Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems
Abstract
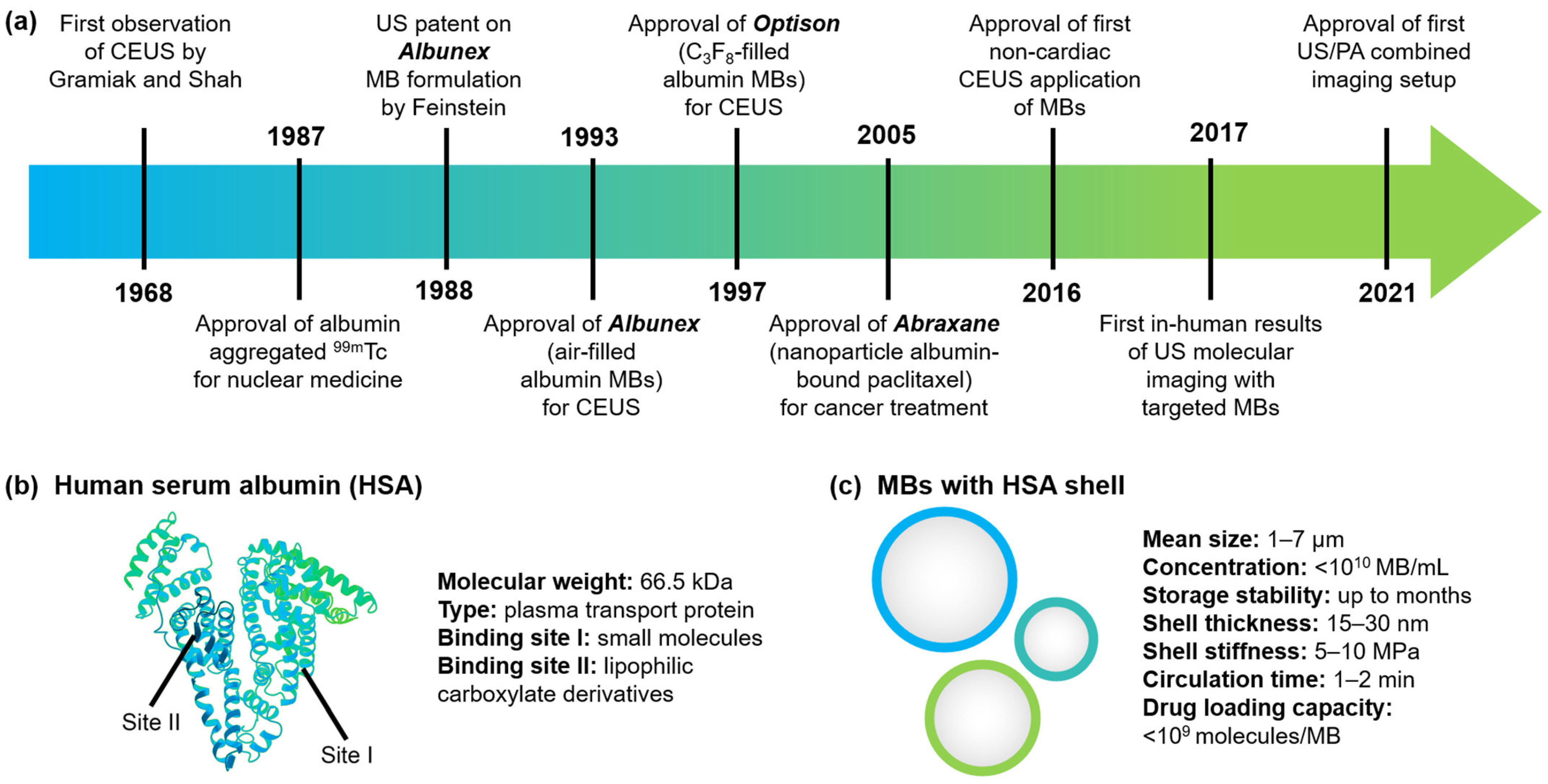
1. Introduction
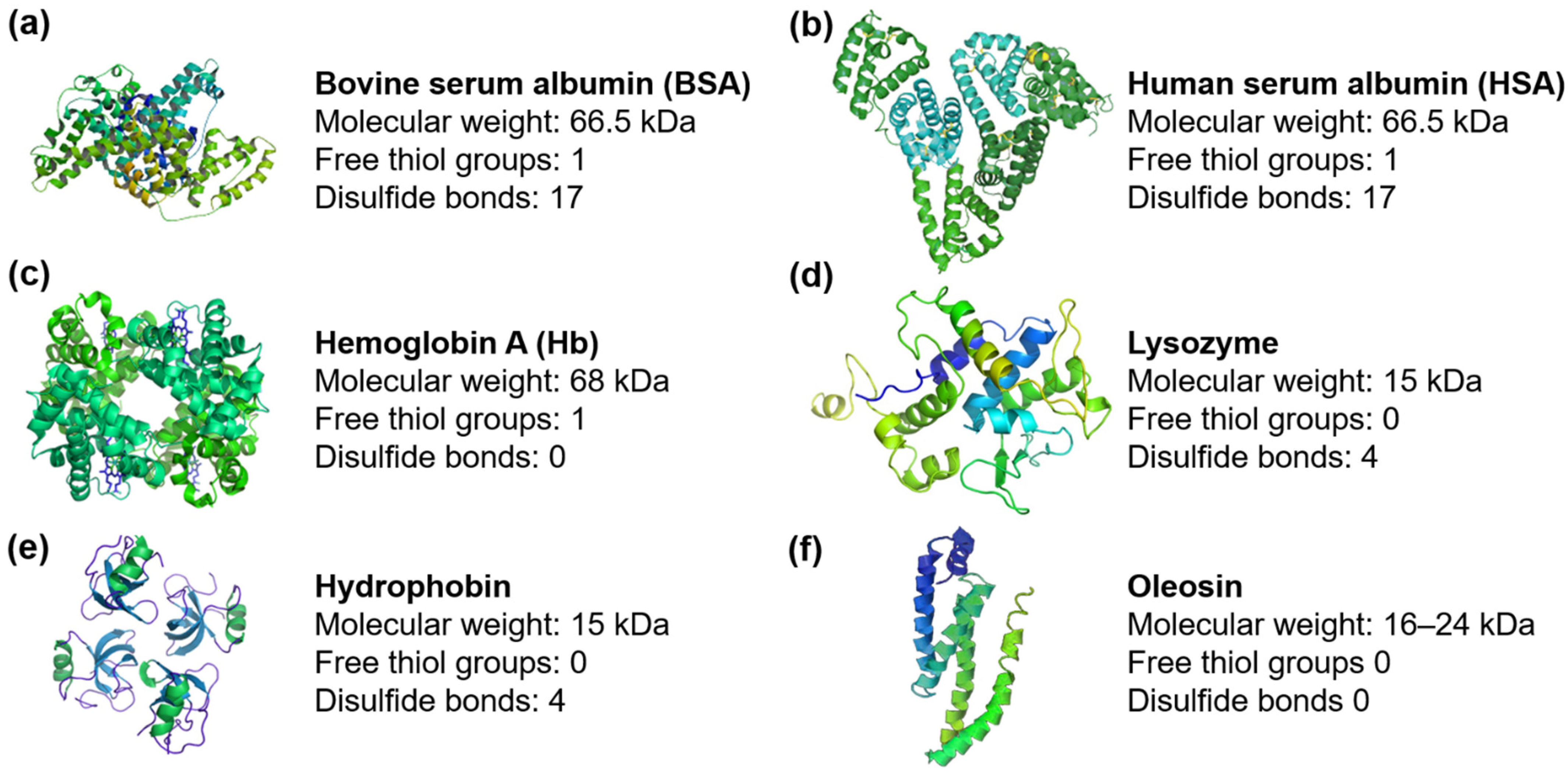
2. Proteins Involved in MB Shell Stabilization
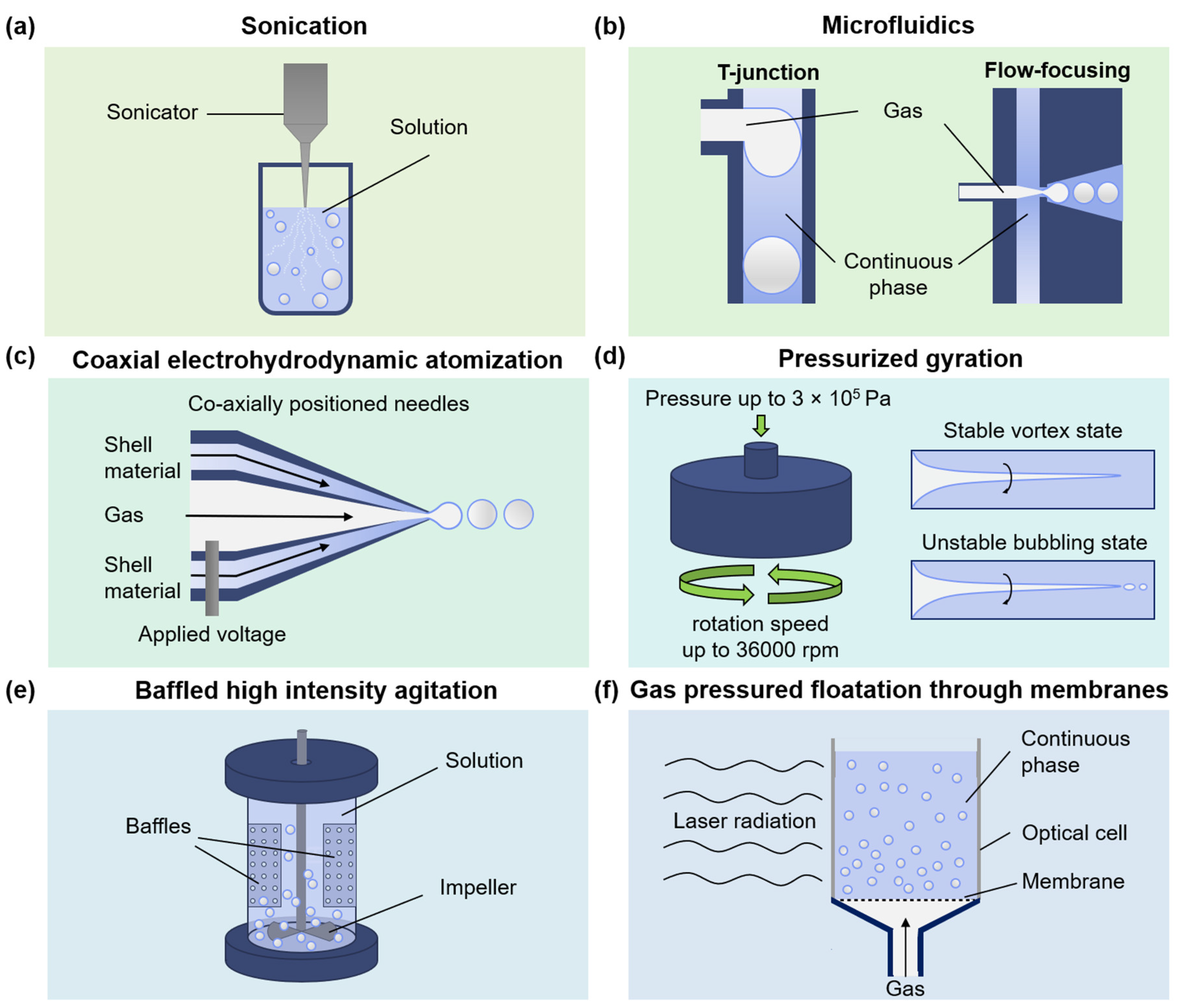
3. Fabrication of MBs with Protein Shell
3.1. Sonication
3.2. Microfluidics
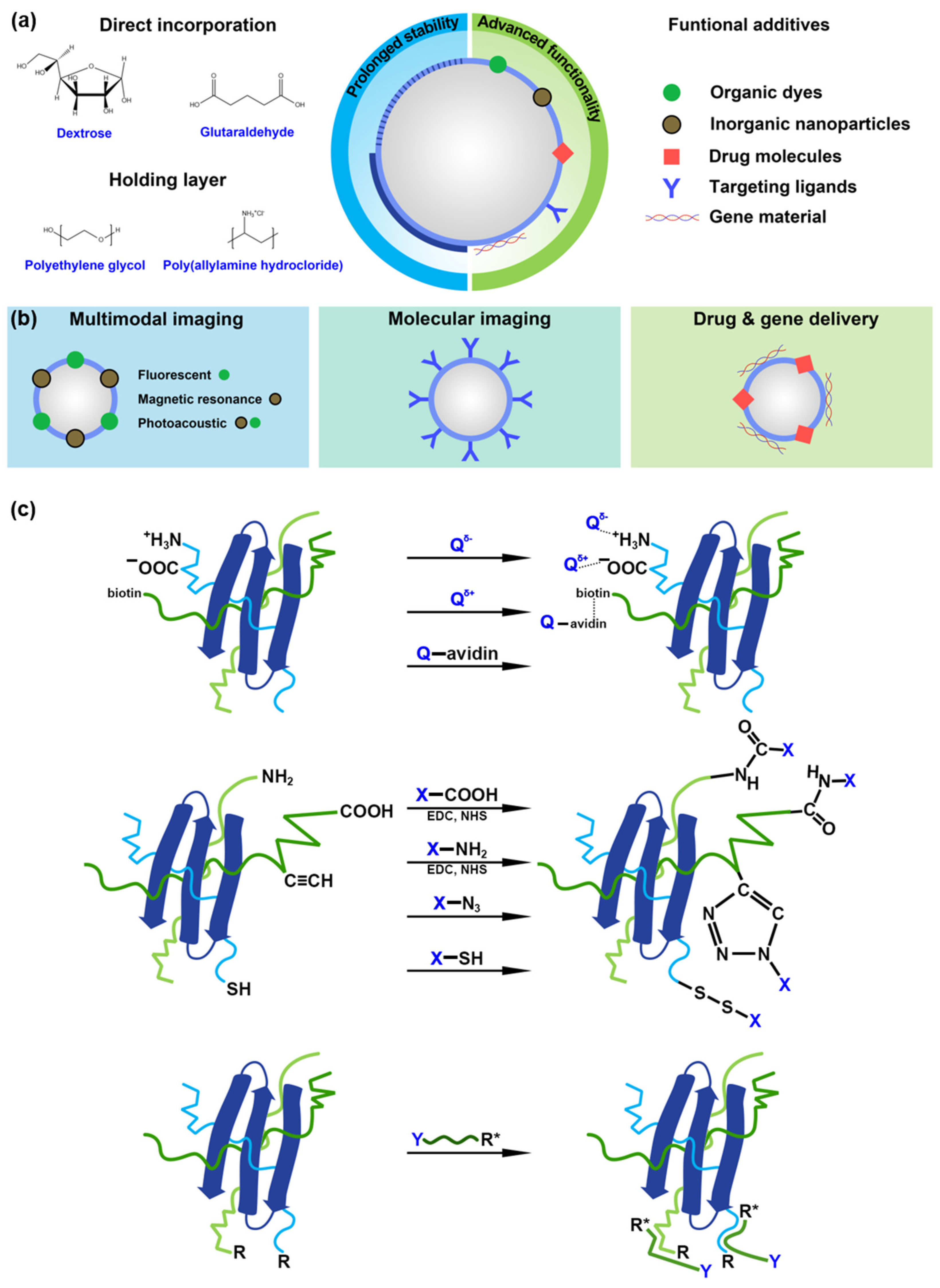
4. Chemical Routes for Stabilization and Functionalization of MBs with Protein Shell
4.1. Prolonged Stability
4.2. Advanced Functionality
5. Advanced Characterization of MBs with a Protein Shell

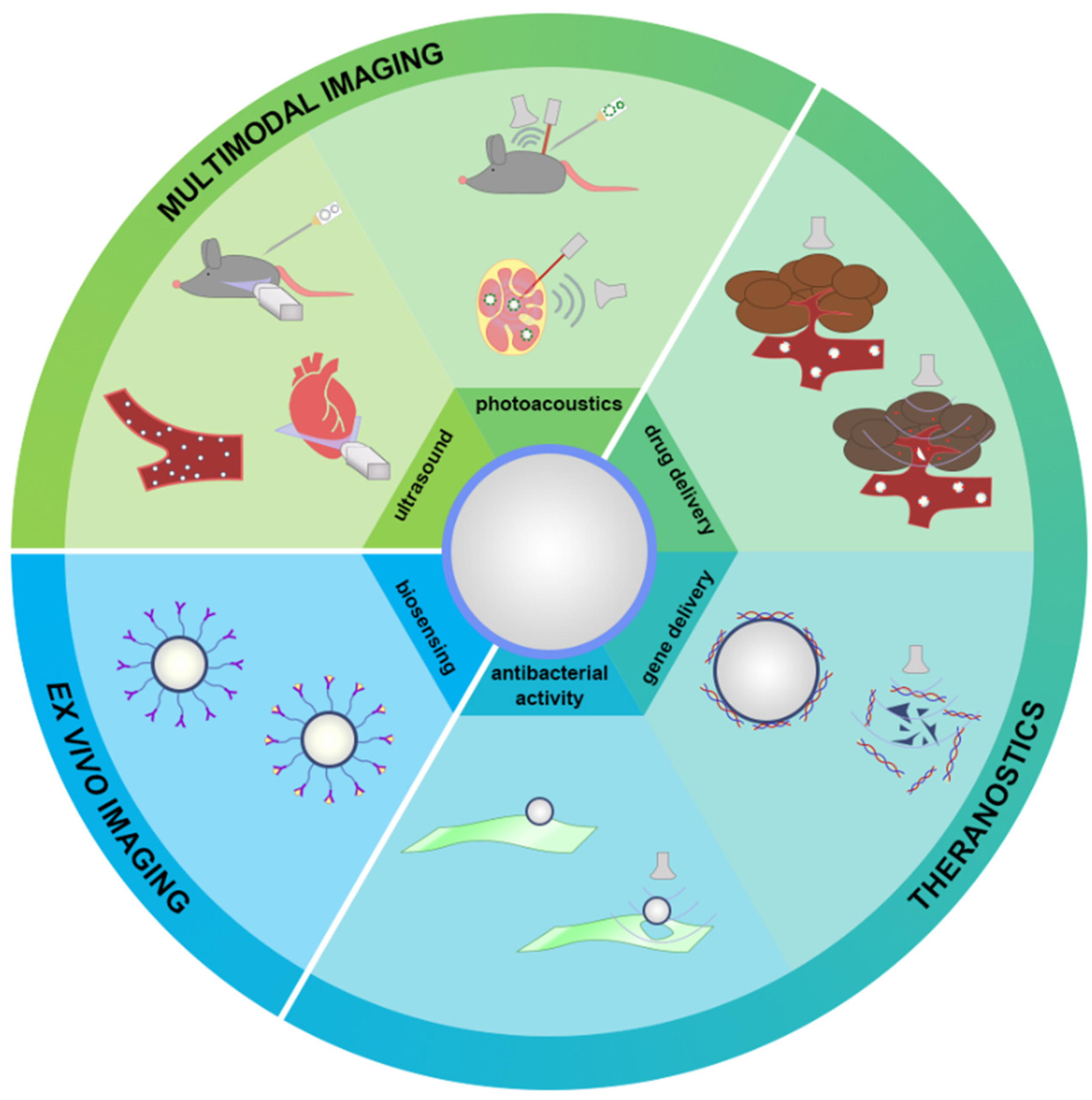
6. Applications of MBs with Protein Shell beyond US Imaging
6.1. Imaging Applications
6.2. Drug/Gene Delivery
6.3. Antibacterial Activity
6.4. Biosensing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N.T. Ultrasound imaging. Phys. Med. Biol. 2006, 51, R83. [Google Scholar] [CrossRef] [PubMed]
- NHS England and NHS Improvement Diagnostic Imaging Dataset Statistical Release. Available online: https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2020/01/Provisional-Monthly-Diagnostic-Imaging-Dataset-Statistics-2020-01-23.pdf (accessed on 1 April 2022).
- Smith-Bindman, R.; Miglioretti, D.L.; Larson, E.B. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff. 2008, 27, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Kwan, M.L.; Marlow, E.C.; Theis, M.K.; Bolch, W.; Cheng, S.Y.; Bowles, E.J.A.; Duncan, J.R.; Greenlee, R.T.; Kushi, L.H.; et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. J. Am. Med. Assoc. 2019, 322, 843–856. [Google Scholar] [CrossRef]
- Lieu, D. Ultrasound physics and instrumentation for pathologists. Arch. Pathol. Lab. Med. 2010, 134, 1541–1556. [Google Scholar] [CrossRef]
- Aldrich, J.E. Basic physics of ultrasound imaging. Crit. Care Med. 2007, 35, 131–137. [Google Scholar] [CrossRef]
- Cosgrove, D. Ultrasound contrast agents: An overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef]
- Wheatley, M.A.; Schrope, B.; Shen, P. Contrast agents for diagnostic ultrasound: Development and evaluation of polymer-coated microbubbles. Biomaterials 1990, 11, 713–717. [Google Scholar] [CrossRef]
- Mujtaba, J.; Liu, J.; Dey, K.K.; Li, T.; Chakraborty, R.; Xu, K.; Makarov, D.; Barmin, R.A.; Gorin, D.A.; Tolstoy, V.P.; et al. Micro-Bio-Chemo-Mechanical-Systems: Micromotors, Microfluidics, and Nanozymes for Biomedical Applications. Adv. Mater. 2021, 33, 2007465. [Google Scholar] [CrossRef]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef] [PubMed]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Batchelor, D.V.B.; Loadman, P.M.; McLaughlan, J.R.; Markham, A.F.; Evans, S.D.; Coletta, P.L. A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles. Pharmaceutics 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Rousou, C.; de Maar, J.; Qiu, B.; van der Wurff-Jacobs, K.; Ruponen, M.; Urtti, A.; Oliveira, S.; Moonen, C.; Storm, G.; Mastrobattista, E.; et al. The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers. Pharmaceutics 2022, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, S.A.G.; Meijlink, B.; Beekers, I.; Olthof, M.; van der Steen, A.F.W.; de Jong, N.; Kooiman, K. Theranostic Microbubbles with Homogeneous Ligand Distribution for Higher Binding Efficacy. Pharmaceutics 2022, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Barmin, R.A.; Rudakovskaya, P.G.; Chernyshev, V.S.; Guslyakova, O.I.; Belcov, P.A.; Obukhova, E.N.; Gayer, A.V.; Shirshin, E.A.; Gorin, D.A. Optoacoustic/Fluorescent/Acoustic Imaging Probe Based on Air-Filled Bubbles Functionalized with Gold Nanorods and Fluorescein Isothiocyanate. ACS Omega 2021, 6, 3809–3821. [Google Scholar] [CrossRef]
- Barmin, R.A.; Rudakovskaya, P.G.; Gusliakova, O.I.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Maksimova, E.A.; Obukhova, E.N.; Chernyshev, V.S.; Khlebtsov, B.N.; Solovev, A.A.; et al. Air-filled bubbles stabilized by gold nanoparticle/photodynamic dye hybrid structures for theranostics. Nanomaterials 2021, 11, 415. [Google Scholar] [CrossRef]
- Liu, M.; Dasgupta, A.; Qu, N.; Rama, E.; Kiessling, F.; Lammers, T. Strategies to Maximize Anthracycline Drug Loading in Albumin Microbubbles. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Maksimova, E.A.; Barmin, R.A.; Rudakovskaya, P.G.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Yashchenok, A.M.; Khlebtsov, B.N.; Solovev, A.A.; Huang, G.; Mei, Y.; et al. Air-Filled Microbubbles Based on Albumin Functionalized with Gold Nanocages and Zinc Phthalocyanine for Multimodal Imaging. Micromachines 2021, 12, 1161. [Google Scholar] [CrossRef]
- Himuro, S. Physicochemical characteristics of microbubbles. Chem. Eng. Jpn. 2007, 71, 165–169. [Google Scholar]
- Koczera, P.; Appold, L.; Shi, Y.; Liu, M.; Dasgupta, A.; Pathak, V.; Ojha, T.; Fokong, S.; Wu, Z.; van Zandvoort, M.; et al. PBCA-based polymeric microbubbles for molecular imaging and drug delivery. J. Control. Release 2017, 259, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Köhler, K.; Möhwald, H.; Sukhorukov, G.B. Gas-filled polyelectrolyte capsules. Angew. Chemie Int. Ed. 2005, 44, 3310–3314. [Google Scholar] [CrossRef] [PubMed]
- Fokong, S.; Siepmann, M.; Liu, Z.; Schmitz, G.; Kiessling, F.; Gätjens, J. Advanced Characterization and Refinement of Poly N-Butyl Cyanoacrylate Microbubbles for Ultrasound Imaging. Ultrasound Med. Biol. 2011, 37, 1622–1634. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Lipid monolayer collapse and microbubble stability. Adv. Colloid Interface Sci. 2012, 183–184, 82–99. [Google Scholar] [CrossRef]
- Fokong, S.; Theek, B.; Wu, Z.; Koczera, P.; Appold, L.; Jorge, S.; Resch-Genger, U.; Van Zandvoort, M.; Storm, G.; Kiessling, F.; et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Control. Release 2012, 163, 75–81. [Google Scholar] [CrossRef]
- Skorb, E.V.; Möhwald, H. “smart” Surface Capsules for Delivery Devices. Adv. Mater. Interfaces 2014, 1, 1400237. [Google Scholar] [CrossRef]
- Korolev, I.; Aliev, T.A.; Orlova, T.; Ulasevich, S.A.; Nosonovsky, M.; Skorb, E.V. When Bubbles Are Not Spherical: Artificial Intelligence Analysis of Ultrasonic Cavitation Bubbles in Solutions of Varying Concentrations. J. Phys. Chem. B 2022, 126, 3161–3169. [Google Scholar] [CrossRef]
- Segers, T.; de Jong, N.; Versluis, M. Uniform scattering and attenuation of acoustically sorted ultrasound contrast agents: Modeling and experiments. J. Acoust. Soc. Am. 2016, 140, 2506–2517. [Google Scholar] [CrossRef]
- Borden, M.A.; Longo, M.L. Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: Effect of lipid hydrophobic chain length. Langmuir 2002, 18, 9225–9233. [Google Scholar] [CrossRef]
- Grishenkov, D.; Kari, L.; Brodin, L.Å.; Brismar, T.B.; Paradossi, G. In vitro contrast-enhanced ultrasound measurements of capillary microcirculation: Comparison between polymer- and phospholipid-shelled microbubbles. Ultrasonics 2011, 51, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, P.V.; Koppolu, S.; Mamou, L.; Chlon, C.; Ketterling, J.A. Influence of Shell Properties on High- Frequency Ultrasound Imaging and Drug. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 53–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lynn, G.M.; Jacobson, O.; Chen, K.; Liu, Y.; Zhang, H.; Ma, Y.; Zhang, F.; Tian, R.; Ni, Q.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1954. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar]
- Zhu, L.; Yang, F.; Chen, L.; Meehan, E.J.; Huang, M. A new drug binding subsite on human serum albumin and drug-drug interaction studied by X-ray crystallography. J. Struct. Biol. 2008, 162, 40–49. [Google Scholar] [CrossRef]
- Curry, S.; Brick, P.; Franks, N.P. Fatty acid binding to human serum albumin: New insights from crystallographic studies. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 1999, 1441, 131–140. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Keller, M.W.; Feinstein, S.B.; Watson, D.D. Successful left ventricular opacification following peripheral venous injection of sonicated contrast agent: An experimental evaluation. Am. Heart J. 1987, 114, 570–575. [Google Scholar] [CrossRef]
- Keller, M.W.; Segal, S.S.; Kaul, S.; Duling, B. The behavior of sonicated albumin microbubbles within the microcirculation: A basis for their use during myocardial contrast echocardiography. Circ. Res. 1989, 65, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.W.; Glasheen, W.; Kaul, S. Albunex: A Safe and Effective Commercially Produced Agent for Myocardial Contrast Echocardiography. J. Am. Soc. Echocardiogr. 1989, 2, 48–52. [Google Scholar] [CrossRef]
- Ward, M.; Wu, J.; Chiu, J.F. Experimental study of the effects of optison® concentration on sonoporation in vitro. Ultrasound Med. Biol. 2000, 26, 1169–1175. [Google Scholar] [CrossRef]
- Wei, K.; Mulvagh, S.L.; Carson, L.; Davidoff, R.; Gabriel, R.; Grimm, R.A.; Wilson, S.; Fane, L.; Herzog, C.A.; Zoghbi, W.A.; et al. The Safety of Definity and Optison for Ultrasound Image Enhancement: A Retrospective Analysis of 78,383 Administered Contrast Doses. J. Am. Soc. Echocardiogr. 2008, 21, 1202–1206. [Google Scholar] [CrossRef]
- Hashiya, N.; Aoki, M.; Tachibana, K.; Taniyama, Y.; Yamasaki, K.; Hiraoka, K.; Makino, H.; Yasufumi, K.; Ogihara, T.; Morishita, R. Local delivery of E2F decoy oligodeoxynucleotides using ultrasound with microbubble agent (Optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem. Biophys. Res. Commun. 2004, 317, 508–514. [Google Scholar] [CrossRef]
- Li, T.; Tachibana, K.; Kuroki, M.; Kuroki, M. Gene Transfer with Echo—Enhanced Contrast Agents: Comparison between Albunex, Optison, and Levovist in Mice—Initial Results. Radiology 2003, 229, 423–428. [Google Scholar] [CrossRef]
- Chumakova, O.V.; Liopo, A.V.; Mark Evers, B.; Esenaliev, R.O. Effect of 5-fluorouracil, optison and ultrasound on MCF-7 cell viability. Ultrasound Med. Biol. 2006, 32, 751–758. [Google Scholar] [CrossRef]
- Sontum, P.C. Physicochemical Characteristics of SonazoidTM, A New Contrast Agent for Ultrasound Imaging. Ultrasound Med. Biol. 2008, 34, 824–833. [Google Scholar] [CrossRef]
- Schneider, M. Sono Vue, a new ultrasound contrast agent. Eur. Radiol. 1999, 9, 347–348. [Google Scholar] [CrossRef]
- Greis, C. Technology overview: SonoVue (Bracco, Milan). Eur. Radiol. Suppl. 2004, 14, 11–15. [Google Scholar] [CrossRef]
- Porter, T.R.; Iversen, P.L.; Li, S.; Xie, F. Interaction of diagnostic ultrasound with synthetic oligonucleotide-labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J. Ultrasound Med. 1996, 15, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Wu, S.Y.; Wang, H.E.; Weng, C.H.; Wu, M.F.; Li, P.C. Evaluation of 18F-labeled targeted perfluorocarbon-filled albumin microbubbles as a probe for microUS and microPET in tumor-bearing mice. Ultrasonics 2013, 53, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Florinas, S.; Nam, H.Y.; Kim, S.W. Enhanced siRNA delivery using a combination of an Arginine-grafted bioreducible polymer, ultrasound, and microbubbles in cancer cells. Mol. Pharm. 2013, 10, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Bonomo, L.; Testa, A.C.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound molecular imaging with BR55 in patients with breast & ovarian lesions: First-in-human results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef]
- Smeenge, M.; Tranquart, F.; Mannaerts, C.K.; de Reijke, T.M.; van de Vijver, M.J.; Laguna, M.P.; Pochon, S.; de la Rosette, J.J.M.C.H.; Wijkstra, H. First-in-human ultrasound molecular imaging with a VEGFR2-specific ultrasound molecular contrast agent (BR55) in prostate cancer a safety and feasibility pilot study. Investig. Radiol. 2017, 52, 419–427. [Google Scholar] [CrossRef]
- Köse, G.; Darguzyte, M.; Kiessling, F. Molecular ultrasound imaging. Nanomaterials 2020, 10, 1935. [Google Scholar] [CrossRef]
- Manohar, S.; Dantuma, M. Current and future trends in photoacoustic breast imaging. Photoacoustics 2019, 16, 100134. [Google Scholar] [CrossRef]
- Premarket Approval (PMA). Imagio Breast Imaging System. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P200003 (accessed on 1 April 2022).
- Ando, Y.; Tabata, H.; Sanchez, M.; Cagna, A.; Koyama, D.; Krafft, M.P. Microbubbles with a Self-Assembled Poloxamer Shell and a Fluorocarbon Inner Gas. Langmuir 2016, 32, 12461–12467. [Google Scholar] [CrossRef]
- Justeau, C.; Vela-Gonzalez, A.V.; Jourdan, A.; Riess, J.G.; Krafft, M.P. Adsorption of Cerium Salts and Cerium Oxide Nanoparticles on Microbubbles Can Be Induced by a Fluorocarbon Gas. ACS Sustain. Chem. Eng. 2018, 6, 11450–11456. [Google Scholar] [CrossRef]
- Mendoza-Ortega, E.E.; Dubois, M.; Krafft, M.P. Fluorocarbon Gas Exposure Induces Disaggregation of Nanodiamond Clusters and Enhanced Adsorption, Enabling Medical Microbubble Formation. ACS Appl. Nano Mater. 2020, 3, 8897–8905. [Google Scholar] [CrossRef]
- Lindner, J.R. Microbubbles in medical imaging: Current applications and future directions. Nat. Rev. Drug Discov. 2004, 3, 527–532. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V. Microbubble Formulations: Synthesis, Stability, Modeling and Biomedical Applications. Ultrasound Med. Biol. 2019, 45, 301–343. [Google Scholar] [CrossRef]
- Ma, X.; Bussonniere, A.; Liu, Q. A facile sonochemical synthesis of shell-stabilized reactive microbubbles using surface-thiolated bovine serum albumin with the Traut’s reagent. Ultrason. Sonochem. 2017, 36, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Narihira, K.; Watanabe, A.; Sheng, H.; Endo, H.; Feril, L.B.; Irie, Y.; Ogawa, K.; Moosavi-Nejad, S.; Kondo, S.; Kikuta, T.; et al. Enhanced cell killing and apoptosis of oral squamous cell carcinoma cells with ultrasound in combination with cetuximab coated albumin microbubbles. J. Drug Target. 2018, 26, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Coggins, M.P.; Kaul, S.; Klibanov, A.L.; Brandenburger, G.H.; Ley, K. Microbubble Persistence in the Microcirculation During Ischemia/Reperfusion and Inflammation Is Caused by Integrin- and Complement-Mediated Adherence to Activated Leukocytes. Circulation 2000, 101, 668–675. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, C.; Zhao, H.; Qin, Y.; Zhang, X.; Li, Y. The fabrication of protein microbubbles with diverse gas core and the novel exploration on the role of interface introduction in protein crystallization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124471. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, S.P.; Liao, A.H.; Yang, Y.C.; Lee, C.R.; Wu, C.H.; Wu, P.C.; Liu, T.M.; Wang, C.R.C.; Li, P.C. Synergistic delivery of gold nanorods using multifunctional microbubbles for enhanced plasmonic photothermal therapy. Sci. Rep. 2014, 4, 5685. [Google Scholar] [CrossRef]
- Villanueva, F.S.; Jankowski, R.J.; Manaugh, C.; Wagner, W.R. Albumin microbubble adherence to human coronary endothelium: Implications for assessment of endothelial function using myocardial contrast echocardiography. J. Am. Coll. Cardiol. 1997, 30, 689–693. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V. Synthesis, characterization and stability of BSA-encapsulated microbubbles. RSC Adv. 2016, 6, 15016–15026. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V.; Gupta, G.; Khanna, N. Effect of PEGylation on performance of protein microbubbles and its comparison with lipid microbubbles. Mater. Sci. Eng. C 2017, 71, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Rovers, T.A.M.; Sala, G.; van der Linden, E.; Meinders, M.B.J. Effect of Temperature and Pressure on the Stability of Protein Microbubbles. ACS Appl. Mater. Interfaces 2016, 8, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rovers, T.A.M.; Sala, G.; van der Linden, E.; Meinders, M.B.J. Disintegration of protein microbubbles in presence of acid and surfactants: A multi-step process. Soft Matter 2015, 11, 6403–6411. [Google Scholar] [CrossRef]
- Rovers, T.A.M.; Sala, G.; van der Linden, E.; Meinders, M.B.J. Temperature is key to yield and stability of BSA stabilized microbubbles. Food Hydrocoll. 2016, 52, 106–115. [Google Scholar] [CrossRef]
- Borrelli, M.J.; O’Brien, W.D.; Bernock, L.J.; Williams, H.R.; Hamilton, E.; Wu, J.; Oelze, M.L.; Culp, W.C. Production of uniformly sized serum albumin and dextrose microbubbles. Ultrason. Sonochem. 2012, 19, 198–208. [Google Scholar] [CrossRef]
- Khan, A.H.; Surwase, S.; Jiang, X.; Edirisinghe, M.; Dalvi, S.V. Enhancing in Vitro Stability of Albumin Microbubbles Produced Using Microfluidic T-Junction Device. Langmuir 2022, 38, 5052–5062. [Google Scholar] [CrossRef]
- Ji, J.; Ji, S.-Y.; He, X.; Ling, W.-P. Preparation of ultrasound microbubbles crosslinked to albumin nanoparticles packaged with tissue-type plasminogen activator gene plasmid and method of in vivo transfection. J. Exp. Pharmacol. 2011, 3, 35–41. [Google Scholar] [CrossRef]
- Du, A.J.; Zhao, X.; Li, B.; Mou, Y.; Wang, Y. DNA-loaded microbubbles with crosslinked bovine serum albumin shells for ultrasound-promoted gene delivery and transfection. Colloids Surf. B Biointerfaces 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Liou, Y.R.; Wang, Y.H.; Lee, C.Y.; Li, P.C. Buoyancy-activated cell sorting using targeted biotinylated albumin microbubbles. PLoS ONE 2015, 10, e0125036. [Google Scholar] [CrossRef]
- Liu, X.; Gong, P.; Song, P.; Xie, F.; Miller, A.L.; Chen, S.; Lu, L. Rapid conjugation of nanoparticles, proteins and siRNAs to microbubbles by strain-promoted click chemistry for ultrasound imaging and drug delivery. Polym. Chem. 2019, 10, 705–717. [Google Scholar] [CrossRef]
- Liu, X.; Gong, P.; Song, P.; Xie, F.; Miller, A.L.; Chen, S.; Lu, L. Fast functionalization of ultrasound microbubbles using strain promoted click chemistry. Biomater. Sci. 2018, 6, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Zhou, M.; Tortora, M.; Paci, M.; Cavalieri, F.; Ashokkumar, M. Molecular properties of lysozyme-microbubbles: Towards the protein and nucleic acid delivery. Amino Acids 2012, 43, 885–896. [Google Scholar] [CrossRef]
- Mahalingam, S.; Raimi-Abraham, B.T.; Craig, D.Q.M.; Edirisinghe, M. Formation of protein and protein-gold nanoparticle stabilized microbubbles by pressurized gyration. Langmuir 2015, 31, 659–666. [Google Scholar] [CrossRef]
- Liao, A.H.; Hung, C.R.; Lin, C.F.; Lin, Y.C.; Chen, H.K. Treatment effects of lysozyme-shelled microbubbles and ultrasound in inflammatory skin disease. Sci. Rep. 2017, 7, 41325. [Google Scholar] [CrossRef]
- Cavalieri, F.; Micheli, L.; Kaliappan, S.; Teo, B.M.; Zhou, M.; Palleschi, G.; Ashokkumar, M. Antimicrobial and biosensing ultrasound-responsive lysozyme-shelled microbubbles. ACS Appl. Mater. Interfaces 2013, 5, 464–471. [Google Scholar] [CrossRef]
- Zhou, M.; Cavalieri, F.; Ashokkumar, M. Tailoring the properties of ultrasonically synthesised microbubbles. Soft Matter 2011, 7, 623–630. [Google Scholar] [CrossRef]
- Zhou, M.; Leong, T.S.H.; Melino, S.; Cavalieri, F.; Kentish, S.; Ashokkumar, M. Sonochemical synthesis of liquid-encapsulated lysozyme microspheres. Ultrason. Sonochem. 2010, 17, 333–337. [Google Scholar] [CrossRef]
- Zhou, M.; Cavalieri, F.; Ashokkumar, M. Modification of the size distribution of lysozyme microbubbles using a post-sonication technique. Instrum. Sci. Technol. 2012, 40, 51–60. [Google Scholar] [CrossRef]
- Vong, F.; Son, Y.; Bhuiyan, S.; Zhou, M.; Cavalieri, F.; Ashokkumar, M. A comparison of the physical properties of ultrasonically synthesized lysozyme- and BSA-shelled microbubbles. Ultrason. Sonochem. 2014, 21, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tchuenbou-Magaia, F.L.; Cox, P.W. Tribological study of suspensions of cysteine-rich protein stabilized microbubbles and subsequent triphasic A/O/W emulsions. J. Texture Stud. 2011, 42, 185–196. [Google Scholar] [CrossRef]
- Chen, Z.; Pulsipher, K.W.; Chattaraj, R.; Hammer, D.A.; Sehgal, C.M.; Lee, D. Engineering the Echogenic Properties of Microfluidic Microbubbles Using Mixtures of Recombinant Protein and Amphiphilic Copolymers. Langmuir 2019, 35, 10079–10086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chattaraj, R.; Pulsipher, K.W.; Karmacharya, M.B.; Hammer, D.A.; Lee, D.; Sehgal, C.M. Photoacoustic and Ultrasound Dual-Mode Imaging via Functionalization of Recombinant Protein-Stabilized Microbubbles with Methylene Blue. ACS Appl. Bio Mater. 2019, 2, 4020–4026. [Google Scholar] [CrossRef] [PubMed]
- Grinstaff, M.W.; Suslick, K.S. Air-filled proteinaceous microbubbles: Synthesis of an echo-contrast agent. Proc. Natl. Acad. Sci. USA 1991, 88, 7708–7710. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Grinstaff, M.W.; Kolbeck, K.J.; Wong, M. Characterization of sonochemically prepared proteinaceous microspheres. Ultrason. Sonochem. 1994, 1, 65–68. [Google Scholar] [CrossRef]
- Kohno, M.; Mokudai, T.; Ozawa, T.; Niwano, Y. Free radical formation from sonolysis of water in the presence of different gases. J. Clin. Biochem. Nutr. 2011, 49, 96–101. [Google Scholar] [CrossRef]
- Asada, K.; Kanematsu, S. Reactivity of Thiols with Superoxide Radicals. Agric. Biol. Chem. 1976, 40, 1891–1892. [Google Scholar] [CrossRef]
- Avivi, S.; Gedanken, A. S–S Bonds Are not Required for the Sonochemical Formation of Proteinaceous Microspheres: The Case of Streptavidin. Biochem. J. 2002, 366, 705–707. [Google Scholar] [CrossRef]
- Murayama, K.; Tomida, M. Heat-induced secondary structure and conformation change of bovine serum albumin investigated by Fourier transform infrared spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Cavalieri, F.; Ashokkumar, M. Exploring New Applications of Lysozyme-Shelled Microbubbles. Langmuir 2019, 35, 9997–10006. [Google Scholar] [CrossRef] [PubMed]
- Reisner, S.A.; Ong, L.S.; Lichtenberg, G.S.; Amico, A.F.; Shapiro, J.R.; Allen, M.N.; Meltzer, R.S. Myocardial perfusion imaging by contrast echocardiography with use of intracoronary sonicated albumin in humans. J. Am. Coll. Cardiol. 1989, 14, 660–665. [Google Scholar] [CrossRef]
- Porter, T.R.; Abdelmoneim, S.; Belcik, J.T.; McCulloch, M.L.; Mulvagh, S.L.; Olson, J.J.; Porcelli, C.; Tsutsui, J.M.; Wei, K. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Z.; Zhou, Y.T.; Grayburn, P.A. Optimization of the size distribution and myocardial contrast effect of perfluorocarbon-filled albumin microbubbles by lyophilization under continuous negative pressure. J. Am. Soc. Echocardiogr. 2000, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Song, J.; Christiansen, J.; Klibanov, A.L.; Xu, F.; Ley, K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001, 104, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; de Geest, B.G.; Vandenbroucke, R.E.; Peeters, L.; Demeester, J.; de Smedt, S.C.; Sanders, N.N. Ultrasound-responsive polymer-coated microbubbles that bind and protect DNA. Langmuir 2006, 22, 7273–7278. [Google Scholar] [CrossRef]
- Anderson, D.R.; Tsutsui, J.M.; Xie, F.; Radio, S.J.; Porter, T.R. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc. Res. 2007, 73, 597–606. [Google Scholar] [CrossRef]
- Anderson, D.R.; Duryee, M.J.; Anchan, R.K.; Garvin, R.P.; Johnston, M.D.; Porter, T.R.; Thiele, G.M.; Klassen, L.W. Albumin-based microbubbles bind up-regulated scavenger receptors following vascular injury. J. Biol. Chem. 2010, 285, 40645–40653. [Google Scholar] [CrossRef] [PubMed]
- Talu, E.; Hettiarachchi, K.; Zhao, S.; Powell, R.L.; Lee, A.P.; Longo, M.L.; Dayton, P.A. Tailoring the size distribution of ultrasound contrast agents: Possible method for improving sensitivity in molecular imaging. Mol. Imaging 2007, 6, 384–392. [Google Scholar] [CrossRef]
- Pulsipher, K.W.; Hammer, D.A.; Lee, D.; Sehgal, C.M. Engineering Theranostic Microbubbles Using Microfluidics for Ultrasound Imaging and Therapy: A Review. Ultrasound Med. Biol. 2018, 44, 2441–2460. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Gorelikov, I.; Williams, R.; Matsuura, N. Microfluidic assembly of monodisperse, nanoparticle-incorporated perfluorocarbon microbubbles for medical imaging and therapy. Langmuir 2010, 26, 13855–13860. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef]
- Dixon, A.J.; Dhanaliwala, A.H.; Chen, J.L.; Hossack, J.A. Enhanced Intracellular Delivery of a Model Drug Using Microbubbles Produced by a Microfluidic Device. Ultrasound Med. Biol. 2013, 39, 1267–1276. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, X.; Zhao, C.; Fu, X.; Zhang, W.; Kong, W.; Zhang, B.; Zhao, Y. Ultrasound-Responsive Microfluidic Microbubbles for Combination Tumor Treatment. Adv. Ther. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Farook, U.; Stride, E.; Edirisinghe, M.J.; Moaleji, R. Microbubbling by co-axial electrohydrodynamic atomization. Med. Biol. Eng. Comput. 2007, 45, 781–789. [Google Scholar] [CrossRef]
- Mahalingam, S.; Meinders, M.B.J.; Edirisinghe, M. Formation, stability, and mechanical properties of bovine serum albumin stabilized air bubbles produced using coaxial electrohydrodynamic atomization. Langmuir 2014, 30, 6694–6703. [Google Scholar] [CrossRef]
- Yan, W.C.; Ong, X.J.; Pun, K.T.; Tan, D.Y.; Sharma, V.K.; Tong, Y.W.; Wang, C.H. Preparation of tPA-loaded microbubbles as potential theranostic agents: A novel one-step method via coaxial electrohydrodynamic atomization technique. Chem. Eng. J. 2017, 307, 168–180. [Google Scholar] [CrossRef]
- Mahalingam, S.; Xu, Z.; Edirisinghe, M. Antibacterial Activity and Biosensing of PVA-Lysozyme Microbubbles Formed by Pressurized Gyration. Langmuir 2015, 31, 9771–9780. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Spontaneous formation behavior of uniform-sized microbubbles from Shirasu porous glass (SPG) membranes in the absence of water-phase flow. Colloids Surf. A Physicochem. Eng. Asp. 2007, 296, 174–181. [Google Scholar] [CrossRef]
- Jang, Y.; Jang, W.S.; Gao, C.; Shim, T.S.; Crocker, J.C.; Hammer, D.A.; Lee, D. Tuning the Mechanical Properties of Recombinant Protein-Stabilized Gas Bubbles Using Triblock Copolymers. ACS Macro Lett. 2016, 5, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Dhanaliwala, A.H.; Dixon, A.J.; Klibanov, A.L.; Hossack, J.A. Synthesis and characterization of transiently stable albumin-coated microbubbles via a flow-focusing microfluidic device. Ultrasound Med. Biol. 2014, 40, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Angilè, F.E.; Vargo, K.B.; Sehgal, C.M.; Hammer, D.A.; Lee, D. Recombinant protein-stabilized monodisperse microbubbles with tunable size using a valve-based microfluidic device. Langmuir 2014, 30, 12610–12618. [Google Scholar] [CrossRef] [PubMed]
- Dhanaliwala, A.H.; Dixon, A.J.; Lin, D.; Chen, J.L.; Klibanov, A.L.; Hossack, J.A. In vivo imaging of microfluidic-produced microbubbles. Biomed. Microdevices 2015, 17, 23. [Google Scholar] [CrossRef]
- Dixon, A.J.; Rickel, J.M.R.; Shin, B.D.; Klibanov, A.L.; Hossack, J.A. In Vitro Sonothrombolysis Enhancement by Transiently Stable Microbubbles Produced by a Flow-Focusing Microfluidic Device. Ann. Biomed. Eng. 2018, 46, 222–232. [Google Scholar] [CrossRef]
- Parhizkar, M.; Sofokleous, P.; Stride, E.; Edirisinghe, M. Novel preparation of controlled porosity particle/fibre loaded scaffolds using a hybrid micro-fluidic and electrohydrodynamic technique. Biofabrication 2014, 6, 45010. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Edirisinghe, M.; Parhizkar, M. Combining microfluidic devices with coarse capillaries to reduce the size of monodisperse microbubbles. RSC Adv. 2016, 6, 63568–63577. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Drude, N.; Weiler, M.; Rommel, D.; Rütten, S.; Geinitz, B.; Van Steenbergen, M.J.; Storm, G.; Kiessling, F.; et al. Shelf-life evaluation and lyophilization of PBCA-based polymeric microbubbles. Pharmaceutics 2019, 11, 433. [Google Scholar] [CrossRef]
- Miller, M.W. Gene transfection and drug delivery. Ultrasound Med. Biol. 2000, 26, 59–62. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Sonochemical nanosynthesis at the engineered interface of a cavitation microbubble. Phys. Chem. Chem. Phys. 2006, 8, 3496–3506. [Google Scholar] [CrossRef]
- Cavalieri, F.; Ashokkumar, M.; Grieser, F.; Caruso, F. Ultrasonic synthesis of stable, functional lysozyme microbubbles. Langmuir 2008, 24, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Micheli, L.; Zhou, M.; Tortora, M.; Palleschi, G.; Ashokkumar, M. Electrochemical investigation of the interaction between lysozyme-shelled microbubbles and vitamin C. Anal. Bioanal. Chem. 2013, 405, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Zhou, M.; Ashokkumar, M.; Caruso, F. One-pot ultrasonic synthesis of multifunctional microbubbles and microcapsules using synthetic thiolated macromolecules. Chem. Commun. 2011, 47, 4096–4098. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shohet, R.V.; Bekeredjian, R.; Frenkel, P.; Grayburn, P.A. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J. Am. Coll. Cardiol. 2003, 42, 301–308. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef]
- Barmin, R.A.; Rudakovskaya, P.G.; Chernyshev, V.S.; Guslyakova, O.I.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Bratashov, D.N.; Abdurashitov, A.S.; Maksimova, E.A.; Demina, P.A.; et al. Impact of fluorescent dyes on the physicochemical parameters of microbubbles stabilized by albumin-dye complex. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129095. [Google Scholar] [CrossRef]
- Gorce, J.M.; Arditi, M.; Schneider, M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: A study of sonovue(TM). Investig. Radiol. 2000, 35, 661–671. [Google Scholar] [CrossRef]
- Newsome, I.G.; Kierski, T.M.; Dayton, P.A. Assessment of the Superharmonic Response of Microbubble Contrast Agents for Acoustic Angiography as a Function of Microbubble Parameters. Ultrasound Med. Biol. 2019, 45, 2515–2524. [Google Scholar] [CrossRef]
- Park, J.I.; Jagadeesan, D.; Williams, R.; Oakden, W.; Chung, S.; Stanisz, G.J.; Kumacheva, E. Microbubbles loaded with nanoparticles: A route to multiple imaging modalities. ACS Nano 2010, 4, 6579–6586. [Google Scholar] [CrossRef]
- Gazzera, L.; Milani, R.; Pirrie, L.; Schmutz, M.; Blanck, C.; Resnati, G.; Metrangolo, P.; Krafft, M.P. Design of Highly Stable Echogenic Microbubbles through Controlled Assembly of Their Hydrophobin Shell. Angew. Chem. Int. Ed. 2016, 55, 10263–10267. [Google Scholar] [CrossRef]
- Lukáč, R.; Kauerová, Z.; Mašek, J.; Bartheldyová, E.; Kulich, P.; Koudelka, Š.; Korvasová, Z.; Plocková, J.; Papoušek, F.; Kolář, F.; et al. Preparation of metallochelating microbubbles and study on their site-specific interaction with rGFP-HisTag as a model protein. Langmuir 2011, 27, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, E.C.; Bederman, I.; de Leon, A.C.; Zhu, J.; Hadley, J.; Kolios, M.C.; Exner, A.A. Theoretical and experimental gas volume quantification of micro-and nanobubble ultrasound contrast agents. Pharmaceutics 2020, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Liao, A.-H.; Chen, J.-H.; Chris Wang, C.-R.; Li, P.-C. Photoacoustic/ultrasound dual-modality contrast agent and its application to thermotherapy. J. Biomed. Opt. 2012, 17, 045001. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Tu, J.; Guo, X.; Huang, P.; Wu, J.; Zhang, D. Characterization of mechanical properties of hybrid contrast agents by combining atomic force microscopy with acoustic/optic assessments. J. Biomech. 2016, 49, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, J.A.; Tinkler, J.M.; Johnson, B.R.G.; McDowall, K.J.; Alsulaimani, F.; Tiede, C.; Tomlinson, D.C.; Freear, S.; Turnbull, W.B.; Evans, S.D.; et al. Protein-conjugated microbubbles for the selective targeting of S. aureus biofilms. Biofilm 2022, 4, 100074. [Google Scholar] [CrossRef]
- Hosny, N.A.; Mohamedi, G.; Rademeyer, P.; Owen, J.; Wu, Y.; Tang, M.X.; Eckersley, R.J.; Stride, E.; Kuimova, M.K. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl. Acad. Sci. USA 2013, 110, 9225–9230. [Google Scholar] [CrossRef]
- Park, B.; Yoon, S.; Choi, Y.; Jang, J.; Park, S.; Choi, J. Stability of engineered micro or nanobubbles for biomedical applications. Pharmaceutics 2020, 12, 1089. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O’Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Lu, W.; Huang, Q.; Ku, G.; Wen, X.; Zhou, M.; Guzatov, D.; Brecht, P.; Su, R.; Oraevsky, A.; Wang, L.V.; et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 2010, 31, 2617–2626. [Google Scholar] [CrossRef]
- Hannah, A.; Luke, G.; Wilson, K.; Homan, K.; Emelianov, S. Indocyanine green-loaded photoacoustic nanodroplets: Dual contrast nanoconstructs for enhanced photoacoustic and ultrasound imaging. ACS Nano 2014, 8, 250–259. [Google Scholar] [CrossRef]
- Dumani, D.S.; Sun, I.C.; Emelianov, S.Y. Ultrasound-guided immunofunctional photoacoustic imaging for diagnosis of lymph node metastases. Nanoscale 2019, 11, 11649–11659. [Google Scholar] [CrossRef] [PubMed]
- Paproski, R.J.; Forbrich, A.; Huynh, E.; Chen, J.; Lewis, J.D.; Zheng, G.; Zemp, R.J. Porphyrin Nanodroplets: Sub-micrometer Ultrasound and Photoacoustic Contrast Imaging Agents. Small 2016, 12, 371–380. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.G.; Reinders, J.H.; Sixma, J.J. Perturbation of human endothelial cells by thrombin or PMA changes the reactivity of their extracellular matrix towards platelets. J. Cell Biol. 1987, 104, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.B.; Hegge-Paping, K.S.M.; Magielse, C.P.E.; Blom, N.R.; Smit, J.W.; van der Meer, J.; Halie, M.R.; Bom, V.J.J. Tumor necrosis factor α-induced endothelial tissue factor is located on the cell surface rather than in the subendothelial matrix. Blood 1994, 84, 1559–1566. [Google Scholar] [CrossRef]
- Bevilacqua, M.P.; Pober, J.S.; Wheeler, M.E.; Cotran, R.S.; Gimbrone, M.A.J. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J. Clin. Investig. 1985, 76, 2003–2011. [Google Scholar] [CrossRef]
- Zwaginga, J. Activation of Endothelial Cells Induces Platelet Thrombus Formatiion on their Matrix. Atherosclerosis 1989, 10, 49–61. [Google Scholar]
- Schnitzer, J.E.; Carley, W.W.; Palade, G.E. Specific albumin binding to microvascular endothelium in culture. Am. J. Physiol.-Heart Circ. Physiol. 1988, 254, H425–H437. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am. J. Physiol.-Heart Circ. Physiol. 1992, 263, H1872–H1879. [Google Scholar] [CrossRef]
- Gimbrone, M.A. Vascular endothelium: An integrator of pathophysiologic stimuli in atherosclerosis. Am. J. Cardiol. 1995, 75, 67B–70B. [Google Scholar] [CrossRef]
- Meredith, I.T.; Anderson, T.J.; Uehata, A.; Yeung, A.C.; Selwyn, A.P.; Ganz, P. Role of endothelium in ischemic coronary syndromes. Am. J. Cardiol. 1993, 72, 27C–31C. [Google Scholar] [CrossRef]
- Anderson, T.J.; Gerhard, M.D.; Meredith, I.T.; Charbonneau, F.; Delagrange, D.; Creager, M.A.; Selwyn, A.P.; Ganz, P. Systemic nature of endothelial dysfunction in atherosclerosis. Am. J. Cardiol. 1995, 75, 71B–74B. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Gimbrone, M.A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liang, X.; Yin, T.; Chen, M.; Qiu, C.; Gao, C.; Wang, X.; Mao, Y.; Qu, E.; Dai, Z.; et al. Porphyrin-grafted lipid microbubbles for the enhanced efficacy of photodynamic therapy in prostate cancer through ultrasound-controlled in situ accumulation. Theranostics 2018, 8, 1665–1677. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D. V Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Cavalieri, F.; Zhou, M.; Ashokkumar, M. The Design of Multifunctional Microbubbles for Ultrasound Image-Guided Cancer Therapy. Curr. Top. Med. Chem. 2010, 10, 1198–1210. [Google Scholar] [CrossRef]
- Cavalieri, F.; Zhou, M.; Tortora, M.; Lucilla, B.; Ashokkumar, M. Methods of Preparation of Multifunctional Microbubbles and their In Vitro/In Vivo Assessment of Stability, Functional and Structural Properties. Curr. Pharm. Des. 2012, 18, 2135–2151. [Google Scholar] [CrossRef]
- Tsutsui, J.M.; Xie, F.; Porter, R.T. The use of microbubbles to target drug delivery. Cardiovasc. Ultrasound 2004, 2, 23. [Google Scholar] [CrossRef]
- Bao, S.; Thrall, B.D.; Miller, D.L. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med. Biol. 1997, 23, 953–959. [Google Scholar] [CrossRef]
- Shohet, R.V.; Chen, S.; Zhou, Y.T.; Wang, Z.; Meidell, R.S.; Unger, R.H.; Grayburn, P.A. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation 2000, 101, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian, R.; Chen, S.; Frenkel, P.A.; Grayburn, P.A.; Shohet, R.V. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation 2003, 108, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Tachibana, K.; Hiraoka, K.; Namba, T.; Yamasaki, K.; Hashiya, N.; Aoki, M.; Ogihara, T.; Yasufumi, K.; Morishita, R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 2002, 105, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, P.A.; Chen, S.; Thai, T.; Shohet, R.V.; Grayburn, P.A. DNA-loaded albumin microbubbles enhance ultrasound-mediated transfection in vitro. Ultrasound Med. Biol. 2002, 28, 817–822. [Google Scholar] [CrossRef]
- Fleming, A.; Allison, V.D. Observations on a Bacteriolytic Substance (“Lysozyme”) Found in Secretions and Tissues. Br. J. Exp. Pathol. 1922, 3, 252–260. [Google Scholar]
- Varahan, S.; Iyer, V.S.; Moore, W.T.; Hancock, L.E. Eep confers lysozyme resistance to enterococcus faecalis via the activation of the extracytoplasmic function sigma factor SigV. J. Bacteriol. 2013, 195, 3125–3134. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Cheung, B.P.K.; Parahitiyawa, N.; Seneviratne, C.J.; Yau, J.Y.Y.; Yeung, K.W.S.; Samaranayake, L.P. Synergistic activity of lysozyme and antifungal agents against Candida albicans biofilms on denture acrylic surfaces. Arch. Oral Biol. 2009, 54, 115–126. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Lammers, T.; Koczera, P.; Fokong, S.; Gremse, F.; Ehling, J.; Vogt, M.; Pich, A.; Storm, G.; Van Zandvoort, M.; Kiessling, F. Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv. Funct. Mater. 2015, 25, 36–43. [Google Scholar] [CrossRef]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused ultrasound and microbubbles-mediated drug delivery to brain tumor. Pharmaceutics 2021, 13, 15. [Google Scholar] [CrossRef]
- Kost, J.; Mitragotri, S.; Gabbay, R.A.; Pishko, M.; Langer, R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat. Med. 2000, 6, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Azagury, A.; Khoury, L.; Enden, G.; Kost, J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Schnaider, L.; Shimonov, L.; Kreiser, T.; Zaguri, D.; Bychenko, D.; Brickner, I.; Kolusheva, S.; Lichtenstein, A.; Kost, J.; Gazit, E. Ultrashort Cell-Penetrating Peptides for Enhanced Sonophoresis-Mediated Transdermal Transport. ACS Appl. Bio Mater. 2020, 3, 8395–8401. [Google Scholar] [CrossRef] [PubMed]
| Microfluidics Type | Primary Shell Material | Additives | Gaseous Core | Size (µm) | Ref. |
|---|---|---|---|---|---|
| Flow-focusing | BSA (3%, 5%) | None/ Dextrose/Glycerol, propylene glycol, and isotonic saline | N2 | 10–20 | [124] |
| Oleosin | Pluronic F68/Pluronic F127 | N2, C4F8 | 3.9 ± 0.2 | [125] | |
| BSA (3%) | Dextrose (10%) | N2 | 9.1–19.8 | [126] | |
| BSA (4%) | Dextrose (10%) | N2 | 9.8 ± 0.3–31.1 ± 1.4 | [127] | |
| Oleosin | Pluronic F68/Pluronic F77/ Pluronic F105/ Pluronic P65 | N2 | 2–4 | [95] | |
| Oleosin | Pluronic F68, Methylene Blue | N2 | 2–4 | [96] | |
| T-junction | BSA (15%) | None/ Tween 40/phospholipid solution | Air | 81 ± 2–555 ± 3 | [128] |
| BSA (15%) | - | N2 | 272 ± 5 | [129] | |
| BSA (15%) | None/Glutaraldehyde (0.75%) | N2 | 270 ± 2 | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudakovskaya, P.G.; Barmin, R.A.; Kuzmin, P.S.; Fedotkina, E.P.; Sencha, A.N.; Gorin, D.A. Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems. Pharmaceutics 2022, 14, 1236. https://doi.org/10.3390/pharmaceutics14061236
Rudakovskaya PG, Barmin RA, Kuzmin PS, Fedotkina EP, Sencha AN, Gorin DA. Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems. Pharmaceutics. 2022; 14(6):1236. https://doi.org/10.3390/pharmaceutics14061236
Chicago/Turabian StyleRudakovskaya, Polina G., Roman A. Barmin, Pavel S. Kuzmin, Elena P. Fedotkina, Alexander N. Sencha, and Dmitry A. Gorin. 2022. "Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems" Pharmaceutics 14, no. 6: 1236. https://doi.org/10.3390/pharmaceutics14061236
APA StyleRudakovskaya, P. G., Barmin, R. A., Kuzmin, P. S., Fedotkina, E. P., Sencha, A. N., & Gorin, D. A. (2022). Microbubbles Stabilized by Protein Shell: From Pioneering Ultrasound Contrast Agents to Advanced Theranostic Systems. Pharmaceutics, 14(6), 1236. https://doi.org/10.3390/pharmaceutics14061236








