Cannabis-Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Active Compounds
2.2. Oils and Emulsions Preparation
2.3. Quantitative Analysis
2.4. Evaluation of Emulsions Stability
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | cannabidiol |
| CBDA | cannabidiolic acid |
| O/W | oil-in-water |
| q.s. | quantum sufficit |
| SD | standard deviation |
| T0 | initial conditions |
| T28 | 28 days of storage at room temperature |
| THC | delta-9-tetrahydrocannabinol |
| THCA | delta-9-tetrahydrocannabinolic acid |
References
- Arias, S.; Leon, M.; Jaimes, D.; Bustos, R.H. Clinical evidence of magistral preparations based on medicinal Cannabis. Pharmaceuticals 2021, 14, 78. [Google Scholar] [CrossRef]
- Romano, L.; Hazekamp, A. An Overview of Galenic Preparation Methods for Medicinal Cannabis. Curr. Bioact. Compd. 2019, 15, 174–195. [Google Scholar] [CrossRef]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, C. Cannabis research data reveals a focus on harms of the drug. Science 2020, 369, 1105. [Google Scholar] [CrossRef]
- Gould, J. The cannabis crop. Nature 2015, 525, S2–S3. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Baratta, F.; Simiele, M.; Pignata, I.; Ravetto Enri, L.; Torta, R.; De Luca, A.; Collino, M.; D’Avolio, A.; Brusa, P. Development of Standard Operating Protocols for the Optimization of Cannabis-Based Formulations for Medical Purposes. Front. Pharmacol. 2019, 10, 701. [Google Scholar] [CrossRef]
- President of the Italian Republic. Decree of the President of the Italian Republic, 309/1990. Testo unico delle leggi in materia di disciplina degli stupefacenti e sostanze psicotrope, prevenzione, cura e riabilitazione dei relativi stati di tossicodipendenza. GU n.255 del 31-10-1990-Suppl. Ordinario n. 67. Available online: https://www.normattiva.it/atto/caricaDettaglioAtto?atto.dataPubblicazioneGazzetta=1990-10-31&atto.codiceRedazionale=090G0363&atto.articolo.numero=0&atto.articolo.sottoArticolo=1&atto.articolo.sottoArticolo1=10&qId=a48eef63-1aea-47a9-acd2-9edb5b6ee34c&tabID=0.057173987633775125&title=lbl.dettaglioAtto (accessed on 14 December 2021).
- Ministry of Health. Ministerial Decree November, 2015. Funzioni di Organismo Statale Per la Cannabis Previsto Dagli Articoli 23 e 28 Della Convenzione Unica Sugli Stupefacenti del 1961, Come Modificata nel 1972. Allegato Rettificato Dal Comunicato 07 Gennaio 2016. G.U. Serie Generale, n. 279 del 30 Novembre 2015. Available online: https://www.gazzettaufficiale.it/eli/id/1990/10/31/090G0363/sg (accessed on 14 December 2021).
- Pharmaceutical Chemical Military Facility in Florence Website. Available online: http://www.farmaceuticomilitare.it/cannabis.aspx?lnrid=25 (accessed on 14 December 2021).
- Ministry of Health (2017). Raccomandazioni per il Medico Prescrittore di Sostanza Vegetale Cannabis fm2 Infiorescenze. Documento Approvato Dal GRUPPO di Lavoro Previsto dall’Accordo di Collaborazione del Ministero Della Salute e del Ministero Della Difesa del 18 Settembre 2014. Available online: http://www.salute.gov.it/imgs/C_17_pagineAree_4589_listaFile_itemName_2_file.pdf (accessed on 14 December 2021).
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Carcieri, C.; Tomasello, C.; Simiele, M.; De Nicolò, A.; Avataneo, V.; Canzoneri, L.; Cusato, J.; Di Perri, G.; D’Avolio, A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm. Pharmacol. 2018, 70, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Lombardi, N.; Crescioli, G.; Maggini, V.; Gallo, E.; Mugelli, A.; Firenzuoli, F.; Baronti, R.; Vannacci, A. Galenic preparations of therapeutic Cannabis sativa differ in cannabinoids concentration: A quantitative analysis of variability and possible clinical implications. Front. Pharmacol. 2019, 9, 1543. [Google Scholar] [CrossRef] [Green Version]
- Dei Cas, M.; Casagni, E.; Casiraghi, A.; Minghetti, P.; Fornasari, D.M.M.; Ferri, F.; Arnoldi, S.; Verniero, G.; Roda, G. Phytocannabinoids profile in medicinal Cannabis oils: The impact of plant varieties and preparation methods. Front. Pharm. 2020, 11, 570616. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Simiele, M.; Pignata, I.; Ravetto Enri, L.; D’Avolio, A.; Torta, R.; De Luca, A.; Collino, M.; Brusa, P. Cannabis-based oral formulations for medical purposes: Preparation, quality and stability. Pharmaceuticals 2021, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Geneva, Switzerland, 2020. [Google Scholar]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; Van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Fuentes, J.; Martín-Banderas, L.; Muñoz-Rubio, I.; Holgado, M.A.; Fernández-Arévalo, M. Development and validation of an RP-HPLC method for CB13 evaluation in several PLGA nanoparticle systems. Sci. World J. 2012, 2012, 737526. [Google Scholar] [CrossRef]
- Križman, M. A simplified approach for isocratic HPLC analysis of cannabinoids by fine tuning chromatographic selectivity. Eur Food Res. Technol 2020, 246, 315–322. [Google Scholar] [CrossRef]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef]
- Maida, V.; Daeninck, P.J. A user’s guide to cannabinoid therapies in oncology. Curr. Oncol. 2016, 23, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.U.; Baum, C.R. Acute Cannabis Toxicity. Pediatr. Emerg. Care 2019, 35, 799–806. [Google Scholar]
- Lucas, C.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharm. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [Green Version]
- Baratta, F.; Germano, A.; Brusa, P. Diffusion of counterfeit drugs in developing countries and stability of galenics stored for months under different conditions of temperature and relative humidity. Croat. Med. J. 2012, 53, 173–184. [Google Scholar] [CrossRef] [Green Version]
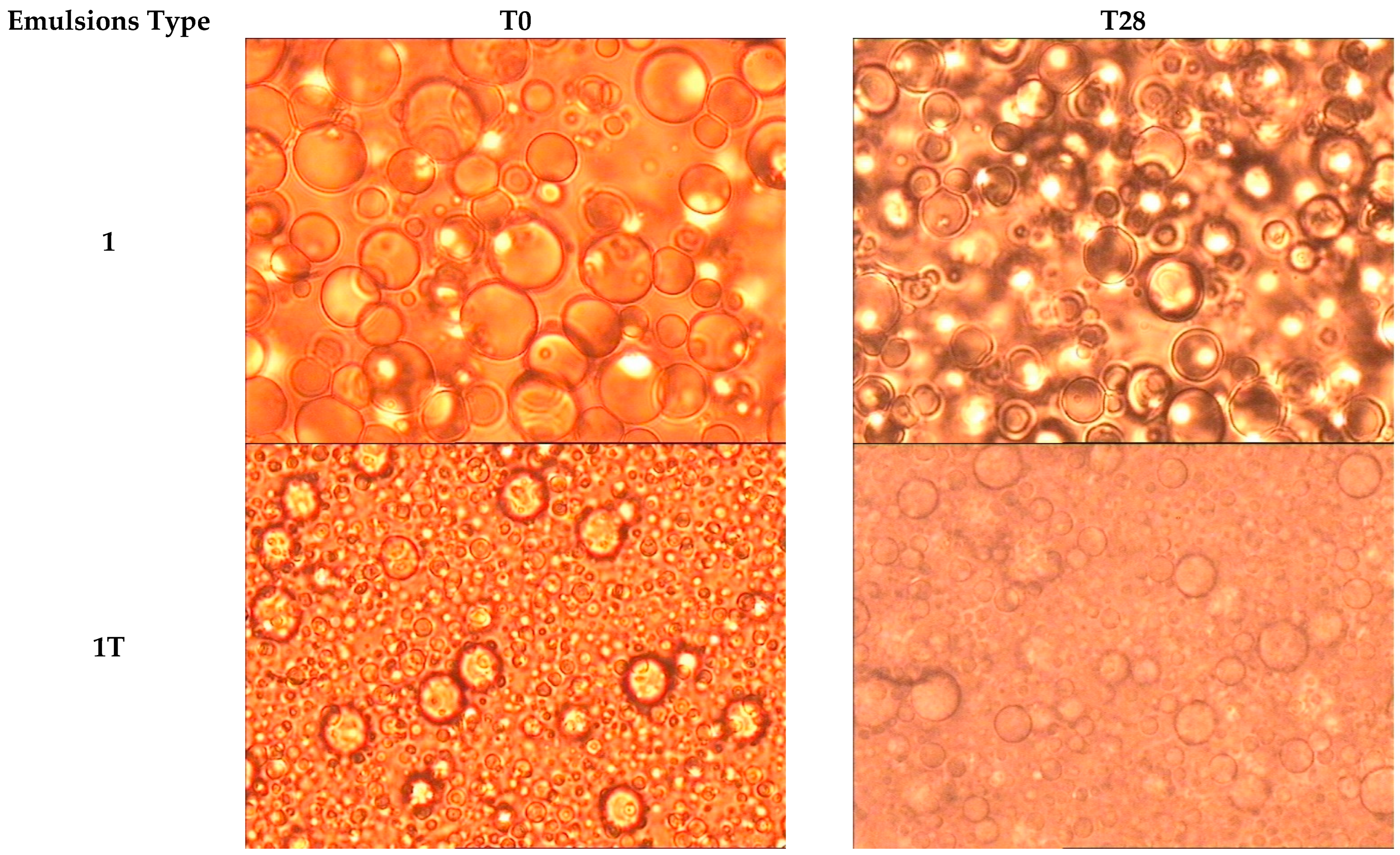
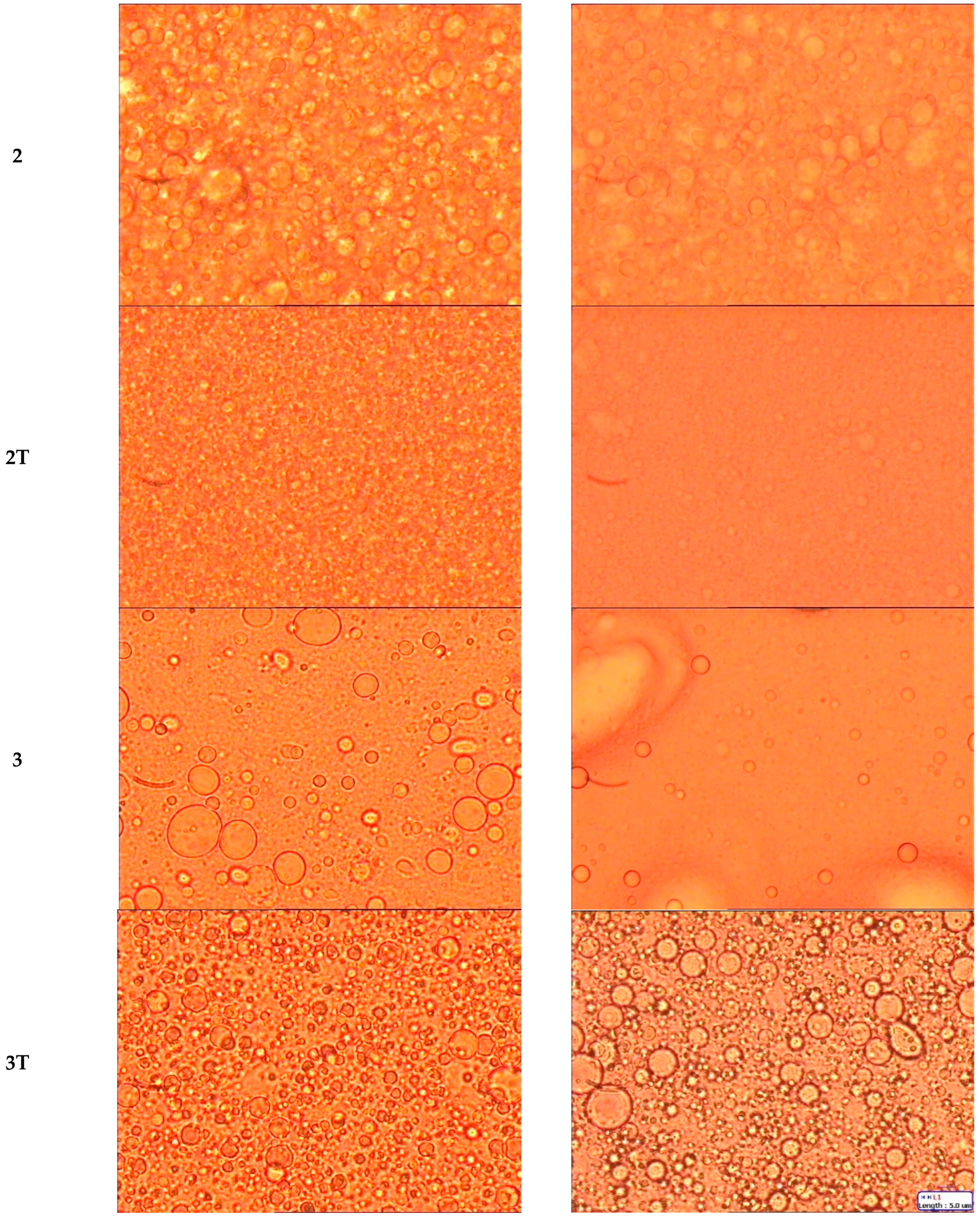
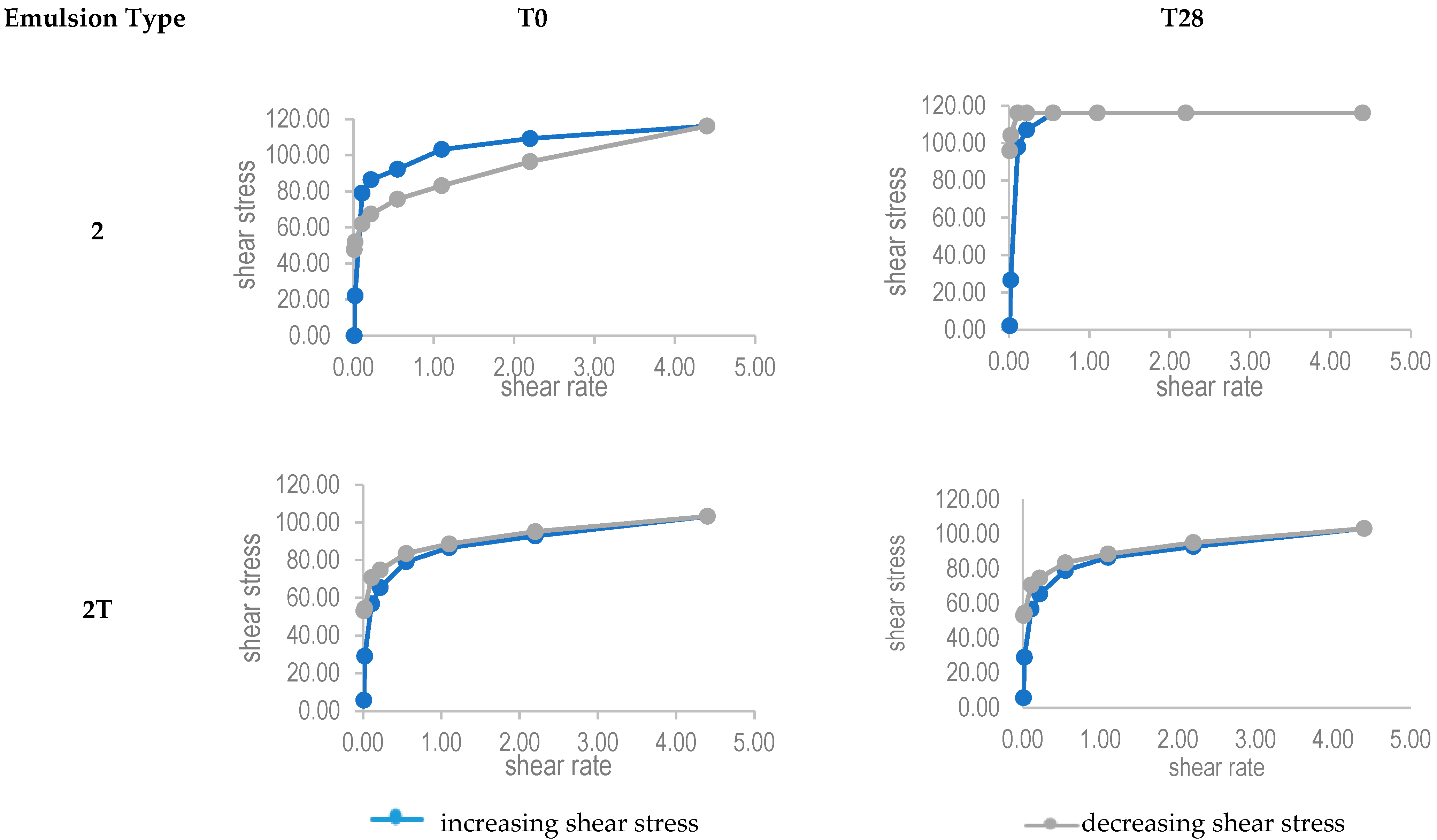
| Emulsion Type | |||
|---|---|---|---|
| Ingredients | 1 and 1T | 2 and 2T | 3 and 3T |
| Cannabis β-4 oil | 10 g | 10 g | 10 g |
| Poloxamer 407 | 15 g | 10 g | / |
| Soy lecithin | / | 10 g | 10 g |
| Glycerol | 10 g | 10 g | / |
| Sodium methyl parahydroxybenzoate | 0.1 g | 0.1 g | 0.1 g |
| Tocopherol | 0.05 g | 0.05 g | 0.05 g |
| Water-soluble lemon flavour | 1 g | 1 g | 1 g |
| Depured water | q.s. to 100 g | q.s. to 100 g | q.s. to 100 g |
| Emulsion Type | T0 | T28 |
|---|---|---|
| Mean Diameter ± SD (µm) | Mean Diameter ± SD. (µm) | |
| 1 | 7.70 ± 4.15 | 8.10 ± 3.18 |
| 1T | 3.81 ± 1.98 | 3.82 ± 1.26 |
| 2 | 3.90 ± 1.40 | 4.41 ± 1.26 |
| 2T | 2.12 ± 0.64 | 2.26 ± 0.78 |
| 3 | 5.81 ± 3.00 | / |
| 3T | 3.39 ± 1.12 | 7.57 ± 2.05 |
| Emulsion Type | T0 | T28 | |
|---|---|---|---|
| Mean Viscosity Values (mPa s) | Mean Viscosity Values (mPa s) | Δ% Compared to T0 | |
| 1 | 1300.523 | 993.388 | −23.62 |
| 1T | 1218.940 | 1276.528 | 4.72 |
| 2 | 1002.986 | 1214.141 | 21.05 |
| 2T | 1295.724 | 1319.718 | 1.85 |
| 3 | 44 | / | / |
| 3T | 10.96 | 11.03 | 0.64 |
| Emulsion Type | T0 | T28 | |
|---|---|---|---|
| Mean pH Values | Mean pH Values | Δ% Compared to T0 | |
| 1 | 8.26 | 7.95 | −3.75 |
| 1T | 9 | 8.86 | −1.56 |
| 2 | 7.44 | 7.26 | −2.42 |
| 2T | 7.53 | 6.94 | −7.84 |
| 3 | 7.37 | 6.22 | −15.60 |
| 3T | 7.42 | 5.6 | −24.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, F.; Peira, E.; Maza, C.; Gallarate, M.; Brusa, P. Cannabis-Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability. Pharmaceutics 2022, 14, 513. https://doi.org/10.3390/pharmaceutics14030513
Baratta F, Peira E, Maza C, Gallarate M, Brusa P. Cannabis-Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability. Pharmaceutics. 2022; 14(3):513. https://doi.org/10.3390/pharmaceutics14030513
Chicago/Turabian StyleBaratta, Francesca, Elena Peira, Carola Maza, Marina Gallarate, and Paola Brusa. 2022. "Cannabis-Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability" Pharmaceutics 14, no. 3: 513. https://doi.org/10.3390/pharmaceutics14030513
APA StyleBaratta, F., Peira, E., Maza, C., Gallarate, M., & Brusa, P. (2022). Cannabis-Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability. Pharmaceutics, 14(3), 513. https://doi.org/10.3390/pharmaceutics14030513