Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Chemical Compounds
2.1.2. Synthesis of Hydrogel Discs and Contact Lenses
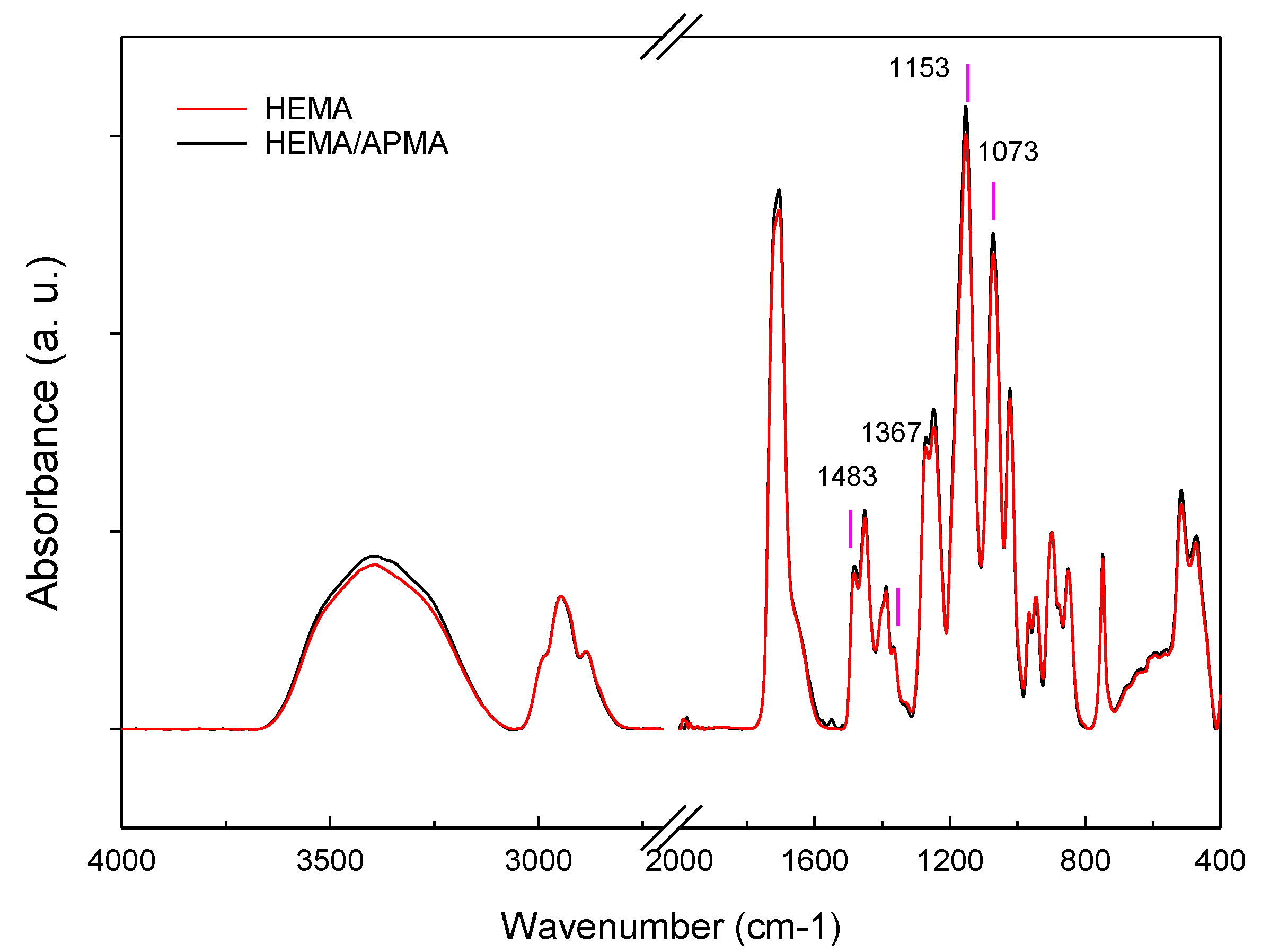
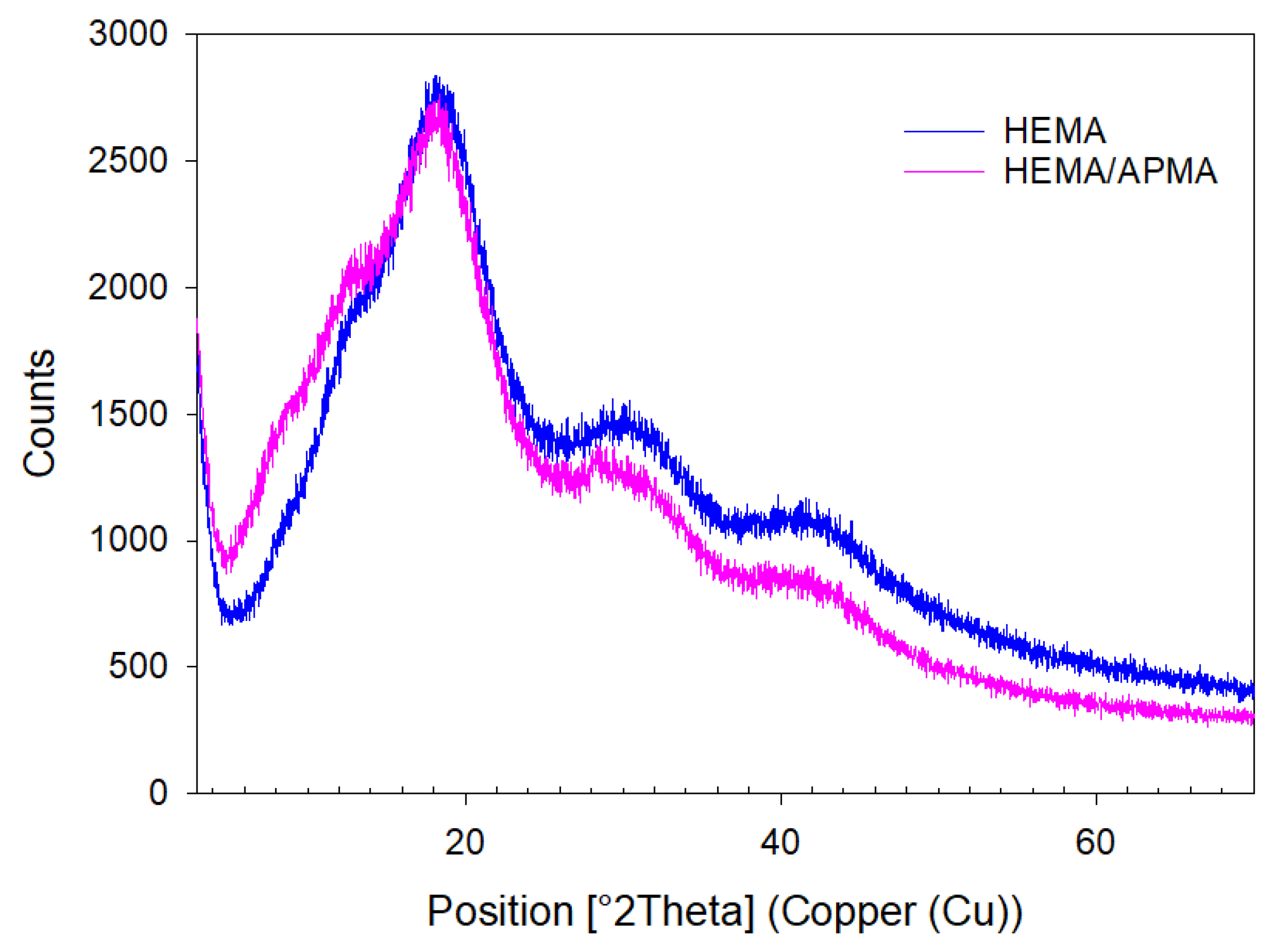
2.1.3. Attenuated Total Reflectance (ATR) and X-ray Powder Diffraction (XRPD)
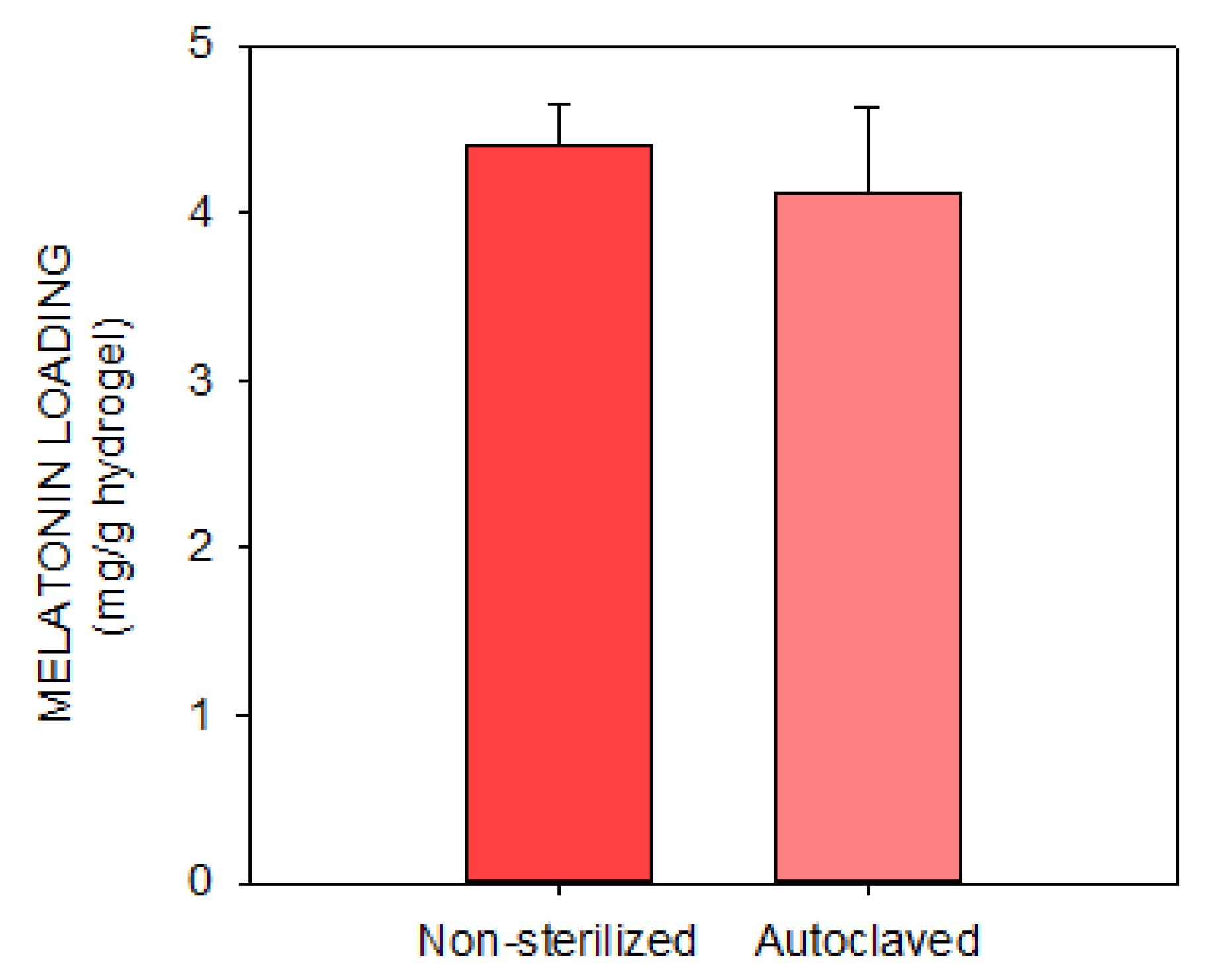
2.1.4. Melatonin Loading and Release
2.2. In Vivo Experiments
2.2.1. Study Design and Animals
2.2.2. Sample Collection and Processing
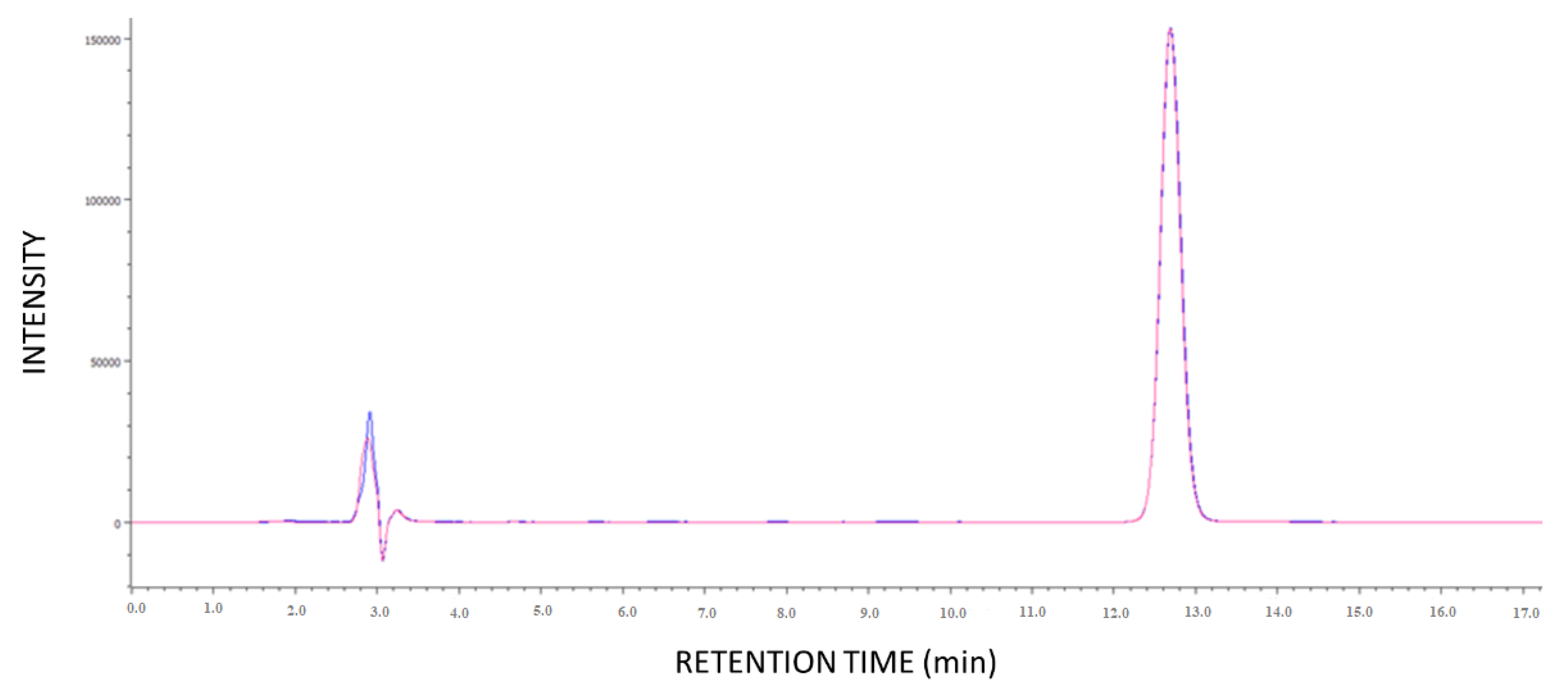
2.2.3. HPLC Analysis
2.2.4. Ocular Measurements
2.3. Statistical Analysis
3. Results
3.1. In Vitro Experiments
3.2. In Vivo Experiments
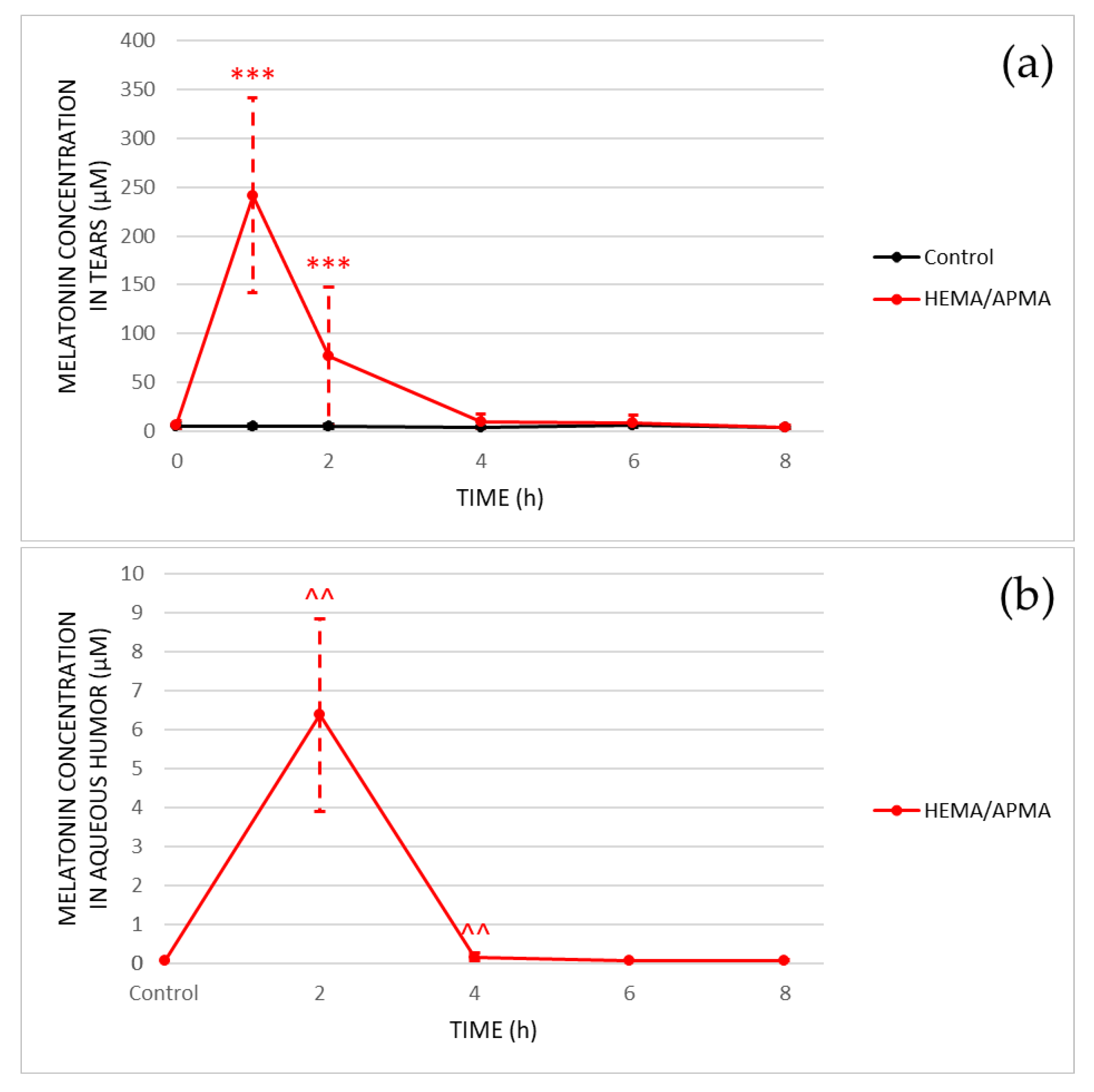
3.2.1. Ocular Kinetics
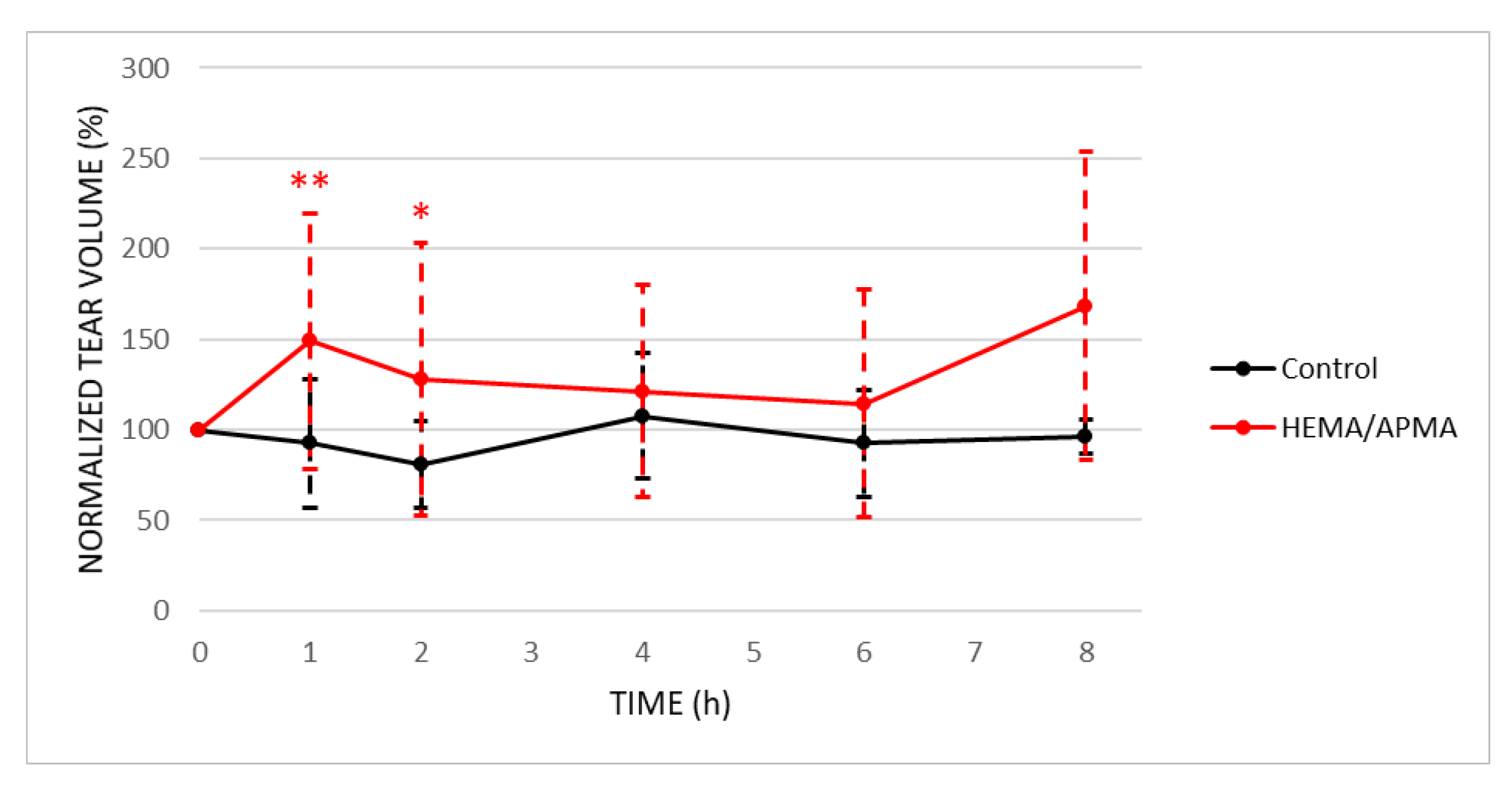
3.2.2. Ocular Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog. Retin. Eye Res. 2020, 75, 100798. [Google Scholar] [CrossRef]
- Ostrin, L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Águila, A.; Martín-Gil, A.; Carpena-Torres, C.; Pastrana, C.; Carracedo, G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules 2021, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: Involvement of Mt2 melatonin receptor. Curr. Eye Res. 2015, 40, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Carpena, C.; Concepción, P.; Díaz, V.; García-García, M.; Jemni, N.; Lledó, V.E.; Martín, M.; Pastrana, C.; Pelissier, R.; et al. Presence of melatonin in human tears. J. Optom. 2017, 10, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Navarro Gil, F.J.; Huete-Toral, F.; Crooke, A.; Dominguez Godinez, C.O.; Carracedo, G.; Pintor, J. Effect of Melatonin and Its Analogs on Tear Secretion. J. Pharmacol. Exp. Ther. 2019, 371, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, P.; Zhao, G.; Wei, S.; Li, Q.; Guo, C.; Cao, Q.; Wu, X.; Di, G. Copolymer Micelle-administered Melatonin Ameliorates Hyperosmolarity-induced Ocular Surface Damage through Regulating PINK1-mediated Mitophagy. Curr. Eye Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Pintor, J.; Peláez, T.; Hoyle, C.H.; Peral, A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: Further evidence for an MT3 receptor. Br. J. Pharmacol. 2003, 138, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Águila, A.; Fonseca, B.; Bergua, A.; Pintor, J. Melatonin analogue agomelatine reduces rabbit’s intraocular pressure in normotensive and hypertensive conditions. Eur. J. Pharmacol. 2013, 701, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Fonseca, B.; Pérez de Lara, M.J.; Pintor, J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. J. Pharmacol. Exp. Ther. 2016, 357, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiovì, F.; Di Prima, G.; Palumbo, F.S.; Licciardi, M.; Pitarresi, G.; Giammona, G. Hyaluronic Acid-Based Micelles as Ocular Platform to Modulate the Loading, Release, and Corneal Permeation of Corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef]
- Carracedo, G.; Pastrana, C.; Serramito, M.; Rodriguez-Pomar, C. Evaluation of tear meniscus by optical coherence tomography after different sodium hyaluronate eyedrops instillation. Acta Ophthalmol. 2019, 97, e162–e169. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Peral, A.; Martinez-Aguila, A.; Pastrana, C.; Huete-Toral, F.; Carpena-Torres, C.; Carracedo, G. Contact Lenses as Drug Delivery System for Glaucoma: A Review. Appl. Sci. 2020, 10, 5151. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Cytosine-functionalized bioinspired hydrogels for ocular delivery of antioxidant transferulic acid. Biomater. Sci. 2020, 8, 1171–1180. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lledo, V.E.; Alkozi, H.A.; Pintor, J. Yellow Filter Effect on Melatonin Secretion in the Eye: Role in IOP Regulation. Curr. Eye Res. 2019, 44, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar] [CrossRef]
- Dominguez-Godinez, C.O.; Martin-Gil, A.; Carracedo, G.; Guzman-Aranguez, A.; González-Méijome, J.M.; Pintor, J. In vitro and in vivo delivery of the secretagogue diadenosine tetraphosphate from conventional and silicone hydrogel soft contact lenses. J. Optom. 2013, 6, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Godinez, C.; Carracedo, G.; Pintor, J. Diquafosol Delivery from Silicone Hydrogel Contact Lenses: Improved Effect on Tear Secretion. J. Ocul. Pharmacol. Ther. 2018, 34, 170–176. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Q.; Wu, W.; Zeng, W.; Feng, Y. Therapeutic Effects of Melatonin on Ocular Diseases: Knowledge Map and Perspective. Front. Pharmacol. 2021, 12, 721869. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int. J. Mol. Sci. 2020, 21, 9267. [Google Scholar] [CrossRef]
- Crespo-Morales, M.; Alkozi, H.A.; López-García, A.; Pintor, J.J.; Diebold, Y. Melatonin receptors are present in the porcine ocular surface and are involved in ex vivo corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4371. [Google Scholar]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Yağci, R.; Aydin, B.; Erdurmuş, M.; Karadağ, R.; Gürel, A.; Durmuş, M.; Yiğitoğlu, R. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr. Eye Res. 2006, 31, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Memisogullari, R.; Koc, M.; Yazici, A.T.; Aslankurt, M.; Gumustekin, K.; Al, B.; Ozabacigil, F.; Yilmaz, A.; Tahsin Ozder, H. Melatonin reduces oxidative stress in the rat lens due to radiation-induced oxidative injury. Int. J. Radiat. Biol. 2008, 84, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Ergün, Y.; Bakariş, S.; Kılınç, M.; Durdu, H.; Ganiyusufoğlu, E. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye 2014, 28, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective Effects of Melatonin on Retinal Inflammation and Oxidative Stress in Experimental Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2016, 2016, 3528274. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhou, J.; Lu, Y.; Chu, R. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr. Eye Res. 2011, 36, 103–111. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Zhou, X.; Zhang, X.; Guan, X.; Mao, J. Regulation of Retinal Melanopsin on Lens-induced Myopia in Guinea Pigs. Optom. Vis. Sci. 2020, 97, 489–495. [Google Scholar] [CrossRef]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann. Clin. Biochem. 2017, 54, 521–529. [Google Scholar] [CrossRef] [Green Version]


| Variable | Group | Time (h) | Mean ± SD | p-Value | |
| Baseline | Post | ||||
| Tear volume (µL) | Control | 1 | 4.3 ± 1.9 | 4.0 ± 1.6 | 0.680 |
| 2 | 4.3 ± 1.9 | 3.5 ± 1.1 | 0.197 | ||
| 4 | 4.3 ± 1.9 | 4.7 ± 1.5 | 0.680 | ||
| 6 | 4.3 ± 1.9 | 4.0 ± 1.3 | 0.480 | ||
| 8 | 4.3 ± 1.9 | 4.2 ± 0.4 | 0.705 | ||
| Contact lens (HEMA/APMA) | 1 | 3.9 ± 2.2 | 5.8 ± 2.8 | 0.009 * | |
| 2 | 3.9 ± 2.2 | 5.0 ± 3.0 | 0.035 * | ||
| 4 | 4.2 ± 2.2 | 5.1 ± 2.4 | 0.129 | ||
| 6 | 4.6 ± 2.5 | 5.3 ± 2.9 | 0.442 | ||
| 8 | 3.7 ± 1.9 | 6.2 ± 3.1 | 0.072 | ||
| Intraocular pressure (mmHg) | Control | 1 | 16.1 ± 3.5 | 17.6 ± 1.8 | 0.225 |
| 2 | 16.1 ± 3.5 | 15.5 ± 5.4 | 0.686 | ||
| 4 | 16.1 ± 3.5 | 16.4 ± 7.0 | 0.893 | ||
| 6 | 16.1 ± 3.5 | 11.7 ± 4.5 | 0.080 | ||
| 8 | 16.1 ± 3.5 | 12.7 ± 2.5 | 0.225 | ||
| Contact lens (HEMA/APMA) | 1 | 12.1 ± 2.9 | 13.7 ± 4.6 | 0.115 | |
| 2 | 12.1 ± 2.9 | 13.7 ± 3.4 | 0.103 | ||
| 4 | 12.2 ± 3.0 | 12.8 ± 2.9 | 0.295 | ||
| 6 | 11.6 ± 2.6 | 13.7 ± 3.8 | 0.065 | ||
| 8 | 12.6 ± 3.0 | 15.3 ± 3.9 | 0.249 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serramito, M.; Pereira-da-Mota, A.F.; Carpena-Torres, C.; Huete-Toral, F.; Alvarez-Lorenzo, C.; Carracedo, G. Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics 2022, 14, 1019. https://doi.org/10.3390/pharmaceutics14051019
Serramito M, Pereira-da-Mota AF, Carpena-Torres C, Huete-Toral F, Alvarez-Lorenzo C, Carracedo G. Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics. 2022; 14(5):1019. https://doi.org/10.3390/pharmaceutics14051019
Chicago/Turabian StyleSerramito, María, Ana F. Pereira-da-Mota, Carlos Carpena-Torres, Fernando Huete-Toral, Carmen Alvarez-Lorenzo, and Gonzalo Carracedo. 2022. "Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments" Pharmaceutics 14, no. 5: 1019. https://doi.org/10.3390/pharmaceutics14051019
APA StyleSerramito, M., Pereira-da-Mota, A. F., Carpena-Torres, C., Huete-Toral, F., Alvarez-Lorenzo, C., & Carracedo, G. (2022). Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics, 14(5), 1019. https://doi.org/10.3390/pharmaceutics14051019









