P.; Keller, N.P. Pka, Ras and RGS Protein Interactions Regulate Activity of AflR, a Zn(II)2Cys6 Transcription Factor in Aspergillus nidulans. Genetics 2003, 165, 1095–1104. [Google Scholar] [CrossRef]
- Zehetbauer, F.; Seidl, A.; Berger, H.; Sulyok, M.; Kastner, F.; Strauss, J. RimO (SrrB) is required for carbon starvation signaling and production of secondary metabolites in Aspergillus nidulans. Fungal Genet. Biol. 2022, 162, 103726. [Google Scholar] [CrossRef]
- Grau, M.F.; Entwistle, R.; Chiang, Y.-M.; Ahuja, M.; Oakley, C.E.; Akashi, T.; Wang, C.C.C.; Todd, R.B.; Oakley, B.R. Hybrid Transcription Factor Engineering Activates the Silent Secondary Metabolite Gene Cluster for (+)-Asperlin in Aspergillus nidulans. ACS Chem. Biol. 2018, 13, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.T.; Chang, S.-L.; Entwistle, R.; Ahn, G.; Lin, T.-S.; Petrova, V.; Yeh, H.-H.; Praseuth, M.B.; Chiang, Y.-M.; Oakley, B.R.; et al. Overexpression of a three-gene conidial pigment biosynthetic pathway in Aspergillus nidulans reveals the first NRPS known to acetylate tryptophan. Fungal Genet. Biol. 2017, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-H.; Ahuja, M.; Chiang, Y.-M.; Oakley, C.E.; Moore, S.; Yoon, O.; Hajovsky, H.; Bok, J.-W.; Keller, N.P.; Wang, C.C.C.; et al. Resistance Gene-Guided Genome Mining: Serial Promoter Exchanges in Aspergillus nidulans Reveal the Biosynthetic Pathway for Fellutamide B, a Proteasome Inhibitor. ACS Chem. Biol. 2016, 11, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Baccile, J.A.; Spraker, J.E.; Le, H.H.; Brandenburger, E.; Gomez, C.; Bok, J.W.; Macheleidt, J.; Brakhage, A.A.; Hoffmeister, D.; Keller, N.P.; et al. Plant-like biosynthesis of isoquinoline alkaloids in Aspergillus fumigatus. Nat. Chem. Biol. 2016, 12, 419–424. [Google Scholar] [CrossRef]
- Lim, F.Y.; Won, T.H.; Raffa, N.; Baccile, J.A.; Wisecaver, J.; Rokas, A.; Schroeder, F.C.; Keller, N.P.; Goldman, G.H. Fungal Isocyanide Synthases and Xanthocillin Biosynthesis in Aspergillus fumigatus. mBio 2018, 9, e00785-18. [Google Scholar] [CrossRef]
- Schüller, A.; Wolansky, L.; Berger, H.; Studt, L.; Gacek-Matthews, A.; Sulyok, M.; Strauss, J. A novel fungal gene regulation system based on inducible VPR-dCas9 and nucleosome map-guided sgRNA positioning. Appl. Microbiol. Biotechnol. 2020, 104, 9801–9822. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Pan, L.; Wang, B.; Pan, L. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiol. Res. 2021, 245, 126694. [Google Scholar] [CrossRef]
- Roux, I.; Woodcraft, C.; Hu, J.; Wolters, R.; Gilchrist, C.L.M.; Chooi, Y.-H. CRISPR-Mediated Activation of Biosynthetic Gene Clusters for Bioactive Molecule Discovery in Filamentous Fungi. ACS Synth. Biol. 2020, 9, 1843–1854. [Google Scholar] [CrossRef]
- Bromann, K.; Toivari, M.; Viljanen, K.; Vuoristo, A.; Ruohonen, L.; Nakari-Setälä, T. Identification and Characterization of a Novel Diterpene Gene Cluster in Aspergillus nidulans. PLoS ONE 2012, 7, e35450. [Google Scholar] [CrossRef]
- Yeh, H.-H.; Chiang, Y.-M.; Entwistle, R.; Ahuja, M.; Lee, K.-H.; Bruno, K.S.; Wu, T.-K.; Oakley, B.R.; Wang, C.C.C. Molecular genetic analysis reveals that a nonribosomal peptide synthetase-like (NRPS-like) gene in Aspergillus nidulans is responsible for microperfuranone biosynthesis. Appl. Microbiol. Biotechnol. 2012, 96, 739–748. [Google Scholar] [CrossRef]
- Ishikawa, N.; Tanaka, H.; Koyama, F.; Noguchi, H.; Wang, C.C.C.; Hotta, K.; Watanabe, K. Non-Heme Dioxygenase Catalyzes Atypical Oxidations of 6,7-Bicyclic Systems To Form the 6,6-Quinolone Core of Viridicatin-Type Fungal Alkaloids. Angew. Chem. Int. Ed. 2014, 53, 12880–12884. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.S.; Chen, B.; Chiang, Y.M.; Wang, C.C.C. Discovery and Elucidation of the Biosynthesis of Aspernidgulenes: Novel Polyenes from Aspergillus nidulans by using Serial Promoter Replacement. Chembiochem 2019, 20, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zabala, A.O.; Xu, W.; Chooi, Y.-H.; Tang, Y. Characterization of a Silent Azaphilone Gene Cluster from Aspergillus niger ATCC 1015 Reveals a Hydroxylation-Mediated Pyran-Ring Formation. Chem. Biol. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Ehrlich, K.C.; Yu, J.; Bhatnagar, D.; Cleveland, T.E. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 1995, 61, 2372–2377. [Google Scholar] [CrossRef]
- Tang, S.; Men, P.; Zhang, W.; Li, H.; Li, Z.; Huang, X.; Lu, X. Identification of a polyketide biosynthesis gene cluster by transcriptional regulator activation in Aspergillus terreus. Fungal Genet. Biol. 2022, 160, 103690. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, C.B.; Li, C.W.; Hua, W.; Wu, C.J.; Zhu, T.J.; Gu, Q.Q. Activation of dormant secondary metabolite production by introducing neomycin resistance into the deep-sea fungus, Aspergillus versicolor ZBY-3. Mar. Drugs 2014, 12, 4326–4352. [Google Scholar] [CrossRef]
- Niehaus, E.M.; Janevska, S.; von Bargen, K.W.; Sieber, C.M.; Harrer, H.; Humpf, H.U.; Tudzynski, B. Apicidin F: Characterization and genetic manipulation of a new secondary metabolite gene cluster in the rice pathogen Fusarium fujikuroi. PLoS ONE 2014, 9, e103336. [Google Scholar] [CrossRef]
- Von Bargen, K.W.; Niehaus, E.M.; Krug, I.; Bergander, K.; Wurthwein, E.U.; Tudzynski, B.; Humpf, H.U. Isolation and Structure Elucidation of Fujikurins A-D: Products of the PKS19 Gene Cluster in Fusarium fujikuroi. J. Nat. Prod. 2015, 78, 1809–1815. [Google Scholar] [CrossRef]
- Arndt, B.; Studt, L.; Wiemann, P.; Osmanov, H.; Kleigrewe, K.; Köhler, J.; Krug, I.; Tudzynski, B.; Humpf, H.-U. Genetic engineering, high resolution mass spectrometry and nuclear magnetic resonance spectroscopy elucidate the bikaverin biosynthetic pathway in Fusarium fujikuroi. Fungal Genet. Biol. 2015, 84, 26–36. [Google Scholar] [CrossRef]
- Arndt, B.; Janevska, S.; Schmid, R.; Hubner, F.; Tudzynski, B.; Humpf, H.U. A Fungal N-Dimethylallyltryptophan Metabolite from Fusarium fujikuroi. Chembiochem 2017, 18, 899–904. [Google Scholar] [CrossRef]
- Sorensen, J.L.; Hansen, F.T.; Sondergaard, T.E.; Staerk, D.; Lee, T.V.; Wimmer, R.; Klitgaard, L.G.; Purup, S.; Giese, H.; Frandsen, R.J. Production of novel fusarielins by ectopic activation of the polyketide synthase 9 cluster in Fusarium graminearum. Environ. Microbiol. 2012, 14, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.J.; Tang, H.Y.; Wang, W.Q.; Yuan, T.L.; Wei, W.Q.; Pang, B.; Gong, X.M.; Wang, S.F.; Li, Y.J.; Zhang, D.; et al. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. Nat. Commun. 2019, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Kakule, T.B.; Sardar, D.; Lin, Z.; Schmidt, E.W. Two related pyrrolidinedione synthetase loci in Fusarium heterosporum ATCC 74349 produce divergent metabolites. ACS Chem. Biol. 2013, 8, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Drott, M.; Greco, C.; Luciano-Rosario, D.; Wang, P.; Keller, N.P. Transcription Factor Repurposing Offers Insights into Evolution of Biosynthetic Gene Cluster Regulation. mBio 2021, 12, e0139921. [Google Scholar] [CrossRef]
- Wang, N.; Cui, C.-B.; Li, C.-W. A new cyclic dipeptide penicimutide: The activated production of cyclic dipeptides by introduction of neomycin-resistance in the marine-derived fungus Penicillium purpurogenum G59. Arch. Pharmacal Res. 2016, 39, 762–770. [Google Scholar] [CrossRef]
- Mózsik, L.; Hoekzema, M.; de Kok, N.A.W.; Bovenberg, R.A.L.; Nygård, Y.; Driessen, A.J.M. CRISPR-based transcriptional activation tool for silent genes in filamentous fungi. Sci. Rep. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Zhou, H.; Zhan, J.; Watanabe, K.; Xie, X.; Tang, Y. A polyketide macrolactone synthase from the filamentous fungus Gibberella zeae. Proc. Natl. Acad. Sci. USA 2008, 105, 6249–6254. [Google Scholar] [CrossRef]
- Haynes, S.W.; Ames, B.D.; Gao, X.; Tang, Y.; Walsh, C.T. Unraveling Terminal C-Domain-Mediated Condensation in Fungal Biosynthesis of Imidazoindolone Metabolites. Biochemistry 2011, 50, 5668–5679. [Google Scholar] [CrossRef]
- Meng, X.; Fang, Y.; Ding, M.; Zhang, Y.; Jia, K.; Li, Z.; Collemare, J.; Liu, W. Developing fungal heterologous expression platforms to explore and improve the production of natural products from fungal biodiversity. Biotechnol. Adv. 2022, 54, 107866. [Google Scholar] [CrossRef]
- Vassaux, A.; Meunier, L.; Vandenbol, M.; Baurain, D.; Fickers, P.; Jacques, P.; Leclère, V. Nonribosomal peptides in fungal cell factories: From genome mining to optimized heterologous production. Biotechnol. Adv. 2019, 37, 107449. [Google Scholar] [CrossRef]
- Liu, C.; Minami, A.; Ozaki, T.; Wu, J.; Kawagishi, H.; Maruyama, J.-i.; Oikawa, H. Efficient Reconstitution of Basidiomycota Diterpene Erinacine Gene Cluster in Ascomycota Host Aspergillus oryzae Based on Genomic DNA Sequences. J. Am. Chem. Soc. 2019, 141, 15519–15523. [Google Scholar] [CrossRef] [PubMed]
- Okorafor, I.C.; Chen, M.; Tang, Y. High-Titer Production of Olivetolic Acid and Analogs in Engineered Fungal Host using a Nonplant Biosynthetic Pathway. ACS Synth. Biol. 2021, 10, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Boecker, S.; Grätz, S.; Kerwat, D.; Adam, L.; Schirmer, D.; Richter, L.; Schütze, T.; Petras, D.; Süssmuth, R.D.; Meyer, V. Aspergillus niger is a superior expression host for the production of bioactive fungal cyclodepsipeptides. Fungal Biol. Biotechnol. 2018, 5, 4. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Sørensen, J.L. Investigating Fungal Biosynthetic Pathways using Heterologous Gene Expression: Fusarium sp. as a Heterologous Host. In Engineering Natural Product Biosynthesis: Methods and Protocols; Skellam, E., Ed.; Springer: New York, NY, USA, 2022; pp. 53–74. [Google Scholar]
- Nielsen, M.R.; Wollenberg, R.D.; Westphal, K.R.; Sondergaard, T.E.; Wimmer, R.; Gardiner, D.M.; Sørensen, J.L. Heterologous expression of intact biosynthetic gene clusters in Fusarium graminearum. Fungal Genet. Biol. 2019, 132, 103248. [Google Scholar] [CrossRef]
- Kindinger, F.; Nies, J.; Becker, A.; Zhu, T.; Li, S.-M. Genomic Locus of a Penicillium crustosum Pigment as an Integration Site for Secondary Metabolite Gene Expression. ACS Chem. Biol. 2019, 14, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Polli, F.; Schütze, T.; Viggiano, A.; Mózsik, L.; Jung, S.; de Vries, M.; Bovenberg, R.A.L.; Meyer, V.; Driessen, A.J.M. A Penicillium rubens platform strain for secondary metabolite production. Sci. Rep. 2020, 10, 7630. [Google Scholar] [CrossRef]
- Bok, J.W.; Ye, R.; Clevenger, K.D.; Mead, D.; Wagner, M.; Krerowicz, A.; Albright, J.C.; Goering, A.W.; Thomas, P.M.; Kelleher, N.L.; et al. Fungal artificial chromosomes for mining of the fungal secondary metabolome. BMC Genom. 2015, 16, 343. [Google Scholar] [CrossRef]
- Clevenger, K.D.; Bok, J.W.; Ye, R.; Miley, G.P.; Verdan, M.H.; Velk, T.; Chen, C.; Yang, K.; Robey, M.T.; Gao, P.; et al. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat. Chem. Biol. 2017, 13, 895–901. [Google Scholar] [CrossRef]
- Harvey, C.J.B.; Tang, M.; Schlecht, U.; Horecka, J.; Fischer, C.R.; Lin, H.C.; Li, J.; Naughton, B.; Cherry, J.; Miranda, M.; et al. HEx: A heterologous expression platform for the discovery of fungal natural products. Sci. Adv. 2018, 4, eaar5459. [Google Scholar] [CrossRef]
- Wiemann, P.; Soukup, A.A.; Folz, J.S.; Wang, P.M.; Noack, A.; Keller, N.P. CoIN: Co-inducible nitrate expression system for secondary metabolites in Aspergillus nidulans. Fungal Biol. Biotechnol. 2018, 5, 6. [Google Scholar] [CrossRef]
- Robey, M.T.; Ye, R.; Bok, J.W.; Clevenger, K.D.; Islam, M.N.; Chen, C.; Gupta, R.; Swyers, M.; Wu, E.; Gao, P.; et al. Identification of the First Diketomorpholine Biosynthetic Pathway using FAC-MS Technology. ACS Chem. Biol. 2018, 13, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Maiya, S.; Grundmann, A.; Li, S.-M.; Turner, G. The Fumitremorgin Gene Cluster of Aspergillus fumigatus: Identification of a Gene Encoding Brevianamide F Synthetase. ChemBioChem 2006, 7, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Tokunaga, K.; Matsuda, Y.; Fujii, I.; Abe, I.; Ebizuka, Y.; Kushiro, T. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2010, 2, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Fujii, I.; Sankawa, U.; Mayorga, M.E.; Timberlake, W.E.; Ebizuka, Y. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Lett. 1999, 40, 91–94. [Google Scholar] [CrossRef]
- Caesar, L.K.; Robey, M.T.; Swyers, M.; Islam, M.N.; Ye, R.; Vagadia, P.P.; Schiltz, G.E.; Thomas, P.M.; Wu, C.C.; Kelleher, N.L.; et al. Heterologous Expression of the Unusual Terreazepine Biosynthetic Gene Cluster Reveals a Promising Approach for Identifying New Chemical Scaffolds. mBio 2020, 11. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, S.; Bian, G.; Yan, P.; Ma, Z.; Dai, W.; Chen, R.; Fu, S.; Huang, H.; Chi, H.; et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nat. Catal. 2022, 5, 277–287. [Google Scholar] [CrossRef]
- Bian, G.; Hou, A.; Yuan, Y.; Hu, B.; Cheng, S.; Ye, Z.; Di, Y.; Deng, Z.; Liu, T. Metabolic Engineering-Based Rapid Characterization of a Sesquiterpene Cyclase and the Skeletons of Fusariumdiene and Fusagramineol from Fusarium graminearum. Org. Lett. 2018, 20, 1626–1629. [Google Scholar] [CrossRef]
- Pedersen, T.B.; Nielsen, M.R.; Kristensen, S.B.; Spedtsberg, E.M.L.; Yasmine, W.; Matthiesen, R.; Kaniki, S.E.K.; Sørensen, T.; Petersen, C.; Muff, J.; et al. Heterologous Expression of the Core Genes in the Complex Fusarubin Gene Cluster of Fusarium Solani. Int. J. Mol. Sci. 2020, 21, 7601. [Google Scholar] [CrossRef]
- Tan, D.; Jamieson, C.S.; Ohashi, M.; Tang, M.C.; Houk, K.N.; Tang, Y. Genome-Mined Diels-Alderase Catalyzes Formation of the cis-Octahydrodecalins of Varicidin A and B. J. Am. Chem. Soc. 2019, 141, 769–773. [Google Scholar] [CrossRef]
- Hoefgen, S.; Lin, J.; Fricke, J.; Stroe, M.C.; Mattern, D.J.; Kufs, J.E.; Hortschansky, P.; Brakhage, A.A.; Hoffmeister, D.; Valiante, V. Facile assembly and fluorescence-based screening method for heterologous expression of biosynthetic pathways in fungi. Metab. Eng. 2018, 48, 44–51. [Google Scholar] [CrossRef]
- Yin, W.B.; Chooi, Y.H.; Smith, A.R.; Cacho, R.A.; Hu, Y.; White, T.C.; Tang, Y. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth. Biol. 2013, 2, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Kornberg, R.D. Action of micrococcal nuclease on chromatin and the location of histone H1. J. Mol. Biol. 1977, 109, 393–404. [Google Scholar] [CrossRef]
- Richmond, T.J.; Finch, J.T.; Rushton, B.; Rhodes, D.; Klug, A. Structure of the nucleosome core particle at 7 Å resolution. Nature 1984, 311, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M. 5 Fungal Chromatin and Its Role in Regulation of Gene Expression. In Fungal Genomics; Nowrousian, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–120. [Google Scholar]
- Buscaino, A. Chromatin-Mediated Regulation of Genome Plasticity in Human Fungal Pathogens. Genes 2019, 10, 855. [Google Scholar] [CrossRef]
- Mivelaz, M.; Cao, A.-M.; Kubik, S.; Zencir, S.; Hovius, R.; Boichenko, I.; Stachowicz, A.M.; Kurat, C.F.; Shore, D.; Fierz, B. Chromatin Fiber Invasion and Nucleosome Displacement by the Rap1 Transcription Factor. Mol. Cell 2020, 77, 488–500. [Google Scholar] [CrossRef]
- Zaret, K.S. Pioneer Transcription Factors Initiating Gene Network Changes. Annu. Rev. Genet. 2020, 54, 367–385. [Google Scholar] [CrossRef]
- Allis, C.D.; Berger, S.L.; Cote, J.; Dent, S.; Jenuwien, T.; Kouzarides, T.; Pillus, L.; Reinberg, D.; Shi, Y.; Shiekhattar, R.; et al. New Nomenclature for Chromatin-Modifying Enzymes. Cell 2007, 131, 633–636. [Google Scholar] [CrossRef]
- Reyes-Dominguez, Y.; Bok, J.W.; Berger, H.; Shwab, E.K.; Basheer, A.; Gallmetzer, A.; Scazzocchio, C.; Keller, N.; Strauss, J. Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol. Microbiol. 2010, 76, 1376–1386. [Google Scholar] [CrossRef]
- Tamaru, H.; Zhang, X.; McMillen, D.; Singh, P.B.; Nakayama, J.-i.; Grewal, S.I.; Allis, C.D.; Cheng, X.; Selker, E.U. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 2003, 34, 75–79. [Google Scholar] [CrossRef]
- Chujo, T.; Scott, B. Histone H3K9 and H3K27 methylation regulates fungal alkaloid biosynthesis in a fungal endophyte–plant symbiosis. Mol. Microbiol. 2014, 92, 413–434. [Google Scholar] [CrossRef]
- Atanasoff-Kardjalieff, A.K.; Lünne, F.; Kalinina, S.; Strauss, J.; Humpf, H.-U.; Studt, L. Biosynthesis of Fusapyrone Depends on the H3K9 Methyltransferase, FmKmt1, in Fusarium mangiferae. Front. Fungal Biol. 2021, 2, 671796. [Google Scholar] [CrossRef]
- Zhang, X.; Noberini, R.; Bonaldi, T.; Collemare, J.; Seidl, M.F. The histone code of the fungal genus Aspergillus uncovered by evolutionary and proteomic analyses. bioRxiv 2022. [Google Scholar] [CrossRef]
- Elías-Villalobos, A.; Barrales, R.R.; Ibeas, J.I. Chromatin modification factors in plant pathogenic fungi: Insights from Ustilago maydis. Fungal Genet. Biol. 2019, 129, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Lachner, M.; Sengupta, R.; Schotta, G.; Jenuwein, T. Trilogies of histone lysine methylation as epigenetic landmarks of the eukaryotic genome. Cold Spring Harb. Symp. Quant. Biol. 2004, 69, 209–218. [Google Scholar] [CrossRef]
- Connolly, L.R.; Smith, K.M.; Freitag, M. The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet 2013, 9, e1003916. [Google Scholar] [CrossRef]
- Jamieson, K.; Rountree, M.R.; Lewis, Z.A.; Stajich, J.E.; Selker, E.U. Regional control of histone H3 lysine 27 methylation in Neurospora. Proc. Natl. Acad. Sci. USA 2013, 110, 6027–6032. [Google Scholar] [CrossRef]
- Schwartz, Y.B.; Kahn, T.G.; Nix, D.A.; Li, X.-Y.; Bourgon, R.; Biggin, M.; Pirrotta, V. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 2006, 38, 700–705. [Google Scholar] [CrossRef]
- Studt, L.; Rösler, S.M.; Burkhardt, I.; Arndt, B.; Freitag, M.; Humpf, H.U.; Dickschat, J.S.; Tudzynski, B. Knock-down of the methyltransferase Kmt6 relieves H3K27me3 and results in induction of cryptic and otherwise silent secondary metabolite gene clusters in Fusarium fujikuroi. Environ. Microbiol. 2016, 18, 4037–4054. [Google Scholar] [CrossRef]
- Kramer, H.M.; Seidl, M.F.; Thomma, B.; Cook, D.E. Local Rather than Global H3K27me3 Dynamics Are Associated with Differential Gene Expression in Verticillium dahliae. mBio 2022, 13, e0356621. [Google Scholar] [CrossRef]
- Jamieson, K.; McNaught, K.J.; Ormsby, T.; Leggett, N.A.; Honda, S.; Selker, E.U. Telomere repeats induce domains of H3K27 methylation in Neurospora. eLife 2018, 7, e31216. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Cook, D.E. Histone modification dynamics at H3K27 are associated with altered transcription of in planta induced genes in Magnaporthe oryzae. PLOS Genet. 2021, 17, e1009376. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Chen, Z.; Zhang, K.; Whitaker, J.W.; Wang, M.; Wang, W. Epigenomic analysis reveals DNA motifs regulating histone modifications in human and mouse. Proc. Natl. Acad. Sci. USA 2019, 116, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Demers, D.H.; Knestrick, M.A.; Fleeman, R.; Tawfik, R.; Azhari, A.; Souza, A.; Vesely, B.; Netherton, M.; Gupta, R.; Colon, B.L.; et al. Exploitation of Mangrove Endophytic Fungi for Infectious Disease Drug Discovery. Mar. Drugs 2018, 16, 376. [Google Scholar] [CrossRef] [PubMed]
- Aldholmi, M.; Wilkinson, B.; Ganesan, A. Epigenetic modulation of secondary metabolite profiles in Aspergillus calidoustus and Aspergillus westerdijkiae through histone deacetylase (HDAC) inhibition by vorinostat. J. Antibiot. 2020, 73, 410–413. [Google Scholar] [CrossRef]
- Zutz, C.; Gacek, A.; Sulyok, M.; Wagner, M.; Strauss, J.; Rychli, K. Small Chemical Chromatin Effectors Alter Secondary Metabolite Production in Aspergillus clavatus. Toxins 2013, 5, 1723–1741. [Google Scholar] [CrossRef]
- Bok, J.W.; Chiang, Y.M.; Szewczyk, E.; Reyes-Dominguez, Y.; Davidson, A.D.; Sanchez, J.F.; Lo, H.C.; Watanabe, K.; Strauss, J.; Oakley, B.R.; et al. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 2009, 5, 462–464. [Google Scholar] [CrossRef]
- Pidroni, A.; Faber, B.; Brosch, G.; Bauer, I.; Graessle, S. A Class 1 Histone Deacetylase as Major Regulator of Secondary Metabolite Production in Aspergillus nidulans. Front. Microbiol. 2018, 9, 2212. [Google Scholar] [CrossRef]
- Henke, M.T.; Soukup, A.A.; Goering, A.W.; McClure, R.A.; Thomson, R.J.; Keller, N.P.; Kelleher, N.L. New Aspercryptins, Lipopeptide Natural Products, Revealed by HDAC Inhibition in Aspergillus nidulans. ACS Chem. Biol. 2016, 11, 2117–2123. [Google Scholar] [CrossRef]
- Albright, J.C.; Henke, M.T.; Soukup, A.A.; McClure, R.A.; Thomson, R.J.; Keller, N.P.; Kelleher, N.L. Large-Scale Metabolomics Reveals a Complex Response of Aspergillus nidulans to Epigenetic Perturbation. ACS Chem. Biol. 2015, 10, 1535–1541. [Google Scholar] [CrossRef]
- Henrikson, J.C.; Hoover, A.R.; Joyner, P.M.; Cichewicz, R.H. A chemical epigenetics approach for engineering the in situbiosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009, 7, 435–438. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Yu, D.; Chen, X.; Tabudravu, J.; Deng, H.; Pan, L. Deletion of the epigenetic regulator GcnE in Aspergillus niger FGSC A1279 activates the production of multiple polyketide metabolites. Microbiol. Res. 2018, 217, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, Z.; Tang, J.; Wu, J.; Tong, J.; Li, M.; Ju, J.; Chen, H.; Wang, L. Identification and Biological Evaluation of Secondary Metabolites from Marine Derived Fungi-Aspergillus sp. SCSIOW3, Cultivated in the Presence of Epigenetic Modifying Agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef] [PubMed]
- Triastuti, A.; Vansteelandt, M.; Barakat, F.; Trinel, M.; Jargeat, P.; Fabre, N.; Amasifuen Guerra, C.A.; Mejia, K.; Valentin, A.; Haddad, M. How Histone Deacetylase Inhibitors Alter the Secondary Metabolites of Botryosphaeria mamane, an Endophytic Fungus Isolated from Bixa orellana. Chem. Biodivers 2019, 16, e1800485. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-M.; Xu, W.; Li, D.; Yin, W.-B.; Chooi, Y.-H.; Li, Y.-Q.; Tang, Y.; Hu, Y. Epigenetic Genome Mining of an Endophytic Fungus Leads to the Pleiotropic Biosynthesis of Natural Products. Angew. Chem. Int. Ed. 2015, 54, 7592–7596. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Stalheim, K.J.; Proteau, P.J.; Loesgen, S. Unexpected Biotransformation of the HDAC Inhibitor Vorinostat Yields Aniline-Containing Fungal Metabolites. ACS Chem. Biol. 2017, 12, 1842–1847. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhou, L.; Chen, H.P.; Liu, J.K. Sesquiterpenes with diverse skeletons from histone deacetylase inhibitor modified cultures of the basidiomycete Cyathus stercoreus (Schwein.) de Toni HFG134. Phytochemistry 2022, 195, 113048. [Google Scholar] [CrossRef]
- Chettri, P.; Dupont, P.-Y.; Bradshaw, R.E. Chromatin-level regulation of the fragmented dothistromin gene cluster in the forest pathogen Dothistroma septosporum. Mol. Microbiol. 2018, 107, 508–522. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Studt, L.; von Bargen, K.W.; Kummer, W.; Humpf, H.-U.; Reuter, G.; Tudzynski, B. Sound of silence: The beauvericin cluster in Fusarium fujikuroi is controlled by cluster-specific and global regulators mediated by H3K27 modification. Environ. Microbiol. 2016, 18, 4282–4302. [Google Scholar] [CrossRef]
- Janevska, S.; Baumann, L.; Sieber, C.M.K.; Munsterkotter, M.; Ulrich, J.; Kamper, J.; Guldener, U.; Tudzynski, B. Elucidation of the Two H3K36me3 Histone Methyltransferases Set2 and Ash1 in Fusarium fujikuroi Unravels Their Different Chromosomal Targets and a Major Impact of Ash1 on Genome Stability. Genetics 2018, 208, 153–171. [Google Scholar] [CrossRef]
- Janevska, S.; Güldener, U.; Sulyok, M.; Tudzynski, B.; Studt, L. Set1 and Kdm5 are antagonists for H3K4 methylation and regulators of the major conidiation-specific transcription factor gene ABA1 in Fusarium fujikuroi. Environ. Microbiol. 2018, 20, 3343–3362. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Connolly, L.R.; Konkel, Z.M.; Neuhaus, G.F.; Chang, X.L.; Pierce, B.R.; Smith, K.M.; Freitag, M.; Loesgen, S. A metabolomics-guided approach to discover Fusarium graminearum metabolites after removal of a repressive histone modification. Fungal Genet. Biol. 2019, 132, 103256. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Ji, T.; Sun, X.; Huang, H.; Zhang, H.; Lu, X.; Wu, L.; Huo, R.; Wu, H.; Gao, X. Histone H3 lysine 9 methyltransferase FvDim5 regulates fungal development, pathogenicity and osmotic stress responses in Fusarium verticillioides. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Fan, A.; Mi, W.; Liu, Z.; Zeng, G.; Zhang, P.; Hu, Y.; Fang, W.; Yin, W.B. Deletion of a Histone Acetyltransferase Leads to the Pleiotropic Activation of Natural Products in Metarhizium robertsii. Org. Lett. 2017, 19, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Qadri, M.; Nalli, Y.; Jain, S.K.; Chaubey, A.; Ali, A.; Strobel, G.A.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. An Insight into the Secondary Metabolism of Muscodor yucatanensis: Small-Molecule Epigenetic Modifiers Induce Expression of Secondary Metabolism-Related Genes and Production of New Metabolites in the Endophyte. Microb. Ecol. 2017, 73, 954–965. [Google Scholar] [CrossRef]
- Ramesha, K.P.; Chandra Mohana, N.; Chandra Nayaka, S.; Satish, S. Epigenetic Modifiers Revamp Secondary Metabolite Production in Endophytic Nigrospora sphaerica. Front. Microbiol. 2021, 12, 730355. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic Modifiers Induce Bioactive Phenolic Metabolites in the Marine-Derived Fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Chavez, F.; Salo, O.; Samol, M.; Ries, M.; Kuipers, J.; Bovenberg, R.A.L.; Vreeken, R.J.; Driessen, A.J.M. Deregulation of secondary metabolism in a histone deacetylase mutant of Penicillium chrysogenum. MicrobiologyOpen 2018, 7, e00598. [Google Scholar] [CrossRef]
- Gubiani, J.R.; Wijeratne, E.M.K.; Shi, T.; Araujo, A.R.; Arnold, A.E.; Chapman, E.; Gunatilaka, A.A.L. An epigenetic modifier induces production of (10′S)-verruculide B, an inhibitor of protein tyrosine phosphatases by Phoma sp. nov. LG0217, a fungal endophyte of Parkinsonia microphylla. Bioorganic Med. Chem. 2017, 25, 1860–1866. [Google Scholar] [CrossRef]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the Coral-Derived Fungus Trichoderma harzianum (XS-20090075) Induced by Chemical Epigenetic Manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Jeon, J.; Kwon, S.; Lee, Y.-H. Histone acetylation in fungal pathogens of plants. Plant Pathol. J. 2014, 30, 1–9. [Google Scholar] [CrossRef]
- Gacek, A.; Strauss, J. The chromatin code of fungal secondary metabolite gene clusters. Appl. Microbiol. Biotechnol. 2012, 95, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Wu, L.; Sun, R.; Keller, N.P.; Yang, K.; Ye, L.; He, S.; Zhang, F.; Wang, S. The HosA Histone Deacetylase Regulates Aflatoxin Biosynthesis Through Direct Regulation of Aflatoxin Cluster Genes. Mol. Plant-Microbe Interact. 2019, 32, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone Deacetylase Activity Regulates Chemical Diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Price, R.J.; Weindling, E.; Berman, J.; Buscaino, A.; Pietro, A.D. Chromatin Profiling of the Repetitive and Nonrepetitive Genomes of the Human Fungal Pathogen Candida albicans. mBio 2019, 10, e01376-19. [Google Scholar] [CrossRef]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef]
- Freitag, M. Histone Methylation by SET Domain Proteins in Fungi. Annu. Rev. Microbiol. 2017, 71, 413–439. [Google Scholar] [CrossRef]
- Gacek-Matthews, A.; Berger, H.; Sasaki, T.; Wittstein, K.; Gruber, C.; Lewis, Z.A.; Strauss, J. KdmB, a Jumonji Histone H3 Demethylase, Regulates Genome-Wide H3K4 Trimethylation and Is Required for Normal Induction of Secondary Metabolism in Aspergillus nidulans. PLOS Genet. 2016, 12, e1006222. [Google Scholar] [CrossRef]
- Bachleitner, S.; Sørensen, J.L.; Gacek-Matthews, A.; Sulyok, M.; Studt, L.; Strauss, J. Evidence of a Demethylase-Independent Role for the H3K4-Specific Histone Demethylases in Aspergillus nidulans and Fusarium graminearum Secondary Metabolism. Front. Microbiol. 2019, 10, 1759. [Google Scholar] [CrossRef]
- Morgan, M.A.J.; Shilatifard, A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat. Genet. 2020, 52, 1271–1281. [Google Scholar] [CrossRef]
- Giles, S.S.; Soukup, A.A.; Lauer, C.; Shaaban, M.; Lin, A.; Oakley, B.R.; Wang, C.C.C.; Keller, N.P. Cryptic Aspergillus nidulans Antimicrobials. Appl. Environ. Microbiol. 2011, 77, 3669–3675. [Google Scholar] [CrossRef]
- Krogan, N.J.; Dover, J.; Khorrami, S.; Greenblatt, J.F.; Schneider, J.; Johnston, M.; Shilatifard, A. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 2002, 277, 10753–10755. [Google Scholar] [CrossRef]
- Palmer, J.M.; Bok, J.W.; Lee, S.; Dagenais, T.R.T.; Andes, D.R.; Kontoyiannis, D.P.; Keller, N.P. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 2013, 1, e4. [Google Scholar] [CrossRef]
- Studt, L.; Janevska, S.; Arndt, B.; Boedi, S.; Sulyok, M.; Humpf, H.-U.; Tudzynski, B.; Strauss, J. Lack of the COMPASS Component Ccl1 Reduces H3K4 Trimethylation Levels and Affects Transcription of Secondary Metabolite Genes in Two Plant–Pathogenic Fusarium Species. Front. Microbiol. 2017, 7, 2144. [Google Scholar] [CrossRef] [PubMed]
- Soukup, A.A.; Chiang, Y.-M.; Bok, J.W.; Reyes-Dominguez, Y.; Oakley, B.R.; Wang, C.C.C.; Strauss, J.; Keller, N.P. Overexpression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol. Microbiol. 2012, 86, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Arthur, A.E.; Hong, S.Y.; Chanda, A.; Linz, J.E. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol. Microbiol. 2007, 66, 713–726. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Kleigrewe, K.; Wiemann, P.; Studt, L.; Sieber, C.M.K.; Connolly, L.R.; Freitag, M.; Güldener, U.; Tudzynski, B.; Humpf, H.-U. Genetic Manipulation of the Fusarium fujikuroi Fusarin Gene Cluster Yields Insight into the Complex Regulation and Fusarin Biosynthetic Pathway. Chem. Biol. 2013, 20, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Schmidt, F.J.; Jahn, L.; Sieber, C.M.K.; Connolly, L.R.; Niehaus, E.-M.; Freitag, M.; Humpf, H.-U.; Tudzynski, B. Two Histone Deacetylases, FfHda1 and FfHda2, Are Important for Fusarium fujikuroi Secondary Metabolism and Virulence. Appl. Environ. Microbiol. 2013, 79, 7719–7734. [Google Scholar] [CrossRef]
- Kong, X.; van Diepeningen, A.D.; van der Lee, T.A.J.; Waalwijk, C.; Xu, J.; Xu, J.; Zhang, H.; Chen, W.; Feng, J. The Fusarium graminearum Histone Acetyltransferases Are Important for Morphogenesis, DON Biosynthesis, and Pathogenicity. Front. Microbiol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Rösler, S.M.; Kramer, K.; Finkemeier, I.; Humpf, H.-U.; Tudzynski, B. The SAGA complex in the rice pathogen Fusarium fujikuroi: Structure and functional characterization. Mol. Microbiol. 2016, 102, 951–974. [Google Scholar] [CrossRef]
- Fischer, J.; Müller, S.Y.; Netzker, T.; Jäger, N.; Gacek-Matthews, A.; Scherlach, K.; Stroe, M.C.; García-Altares, M.; Pezzini, F.; Schoeler, H.; et al. Chromatin mapping identifies BasR, a key regulator of bacteria-triggered production of fungal secondary metabolites. eLife 2018, 7, e40969. [Google Scholar] [CrossRef]
- Nützmann, H.-W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schümann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef] [PubMed]
- Atanasoff-Kardjalieff, A.K.; Studt, L. Secondary Metabolite Gene Regulation in Mycotoxigenic Fusarium Species: A Focus on Chromatin. Toxins 2022, 14, 96. [Google Scholar] [CrossRef]
- Sun, R.; Wen, M.; Wu, L.; Lan, H.; Yuan, J.; Wang, S. The Fungi-specific histone Acetyltransferase Rtt109 mediates morphogenesis, Aflatoxin synthesis and pathogenicity in Aspergillus flavus by acetylating H3K9. IMA Fungus 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.R.; Ameri, A.J.; Lu, Z.; Kamei, M.; Schmitz, R.J.; Lewis, Z.A. Chromatin accessibility profiling in Neurospora crassa reveals molecular features associated with accessible and inaccessible chromatin. BMC Genom. 2021, 22, 459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, Y.; Wang, Y.; Gao, D.; King, J.; Xu, Y.; Liang, F.S. Investigating crosstalk between H3K27 acetylation and H3K4 trimethylation in CRISPR/dCas-based epigenome editing and gene activation. Sci. Rep. 2021, 11, 15912. [Google Scholar] [CrossRef]
- Nai, Y.-S.; Huang, Y.-C.; Yen, M.-R.; Chen, P.-Y. Diversity of Fungal DNA Methyltransferases and Their Association with DNA Methylation Patterns. Front. Microbiol. 2021, 11, 616922. [Google Scholar] [CrossRef]
- Pillay, L.C.; Nekati, L.; Makhwitine, P.J.; Ndlovu, S.I. Epigenetic Activation of Silent Biosynthetic Gene Clusters in Endophytic Fungi using Small Molecular Modifiers. Front. Microbiol. 2022, 13, 815008. [Google Scholar] [CrossRef]
- Basenko, E.Y.; Sasaki, T.; Ji, L.; Prybol, C.J.; Burckhardt, R.M.; Schmitz, R.J.; Lewis, Z.A. Genome-wide redistribution of H3K27me3 is linked to genotoxic stress and defective growth. Proc. Natl. Acad. Sci. USA 2015, 112, E6339–E6348. [Google Scholar] [CrossRef]
- Freitag, M.; Hickey, P.C.; Khlafallah, T.K.; Read, N.D.; Selker, E.U. HP1 is essential for DNA methylation in neurospora. Mol. Cell 2004, 13, 427–434. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhao, Y.; Cheng, J.; Xie, J.; Fu, Y.; Jiang, D.; Chen, T. Histone H3 Lysine 9 Methyltransferase DIM5 Is Required for the Development and Virulence of Botrytis cinerea. Front. Microbiol. 2016, 7, 1289. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, J.-P.; Tsang, A. CRISPR_Cas systems for fungal research. Fungal Biol. Rev. 2020, 34, 189–201. [Google Scholar] [CrossRef]
- Jiang, C.; Lv, G.; Tu, Y.; Cheng, X.; Duan, Y.; Zeng, B.; He, B. Applications of CRISPR/Cas9 in the Synthesis of Secondary Metabolites in Filamentous Fungi. Front. Microbiol. 2021, 12, 638096. [Google Scholar] [CrossRef]
- Chavez, A.; Tuttle, M.; Pruitt, B.W.; Ewen-Campen, B.; Chari, R.; Ter-Ovanesyan, D.; Haque, S.J.; Cecchi, R.J.; Kowal, E.J.K.; Buchthal, J.; et al. Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563–567. [Google Scholar] [CrossRef]
- Wensing, L.; Sharma, J.; Uthayakumar, D.; Proteau, Y.; Chavez, A.; Shapiro, R.S. A CRISPR Interference Platform for Efficient Genetic Repression in Candida albicans. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Wensing, L.; Shapiro, R.S. Design and Generation of a CRISPR Interference System for Genetic Repression and Essential Gene Analysis in the Fungal Pathogen Candida albicans. Methods Mol. Biol. 2022, 2377, 69–88. [Google Scholar] [CrossRef]
- Schmidtmann, E.; Anton, T.; Rombaut, P.; Herzog, F.; Leonhardt, H. Determination of local chromatin composition by CasID. Nucleus 2016, 7, 476–484. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Nødvig, C.S.; Nielsen, J.B.; Kogle, M.E.; Mortensen, U.H. A CRISPR-Cas9 System for Genetic Engineering of Filamentous Fungi. PLoS ONE 2015, 10, e0133085. [Google Scholar] [CrossRef] [PubMed]
- Nødvig, C.S.; Hoof, J.B.; Kogle, M.E.; Jarczynska, Z.D.; Lehmbeck, J.; Klitgaard, D.K.; Mortensen, U.H. Efficient oligo nucleotide mediated CRISPR-Cas9 gene editing in Aspergilli. Fungal Genet. Biol. 2018, 115, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, R.; Buiting-Wiessenhaan, N.; Dalhuijsen, S.; Roubos, J.A. CRISPR/Cpf1 enables fast and simple genome editing of Saccharomyces cerevisiae. Yeast 2018, 35, 201–211. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; Bratovič, M.; Le Rhun, A.; Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 2017, 35, 31–34. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kancharla, N.; Javalkote, V.S.; Dasgupta, S.; Brutnell, T.P. CRISPR-Cas12a (Cpf1): A Versatile Tool in the Plant Genome Editing Tool Box for Agricultural Advancement. Front. Plant Sci. 2020, 11, 584151. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Song, R.; Zhai, Q.; Sun, L.; Huang, E.; Zhang, Y.; Zhu, Y.; Guo, Q.; Tian, Y.; Zhao, B.; Lu, H. CRISPR/Cas9 genome editing technology in filamentous fungi: Progress and perspective. Appl. Microbiol. Biotechnol. 2019, 103, 6919–6932. [Google Scholar] [CrossRef]
- Kim, H.K.; Song, M.; Lee, J.; Menon, A.V.; Jung, S.; Kang, Y.M.; Choi, J.W.; Woo, E.; Koh, H.C.; Nam, J.W.; et al. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat. Methods 2017, 14, 153–159. [Google Scholar] [CrossRef]
- Jacobsen, T.; Ttofali, F.; Liao, C.; Manchalu, S.; Gray, B.N.; Beisel, C.L. Characterization of Cas12a nucleases reveals diverse PAM profiles between closely-related orthologs. Nucleic Acids Res. 2020, 48, 5624–5638. [Google Scholar] [CrossRef]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Cofsky, J.C.; Karandur, D.; Huang, C.J.; Witte, I.P.; Kuriyan, J.; Doudna, J.A. CRISPR-Cas12a exploits R-loop asymmetry to form double-strand breaks. eLife 2020, 9, e55143. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Smith, J.D.; Suresh, S.; Schlecht, U.; Wu, M.; Wagih, O.; Peltz, G.; Davis, R.W.; Steinmetz, L.M.; Parts, L.; St. Onge, R.P. Quantitative CRISPR interference screens in yeast identify chemical-genetic interactions and new rules for guide RNA design. Genome Biol. 2016, 17, 45. [Google Scholar] [CrossRef]
- Malzahn, A.A.; Tang, X.; Lee, K.; Ren, Q.; Sretenovic, S.; Zhang, Y.; Chen, H.; Kang, M.; Bao, Y.; Zheng, X.; et al. Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wu, J.; Zhao, G.; Wang, J. CRISPR-Cas12a-Assisted Genome Editing in Amycolatopsis mediterranei. Front. Bioeng. Biotechnol. 2020, 8, 698. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Li, F.; Li, J.; Sun, W.; Tian, C. Upgrading of efficient and scalable CRISPR–Cas-mediated technology for genetic engineering in thermophilic fungus Myceliophthora thermophila. Biotechnol. Biofuels 2019, 12, 293. [Google Scholar] [CrossRef]
- Lightfoot, J.D.; Fuller, K.K. CRISPR/Cas9-Mediated Gene Replacement in the Fungal Keratitis Pathogen Fusarium solani var. petroliphilum. Microorganisms 2019, 7, 457. [Google Scholar] [CrossRef]
- Fuller, K.K.; Chen, S.; Loros, J.J.; Dunlap, J.C. Development of the CRISPR/Cas9 System for Targeted Gene Disruption in Aspergillus fumigatus. Eukaryot. Cell 2015, 14, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Pachlinger, R.; Mitterbauer, R.; Adam, G.; Strauss, J. Metabolically independent and accurately adjustable Aspergillus sp. expression system. Appl. Environ. Microbiol. 2005, 71, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Valiante, V.; Nødvig, C.S.; Mattern, D.J.; Slotkowski, R.A.; Mortensen, U.H.; Brakhage, A.A. Functional Reconstitution of a Fungal Natural Product Gene Cluster by Advanced Genome Editing. ACS Synth. Biol. 2017, 6, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cook, D.E. CRISPR-Cas12a ribonucleoprotein-mediated gene editing in the plant pathogenic fungus Magnaporthe oryzae. STAR Protoc. 2022, 3, 101072. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwe, T.M.; Arentshorst, M.; Ernst, T.; Alazi, E.; Punt, P.J.; Ram, A.F.J. Efficient marker free CRISPR/Cas9 genome editing for functional analysis of gene families in filamentous fungi. Fungal Biol. Biotechnol. 2019, 6, 13. [Google Scholar] [CrossRef]
- Schuster, M.; Kahmann, R. CRISPR-Cas9 genome editing approaches in filamentous fungi and oomycetes. Fungal Genet. Biol. 2019, 130, 43–53. [Google Scholar] [CrossRef]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iye, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J.; et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef]
- Hall, D.B.; Struhl, K. The VP16 Activation Domain Interacts with Multiple Transcriptional Components as Determined by Protein-Protein Cross-linking in Vivo. J. Biol. Chem. 2002, 277, 46043–46050. [Google Scholar] [CrossRef]
- Hinz, J.M.; Laughery, M.F.; Wyrick, J.J. Nucleosomes Inhibit Cas9 Endonuclease Activity in Vitro. Biochemistry 2015, 54, 7063–7066. [Google Scholar] [CrossRef]
- Thakore, P.I.; D’Ippolito, A.M.; Song, L.; Safi, A.; Shivakumar, N.K.; Kabadi, A.M.; Reddy, T.E.; Crawford, G.E.; Gersbach, C.A. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 2015, 12, 1143–1149. [Google Scholar] [CrossRef]
- Klann, T.S.; Black, J.B.; Chellappan, M.; Safi, A.; Song, L.; Hilton, I.B.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. CRISPR–Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat. Biotechnol. 2017, 35, 561–568. [Google Scholar] [CrossRef]
- Li, J.; Mahata, B.; Escobar, M.; Goell, J.; Wang, K.; Khemka, P.; Hilton, I.B. Programmable human histone phosphorylation and gene activation using a CRISPR/Cas9-based chromatin kinase. Nat. Commun. 2021, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Lickwar, C.R.; Mueller, F.; Hanlon, S.E.; McNally, J.G.; Lieb, J.D. Genome-wide protein–DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 2012, 484, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, S.; Chen, R.; Xie, A. Target binding and residence: A new determinant of DNA double-strand break repair pathway choice in CRISPR/Cas9 genome editing. J. Zhejiang Univ.—Sci. B 2021, 22, 73–86. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Zhang, T. Computational approaches for effective CRISPR guide RNA design and evaluation. Comput. Struct. Biotechnol. J. 2020, 18, 35–44. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, T.; Chen, C.H.; Li, W.; Meyer, C.A.; Wu, Q.; Wu, D.; Cong, L.; Zhang, F.; Liu, J.S.; et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015, 25, 1147–1157. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J.-S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef]
- Mendoza, B.J.; Trinh, C.T. Enhanced guide-RNA design and targeting analysis for precise CRISPR genome editing of single and consortia of industrially relevant and non-model organisms. Bioinformatics 2017, 34, 16–23. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Xiong, X.; Yan, B.; Xie, W.; Sheen, J.; Li, J.-F. A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants 2017, 3, 930–936. [Google Scholar] [CrossRef]
- Hwang, G.-H.; Song, B.; Bae, S. Current widely-used web-based tools for CRISPR nucleases, base editors, and prime editors. Gene Genome Ed. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, F.; Yan, W.; Dai, Z.; Dong, W.; Zhou, J.; Zhang, W.; Xin, F.; Jiang, M. Recent Advances of CRISPR/Cas9-Based Genetic Engineering and Transcriptional Regulation in Industrial Biology. Front. Bioeng. Biotechnol. 2020, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tu, L.-C.; Naseri, A.; Huisman, M.; Zhang, S.; Grunwald, D.; Pederson, T. CRISPR-Cas9 nuclear dynamics and target recognition in living cells. J. Cell Biol. 2016, 214, 529–537. [Google Scholar] [CrossRef]
- Palermo, G.; Miao, Y.; Walker, R.C.; Jinek, M.; McCammon, J.A. CRISPR-Cas9 conformational activation as elucidated from enhanced molecular simulations. Proc. Natl. Acad. Sci. USA 2017, 114, 7260–7265. [Google Scholar] [CrossRef] [PubMed]
- Mekler, V.; Minakhin, L.; Semenova, E.; Kuznedelov, K.; Severinov, K. Kinetics of the CRISPR-Cas9 effector complex assembly and the role of 3′-terminal segment of guide RNA. Nucleic Acids Res. 2016, 44, 2837–2845. [Google Scholar] [CrossRef]
- Raper, A.T.; Stephenson, A.A.; Suo, Z. Functional Insights Revealed by the Kinetic Mechanism of CRISPR/Cas9. J. Am. Chem. Soc. 2018, 140, 2971–2984. [Google Scholar] [CrossRef]
- Strohkendl, I.; Saifuddin, F.A.; Rybarski, J.R.; Finkelstein, I.J.; Russell, R. Kinetic Basis for DNA Target Specificity of CRISPR-Cas12a. Mol. Cell 2018, 71, 816–824.e3. [Google Scholar] [CrossRef]
- Sundaresan, R.; Parameshwaran, H.P.; Yogesha, S.D.; Keilbarth, M.W.; Rajan, R. RNA-Independent DNA Cleavage Activities of Cas9 and Cas12a. Cell Rep. 2017, 21, 3728–3739. [Google Scholar] [CrossRef]
- Swarts, D.C.; van der Oost, J.; Jinek, M. Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol. Cell 2017, 66, 221–233.e4. [Google Scholar] [CrossRef]
- Dong, D.; Ren, K.; Qiu, X.; Zheng, J.; Guo, M.; Guan, X.; Liu, H.; Li, N.; Zhang, B.; Yang, D.; et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 2016, 532, 522–526. [Google Scholar] [CrossRef]
- Mekler, V.; Kuznedelov, K.; Severinov, K. Quantification of the affinities of CRISPR-Cas9 nucleases for cognate protospacer adjacent motif (PAM) sequences. J. Biol. Chem. 2020, 295, 6509–6517. [Google Scholar] [CrossRef]
- Chung, C.H.; Allen, A.G.; Sullivan, N.T.; Atkins, A.; Nonnemacher, M.R.; Wigdahl, B.; Dampier, W. Computational Analysis Concerning the Impact of DNA Accessibility on CRISPR-Cas9 Cleavage Efficiency. Mol. Ther. 2020, 28, 19–28. [Google Scholar] [CrossRef]
- Daer, R.M.; Cutts, J.P.; Brafman, D.A.; Haynes, K.A. The Impact of Chromatin Dynamics on Cas9-Mediated Genome Editing in Human Cells. ACS Synth. Biol. 2017, 6, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Verkuijl, S.A.N.; Rots, M.G. The influence of eukaryotic chromatin state on CRISPR–Cas9 editing efficiencies. Curr. Opin. Biotechnol. 2019, 55, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.S.; Jiang, F.; Doudna, J.A.; Lim, W.A.; Narlikar, G.J.; Almeida, R. Nucleosome breathing and remodeling constrain CRISPR-Cas9 function. eLife 2016, 5, e13450. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef]
- Clarke, R.; Heler, R.; MacDougall, M.S.; Yeo, N.C.; Chavez, A.; Regan, M.; Hanakahi, L.; Church, G.M.; Marraffini, L.A.; Merrill, B.J. Enhanced Bacterial Immunity and Mammalian Genome Editing via RNA-Polymerase-Mediated Dislodging of Cas9 from Double-Strand DNA Breaks. Mol. Cell 2018, 71, 42–55.e8. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Hou, X.-M.; Guo, L.; Wang, F.; Bi, L.; Zhang, X.; Li, H.-H.; Wen, F.; Xi, X.-G.; et al. Dynamics of Staphylococcus aureus Cas9 in DNA target Association and Dissociation. EMBO Rep. 2020, 21, e50184. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, Y.H.; Jang, Y.; Yu, J.; Goo, J.; Lee, G.; Jeong, Y.K.; Lee, S.H.; Kim, I.-S.; Kim, J.-S.; et al. Direct observation of DNA target searching and cleavage by CRISPR-Cas12a. Nat. Commun. 2018, 9, 2777. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, F.; Zhang, S.; Jin, J.; Bi, L.; Lu, Y.; Li, M.; Xi, X.-G.; Huang, X.; Shen, B.; et al. The post-PAM interaction of RNA-guided spCas9 with DNA dictates its target binding and dissociation. Sci. Adv. 2019, 5, eaaw9807. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Künne, T.; Swarts, D.C.; Brouns, S.J. Planting the seed: Target recognition of short guide RNAs. Trends Microbiol. 2014, 22, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Scott, D.A.; Kriz, A.J.; Chiu, A.C.; Hsu, P.D.; Dadon, D.B.; Cheng, A.W.; Trevino, A.E.; Konermann, S.; Chen, S.; et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 2014, 32, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.W.; Li, Z.; Peterson, R.T.; Yeh, J.-R.J.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Topkar, V.V.; Zheng, Z.; Joung, J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 2015, 33, 1293–1298. [Google Scholar] [CrossRef]
- Tóth, E.; Varga, É.; Kulcsár, P.I.; Kocsis-Jutka, V.; Krausz, S.L.; Nyeste, A.; Welker, Z.; Huszár, K.; Ligeti, Z.; Tálas, A.; et al. Improved LbCas12a variants with altered PAM specificities further broaden the genome targeting range of Cas12a nucleases. Nucleic Acids Res. 2020, 48, 3722–3733. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Sousa, A.A.; Walton, R.T.; Tak, Y.E.; Hsu, J.Y.; Clement, K.; Welch, M.M.; Horng, J.E.; Malagon-Lopez, J.; Scarfò, I.; et al. Engineered CRISPR–Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 2019, 37, 276–282. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Zheng, Z.; Nguyen, N.T.; Liebers, M.; Topkar, V.V.; Thapar, V.; Wyvekens, N.; Khayter, C.; Iafrate, A.J.; Le, L.P.; et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015, 33, 187–197. [Google Scholar] [CrossRef]

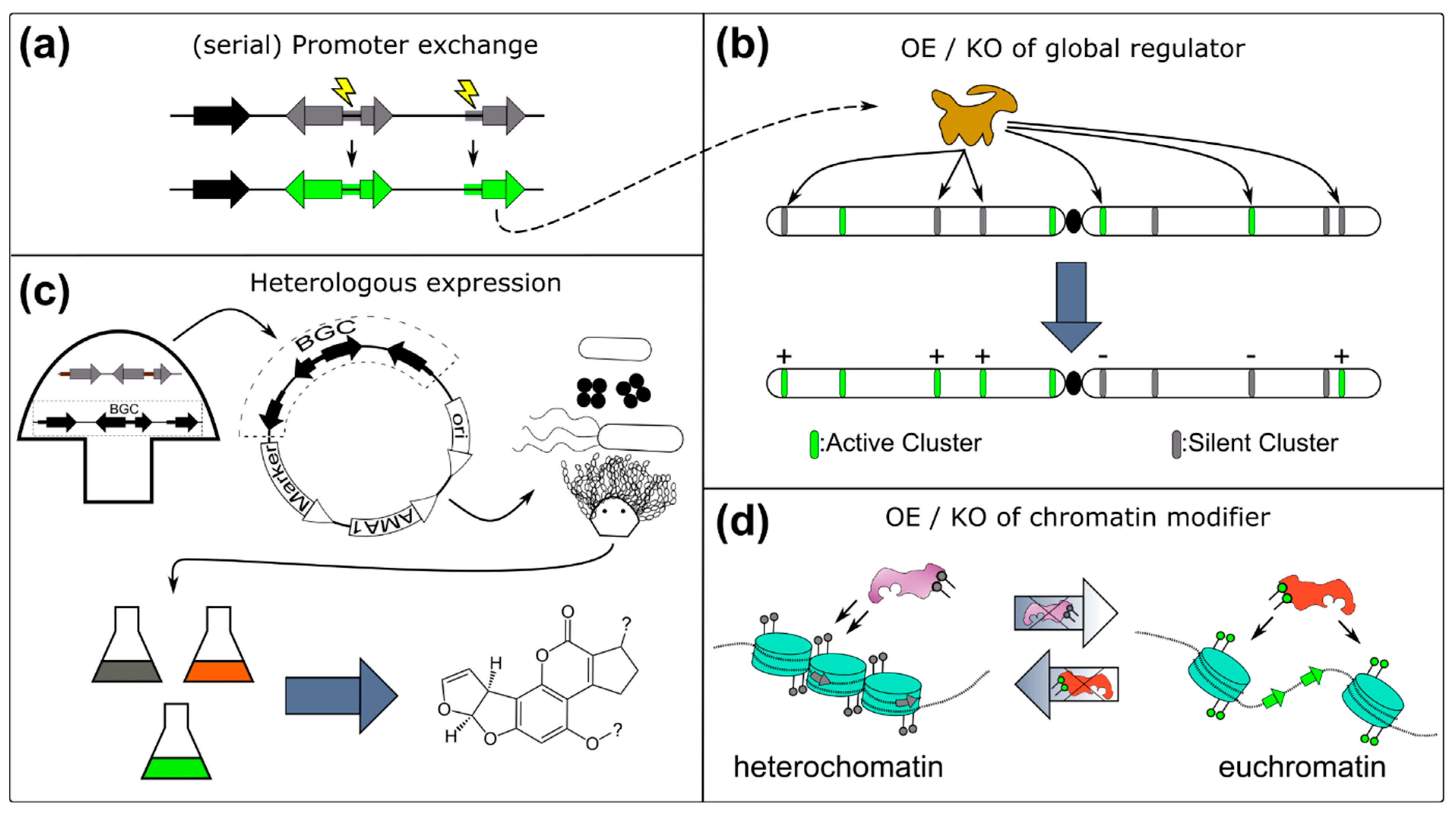
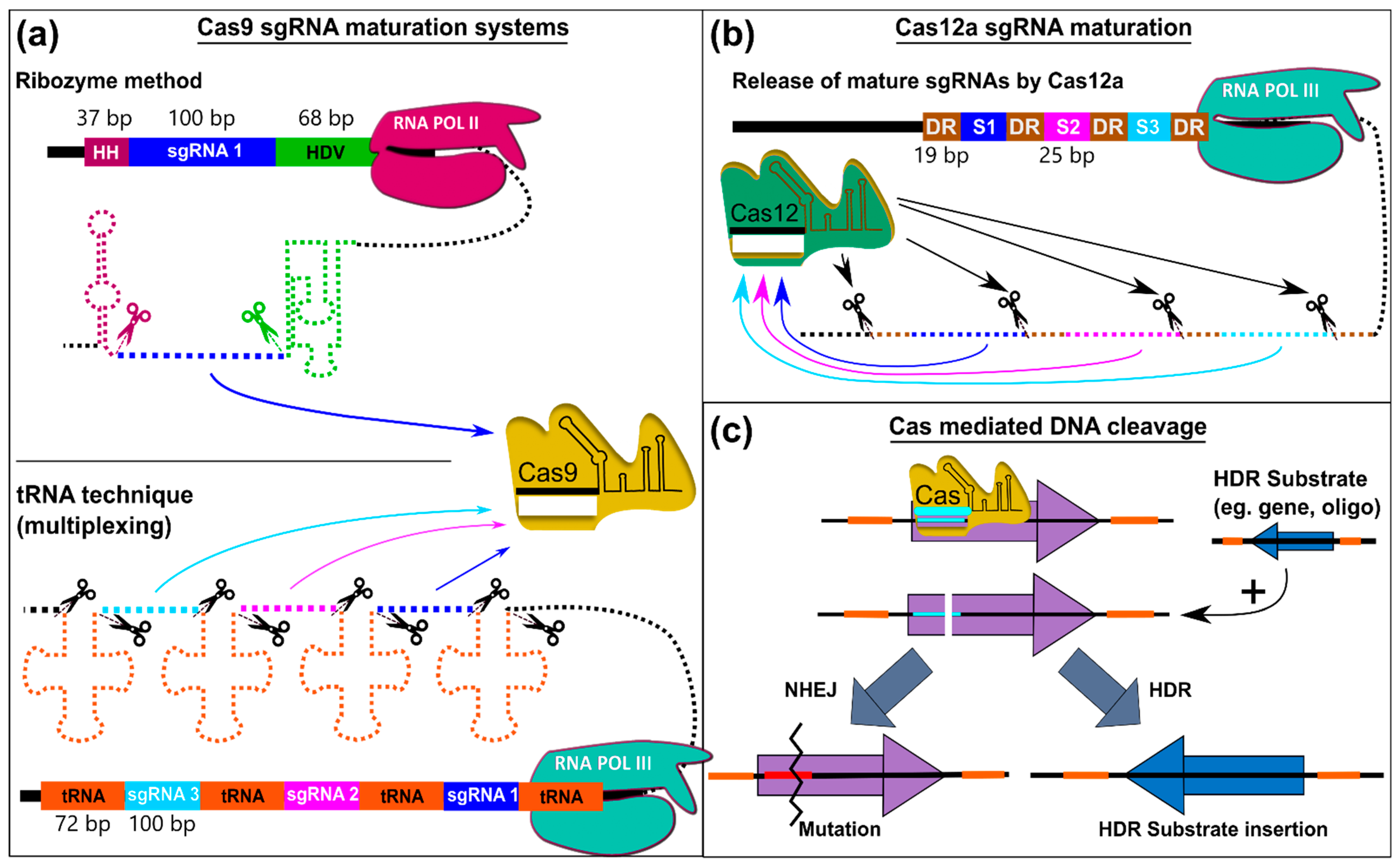
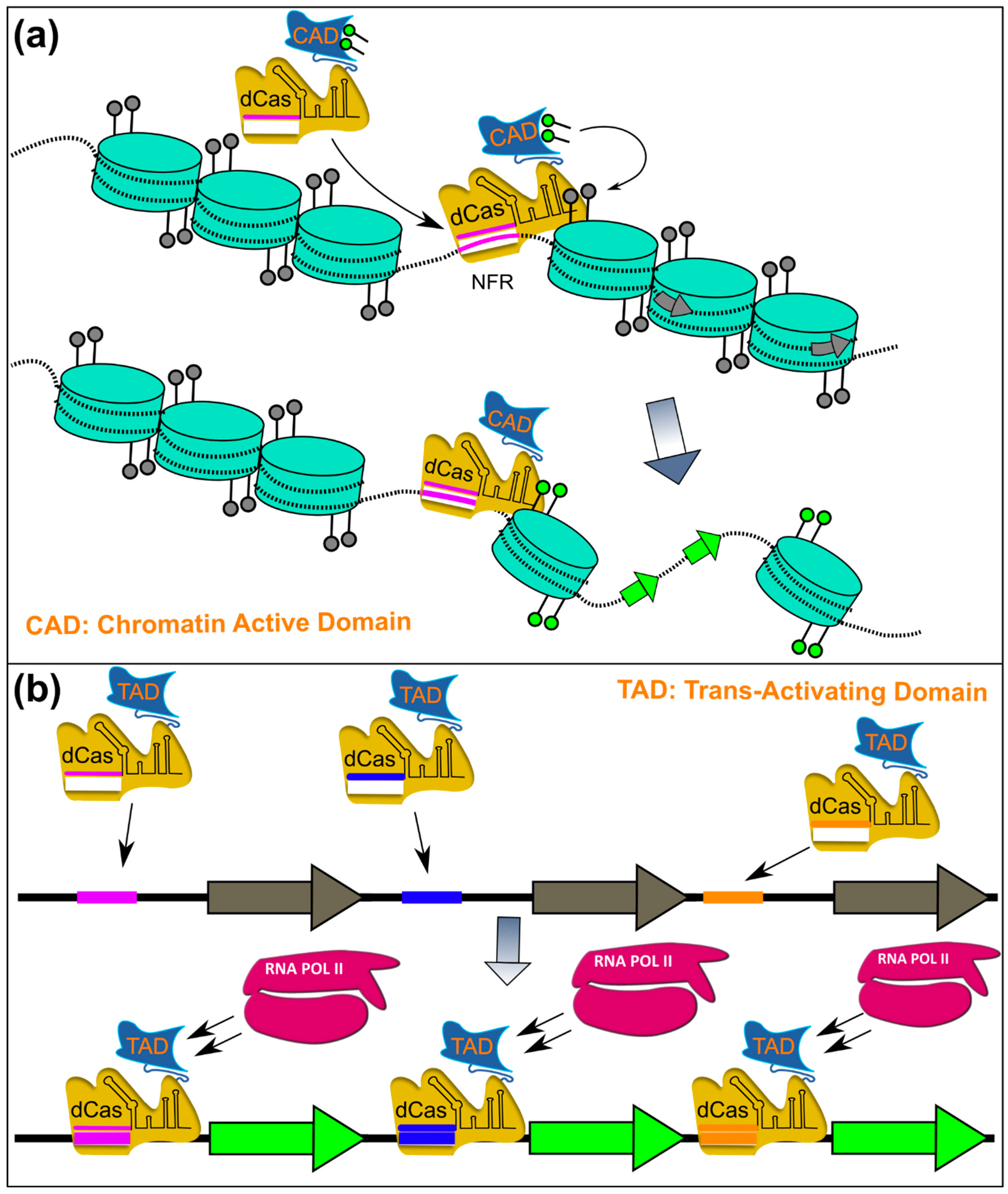
| Tools and Database Used for BGC Predictions | |||||
|---|---|---|---|---|---|
| Tool/ Database | Type | Purposes | Last Update | Link (Accessed on 15 March 2022) | Ref. |
| EMBL-EBI/ENA | DB | uncurated Database (large repository: DNA, RNA, genome annotations) | regular updates | ebi.ac.uk | [87,88] |
| GenBank | DB | uncurated Database (large repository: DNA, RNA, genome annotations) | every 2 month | ncbi.nlm.nih.gov/genbank | [89] |
| GNPS | DB | “Global Natural Products Social Molecular Networking” community-curated mass spectrometry database | 2022 | gnps.ucsd.edu | [90] |
| fungiSMASH | PT | Gold standard for BGC prediction. Allrounder with an extensive variety of integrated tools and algorithms, web-application or stand-alone; Rule-based and rule-independent algorithms. Versions for fungi, bacteria and plants available. Fungal version has additional BGC border detection based on TF-binding sites | 2021 | https://fungismash.secondarymetabolites.org/#!/start | [78] |
| NP-atlas 2.0 | DB | curated community database of microbially-derived SMs compounds from marine macro algae and diatoms are excluded | 2021 | npatlas.org/ | [91] |
| TIGRFAMs | DB | curated database of protein families (mainly prokaryotic) | 2021 | ncbi.nlm.nih.gov/genome/annotation_prok/tigrfams/ | [92] |
| PFAM v35 | DB | partly curated database protein domains and families | 2021 | pfam.xfam.org/ | [93] |
| FunOrder | PT | detection of essential genes within a BGC by a co-evolutionary approach | 2021 | github.com/gvignolle/FunOrder | [72] |
| ARTS 2.0 | PT | Antibiotics cluster prediction by detection of resistance genes; web application | 2020 | arts.ziemertlab.com | [83] |
| HMMER | PT | DNA based Homology search via pHMM; as source code or as web application | 2020 | hmmer.org/ | [94] |
| CO-OCCUR | PT | genome-wide co-occurence of BGC-related gene pairs; source code available | 2020 | github.com/egluckthaler/co-occur | [37] |
| TOUCAN | PT | BGC prediction via phylogeny, function, composition at amino acid level (k-mers, Pfam protein domains, and GO terms); source code (python/perl) available | 2020 | github.com/bioinfoUQAM/TOUCAN | [95] |
| antiSMASH Database v3 | DB | high-quality genomes and BGCs of bacteria, archaea, and fungi | 2020 | antismash-db.secondarymetabolites.org/ | [96] |
| NORINE | DB | manually curated DB for NRPS | 2020 | bioinfo.lifl.fr/norine/ | [97] |
| FRIGG | PT | Antibiotics BGC prediction based on presence of resistance gene; Pipeline that combines different tools; Python and R-based script available | 2019 | zenodo.org/record/2560245#.YjlMHepBw5s | [85] |
| BIG-SCAPE + CORASON | Output refinement | takes output of antismash and generates phylogenic BGC family networks; software application | 2019 | git.wageningenur.nl/medema-group/BiG-SCAPE | [98] |
| MiBIg 2 | DB | curated database (community effort and institute curation) of known BGCs and their products | 2019 | mibig.secondarymetabolites.org/ | [99] |
| IMG-ABC v5.0 | DB | predicted BGCs with environmental metadata (prediction via antiSMASH v5) | 2019 | img.jgi.doe.gov/abc-public | [100] |
| Deep-BGC | PT | Deep learning strategy to determine BGC product classes and chemical activity | 2019 | github.com/Merck/deepbgc | [101] |
| RiPPMiner | PT | RiPPs; web-application | 2017 | nii.ac.in/rippminer.html | [102] |
| SBSPKSv2 | PT | PKS/NRPS (structure/ substrate/ product) | 2017 | nii.ac.in/sbspks2.html | [103,104] |
| SEMPI 2 | PT | genome-based PKS I modular product prediction; web-application | 2017 | pharmaceutical-bioinformatics.de/SeMPI/ | [105] |
| CASSIS and SMIPS | PT | Promoter base- BGC prediction focused around SM-key enzymes, online or as application | 2016 | sbi.hki-jena.de/cassis | [106] |
| FunGene-ClusterS | PT | Transcriptome and genome-assisted BGC prediction; standalone and web application | 2016 | fungiminions.shinyapps.io/FunGeneClusterS | [107,108] |
| 2metDB/SecmetDB | PT | PKS/NRPS-prediction tool; software application | 2015 | sourceforge.net/projects/secmetdb/ | [109] |
| GNP | PT | Structure prediction and LC-MS data peak identification; web-application | 2015 | magarveylab.ca/gnp/ | [110] |
| Smiles2Mono-mers (s2m) | PT | predict monomers from polymeric structure; webserver and standalone | 2015 | bioinfo.lifl.fr/norine/smiles2monomers.jsp | [111] |
| ClusterFinder | PT | BGC detection via Pfam domain occurrence in genome and metagenomic data; software application and integrated into antiSMASH and IMG-ABC | 2014 | github.com/petercim/ClusterFinder | [82] |
| MIPS-CG | PT | Clusters of BGC-related Pfam-domains outside of syntenic blocks; web-application | 2014 | fung-metb.net/ (currently offline) | [47] |
| MIDDAS-M | PT | Motif-Independent transcriptome and genome-assisted BGC prediction | 2013 | 133.242.13.217/MIDDAS-M (currently offline) | [86] |
| ClusterMine-360 | DB | crowd-source, semi and auto-curated database of microbial NRPS and PKS | 2013 | clustermine360.ca/ (currently offline) | [112] |
| NRPS-predictor2 | PT | predict bacterial and fungal NRPS adenylation domain substrate specificity; web-application | 2011 | nrps.informatik.uni-tuebingen.de/ (currently offline) | [113] |
| SMURF | PT | Rule-based-backbone gene search via HMMs in genome data; web-application | 2010 | jcvi.org/smurf/ | [79] |
| CLUSEAN | PT | PKS/NRPS-prediction tool; software application | 2009 | bitbucket.org/tilmweber/clusean | [114] |
| BGC Activation by Natural Triggers and Manipulation of Their Global Regulators | |||
|---|---|---|---|
| Section 1: Aspergillus and Penicillium | |||
| Trigger | Organism | Observation | Ref. |
| ApyapA∆ | Aspergillus parasiticus | ApyapA∆: increased aflatoxin production | [173] |
| csnE∆ | Aspergillus nidulans | csnE∆: production of orsellinic acid-related metabolites: orcinol, diorcinol, cordyol C, violaceol I and violaceol II; activation of DHMBA cluster | [181,182] |
| mcRA∆ (TF) | Aspergillus nidulans Aspergillus terreus Penicillium canescens | mcRA∆: Upregulation of secondary metabolites in all three organisms A. nidulans dereplication strain: two new metabolites from the cichorine pathway, production of felinone A new compounds of other organisms not identified | [175] |
| sumO∆ | Aspergillus nidulans | sumO∆: production of Asperthecin, decrease of austinol/dehydroaustinol and sterigmatocystin | [183] |
| Addditives (supernatant extract) | Aspergillus nidulans Streptomyces rapamycinicus | a novel a guanidine containing macrolide named polaramycin B, produced by S. rapamycinicus, is responsible for the derepression of the ors- cluster and its derivates in A. nidulans | [184] |
| Additives | Penicillium citrinum | Cultivation in presence of rare earth metals (i.e., scandium chloride) and isolation of three new peptide derivatives | [185] |
| C-source ∆/OE of creA | Aspergillus flavus | creA∆ (Carbon catabolite repressor) Growth defects, impaired conidia production, increased amount of sclerotia, near abolishment of aflatoxin production, impaired virulence OE of creA: similar to wild type | [122] |
| C-source | Aspergillus nidulans | Carbon source dependent BGC expression | [186] |
| C-source laeA∆ | Penicillium expansum | sucrose decreases both patulin and laeA levels and increases creA expression LaeA regulates patulin BGC, laeA∆ results in less virulence | [144] |
| Cultivation (chemostat) N starvation | Aspergillus nidulans | Production of antiproliferative compounds sanghaspirodins A and B Convergence of two BGCs pathways (anthraquinone and orsellinic acid-derived) | [128] |
| Light Temperature veA∆ laeA∆ | Aspergillus fumigatus | Connection between temperature and light regulation of 11 BGCs Involvement of VeA at 37 °C and LaeA at 30 °C and 37 °C in BGC regulation | [157] |
| Light velB∆ veA∆ | Aspergillus nidulans | velvet complex VelB/VeA/LaeA regulates developmental regulation and secondary metabolism in a light-dependent manner. velB∆ or veA∆: defects in sexual fruiting-body formation and secondary metabolite production | [138] |
| Light laeA∆, veA∆, velB∆ | Aspergillus ochraceus | laeA∆/veA∆/velB∆: differential regulation of 66% of all BGC backbone genes (majority downregulated) drastic reduction of Ochratoxin A production | [136] |
| Light Velvet: ∆Apc.LaeA∆ Apc.VeA∆ | Aspergillus pachycristatus | Apc.LaeA∆ or Apc.VeA∆ reduce production of echinocandin B and sterigmatocystin Impact of deletions on aerial hyphae, pigmentation, development of conidiophores, conidial germination rate, and ascospore maturation | [134] |
| Media Mimicking of plant and rhizosphere environment Nitrogen, Metals, Amino acids | Aspergillus terreus | (independent) signals for terrain production: methionine, nitrogen limitation, iron starvation (=host rhizosphere) Global regulators AreA and AtfA essential for terrain production | [119] |
| Media composition Carbon source Amino acids CreA | Aspergillus terreus | discovery of isoflavipucine and dihydroisoflavipucine production only in the presence of certain amino acids, alkaline pH, and strictly repressed in the presence of glucose (CreA). | [167] |
| Media C-source | Penicillium citrinum | Increased production of citrinin on glucose compared to sucrose | [187] |
| Media HDACi DNMTi | Aspergillus awamori | Media: MEA, rice, nutrient, triptone soya agar HDACi: valproic acid, nicotinamide, trichostatin A DNMTi: 5-azacytidine Different metabolic profiles between media and epigenetic modifiers Nicotinamide was found to be the best epigenetic inducer Rice grains were the best medium for SM induction | [188] |
| Metals and Trace elements | Aspergillus fumigatus | Xanthocillin BGC-derived isocyanides help accumulate copper and exhibit antimicrobial activity | [166] |
| Metals and Trace elements | Aspergillus fumigatus | HapX and SreA, which oppositely regulate iron homeostasis also control an iron-dependent secondary metabolite network comprising the virulence factor hexadehydroastechrome (tryptophan-derived iron (III)-complex) which causes an iron starvation phenotype upon overexpression | [165] |
| Metals and Trace elements Oxidative stress sidC∆ | Aspergillus nidulans | NRPS SidC involved in iron regulation (excess and starvation) Upregulated by oxidative stress sidC∆: bad iron utilization (reduced growth under iron-starvation and higher iron demand), delayed germination under iron-depleted conditions, higher sensitivity of conidia to oxidative stress, no cleistothecia formation | [169] |
| Metals and Trace elements | Penicillium brasilianum | CuSO4 and MnSO4 alter the SM-profile production of a series of cyclodepsipeptides repression of verruculogen biosynthesis | [163] |
| Metals and Trace elements | Penicillium urticae | eight metal ions tested manganese dependent conversion of 6-methylsylicylic acid to patulin | [164] |
| OE laeA | Aspergillus nidulans | overexpression of laeA: activation of the Terrequinone (tdi) cluster and production of terrequinone A | [189] |
| Osmotic & Saline stress | Aspergillus aculeatus Three ex-type strains from three different collections | Osmotic (glycerol) and saline stress had different impacts on SM profile of fungal strains. Also strain-source specific chemotypes. Same morphology across all treatments | [159] |
| Oxidative stress Carbon source | Aspergillus flavus | Strains that produce less aflatoxin are more prone to oxidative stress and have more differentially regulated genes (also SM genes) under oxidative stress conditions carbon source affects BGC expression and production | [190] |
| Oxidative stress ApyapA∆ | Aspergillus ochraceus | ApyapA∆ shows missing redox balance which triggers aflatoxin synthesis and an involvement of oxidative stress in ochratoxin A regulation | [174] |
| Oxidative stress veA∆ | Aspergillus parasiticus | transcription factor AtfB regulates conidial tolerance towards oxidative stress AtfB binds to promoters with CRE sites in the aflatoxin cluster under inducing conditions. veA∆ abolishes these interactions | [171] |
| pH | A. nidulans A. parasiticus | Sterigmatocystin/aflatoxin expressed at higher levels at acidic pH than on neutral or alkalic pH Constitutive active PacC in A. nidulans reduced sterigmatocystin production | [150] |
| pH Acidic stress | Acid-Tolerant Fungi Penicillium Aspergillus Talaromyces Cladosporium Allophoma Alternaria Trichoderma | Supernatant extracts: ¾ shows cytotoxic activity, <¼ show antimicrobial or anti-H1N1 activity pH-related chemical diversity of P. oxalicum | [149] |
| pH AcpacC∆ | Aspergillus carbonarius | PacC involved in pathogenicity and Ochratoxin A production AcpacC∆: impaired fungal growth at neutral/alkaline pH, diminished gluconic and citric acid production, reduced virulence toward grapes and nectarine fruits | [148] |
| pH Light Temperature | Aspergillus flavus | Aflatoxin production: 5x increase of in darkness pH 4 media produces 30 times more than pH 7.4 strongly reduced production at 18 or 40 °C | [191] |
| pH pacC | Aspergillus nidulans | Penicillin highest at alkaline pH and in mutated pacC (mimics alkaline conditions) Production was lowest at acid pH and in strains that mimic acid pH | [151] |
| Solid-state vs. submerged fermentation Media | Aspergillus oryzae | Solid-state: Increase of coumarins and oxylipins, higher antimicrobial activities submerged: Terpenoids abundant | [178] |
| Solid-state vs. submerged fermentation | Penicillium expansum | Submerged fermentation: strongly increased production of polyketide metabolites (agonodepside B, rotiorin, verrucosidin, and ochrephilone) Solid-state fermentation: exclusive production of meroterpenoid compounds (andrastin A and C) | [177] |
| Temperature | Aspergillus fumigatus | Temperature affects stress tolerance, pigmentation, and trypacidin accumulation in conidia | [192] |
| Temperature | Aspergillus fumigatus | Endocrocin biosynthesis in spores is temperature-dependent and endocrine is connected to pathogenicity | [193] |
| Temperature | Penicillium oxalicum P. citrinum | Unique metabolite profile under temperature stress | [194] |
| Temperature Oxidative stress Culture conditions | Aspergillus flavus | Float culture method Stress conditions: aflatoxin is downregulated under stress secondary metabolites with antioxidant properties (e.g., kojic acid and imizoquins) were upregulated under stress | [118] |
| Velvet: ΔlaeA laeA-OE | Aspergillus spp. | laeA∆: abolishment of sterigmatocystin, penicillin, and lovastatin laeA-OE: increased penicillin and lovastatin production laeA expression is negatively regulated by AflR | [142] |
| mpkA∆ (kinase knockout library) | Aspergillus nidulans | mpkA∆: activation of the pkf-cluster and production of aspernidine A | [195] |
| Section 2: Fusarium | |||
| Trigger | Organism | Observation | Ref. |
| ∆/OE lae1 | Fusarium fujikuroi | Δlae1: downregulation of unknown NRPS4 cluster OE-lae1 induced beauvericin cluster and STC7-cluster (product not identified) generally LaeA has a substantial impact on many SMs | [147] |
| ∆areA, ∆areB | Fusarium fujikuroi | deletions have an impact on >50% of BGCs ∆areB: upregulation of cryptic clusters PKS09, NRPS04, NRPS21 | [127] |
| ∆areA, areB | Fusarium fujikuroi | AreA and AreB are required for gibberellic acid production | [196] |
| ∆pacC | Fusarium fujikuroi | pacC deletion de-represses bikaverin cluster-complementation with active pacC variant represses bikaverin cluster | [153] |
| Global regulator of development Δcsm1 (NsdD) | Fusarium fujikuroi | Δcsm1: elevated conidiation in wt and also in non-sporulating ΔveA strain. Induction of BGC for bikaverin and fusarubins Impact on 19 of 47 BGCs | [176] |
| Light N-source ∆veA, ∆laeA | Fusarium oxysporum | velvet complex and nitrate metabolism intertwined ∆veA or ∆laeA: growth impaired on nitrate/nitrite AREA involved in chromatin accessibility of two velvet-regulated BGCs (beauvericin and siderophore ferricrocin) | [131] |
| Light N- source pH ∆areA, ∆areB ∆vel1, ∆lae1 ∆pacC | Fusarium fujikuroi | ∆areA: reduces fusaric acid production ∆areB: abolished fusaric acid production ∆vel1 and ∆lae1: significant reduction of fusaric acid ∆pacC: abolishment of fusaric acid cluster expression | [197] |
| Light ∆fgwc-1 ∆fgwc-2 | Fusarium graminearum | ∆fgwc-1/∆fgwc-2: impaired carotenogenesis, photoreactivation and maturity of perithecia mutants produced more aurofusarin and trichothecenes independent from light and have derepressed conidiation during constant light | [198] |
| Light ∆vel1, ∆vel2, ∆lae1 | Fusarium fujikuroi | ∆ffvel1 and ∆fflae1: velvet positively regulates gibberellic acid, fumonisins and fusarin C, velvet negatively regulates bikaverin | [141] |
| N-source pH Host infection | Fusarium fujikuroi F. circinatum F. mangiferae F. oxysporum | PKS19 cluster is induced on rice but not maize Gibberelic acid cluster only expressed in F. fujikuroi Different conditions for different metabolites necessary: fusarins: high nitrogen, bikaverin and fusarubins: acidic low nitrogen or alkaline low nitrogen, Fumonisin B1: acidic low nitrogen | [125] |
| N-source ∆areA | Fusarium graminearum | asparagine was found to be a preferential nitrogen source. ∆areA led to poor growth on NaNO3 Utilization of aspartic acid, histidine, isoleucine, leucine, threonine, tyrosine, and valine as nitrogen sources was shown to depend of a functional AREA. AREA is required for the production of deoxynivalenol (DON), zearalenone, and fusarielin H regardless of the medium | [129] |
| N-source ∆areA | Gibberella fujikuroi | ∆areA: significantly reduced gibberellin production | [199], [200] |
| N-source/starvation ∆ of N-pathway regulators | Fusarium Fujikuroi | Effect of N-source (glutamine, nitrate) and starvation in different mutants ΔniaD, ΔnrtA, ΔnirA and ΔareA expression of backbone genes of gibberellic acid and bikaverin BGC after 72 h in starvation in wt and all mutants except for gibberellic acid in ΔareA | [124] |
| pH PAC1 | Fusarium graminearum | Pac1 negatively regulates trichothecene BGC; induction under acidic pH Δfgpac1: reduced development under neutral and alkaline pH, increased sensitivity to H2O2 and early onset of Tri expression and product synthesis | [155] |
| Temperature | Fusarium langsethiae F. sporotrichioides | Impact of temperature and time on spore amount and T-2, HT-2 toxin production | [201] |
| Section 3: Other Species | |||
| Trigger | Organism | Observation | Ref. |
| Additives | Colletotrichum gloeosporioides | Triggering silent BGCs by natural dietry components: Grape skin and turmeric extracts resulted in 20 and 14 additional compounds | [202] |
| C-source | Spicaria elegans | starch vs. glucose novel spicochalasin A and five new aspochalasins (M–Q) | [203] |
| Co-cultivation Additives | Isaria felina | potassium bromide and cocultivation with Aspergillus sulphureus: discovery of Isariketide B and Oxirapentyn L | [204] |
| Cultivation optimization for SM production | Phomopsis sp. Hant25 | Optimization of different media and cultivation parameters for overproduction of mycoepoxydiene Parameters: combination of stationary and agitated, solid support (cellulose paper disc), different medias, cultivation time | [179] |
| Light ∆lreA (=WC-1) | Alternaria alternata | Opposite light regulation of mycotoxin and spore production spores decreased under light and production of alternariol increased two to three times altertoxin production was strictly dependent on light. ∆ white-collar 1 (WC-1) gene (lreA): derepression of spore formation in dark and in light. altertoxin formation strongly induced in the dark alternariol formation partially dependent on LreA still able to partially respond to blue light | [205] |
| Light Velvet ∆velB ∆veA ∆laeA | Cuetootiopsis microspora | Velvet Complex involved in the development, stress tolerance, and secondary metabolism ∆velB: hypersensitive to osmotic stress and congo red pestalotiollide B production requires velB and laeA. veA inhibits pestalotiollide B biosynthesis | [132] |
| Light Velvet Iron starvation | Neurospora crassa | Velvet controls development, secondary metabolism, and light-dependent carotenoid production Heterotrimeric velvet complex (VE-1/VE-2/LAE-1) suppresses the production of the siderophore coprogen under iron starvation | [133] |
| Light C-source ∆CRE1 | Trichoderma reesei | Intertwined regulation of light and carbon pathways CRE1- regulated cluster is responsible for light-dependent production of dihydrotrichotetronin Biosynthesis of the antibiotic paracelsin was influenced | [135] |
| Light ∆SUB1 | Trichoderma reesei | SUB1 is light-regulated SUB1 affects growth and is required for female fertility SM is regulated by SUB1 in a light- and nutrient-dependent manner | [206] |
| Media Salts Cultivation type | Nigrospora sp. MA75 | Discovery of 2,3-didehydro-19a-hydroxy-14-epicochlioquinone B, 6-O-desmethyldechlorogriseofulvin 6′-hydroxygriseofulvin Media: NaCl vs. NaI, static/solid vs. agitated/liquid, rice-based vs. yeast extract | [207] |
| Media HDACi | Drechslera sp., | HDACi: SAHA, valproate, octanoylhydroxamic acid 2 new metabolites: chromanone and prenylhydroxybenzoic acid different metabolome between MEA and minimal media biotransformation of the inhibitors occurred | [208] |
| Media HDACi DNMTi | Cladosporium resinae | Media. Czapek-DOX, YES, PDA, starch, liquid vs. solid HDACi: SAHA DNMTi: 5-AZA Czapek yeast extract broth: more total secondary metabolites DNMTi and HDACi: expressions of silent genes | [209] |
| N-source ∆AcareA | Acremonium chrysogenum | AcareA is required for nitrogen metabolism and cephalosporin production | [130] |
| OE-laeA | Chaetomium globosum | overexpression of laeA: activation of the chaetoglobosin gene cluster and production of chaetoglobosin Z | [145] |
| pH ΔpacC pacCc | Trichoderma virens | ΔpacC: decreased competitiveness against Rhizoctonia solani and Sclerotium rolfsii Constitutive PacC: wt like overgrowth of R. solani | [152] |
| Plant Hormones | Arthrinium sacchari | discovery of the polyketides kinetin, 6-benzylaminopurine, and forchlorfenuron | [210] |
| Salinity | 47 marine fungi isolates | NaCl and KCl had growth-promoting effect on most marine fungi and 15% of isolates showed increased antifungal activity against Candida albicans, NaCl had impact on the metabolite profile | [160] |
| Salinity | Spicaria elegans | Different secondary metabolites on different saline conditions (3% and 10%) Four metabolites, only at 10% salinity: (2E,2’Z)-3,3’-(6,6’-dihydroxybiphenyl-3,3’-diyl) (new compound), diacrylic acid, aspulvinone E, aspochalasin E and trichodermamide B | [162] |
| Solid vs. liquid fermentation Solid growth support | Cylindrocarpon sp. Acremonium sp. Penicillium sp. | Addition of inert absorbent polymeric attachment supports in solid state and submerged fermentation leading to distinct metabolite profiles Impact on culture morphology and relative metabolite yields Solid agar with moist polyester-cellulose paper: Cylindrocarpon sp. (pyrrocidines A and B), Acremonium sp. (acremonidins A–E) Agitated submerged cultures: Complex HPLC metabolome, decrease in target compounds Liquid fermentation with various inert growth supports (polypropylene, polypropylene cellulose, polyester-cellulose, or polyurethane): production of rugulosin, skyrin, flavomannin, and the new compound WF159-A (bisanthracene) | [180] |
| Temperature | 40 polar fungal isolates | 45% of fungal strains showed antimicrobial activity Different fungal metabolite profiles dependent on temperature (4, 10, 15, 28 °C) | [158] |
| ΔvmlaeA | Valsa mali | ΔvmLaeA : reduced virulence and differential regulated secondary metabolism profile (31/60). | [143] |
| BGC Activation by Co-Cultivation | ||||
|---|---|---|---|---|
| Setup | Method | Organism | Observation | Ref. |
| 1 | Co-cultivation of two developmental stages (morphs) of a marine alga-derived fungus Pre-grow both morphs on agar plates (2–3 weeks) → small liquid pre-cultures → combine mono-cultures after 14 days → cultivate for 15–20 days | Aspergillus alliaceus and its teleomorph Petromyces alliaceus | Production of the cytotoxic chlorinated bianthrone allianthrone A (only liquid) Morphs develop new phenotype in co-culture under liquid but not solid fermentation Restreaks after co-culture keep phenotype and can produce bianthrones on liquid and solid media | [218] |
| 1 | Submerse co-culture and membrane-separated culture 5 mL of pre-culture (A. flavipes and Streptomyces sp. in a ratio of 1:4 (v/v)) were added to 200 mL medium 8 days of cultivation | Aspergillus flavipes marine actinomycete Streptomyces sp. | Production of five aspochalasins and rosellichalasin by fungus with cytotoxic effects against Streptomyces. Physical interaction necessary | [219] |
| 1 | 3 media (ISP2, GYE, F) inoculated with 3d old pre-cultures. 4L culture, fungal preculture was incubated 2 d earlier, 6 h before harvest 50 g/L Diaion HP-20 resin was added | Aspergillus fumigatus MR2012 (marine) Streptomyces leeuwenhoekii (C34 and C58) (desert) | Fungus: production of luteoride D (luteoride derivative), pseurotin G (pseurotin derivative), terezine D, 11-O-methylpseurotin A Bacteria C34: Chaxapeptin C58: Chaxapeptin doubled, pentalenic acid | [220] |
| 1 | 16 h old mycelia of A. fumigatus were washed and placed in fresh AMM with 1/20 vol of the streptomyces culture or with 1/20 filtered supernatant. Harvest after 12 h | Aspergillus fumigatus Streptomyces rapamycinicus | induction of fungal PKS FgnA, the discovery of bacteria-specific germination inhibitor fumigermin | [221] |
| 1 | 2 day 30 °C liquid agitated co-culture (93 mL London + 32 mL GYM medium). simultaneous inoculation of both MOs (equal amounts of spores) | Aspergillus nidulans Streptomyces mobaraensis | bacterial glycopeptide (antibiotic) triggers antibacterial and iron-chelating tropolonesanhydrosepedonin and antibiotic C (A. nidulans), polyketide tripyrnidone (novel structure) | [222] |
| 1 | Solid rice medium | Aspergillus versicolor Bacillus subtilis | two new tetralones; aspvanicin A and aspvanicin B several other cryptic SMs; cytotoxic activity against mouse lymphoma cells | [223] |
| 1 | Confrontation assay on ISP2 agar MALDI-TOF straight from agar Inoculation for 48 h at 30 °C | Aspergillus fumigatus Aspergillus niger Bacillus amyloliquefaciens (GA40) | The coral-derived bacteria GA40 produced an antifungal iturin | [224] |
| 1 | Precultures on rice media 1 week then co-culture for 2 weeks. Axenic cultivation with potassium bromide | Aspergillus sulphureus KMM 4640 Isaria feline | discovery of Isariketide B (rice media with KBr) and Oxirapentyn L (Co-cultivation) | [204] |
| 1 | Fungal bacterial stepwise liquid co-culture. Addition of bacterial broth (1/1000) to fungal culture on day 3. Further cultivation for 2 days | Emericella sp. (CNL-878; marine fungus) Salinispora arenicola (CNH-665; ascomycete) | Emericellamides A and B synthesis by Emericella sp. | [225] |
| 1 | Solid agar confrontation assay (5 days) Submerged fermentation (2 mL of each pre-culture and cultivation for 5 days in broth) Co-cultivation of MF028 with five other strains time-course | Chaunopycnis sp. (CMB-MF028) Trichoderma hamatum (CMB-MF030) Co-isolated from mollusc Siphonaria sp. | Chaunopyran A production Cultivation on sterilized mycelia showed no induction | [226] |
| 1 | Extraction of confrontation zone (Agar) Incubation of several weeks Dereplication via monocultures Method development: ~600 cultures by confrontation assay on agar UHPLC–TOF-MS fingerprints of mono and cocultures have been prepared to filter out new peaks | Fusarium isolates (clinical, soil or plant-derived) Aspergillus clavatus, Trichophyton rubrum, Acremonium, Cladosporium sp. Hohenbuehelia reniformis Bionectria ochroleuca Eutypa lata | Chemical novel MS spectra have been recorded Results indicate that a large portion of fungi produce new metabolites in co-culture | [213] |
| 1 | Solid state confrontation cultivation 17 basidiomycetes, 136 co-cultures Molecular network analysis Liquid co-cultivation for 5–30 days | Ganoderma applanatum Trametes versicolor | discovery of novel xylosides 3-phenyllactic acid and orsellinic acid in G. applanatum | [227] |
| 1 | Addition of bacterial broth (1/1000) to fungal culture on day 3. Harvest after 24 h. Screen of 50 fungal strains | Libertella sp. (CNL-523) α-proteobacterium (CNJ-328) | Discovery of Libertellenones A–D, produced by the addition of marine α-proteobacterium to a 3-day Libertella sp. culture. | [228] |
| 1 | Submerged fermentation Different pre-grow times before co-cultivation. Optimal: addition of fungal culture to 2-day bacterial culture and further incubation for 7 days. Other setups showed metabolome of either bacterial or fungal axenic culture | Penicillium sp. (WC-29-5) Streptomyces fradiae (007) | Production of five phenolic polyketides | [229] |
| 1 | Solid rice media, Cultivation parameters Optimal: inoculation of Penicillium sp mycelium plug. and seed medium of Bacillus sp. simultaneously at the center of petri dish 15d culture | Penicillium sp. (DT-F29) Bacillus sp. (B31) | Ten bioactive 2,5-diketopiperazines by Penicillium sp. | [230] |
| 1 | Co-cultivation on solid rice medium | Penicillium strains (IO1 and IO2) isolated from sponge | Accumulation of norlichexanthone and monocerin, biotransformation of the antifungal pyridoxatin to methyl-pyridoxatin | [231] |
| 1 | Liquid co-culture with seawater-based marine nutrient medium. After 24 h, bacterial culture is added to fungal cultivation following further 6d cultivation | Pestalotia (marine fungus) CNJ-328 (marine bacterium) | Discovery of pestaolne (antibiotic) | [216] |
| 1 | Submerged fermentation and sea water agar, inoculation of flasks with spores of both fungi, cultivation for 9 days | Talaromyces aculeatus Penicillium variabile | Four new polyketides (penitalarins A/ B/C and nafuredin B) cytotoxicity against some human cancer cell lines | [232] |
| 2 | Mycelia from A nidulans o/n preculture was combined with 1/20 (v/v) Sreptomyces preculture in fresh AMM medium. Further cultivation at 37 °C for 24 h | Aspergillus nidulans with a collection of 58 soil-dwelling actinomycetes | Physical interaction between the fungus and Streptomyces hygroscopicus. Induction of orsellinic acid and derivatives (lecanoric acid, yellow polyketides) | [215] |
| 2 | Co-culture in Warkingsman’s agar (Confrontation assay) and liquid medium (5 days) | Fusarium graminearum vs. 12.000 bacteria | reduced virulence of F. graminearum due to Pseudomonas piscium (FgGcn5 inhibitor phenazine-1-carboxamide) | [214] |
| 3 | treatment of Aspergillus with extracts of Streptomyces cultures | Aspergillus nidulans Streptomyces rapamycinicus | a guanidine containing macrolide, polaramycin B that is constitutively produced by the bacteria triggers the production of the ORS BGC | [184] |
| 3 | analytical scale microbial cultivations in a 24-well plate cultivation in presence of metabolites from other cultivation | Aspergillus sp. Streptomyces sp. | bacteriostatic cyclo-(L-Phe-trans-4-hydroxy-L-Pro) from Aspergillus stimulates nitric oxide production by Streptomyces which triggers silent BGC of Aspergillus: fungistatic heronapyrrole B | [233] |
| Targeted BGC Activation by Overexpression | ||||
|---|---|---|---|---|
| Method | Target/Parameter | Organism | Observation | Ref. |
| TF | fsqA (TF) | Aspergillus fumigatus | OE of fsqA (fsq gene cluster) production of pyrido [1,2-b]isoquinolines fumisoquin A and fumisoquin B | [249] |
| TF | xanC | Aspergillus fumigatus | OE of xanC: Activation of xan cluster and production of xanthocillin derivatives, the sulfated xanthocillin derivative BU-4704 and xanthocillin X monomethyl ether | [250] |
| CRISPRa | AN8506 AN8507 mdpE | Aspergillus nidulans | Method establishment: upregulation of putative BGC genes (AN8506, AN8507) and the silent mdP cluster via nucleosome map assisted targeting of inducible VPR-dCas9 into accessible chromatin in promoter | [251] |
| CRISPRa | breF, fuml, fwnA | Aspergillus nidulans | Method establishment: fusion of different chromatin active enzymes/domains to dCas9, activation of breF and fuml via p300-dCas9 (HAT), repression of breF by HAT GcnE-dCas9 and HDAC HosA-dCas9 and RpdA-dCas9 activation of fwnA by HosA-dCa9 | [252] |
| CRISPRa (multiplex) | mic- BGC | Aspergillus nidulans | method establishment: activation of mic cluster by dCas12a-VPR (multigene CRISPRa): dehydromicroperfuranone production | [253] |
| Hybrid Activator | (+)-Asperlin cluster | Aspergillus nidulans | fusion of the DNA binding domain of AlnR (pathway-specific TF) and the trans-activation domain of AfoA lead to the activation of the (+)-Asperlin cluster | [246] |
| TF | aspyridone cluster (asp) | Aspergillus nidulans | overexpression of the pathway-specific TF apdR: activation of the silent BGC and production of aspyridone | [237] |
| TF | ctnR | Aspergillus nidulans | overexpression of the TF ctnR: Cluster activation (7 genes) and asperfuranone biosynthesis | [238] |
| TFBB gene | several BGCs | Aspergillus nidulans | Chicorine cluster activation pkg cluster activation: alternariol, citreoisocoumarin, analogs of citreoisocoumarin pki cluster: 6-Hydroxy-7-methyl-3-nonylisoquinoline-5,8-dione pkhB /pkhA: 2,4-dihydroxy-6-[(3E,5E,7E)-2-oxonona-3,5,7-trienyl]benzaldehyde Pkd: 2-ethyl-4,6-dihydroxy-3,5-dimethylbenzaldehyde | [243] |
| TF | pbcR | Aspergillus nidulans | overexpression of pbcR resulted in upregulation of a 7-gene cluster and the production of ent-pimara-8(14),15-diene | [254] |
| BB gene Het. Expr. | micro-perfuranone (micA, BGC) | Aspergillus nidulans | promoter exchange or heterologous expression (A. niger) of backbone gene micA was sufficient to produce microperfuranone | [255] |
| BB gene Het. Expr. | asqK | Aspergillus nidulans | OE of BB gene and heterologous expression for stepwise pathway elucidation: Products: 4′-methoxylated 6,7-benzodiazepinediones and 4′-methoxyviridicatin | [256] |
| Multiple genes | inpE cluster | Aspergillus nidulans | promoter replacement of seven genes (inpC-inpB): production of protease inhibitor fellutamide B | [248] |
| Multiple genes | ivo cluster | Aspergillus nidulans | OE of ivoA, B and C (and combinations): production of a dark pigment with the precursors N-acetyltryptophan and N-acetyl-6-hydroxytryptophan | [247] |
| Multiple genes | sgdA, D, C, F | Aspergillus nidulans | promoter replacements (4 genes): production of aspernidgulene A1 and A2 | [257] |
| TF | azaR (TF) | Aspergillus niger | overexpression of azaR: Activation of aza cluster and production of azanigerones A–F | [258] |
| TF | aflR | Aspergillus parasiticus | overexpression of aflR lead to the production of aflatoxin under repressing conditions | [259] |
| TF | TF of pgm BGC | Aspergillus terreus | OE of pgm cluster TF (ATEG_06205) led to the production of naphthoquinones | [260] |
| Resistance gene | neomycin resistance | Aspergillus versicolor | The introduction of neomycin resistance leads to production of the new anti-tumor bioactive compounds | [261] |
| TF | NRPS31 BGC (APS-like) PKS19- BGC | Fusarium fujikuroi | overexpression of pathway-specific TF (APS2) and complementation of enzyme (F. semitectum APS8) resulted in production of ApicidinF OE of TF and PKS19 resulted in production of four novel compounds (Fujikurin A–D) | [125,262,263] |
| TF | APF2 | Fusarium fujikuroi | overexpression of APF2 increases apicidin F synthesis | [262] |
| Whole BGC | BIK2 or BIK3 | Fusarium fujikuroi | Separate OE of BIK2 and BIK3: revelation of precursor oxo-pre-bikaverin | [264] |
| BB gene | DMATS1, FFUJ_09179 | Fusarium fujikuroi | OE of DMATS1 yielded a reversely N-prenylated tryptophan | [265] |
| TF | TF22 (PKS-NRPS1) | Fusarium fujikuroi | Tet-on activation of TF22 (FFUJ_02222): cluster activation and trichosetin production | [239] |
| TF | FSL7 (TF) | Fusarium graminearum | overexpression of the TF FSL7: activation of the FSL cluster (PKS9) and production of fusarielins, F, G, and H | [266] |
| BB gene | PKS8 | Fusarium graminearum | activation of Gibepyrones and Prolipyrone B production | [33] |
| TF | FGM4 (fg3_54 BGC; NRPS) | Fusarium graminearum | OE triggers the production of fusaoctaxin A. Deletion compromises plant infection | [267] |
| BB gene | FSDS | Fusarium heterosporum | OE of backbone gene resulted in novel Tyrosine-Derived 2,4-Pyrrolidinedione | [268] |
| TF | PEXANC (TF) | Penicillium expansum | OE of PEXANC resulted in the activation of the Citrinin cluster via the TF CtnA and production of Citrinin (evolutionary TF repurposing) The theoretical cluster responsible for xanthocillin remained silent | [269] |
| Resistance gene | neomycin resistance | Penicillium purpurogenum | Introduction of neomycin resistance results in the production of the new cyclic dipeptide penicimutide | [270] |
| CRISPRa (TF) | macR (TF) | Penicillium rubens | induction of macrophorin pathway-specific TF macR: production of macrophorin A, D and 4′-oxomacrophorin D | [271] |
| BGC Activation by Heterologous Expression | |||
|---|---|---|---|
| Target/Parameter | Organisms | Observation | Ref. |
| high throughput BGC activation | 22 Species (Donors) S. cerevisiae (Host) | method establishment: Hex Expression of 22/41 fungal BGCs resulted in detectable compounds | [285] |
| 156 FACs with random genome fragments | Aspergillus aculeatus (Donor) Aspergillus terreus (Donor) Aspergillus wentii (Donor) Aspergillus nidulans (Host) | method establishment: FAC-MS, Identification of 15 new substances Clusters/metabolites: A. terreus: ATEG_07067, ATEG_07500, sesterterpenoid, valactamide A, benzomalvin A/D A. aculeatus: AACU_51108, AACU_59515, A. wentii: ASPWE_027400, ASPWE_034272, ASPWE_042595, ASPWE_044725, ASPWE_085322, ASPWE_151732, ASPWE_163793 | [284] |
| diketomorpholine BGC | Aspergilllus aculeatus (Donor) Aspergillus nidulans (Host) | Elucidation of the biosynthetic pathway of acu-dioxomorpholine, featuring a new type of condensation domain, by FAC-MS | [287] |
| ftmA (NRPS) | Aspergillus fumigatus (Donor/Host) Aspergillus nidulans (Host) | OE of the NRPS ftmA: Production of Brevianamide F (Product of NRPS, product of cluster is putatively fumitremorgin) | [288] |
| pyr Cluster | Aspergillus fumigatus (Donor) Aspergillus. oryzae (Host) | successive expression of the pyr cluster: Characterization of pyr cluster and isolation of the meroterpenoid pyripyropene | [289] |
| micro-perfuranone (micA, BGC) | Aspergillus nidulans (Donor) Aspergillus niger (Host) | promoter exchange or heterologous expression of backbone gene micA was sufficient to produce microperfuranone | [255] |
| asqJ asqE-L | Aspergillus nidulans (Donor) Escherichia coli (Host) | OE of asqE-L in host and elucidation of AsqJ function by expression in E. coli Final products: 4′-methoxylated 6,7-benzodiazepinediones and 4′-methoxyviridicatin | [256] |
| wA (NWA) | Aspergillus nidulans (Donor) Aspergillus oryzae (Host) | expression of the WA-PKS: identification of the YWA1 compound | [290] |
| terreazepine BGC | Aspergillus terreus (Donor) Aspergillus nidulans (Host) | production of terreazepine | [291] |
| 39 BGCs from various species | Donors: Colletotrichum gloeosporioides Alternaria alternata Fusarium graminearum Trichoderma viride Aspergillus flavipes Host: Aspergillus oryzae | method establishment: genome mining assisted automated and high-throughput (auto-HTP) biofoundry workflow refactoring of 39 BGCs and production of 185 distinct terpenoids | [292] |
| STC5, STC3 | Fusarium fujikuroi (Donor) E. coli (Host) | Use of homologues STC3: (+)-eremophilene STC5:(¢)-guaia-6,10(14)-diene | [55] |
| carotenoid BGC of Fusarium fujikuroi | Fusarium fujikuroi (Donor) Aspergillus nidulans (Host) | method establishment: aflR scaffold as heterologous expression matrix; successful production of β-carotene | [286] |
| STC1 | Fusarium fujikuroi (Donor) E. coli (Host) | Heterologous Expression of STC1 identification of (–)-germacrene D | [176] |
| FgJ03939 BGC | Fusarium graminearum (Donor) Saccharomyces cerevisiae (Host) | stepwise synthesis of several new sesquiterpene synthase-derived metabolites such as fusariumdiene, fusagramineol | [293] |
| part of the fusarubin gene cluster | Fusarium solani (Donor) S. cerevisiae (Host) | Expression of FVPPT, fsr1, fsr2, and fsr3 in S. cerevisiae (galactose inducible promoter) and biosynthesis of bostrycoidin | [294] |
| pvhA-E (BGC) | Penicillium variabile (Donor) Aspergillus nidulans (Host) | Heterologous expression of genes pvhA-e: production of varicidin A and B | [295] |
| psilocybin BGC | Psilocybe carpophores (Donor) Aspergillus nidulans (Host) | method establishment: Successful Expression of whole BGC as polycistronic RNA piconavirus assisted expression of the whole basidiomycete derived psilocybin BGC | [296] |
| TESG_6702 to 6705 | Trichophyton tonsurans (Donor) Aspergillus nidulans (Host) | Expression of the whole cluster resulted in the production of neosartoricins | [297] |
| BGC Activation by Interference of Chromatin-Regulation | |||
|---|---|---|---|
| Method | Organism | Observation | Ref. |
| HDACi DNMTi | 162 mangrove endophytic Fungi | HDACi: sodium butyrate, DNMTi: 5-azacytidine Activity against ESKAPE MOs and Mycobacterium tuberculosis and Leishmania donovani: 254/ 1608 extracts showed bioactivity, HDACi or DNMTi: 72 fungi produced active extracts, 40 fungi were active only in the control | [320] |
| Media HDACi DNMTi | Aspergillus awamori | Media: MEA, rice, nutrient, triptone soya agar HDACi: valproic acid, nicotinamide, trichostatin A, DNMTi: 5-azacytidine Different metabolic profiles between media and epigenetic modifiers Nicotinamide was found to be the best epigenetic inducer Rice grains was the best medium for SM induction | [188] |
| HDACi DNMTi | Aspergillus calidoustus, Aspergillus westerdijkiae Aspergillus bombycis Aspergillus carbonarius Aspergillus fischeri | HDACi: SAHA, DNMTi: 5-azacytidine significant changes in secondary metabolite profile with examples of both induction and repression. | [321] |
| HDACi DNMTi | Aspergillus clavatus | HDACi: valproic acid, trichostatin A, butyrate DNMTi: 5-azacytidine. N-acetyl-d-glucosamine Source of peptone strongly influences the SM profile, epigenetic modifier effects SM profile which is metabolite-specific and dependent on growth media and incubation time | [322] |
| Deletion | Aspergillus nidulans | cclA∆ (Bre2; H3K4me): activation of mdp cluster: production of monodictyphenone, emodin, and emodin derivatives activation of anti-osteoporosis polyketides cluster: F9775A and F9775B | [323] |
| Deletion | Aspergillus nidulans | HepA occupancy decreases during sterigmatocystin activation hepA∆/clrD∆ (H3K9me): upregulation of sterigmatocystin, isopenicillin A, terraquinone A LaeA involved in reversing heterochromatic marks at ST BGC, | [305] |
| Deletion | Aspergillus nidulans | hosA∆ (HDAC): impact on H4ac, SM, carbohydrate metabolism, virulence, defense | [324] |
| Knockdown | Aspergillus nidulans | knockdown of rpdA (HDAC): production of aspercryptin A1 and A2 and fellutamides | [325,326] |
| HDACi | Aspergillus niger | HDACi: SAHA, discovery of nygerone A | [327] |
| Deletion | Aspergillus niger | gcnE∆ (SAGA/Ada; acetylation): activation of 12 silent clusters (1 novel compound): nigerpyrone, carbonarone A, pestalamide A, funalenone, aurasperone E, aurasperone A | [328] |
| HDACi DNMTi | Aspergillus sp. | HDACi: suberohydroxamic acid, DNMTi: 5-azacytidine discovery of diphenylether-O-glycoside (diorcinol 3-O-α-D-ribofuranoside) | [329] |
| HDACi | Botryosphaeria mamane | HDACi: SAHA, Valporate, production of five unknown compounds | [330] |
| Deletion | Calcarisporium arbuscula | ∆hdaA (HDAC): upregulation of 75% of BGC genes: isolation of 10 new compounds | [331] |
| HDACi DNMTi | Chalara sp. 6661 | HDACi: sodium butyrate, suberobishydroxamic acid, vorinostat; DNMTi: 5-azacytidine vorinostat: four new modified xanthones, only one out of four inhibitors showed effect Biotransformation of vorinostat | [332] |
| Media HDACi DNMTi | Cladosporium resinae | Media: Czapek-DOX, YES, PDA, starch, liquid vs. solid; HDACi: SAHA; DNMTi: 5-AZA Czapek, yeast extract broth: more total secondary metabolites DNMTi and HDACi: expressions of silent genes | [209] |
| HDACi DNMTi | Cyathus stercoreus | nine previously undescribed sesquiterpenes | [333] |
| Deletion | Dothistroma septosporum | ΔDsKmt1 (H3K9me) or ΔDsKmt6 (H3K27me): Substantial decrease of H3K9me3 or H3K27me3. Increase of production of dothistromin in both cases | [334] |
| Media HDACi | Drechslera sp., | HDACi: SAHA, valproate, octanoylhydroxamic acid; 2 new metabolites: chromanone and prenylhydroxybenzoic acid; different metabolome between MEA and minimal media biotransformation of the inhibitors | [208] |
| Deletion | Epichloe festucae | ΔclrD (H3K9me) and/or ΔezhB (H3K27me): reduction of H3K9me3 and/or H3K27me3 activation of bioprotective lolitrems (ltm) and ergot alkaloids (eas) | [307] |
| Deletion | Fusarium fujikuroi | Δhda1(hdaA; HDAC): production of beauvericin (BEA-cluster; NRPS22) | [335] |
| Deletion | Fusarium fujikuroi | Δset2 and Δash1 (H3K36me): upregulation of PKS-NRPS9 and NRPS4 | [336] |
| Deletion | Fusarium fujikuroi | ∆set1 (H3K4me): activation of PKSNRPS9, PKS type III, NRPS4, NRPS23, and DMATS3 | [337] |
| Deletion | Fusarium graminearum | Δkmt6 (PRC2, H3K27me): Differential regulation of many SM genes, upregulation of: NRPS9, NRPS14, NRPS16, NRPS19, PKS2, PKS3, PKS13, PKS22, PKS28, PKS29, STC5 | [312] |
| Deletion | Fusarium graminearum (dereplication strain) | ∆kmt6 (H3K27me): discovery of Protofusarin and N-ethyl anthranilic acid, N-phenethylacetamide, N-acetyltryptamine ∆kmt6 and ∆fus1: tricinolone, tricinolonic acid | [338] |
| Deletion | Fusarium mangiferae | ∆fmkmt1 (H3K9me): increased beauvericin biosynthesis, nearly abolished fusapyrone production; revelation of fusapyrone BGC (FmPKS40) | [308] |
| Deletion | Fusarium verticillioides | Δfvdim5 (H3K9me): increase of fumonisin B1 and melanin biosynthesis, reduced virulence | [339] |
| Deletion | Metarhizium robertsii | Δhat1: activation of 11 orphan BGCs Meromuside A–I, Meromutides A and B | [340] |
| HDACi DNMTi | Muscodor yucatanensis | HDACi: SAHA, DNMTi: 5-azacytidine different morphology, cultural characteristics, metabolites | [341] |
| HDACi DNMTi | Nigrospora sphaerica | HDACi: SAHA, sodium butyrate, valproic acid, quercetin DNMTi: 5-azacytidine, hydralazine hydrochloride induction of cryptic metabolites by all modifiers used | [342] |
| HDACi | Penicillium brevicompactum | HDACi: nicotinamide, sodium butyrate total phenolic compounds increased drastically upon HDACi treatment Nicotinamide: p-anisic acid, p-anisic acid methyl ester, benzyl anisate, syringic acid, sinapic acid, acetosyringone, phenyl acetic acid, gentisaldehyde and p-hydroxy benzaldehyde Sodium butyrate enhanced the productivity of anthranilic acid and ergosterol peroxide | [343] |
| Deletion | Penicillium chrysogenum | ∆hdaA (HDAC): new compound. Maybe crosstalk with sorbicillinoids cluster | [344] |
| HDACi | Phoma sp. | SAHA triggers the production of (10’S)-verruculide B, vermistatin and dihydrovermistatin Absence of SAHA: discovery of (S,Z)-5-(3′,4′-dihydroxybutyldiene)-3-propylfuran-2(5H)-one | [345] |
| HDACi | Trichoderma harzianum | HDACi: sodium butyrate; diterpenoids changed into cyclonerane sesquiterpenoids. 3 new compounds: cleistanthane diterpenoid, harzianolic acid A, harziane diterpenoid, harzianone E, cyclonerane sesquiterpenoid, 3,7,11-trihydroxy-cycloneran | [346] |
| Knockdown | Fusarium fujikuroi | Knockdown of KMT6 (H3K27me; PRC2): differential regulation of SM-clusters, upregulated SM genes: NRPS22, STC4, tetraterpene cyclase-encoding gene TETC1, PKS-NRPS1, STC5, STC8, DMATS3, NRPS4, PKS2) detection of six yet unidentified cluster-products: STC5: guaia-6,10(14)-diene, STC3: (+)-eremophilene | [55,315] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schüller, A.; Studt-Reinhold, L.; Strauss, J. How to Completely Squeeze a Fungus—Advanced Genome Mining Tools for Novel Bioactive Substances. Pharmaceutics 2022, 14, 1837. https://doi.org/10.3390/pharmaceutics14091837
Schüller A, Studt-Reinhold L, Strauss J. How to Completely Squeeze a Fungus—Advanced Genome Mining Tools for Novel Bioactive Substances. Pharmaceutics. 2022; 14(9):1837. https://doi.org/10.3390/pharmaceutics14091837
Chicago/Turabian StyleSchüller, Andreas, Lena Studt-Reinhold, and Joseph Strauss. 2022. "How to Completely Squeeze a Fungus—Advanced Genome Mining Tools for Novel Bioactive Substances" Pharmaceutics 14, no. 9: 1837. https://doi.org/10.3390/pharmaceutics14091837
APA StyleSchüller, A., Studt-Reinhold, L., & Strauss, J. (2022). How to Completely Squeeze a Fungus—Advanced Genome Mining Tools for Novel Bioactive Substances. Pharmaceutics, 14(9), 1837. https://doi.org/10.3390/pharmaceutics14091837








