Population Pharmacokinetics and Dosing Regimen Optimization of Latamoxef in Chinese Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dosing Regimen and Sampling
2.3. Assay of Serum Latamoxef
2.4. PPK Modeling
2.5. Covariate Analysis
2.6. Validation of Final PPK Model
2.7. Simulation and Dosing Optimization
3. Results
3.1. Study Population
3.2. PPK Modeling
- PPK: population pharmacokinetic; SE (%), percent standard error;
- Bias(%) = (Median Estimate Bootstrap − Estimate Final model)/Estimate Final model × 100%.
3.3. Validation of Final PPK Model
3.4. Simulation and Dosing Regimen Optimization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, R.; Wills, P.J.; Bedford, K.A. Epimers of moxalactam: In vitro comparison of activity and stability. Antimicrob. Agents Chemother. 1981, 20, 30–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, J.J.; Wang, Y.; Ji, J.S.; Wang, Y.F.; Wang, H.P.; Xu, Y.C.; Yu, Y.S. The activity of moxalactam against Enterobacteriaceae and anaerobia in vitro. Zhonghua Yi Xue Za Zhi 2016, 96, 1459–1464. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: M100S, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Ito, A.; Tatsumi, Y.; Wajima, T.; Nakamura, R.; Tsuji, M. Potent antibacterial activities of latamoxef (moxalactam) against ESBL producing Enterobacteriaceae analyzed by Monte Carlo simulation. Jpn. J. Antibiot. 2014, 67, 109–122. [Google Scholar] [PubMed]
- Aronoff, G.R.; Sloan, R.S.; Luft, F.C. Pharmacokinetics of moxalactam in patients with normal and impaired renal function. J. Infect. Dis. 1982, 145, 365–369. [Google Scholar] [CrossRef]
- Lam, M.; Manion, C.V.; Czerwinski, A.W. Pharmacokinetics of moxalactam in patients with renal insufficiency. Antimicrob. Agents Chemother. 1981, 19, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Martinez, O.V.; Levi, J.U.; Livingstone, A.; Malinin, T.I. Biliary excretion of moxalactam. Antimicrob. Agents Chemother. 1981, 20, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Nahata, M.C.; Durrell, D.E.; Barson, W.J. Moxalactam epimer kinetics in children. Clin. Pharmacol. Ther. 1982, 31, 528–532. [Google Scholar] [CrossRef]
- Latif, R.; Thirumoorthi, M.C.; Buckley, J.A.; Kobos, D.M.; Aravind, M.K.; Kauffman, R.E.; Dajani, A.S. Pharmacokinetic and clinical evaluation of moxalactam in infants and children. Dev. Pharmacol. Ther. 1981, 3, 222–231. [Google Scholar]
- Qi, H.; Wu, Y.E.; Liu, Y.L.; Kou, C.; Wang, Z.M.; Peng, X.X.; Chen, L.; Cui, H.; Wang, Y.J.; Li, J.Q.; et al. Latamoxef for Neonates with Early-Onset Neonatal Sepsis: A Study Protocol for a Randomized Controlled Trial. Front. Pharmacol. 2021, 12, 635517. [Google Scholar] [CrossRef]
- Goutelle, S.; Woillard, J.B.; Neely, M.; Yamada, W.; Bourguignon, L. Nonparametric Methods in Population Pharmacokinetics. J. Clin. Pharmacol. 2022, 62, 142–157. [Google Scholar] [CrossRef]
- Guidi, M.; Csajka, C.; Buclin, T. Parametric Approaches in Population Pharmacokinetics. J. Clin. Pharmacol. 2022, 62, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Kou, C.; Qi, Y.J.; Tang, B.H.; Wu, Y.E.; Jin, F.; Luo, X.J.; Shen, Y.H.; Guo, Y.J.; Qi, X.; et al. Population pharmacokinetics and dosing optimization of latamoxef in neonates and young infants. Int. J. Antimicrob. Agents 2019, 53, 347–351. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ichihashi, T.; Hirano, K.; Kinoshita, H. Plasma protein binding and urinary excretion of R- and S-epimers of an arylmalonylamino 1-oxacephem. I: In humans. J. Pharm. Sci. 1981, 70, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Aronoff, S.C.; Myers, C.M.; Husak, M.P.; Bertino, J.S., Jr.; Blumer, J.L. Developmental pharmacokinetics of moxalactam. Antimicrob. Agents Chemother. 1983, 24, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Lüthy, R.; Blaser, J.; Bonetti, A.; Simmen, H.; Wise, R.; Siegenthaler, W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob. Agents Chemother. 1981, 20, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Butler, K.; English, A.R.; Ray, V.A.; Timreck, A.E. Carbenicillin: Chemistry and mode of action. J. Infect. Dis. 1970, 122, S1–S8. [Google Scholar] [CrossRef]
- Morimoto, S.; Nomura, H.; Fugono, T.; Azuma, T.; Minami, J. Semisynthetic -lactam antibiotics. 2. Synthesis and properties of D- and L- -sulfobenzylpenicillins. J. Med. Chem. 1972, 15, 1108–1111. [Google Scholar] [CrossRef]
- Su, M.X.; Liu, M.H.; Di, B.; Huang, L.L.; Jiang, Y.; Ma, P.C.; Hang, T.J. Pharmacokinetic differences between the epimers of cefotetan disodium after single intravenous injection in healthy Chinese volunteers. Eur. J. Drug. Metab. Pharmacokinet. 2011, 36, 223–228. [Google Scholar] [CrossRef]
- Hashimoto, N.; Tanaka, H. Epimerization kinetics of moxalactam, its derivatives, and carbenicillin in aqueous solution. J. Pharm. Sci. 1985, 74, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Ichihashi, T.; Hirano, K.; Yamada, H. Epimerization kinetics of moxalactam in frozen urine and plasma samples. Pharm. Res. 1990, 7, 364–369. [Google Scholar] [CrossRef] [PubMed]


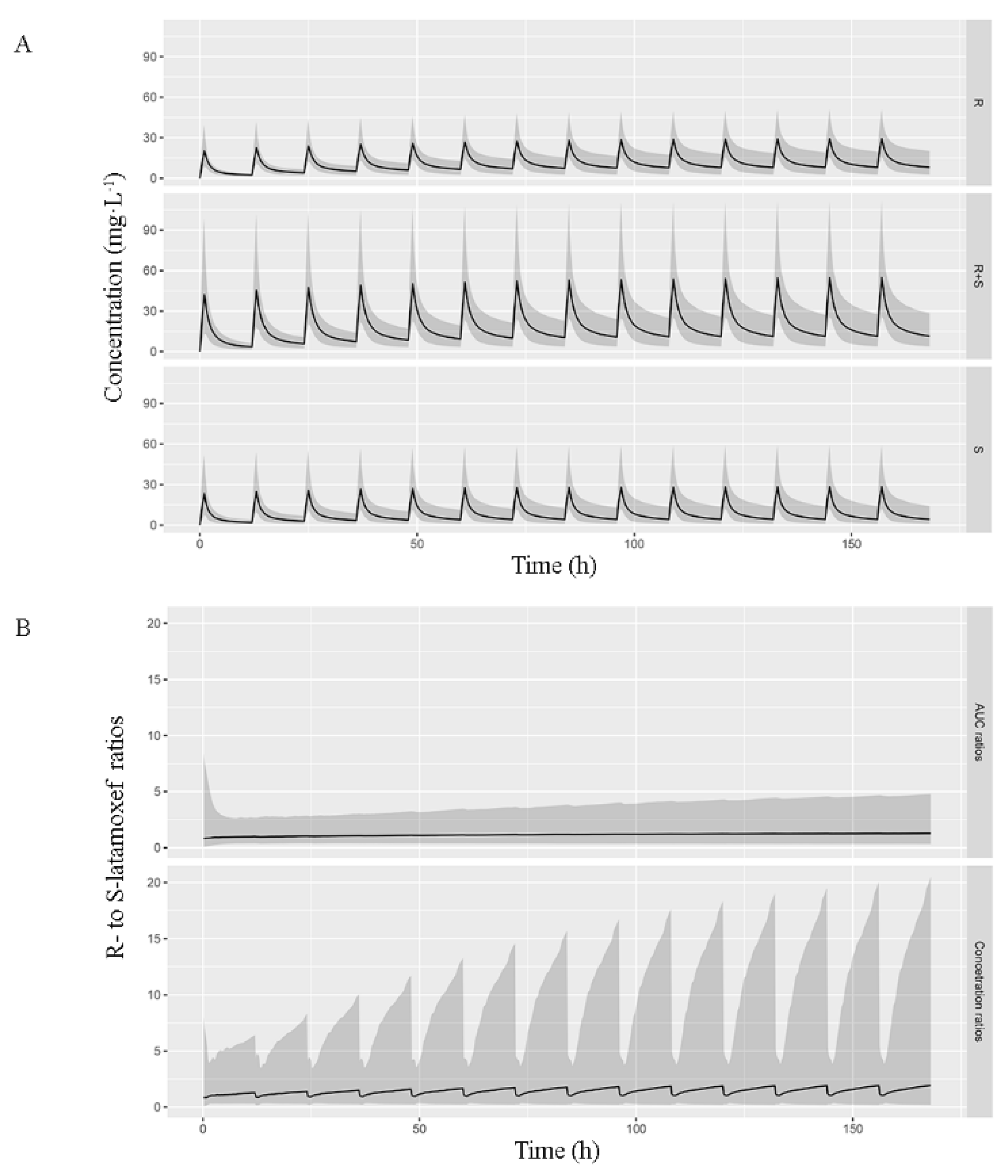
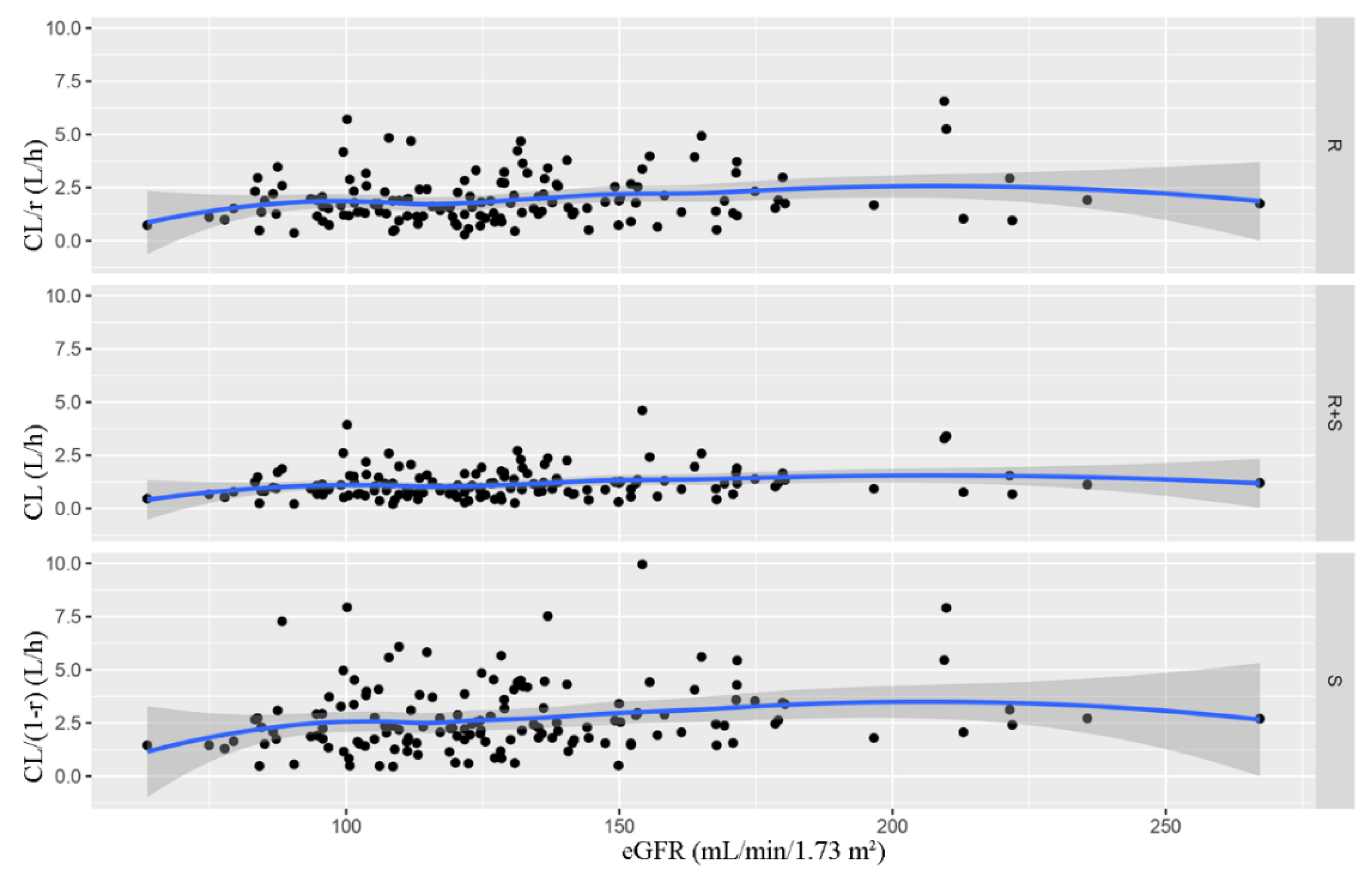
| Number | Mean (SD) | Median (Range) | |
|---|---|---|---|
| Patients | 145 | ||
| Gender (M:F) | 91:54 | ||
| Age (years) | 1.08 (1.63) | 0.60 (0.08–10.58) | |
| Weight (kg) | 8.68 (4.11) | 8 (2.9–27.5) | |
| Height (cm) | 71.80 (16.98) | 68.00 (49.00–140.00) | |
| Body surface area (m2) | 0.41 (0.14) | 0.39 (0.20–1.03) | |
| Blood urea nitrogen (mmol/L) | 3.13 (1.37) | 3.00 (0.60–10.10) | |
| Serum creatinine concentration (μmol/L) | 21.37 (5.97) | 20.90 (9.70–48.10) | |
| Uric acid (μmol/L) | 229.18 (81.15) | 224.50 (61.00–488.40) | |
| Cystatin C (μmol/L) | 1.12 (0.26) | 1.08 (0.51–2.13) | |
| Estimated glomerular filtration rate (mL/min·1.73 m2) | 128.37 (33.79) | 123.76 (63.61–267.24) | |
| R-epimer concentration (μg/mL) | 16.91 (13.94) | 11.86 (0.19–60.92) | |
| S-epimer concentration (μg/mL) | 14.73 (14.52) | 9.44 (0.06–62.97) | |
| Total latamoxef concentration (μg/mL) | 29.08 (26.45) | 19.34 (1.84–117.88) | |
| High sensitive C reaction protein (mg/L) | 13.45 (24.54) | 4.02 (0.78–156.00) | |
| Procalcitonin (ng/mL) | 0.33 (0.53) | 0.14 (0.03–3.31) |
| Candidate Models | Model Description | OFV | ||
|---|---|---|---|---|
| k1 | MF | |||
| Model I | Estimated | 1 | 1408.59 | |
| Model II | Estimated | 1 | 1407.12 | |
| Model III | 0.75 | 1 | 1413.9 | |
| Model IV | 0.75 | 1 | 1420.83 | |
| Model V | 0.75 | 1405.45 | ||
| Model VI | 0.75 | 1408.67 | ||
| Model VII | 1 | 1408.43 | ||
| Model VIII | 1 | 1406.33 | ||
| Model IX | 1 | 1407.84 | ||
| Model X | 1 | 1406.05 | ||
| Group | Parameter | Final Model | Bootstrap Analysis | Bias (%) | |||
|---|---|---|---|---|---|---|---|
| Estimate | SE (%) | 2.5th Percentile | Median Estimate | 97.5th Percentile | |||
| R + S | θV1 (L) | 4.84 | 15.85 | 3.30 | 4.66 | 6.53 | −3.72 |
| θV2 (L) | 16.18 | 47.35 | 9.05 | 16.54 | 26.41 | 2.22 | |
| θCL (L/h) | 1.00 | 9.05 | 0.82 | 0.99 | 1.15 | −1.00 | |
| θQ (L/h) | 0.97 | 15.93 | 0.71 | 0.98 | 1.62 | 1.03 | |
| θ1 | 1.00 (fixed) | ||||||
| θ2 | 1.00 (fixed) | ||||||
| θ3 | 1.49 | 14.69 | 1.05 | 1.46 | 1.92 | −2.01 | |
| θ4 | 0.75 (fixed) | ||||||
| Inter-individual | |||||||
| ωV1 (%) | 105.04 | 29.96 | 26.70 | 110.78 | 194.86 | 5.46 | |
| ωCL (%) | 28.84 | 31.45 | 11.26 | 28.08 | 44.90 | −2.64 | |
| Residual variability | |||||||
| σ (mg/L) | 7.29 | 11.49 | 5.06 | 7.07 | 9.05 | −3.02 | |
| R | θV1/r (L) | 9.69 | 16.00 | 6.69 | 9.51 | 13.35 | −1.86 |
| θV1−R (L) | 4.31–5.65 | ||||||
| θV2/r (L) | 33.00 | 33.75 | 21.54 | 33.23 | 48.37 | 0.70 | |
| θV2−R (L) | 14.67–19.25 | ||||||
| θCL/r (L/h) | 1.68 | 9.71 | 1.37 | 1.67 | 1.93 | −0.60 | |
| θCL−R (L/h) | 0.75–0.98 | ||||||
| θQ/r (L/h) | 3.15 | 21.70 | 2.08 | 3.14 | 5.24 | −0.32 | |
| θQ−R (L/h) | 1.40–1.84 | ||||||
| θ5 | 1.00 (fixed) | ||||||
| θ6 | 1.00 (fixed) | ||||||
| θ7 | 1.42 | 18.07 | 0.97 | 1.42 | 1.89 | 0.00 | |
| θ8 | 0.75 (fixed) | ||||||
| Inter-individual | |||||||
| ωV1/r (%) | 65.11 | 41.41 | 7.03 | 67.79 | 128.55 | 4.12 | |
| ωCL/r (%) | 35.37 | 34.46 | 15.80 | 34.95 | 54.10 | −1.19 | |
| Residual variability | |||||||
| σR (mg/L) | 5.33 | 13.07 | 3.91 | 5.21 | 6.27 | −2.25 | |
| S | θV1/(1−r) (L) | 8.12 | 18.13 | 5.45 | 8.55 | 12.68 | 5.30 |
| θV1−S (L) | 3.38–4.51 | ||||||
| θV2/(1−r) (L) | 19.13 | 48.39 | 9.50 | 17.86 | 58.69 | −6.64 | |
| θV2−S (L) | 7.97–10.63 | ||||||
| θCL/(1−r) (L/h) | 2.36 | 9.65 | 1.81 | 2.32 | 2.85 | −1.69 | |
| θCL−S (L/h) | 0.98–1.31 | ||||||
| θQ/(1−r) (L/h) | 1.89 | 20.26 | 0.93 | 1.81 | 3.46 | −4.23 | |
| θQ−S (L/h) | 0.79–1.05 | ||||||
| θ9 | 1.00 (fixed) | ||||||
| θ10 | 1.00 (fixed) | ||||||
| θ11 | 1.33 | 20.48 | 0.78 | 1.34 | 1.98 | 0.75 | |
| θ12 | 0.75 (fixed) | ||||||
| Inter-individual | |||||||
| ωV1/(1−r) (%) | 116.20 | 33.21 | 25.41 | 129.09 | 232.77 | 11.09 | |
| ωCL/(1−r) (%) | 43.20 | 28.80 | 0.90 | 40.04 | 79.18 | −7.31 | |
| Residual variability | |||||||
| σS (mg/L) | 3.81 | 9.24 | 1.79 | 3.72 | 5.27 | −2.36 | |
| Item | Model | ||
|---|---|---|---|
| R + S | R | S | |
| NPDE mean (SE) | 0.04 (0.08) | 0.03 (0.09) | −0.01 (0.09) |
| Variance (SE) | 1.10 (0.12) | 1.14 (0.13) | 1.20 (0.14) |
| Skewness Value | 0.06 | 0.24 | −0.15 |
| Kurtosis Value | −0.05 | 0.15 | −0.01 |
| t-test p-value | 0.626 | 0.722 | 0.937 |
| Fisher variance test p-value | 0.349 | 0.214 | 0.100 |
| Shapiro–Wilks test of normality p-value | 0.367 | 0.204 | 0.119 |
| Global adjusted p-value | 1.000 | 0.613 | 0.301 |
| BSA Group | MIC90 (μg/mL) | |||
|---|---|---|---|---|
| 0.5 | 1 | 2 | 8 | |
| 0.2–0.4 m2 | 50 mg, q12h | 100 mg, q12h | 150 mg, q12h | 200 mg, q6h |
| 0.41–0.6 m2 | 100 mg, q12h | 200 mg, q12h | 375 mg, q12h | 475 mg, q6h |
| 0.61–0.8 m2 | 200 mg, q12h | 375 mg, q12h | 400 mg, q8h | 625 mg, q6h(2h) |
| 0.81–1.0 m2 | 300 mg, q12h | 550 mg, q12h | 600 mg, q8h | 950 mg, q6h(2h) |
| 1.01–1.2 m2 | 500 mg, q12h | 925 mg, q12h | 900 mg, q8h | 1400 mg, q6h(2h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, D.; Mei, Y.; Wu, S.; Li, X.; Li, S.; Wang, J.; Gao, L.; Xu, H.; Tuo, Y. Population Pharmacokinetics and Dosing Regimen Optimization of Latamoxef in Chinese Children. Pharmaceutics 2022, 14, 1033. https://doi.org/10.3390/pharmaceutics14051033
Wang Y, Sun D, Mei Y, Wu S, Li X, Li S, Wang J, Gao L, Xu H, Tuo Y. Population Pharmacokinetics and Dosing Regimen Optimization of Latamoxef in Chinese Children. Pharmaceutics. 2022; 14(5):1033. https://doi.org/10.3390/pharmaceutics14051033
Chicago/Turabian StyleWang, Yang, Dan Sun, Yan Mei, Sanlan Wu, Xinlin Li, Sichan Li, Jun Wang, Liuliu Gao, Hua Xu, and Yali Tuo. 2022. "Population Pharmacokinetics and Dosing Regimen Optimization of Latamoxef in Chinese Children" Pharmaceutics 14, no. 5: 1033. https://doi.org/10.3390/pharmaceutics14051033
APA StyleWang, Y., Sun, D., Mei, Y., Wu, S., Li, X., Li, S., Wang, J., Gao, L., Xu, H., & Tuo, Y. (2022). Population Pharmacokinetics and Dosing Regimen Optimization of Latamoxef in Chinese Children. Pharmaceutics, 14(5), 1033. https://doi.org/10.3390/pharmaceutics14051033





