Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analytical Method for PTX
2.2.2. Defining the Quality Target Product Profile (QTPP) and Critical Quality Attributes (CQAs)
2.2.3. Initial Risk Assessment and Factor Screening Study
2.2.4. Preparation of αTS-PTX-NLC and αTS-PTX-PEG-NLC
2.2.5. Particle Size Analysis, Poly Dispersity Index (PDI) and Zeta Potential (ZP) Determination
2.2.6. Entrapment Efficiency
Assay of PTX-Loaded NLCs
2.2.7. Sterilization of the Formulation by Autoclave
2.2.8. In Vitro Drug Release
2.2.9. Morphology of the PTX-NLC by Transmission Electron Microscopy (TEM)
3. Results and Discussion
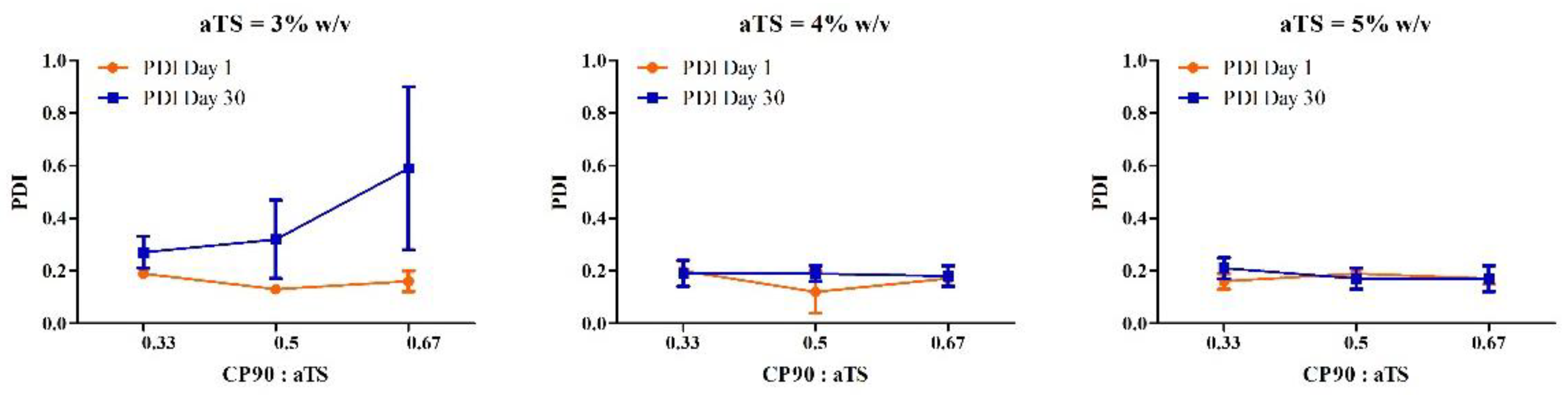
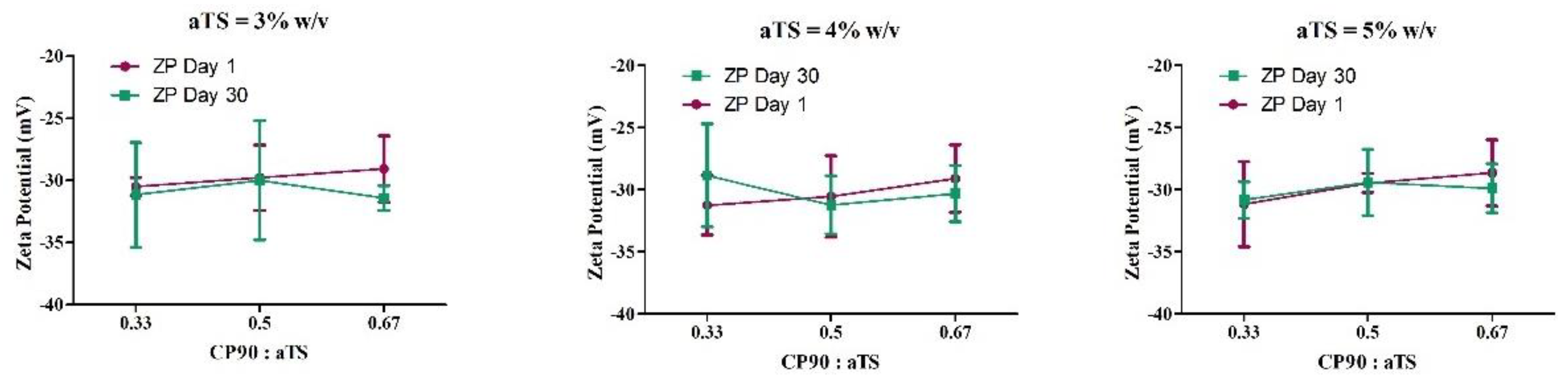
3.1. Optimization of the Lipid Ratio
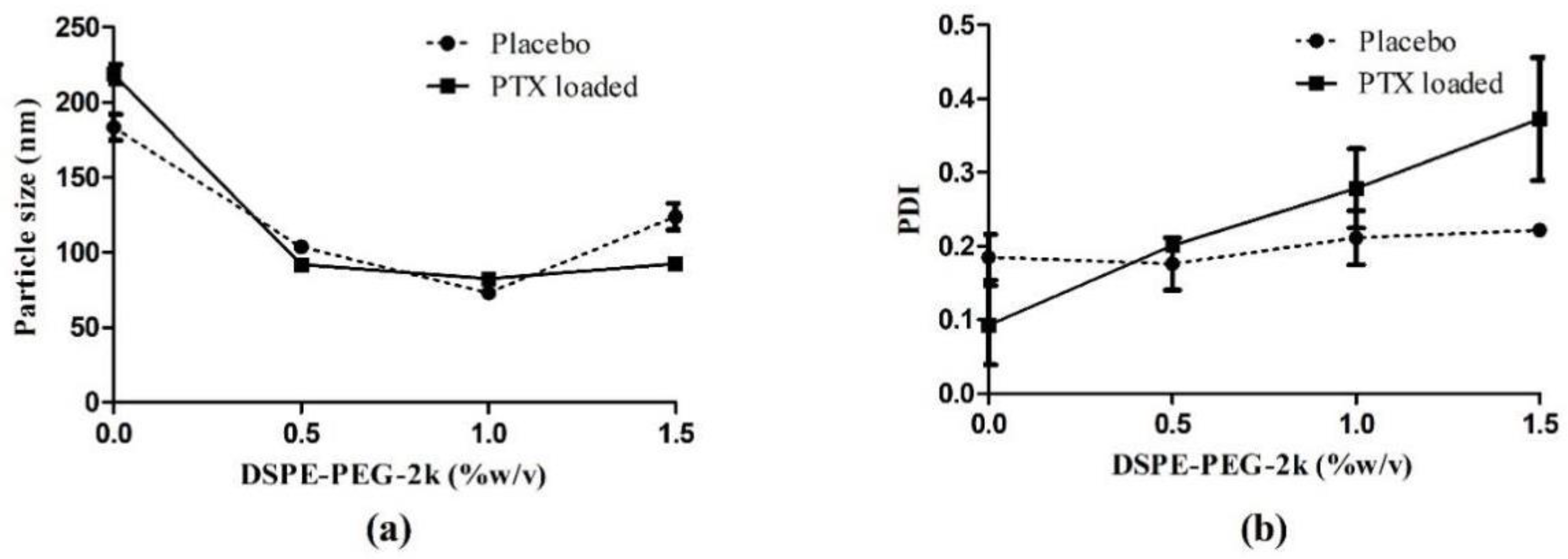
3.2. Optimization of DSPE-PEG-2k Concentration
3.3. Drug Release Study
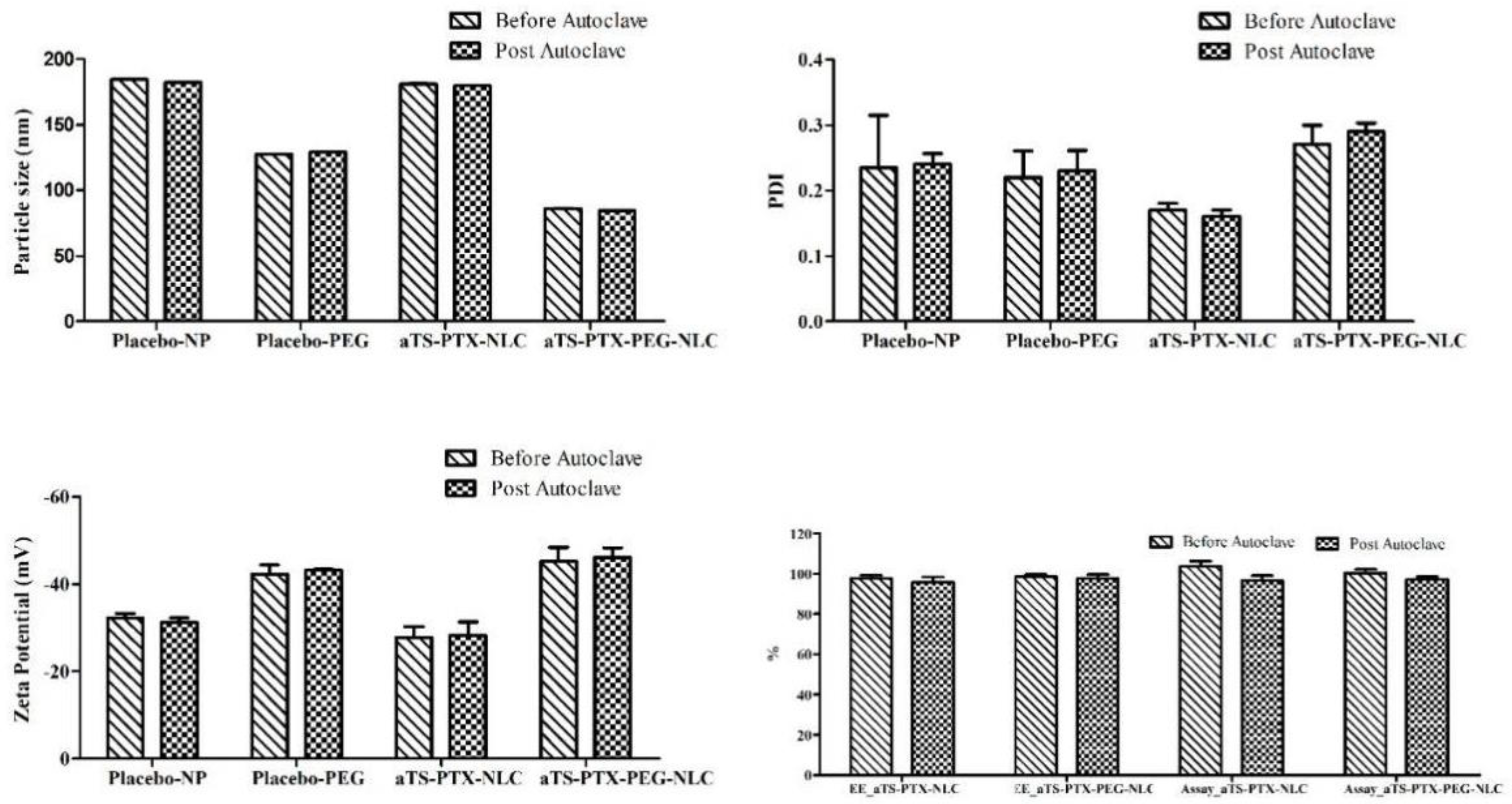
3.4. Sterilization of Formulation
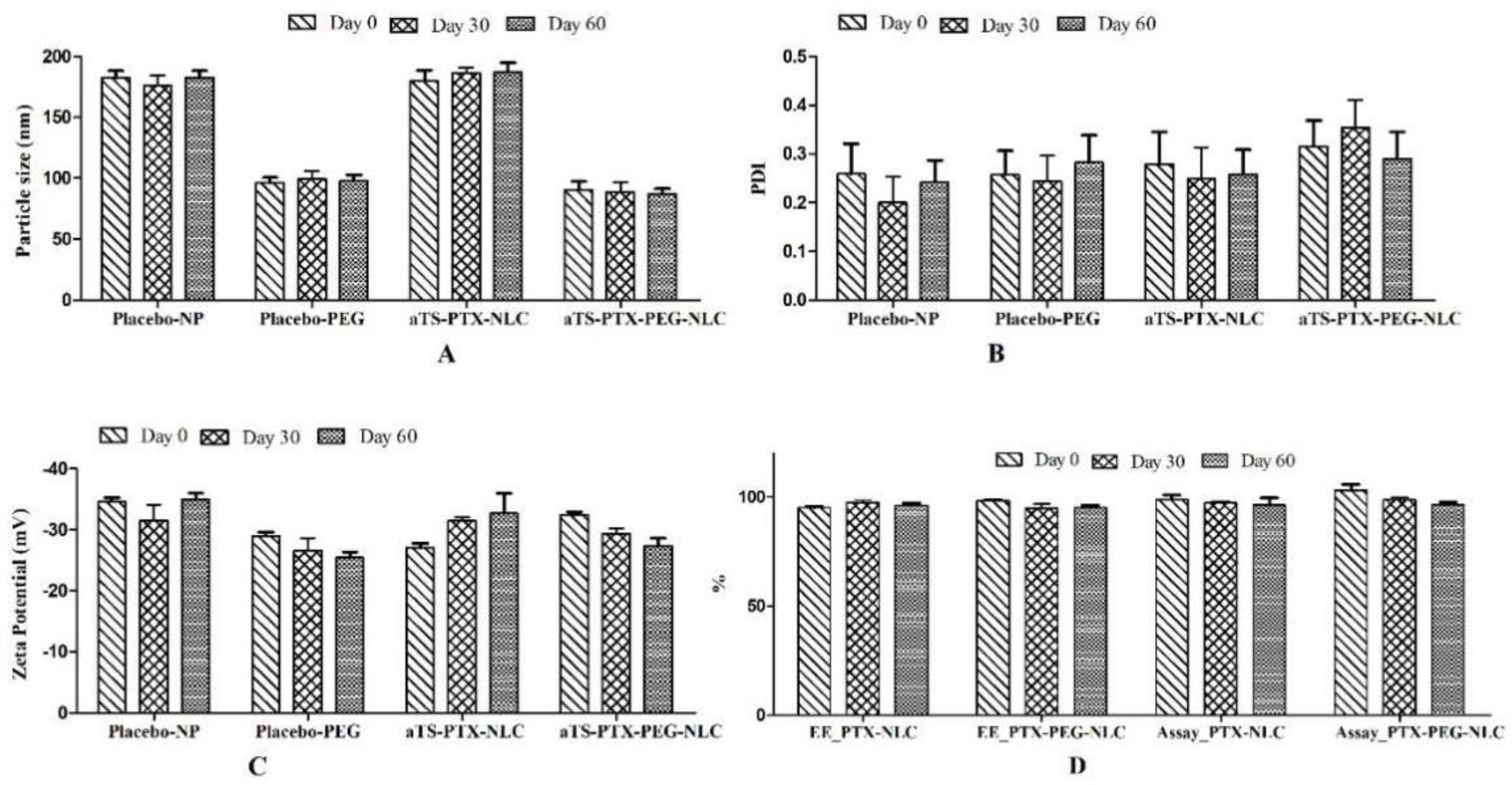
3.5. Stability Studies
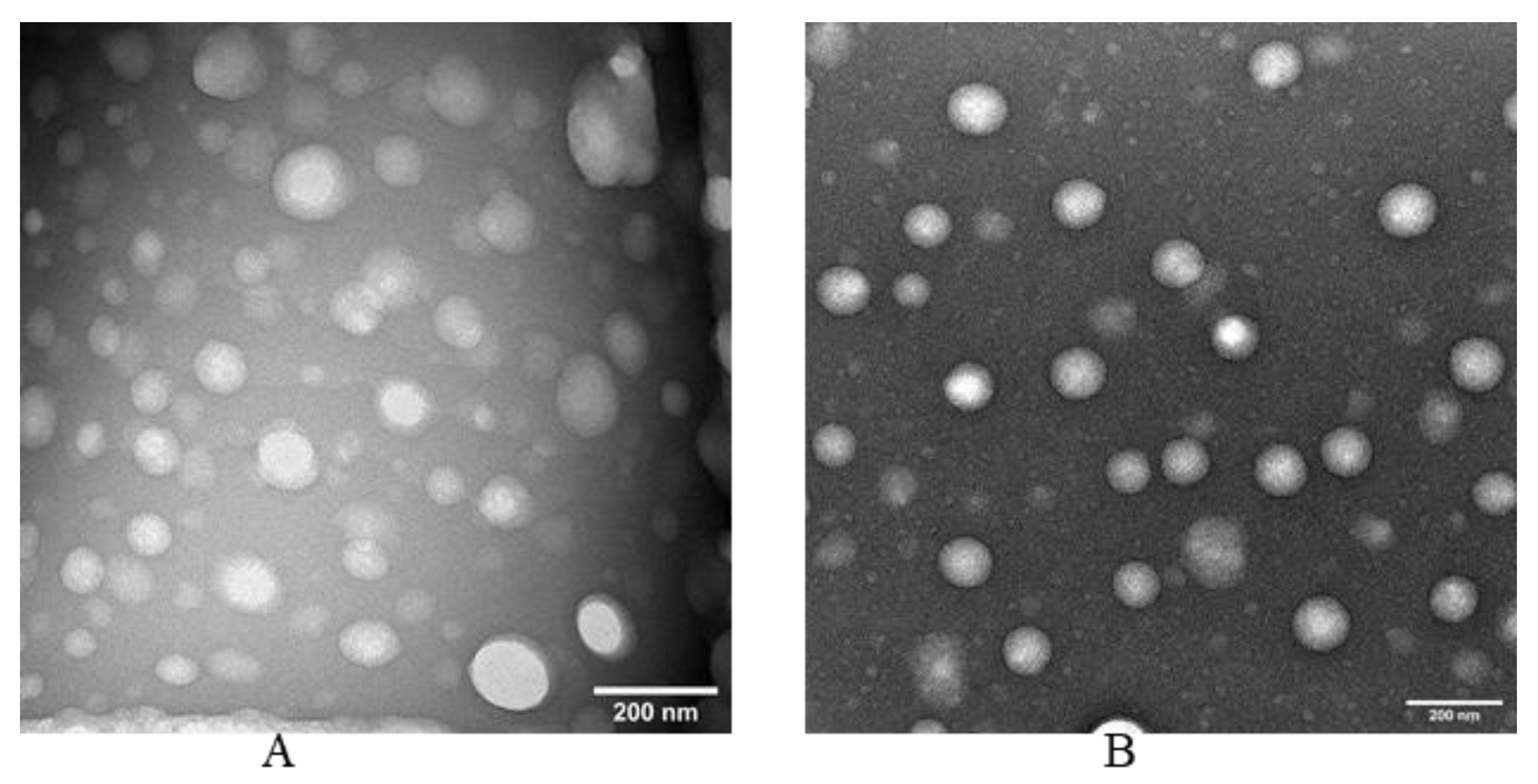
3.6. Electron Microscopy of NLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aerts, I.; Rouic, L.L.; Gauthier-Villars, M.; Brisse, H.; Doz, F.; Desjardins, L. Retinoblastoma. Orphanet J. Rare Dis. 2006, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Fabian, I.D.; Onadim, Z.; Karaa, E.; Duncan, C.; Chowdhury, T.; Scheimberg, I.; Ohnuma, S.I.; Ashwin Reddy, M.; Sagoo, M.S. The Management of Retinoblastoma. Oncogene 2018, 37, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, D.L.; Sage, J. Cellular Mechanisms of Tumour Suppression by the Retinoblastoma Gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Drago-Ferrante, R.; Santulli, A.; Di Fiore, R.; Giuliano, M.; Calvaruso, G.; Tesoriere, G.; Vento, R. Low Doses of Paclitaxel Potently Induce Apoptosis in Human Retinoblastoma Y79 Cells by Up-Regulating E2F1. Int. J. Oncol. 2008, 33, 677–687. [Google Scholar]
- Patil, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-Mediated Simultaneous and Targeted Delivery of Paclitaxel and Tariquidar Overcomes Tumor Drug Resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and Metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Neuzil, J.; Weber, T.; Gellert, N.; Weber, C. Selective Cancer Cell Killing by α-Tocopheryl Succinate. Br. J. Cancer 2001, 84, 87–89. [Google Scholar] [CrossRef]
- Gu, X.; Song, X.; Dong, Y.; Cai, H.; Walters, E.; Zhang, R.; Pang, X.; Xie, T.; Guo, Y.; Sridhar, R. Vitamin E Succinate Induces Ceramide-Mediated Apoptosis in Head and Neck Squamous Cell Carcinoma in Vitro and in Vivo. Clin. Cancer Res. 2008, 14, 1840–1848. [Google Scholar] [CrossRef]
- Traber, M.G.; Packer, L. Vitamin E: Beyond Antioxidant Function. Am. J. Clin. Nutr. 1995, 62, 1501S–1509S. [Google Scholar] [CrossRef]
- Ni, J.; Chen, M.; Zhang, Y.; Li, R.; Huang, J.; Yeh, S. Vitamin E Succinate Inhibits Human Prostate Cancer Cell Growth via Modulating Cell Cycle Regulatory Machinery. Biochem. Biophys. Res. Commun. 2003, 300, 357–363. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Guo, Y.-H.; Gu, X.-B. Anticancer Mechanisms of Vitamin E Succinate. Ai Zheng Aizheng Chin. J. Cancer 2009, 28, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Mussi, S.V.; Gomes, D.A.; Yoshida, M.I.; Frezard, F.; Carregal, V.M.; Ferreira, L.A.M. α-Tocopherol Succinate Improves Encapsulation and Anticancer Activity of Doxorubicin Loaded in Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2016, 140, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Han, J.; Dou, H. Paclitaxel-Loaded Tocopheryl Succinate-Conjugated Chitosan Oligosaccharide Nanoparticles for Synergistic Chemotherapy. J. Mater. Chem. 2012, 22, 8930–8937. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Rostami, M.; Hassanzadeh, F.; Sadeghi, H.; Mostafavi, A.; Minaiyan, M.; Lavasanifar, A. Co-Delivery of Paclitaxel and α-Tocopherol Succinate by Novel Chitosan-Based Polymeric Micelles for Improving Micellar Stability and Efficacious Combination Therapy. Drug Dev. Ind. Pharm. 2015, 41, 1137–1147. [Google Scholar] [CrossRef]
- Duhem, N.; Danhier, F.; Pourcelle, V.; Schumers, J.-M.; Bertrand, O.; LeDuff, C.S.; Hoeppener, S.; Schubert, U.S.; Gohy, J.-F.; Marchand-Brynaert, J. Self-Assembling Doxorubicin—Tocopherol Succinate Prodrug as a New Drug Delivery System: Synthesis, Characterization, and in Vitro and in Vivo Anticancer Activity. Bioconjugate Chem. 2014, 25, 72–81. [Google Scholar] [CrossRef]
- Lu, J.; Liu, C.; Wang, P.; Ghazwani, M.; Xu, J.; Huang, Y.; Ma, X.; Zhang, P.; Li, S. The Self-Assembling Camptothecin-Tocopherol Prodrug: An Effective Approach for Formulating Camptothecin. Biomaterials 2015, 62, 176–187. [Google Scholar] [CrossRef]
- Meher, J.G.; Dixit, S.; Pathan, D.K.; Singh, Y.; Chandasana, H.; Pawar, V.K.; Sharma, M.; Bhatta, R.S.; Konwar, R.; Kesharwani, P.; et al. Paclitaxel-Loaded TPGS Enriched Self-Emulsifying Carrier Causes Apoptosis by Modulating Survivin Expression and Inhibits Tumour Growth in Syngeneic Mammary Tumours. Artif. Cells Nanomed. Biotechnol. 2018, 46, S344–S358. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Muggia, F.M. Complement Activation by Cremophor EL as a Possible Contributor to Hypersensitivity to Paclitaxel: An in Vitro Study. JNCI J. Natl. Cancer Inst. 1998, 90, 300–306. [Google Scholar] [CrossRef]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane®, a Novel Cremophor®-Free, Albumin-Bound Particle Form of Paclitaxel for the Treatment of Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-Infrared Mediated Quantum Dots and Paclitaxel Co-Loaded Nanostructured Lipid Carriers for Cancer Theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.-H.; Na, Y.-G.; Huh, H.W.; Hwang, S.-J.; Kim, M.-S.; Kim, M.; Lee, H.-K.; Cho, C.-W. The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers 2019, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, M.; Zhang, L.; Feng, L.; Zhang, N. Hyaluronic Acid-Coated Nanostructured Lipid Carriers for Targeting Paclitaxel to Cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Koh, G.Y.; Dong, X.; Hollingsworth, J.; Zhang, J.; Russo, P.S.; Yang, P.; Stout, R.W. Cytotoxic and Anti-Angiogenic Paclitaxel Solubilized and Permeation-Enhanced by Natural Product Nanoparticles. Anticancer Drugs 2015, 26, 167–179. [Google Scholar] [CrossRef]
- Shadambikar, G.; Marathe, S.; Ji, N.; Almutairi, M.; Bandari, S.; Zhang, F.; Chougule, M.; Repka, M. Formulation Development of Itraconazole PEGylated Nano-Lipid Carriers for Pulmonary Aspergillosis Using Hot-Melt Extrusion Technology. Int. J. Pharm. X 2021, 3, 100074. [Google Scholar] [CrossRef]
- Patil, A.; Lakhani, P.; Taskar, P.; Wu, K.-W.; Sweeney, C.; Avula, B.; Wang, Y.-H.; Khan, I.A.; Majumdar, S. Formulation Development, Optimization, and In Vitro—In Vivo Characterization of Natamycin-Loaded PEGylated Nano-Lipid Carriers for Ocular Applications. J. Pharm. Sci. 2018, 107, 2160–2171. [Google Scholar] [CrossRef]
- Shao, Z.; Shao, J.; Tan, B.; Guan, S.; Liu, Z.; Zhao, Z.; He, F.; Zhao, J. Targeted Lung Cancer Therapy: Preparation and Optimization of Transferrin-Decorated Nanostructured Lipid Carriers as Novel Nanomedicine for Co-Delivery of Anticancer Drugs and DNA. Int. J. Nanomed. 2015, 10, 1223–1233. [Google Scholar] [CrossRef]
- De Sousa Marcial, S.P.; Carneiro, G.; Leite, E.A. Lipid-Based Nanoparticles as Drug Delivery System for Paclitaxel in Breast Cancer Treatment. J. Nanoparticle Res. 2017, 19, 340. [Google Scholar] [CrossRef]
- Lee, M.K.; Lim, S.J.; Kim, C.K. Preparation, Characterization and in Vitro Cytotoxicity of Paclitaxel-Loaded Sterically Stabilized Solid Lipid Nanoparticles. Biomaterials 2007, 28, 2137–2146. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, J.; Du, Y.Z.; You, J.; Hu, F.Q.; Zeng, S. Cellular Uptake of Solid Lipid Nanoparticles and Cytotoxicity of Encapsulated Paclitaxel in A549 Cancer Cells. Int. J. Pharm. 2008, 348, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Balguri, S.P.; Adelli, G.R.; Janga, K.Y.; Bhagav, P.; Majumdar, S. Ocular Disposition of Ciprofloxacin from Topical, PEGylated Nanostructured Lipid Carriers: Effect of Molecular Weight and Density of Poly (Ethylene) Glycol. Int. J. Pharm. 2017, 529, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Z.; Ho, P.C.; Lee, H.S.; Vaddi, H.K.; Chan, Y.W.; Yung, C.S. Quantitation of Paclitaxel in Micro-Sample Rat Plasma by a Sensitive Reversed-Phase HPLC Assay. J. Pharm. Biomed. Anal. 2003, 31, 283–289. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Pal, T.K. Development and Validation of RP-HPLC Method: Scope of Application in the Determination of Oil Solubility of Paclitaxel. J. Chromatogr. Sci. 2014, 52, 68–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badea, I.; Ciutaru, D.; Lazar, L.; Nicolescu, D.; Tudose, A. Rapid HPLC Method for the Determination of Paclitaxel in Pharmaceutical Forms without Separation. J. Pharm. Biomed. Anal. 2004, 34, 501–507. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Müllertz, A.; Norling, T.; Kristensen, H.G. The Effect of α-Tocopherol on the in Vitro Solubilisation of Lipophilic Drugs. Int. J. Pharm. 2001, 222, 217–224. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Li, X.; Piao, H.; Piao, H.; Cui, F.; Fang, L. α-Tocopherol Succinate-Modified Chitosan as a Micellar Delivery System for Paclitaxel: Preparation, Characterization and in Vitro/in Vivo Evaluations. Int. J. Pharm. 2012, 423, 480–488. [Google Scholar] [CrossRef]
- Sweeney, C.; Dudhipala, N.; Thakkar, R.; Mehraj, T.; Marathe, S.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Effect of Surfactant Concentration and Sterilization Process on Intraocular Pressure–Lowering Activity of Δ9-Tetrahydrocannabinol-Valine-Hemisuccinate (NB1111) Nanoemulsions. Drug Deliv. Transl. Res. 2021, 11, 2096–2107. [Google Scholar] [CrossRef]
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=browseByLetter.page&Letter=P (accessed on 18 January 2022).
- Youssef, A.; Dudhipala, N.; Majumdar, S. Ciprofloxacin Loaded Nanostructured Lipid Carriers Incorporated into In-Situ Gels to Improve Management of Bacterial Endophthalmitis. Pharmaceutics 2020, 12, 572. [Google Scholar] [CrossRef]
- Beg, S.; Kaur, R.; Khurana, R.K.; Rana, V.; Sharma, T.; Singh, B. QbD-Based Development of Cationic Self-Nanoemulsifying Drug Delivery Systems of Paclitaxel with Improved Biopharmaceutical Attributes. AAPS PharmSciTech 2019, 20, 118. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Saha, D.R.; Ulhosna, T.; Sharker, S.M.; Shohag, M.H.; Islam, M.S.; Ray, S.K.; Rahman, G.M.S.; Reza, H.M. QbD Based Development of Resveratrol-Loaded Mucoadhesive Lecithin/Chitosan Nanoparticles for Prolonged Ocular Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102480. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Loboa, J.M.S. Applications of Polymeric and Lipid Nanoparticles in Ophthalmic Pharmaceutical Formulations: Present and Future Considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3—Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. ISBN 978-0-12-814031-4. [Google Scholar]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional Nanocrystals for Cancer Therapy: A Potential Nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. ISBN 978-0-12-816505-8. [Google Scholar]
- Stevens, J.P. Intermediate Statistics: A Modern Approach, 3rd ed.; Routledge: Abingdon-on-Thames, UK, 2013; ISBN 978-1-136-67719-9. [Google Scholar]
- Keselman, H.J.; Algina, J.; Kowalchuk, R.K. The Analysis of Repeated Measures Designs: A Review. Br. J. Math. Stat. Psychol. 2001, 54, 1–20. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, and In Vitro Cytotoxicity. J. Nanomater. 2012, 2012, 358782. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, M.; Jin, S.; Fan, L.; Zhu, W.; Sui, X.; Cao, L.; Yang, C.; Han, C. Combined Using of Paclitaxel and Salinomycin Active Targeting Nanostructured Lipid Carriers against Non-Small Cell Lung Cancer and Cancer Stem Cells. Drug Deliv. 2019, 26, 281–289. [Google Scholar] [CrossRef]
- Pignatello, R.; Leonardi, A.; Pellitteri, R.; Carbone, C.; Caggia, S.; Graziano, A.C.E.; Cardile, V. Evaluation of New Amphiphilic PEG Derivatives for Preparing Stealth Lipid Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 136–144. [Google Scholar] [CrossRef]
- Cho, Y.W.; Lee, J.; Lee, S.C.; Huh, K.M.; Park, K. Hydrotropic Agents for Study of in Vitro Paclitaxel Release from Polymeric Micelles. J. Control. Release 2004, 97, 249–257. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.; García, M. Lipid Nanoparticles (SLN, NLC): Overcoming the Anatomical and Physiological Barriers of the Eye–Part II-Ocular Drug-Loaded Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Arnold, Y.E.; Imanidis, G.; Kuentz, M. In Vitro Digestion Kinetics of Excipients for Lipid-Based Drug Delivery and Introduction of a Relative Lipolysis Half Life. Drug Dev. Ind. Pharm. 2012, 38, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of Solid Lipid Nanoparticles as a Function of Lipase Incubation Time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Gökçe, E.H.; Sandri, G.; Eğrilmez, S.; Bonferoni, M.C.; Güneri, T.; Caramella, C. Cyclosporine A-Loaded Solid Lipid Nanoparticles: Ocular Tolerance and In Vivo Drug Release in Rabbit Eyes. Curr. Eye Res. 2009, 34, 996–1003. [Google Scholar] [CrossRef]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Güneri, T.; Caramella, C. Cyclosporine A Loaded SLNs: Evaluation of Cellular Uptake and Corneal Cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Karuppayil, S.M.; Raut, J.S.; Giansanti, F.; Papucci, L.; Schiavone, N.; Kaur, I.P. Lipid-Polyethylene Glycol Based Nano-Ocular Formulation of Ketoconazole. Int. J. Pharm. 2015, 495, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2019, 9, 33. [Google Scholar] [CrossRef]
- Palem, C.R.; Gannu, R.; Doodipala, N.; Yamsani, V.V.; Yamsani, M.R. Transmucosal delivery of domperidone from bilayered buccal patches: In vitro, ex vivo and in vivo characterization. Arch. Pharmacal Res. 2011, 34, 1701–1710. [Google Scholar] [CrossRef]
- Dudhipala, N.; Ettireddy, S.; Youssef, A.A.; Puchchakayala, G. Cyclodextrin Complexed Lipid Nanoparticles of Irbesartan for Oral Applications: Design, Development, and In Vitro Characterization. Molecules 2021, 26, 7538. [Google Scholar] [CrossRef]
- Nagaraj, B.; Tirumalesh, C.; Dinesh, S.; Narendar, D. Zotepine loaded lipid nanoparticles for oral delivery: Development, characterization, and in vivo pharmacokinetic studies. Future J. Pharm. Sci. 2020, 6, 37. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of Light and Temperature on Zeta Potential and Physical Stability in Solid Lipid Nanoparticle (SLNTM) Dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]



| Attribute | QTPP | Justification |
|---|---|---|
| Dosage form type | Lipid nanoparticles | Biocompatible and helps to increase the bioavailability of the drug with controlled release. Enhance cellular uptake of the drug. |
| Route of administration | Precorneal and intravitreal injection | Administration in the precorneal cul-de-sac is the most patient-friendly route for ocular drug delivery. While the intravitreal injection is a bit challenging, it can deliver a high dose of drug at the site and help achieve drug depot formation. |
| Storage stability | At least 2 months in suspension form | The NLCs need to be stable for a longer duration in suspension form for multiple administration. The stability on dilution to ease dose adjustment. |
| Quality Attributes | Target | Is This a CQA? | Justification |
|---|---|---|---|
| Physical attributes | No | Color, odor, and appearance were not considered as critical as these are not directly linked to patients. | |
| Drug content | More than 95% | Yes | Drug content is vital for any pharmaceutical dosage form for attaining the maximal plasma concentration of a drug. The drug content does not decrease substantially in the selected method and hence was regarded as moderately critical. |
| Particle size | Less than 200 nm | Yes | As NLC was administered through the ocular route, particle size will have a significant impact on the drug absorption. So, it was considered as critical. |
| Entrapment efficiency | More than 95% | Yes | Higher entrapment efficiency is vital for accomplishing maximum drug release regulation from dosage form and hence the therapeutic concentration of the drug. Thus, it was considered as critical. |
| Amount of drug release after 60 min | Less than 50% | Yes | The amount of drug released in the first hour will decide the promptness of the formulation effect; thus, it is taken as critical. The deterred release will help to retain the drug within the NLC. |
| # | αTS (%) | CP90:αTS | PTX (% w/v) | Particle Size (nm) | PDI | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | ||||
| F1 | 3.00 | 0.33 | 0.1 | 182.83 ± 5.26 | 232.92 ± 24.30 | 0.19 ± 0.01 | 0.27 ± 0.06 | −30.51 ± 0.72 | −31.16 ± 4.20 |
| F2 | 3.00 | 0.5 | 0.1 | 166.13 ± 5.53 | 196.47 ± 5.92 | 0.13 ± 0.01 | 0.32 ± 0.15 | −29.77 ± 2.62 | −30.01 ± 4.80 |
| F3 | 3.00 | 0.67 | 0.1 | 153.97 ± 3.95 | 244.81 ± 8.21 | 0.16 ± 0.04 | 0.59 ± 0.31 | −29.08 ± 2.65 | −31.42 ± 0.98 |
| F4 | 4.00 | 0.33 | 0.1 | 208.97 ± 10.52 | 186.16 ± 6.79 | 0.20 ± 0.01 | 0.19 ± 0.05 | −31.25 ± 2.41 | −28.85 ± 4.14 |
| F5 | 4.00 | 0.5 | 0.1 | 214.17 ± 11.34 | 177.51 ± 5.16 | 0.12 ± 0.08 | 0.19 ± 0.03 | −30.54 ± 3.28 | −31.22 ± 2.34 |
| F6 | 4.00 | 0.67 | 0.1 | 195.77 ± 3.67 | 175.28 ± 6.15 | 0.17 ± 0.00 | 0.18 ± 0.04 | −29.11 ± 2.72 | −30.31 ± 2.26 |
| F7 | 5.00 | 0.33 | 0.1 | 237.93 ± 9.25 | 214.21 ± 6.93 | 0.16 ± 0.03 | 0.21 ± 0.04 | −31.18 ± 3.42 | −30.83 ± 1.48 |
| F8 | 5.00 | 0.5 | 0.1 | 223.30 ± 8.95 | 199.92 ± 5.34 | 0.19 ± 0.02 | 0.17 ± 0.04 | −29.47 ± 0.77 | −29.42 ± 2.67 |
| F9 | 5.00 | 0.67 | 0.1 | 198.50 ± 1.47 | 203.66 ± 6.62 | 0.17 ± 0.02 | 0.17 ± 0.05 | −28.65 ± 2.67 | −29.89 ± 1.97 |
| Within-Subject Effects | p-Value | ||
|---|---|---|---|
| Particle Size | PDI | Zeta Potential | |
| Stability | 0.001 | <0.001 | 0.288 |
| Stability*αTS | <0.001 | <0.001 | 0.378 |
| Stability*CP90:αTS | <0.001 | 0.101 | 0.057 |
| Stability*αTS*CP90:αTS | <0.001 | 0.029 | 0.566 |
| Between-Subject Effects | p-Value | ||
|---|---|---|---|
| Particle Size | PDI | Zeta Potential | |
| αTS | <0.001 | 0.009 | 0.941 |
| CP90:αTS | 0.001 | 0.275 | 0.772 |
| αTS*CP90:αTS | 0.02 | 0.233 | 0.938 |
| αTS (% w/v) | CP90 (% w/v) | PTX (% w/v) | DSPE-PEG-2k (% w/v) | Particle Size (nm) | PDI |
|---|---|---|---|---|---|
| 4.0 | 2.0 | - | 0 | 183.30 ± 8.41 | 0.19 ± 0.03 |
| 4.0 | 2.0 | - | 0.5 | 103.73 ± 1.79 | 0.18 ± 0.04 |
| 4.0 | 2.0 | - | 1 | 72.74 ± 0.55 | 0.21 ± 0.04 |
| 4.0 | 2.0 | - | 1.5 | 123.70 ± 8.62 | 0.22 ± 0.01 |
| 4.0 | 2.0 | 0.1 | 0 | 218.83 ± 6.38 | 0.09 ± 0.05 |
| 4.0 | 2.0 | 0.1 | 0.5 | 91.80 ± 1.69 | 0.20 ± 0.00 |
| 4.0 | 2.0 | 0.1 | 1 | 82.37 ± 1.61 | 0.28 ± 0.05 |
| 4.0 | 2.0 | 0.1 | 1.5 | 92.40 ± 4.14 | 0.37 ± 0.08 |
| Variable | p-Value | |
|---|---|---|
| Particle Size | PDI | |
| DPPE-PEG-2k | <0.001 | <0.001 |
| PTX | 0.821 | 0.058 |
| DSPE-PEG-2K*PTX | <0.001 | 0.002 |
| Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas Model | ||
|---|---|---|---|---|---|
| R2 | n | ||||
| αTS-PTX-NLC | 0.9376 | 0.9824 | 0.7445 | 0.9880 | 0.7408 |
| αTS-PTX-PEG-NLC | 0.9102 | 0.9744 | 0.9793 | 0.9991 | 0.7409 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. https://doi.org/10.3390/pharmaceutics14051034
Marathe S, Shadambikar G, Mehraj T, Sulochana SP, Dudhipala N, Majumdar S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics. 2022; 14(5):1034. https://doi.org/10.3390/pharmaceutics14051034
Chicago/Turabian StyleMarathe, Sushrut, Gauri Shadambikar, Tabish Mehraj, Suresh P. Sulochana, Narendar Dudhipala, and Soumyajit Majumdar. 2022. "Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel" Pharmaceutics 14, no. 5: 1034. https://doi.org/10.3390/pharmaceutics14051034
APA StyleMarathe, S., Shadambikar, G., Mehraj, T., Sulochana, S. P., Dudhipala, N., & Majumdar, S. (2022). Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics, 14(5), 1034. https://doi.org/10.3390/pharmaceutics14051034