Effect of Thrombopoietin Receptor Agonist on Pregnant Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Tissue Preparation
2.3. Histology
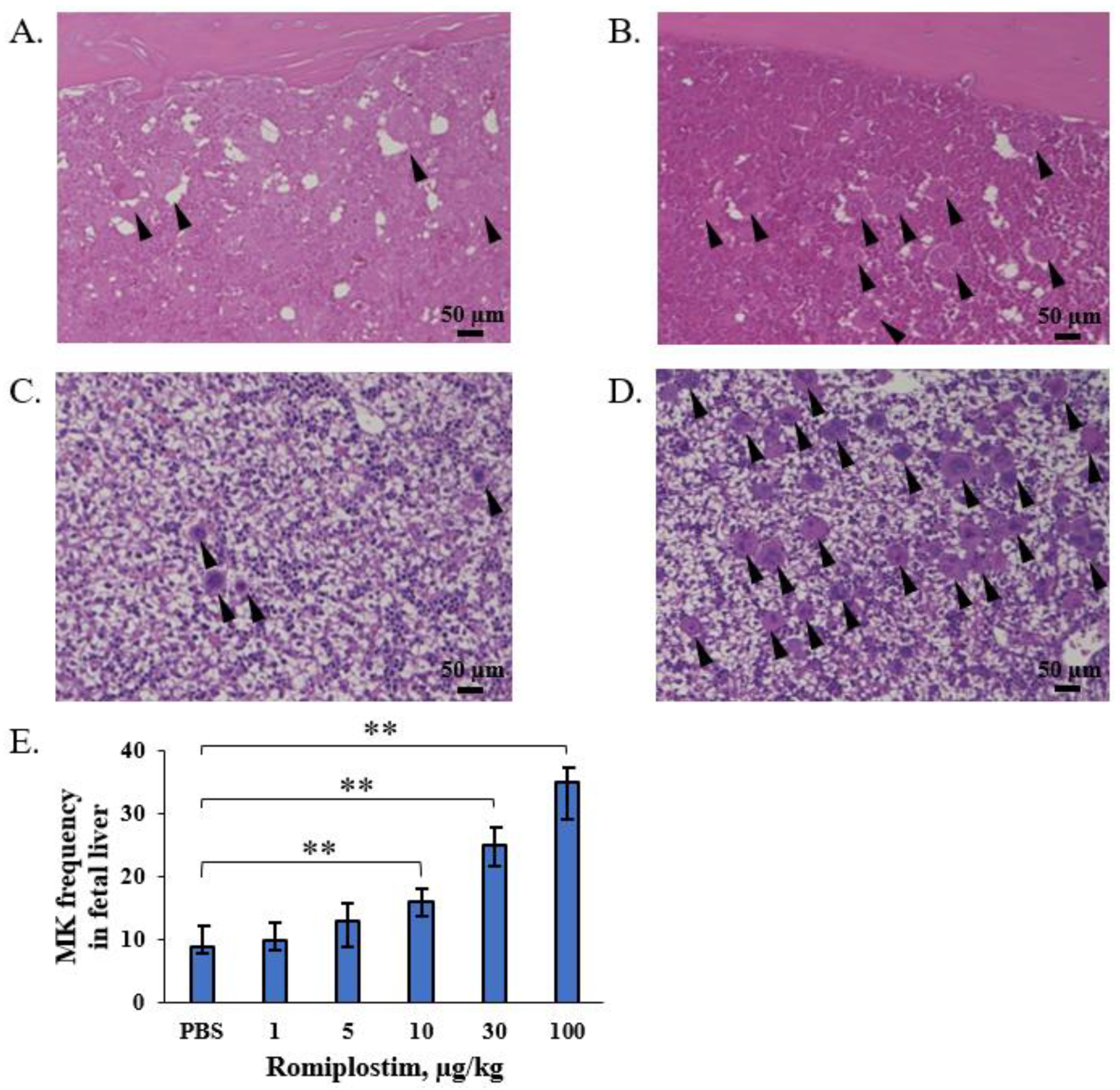
2.4. Counting of Megakaryocytes
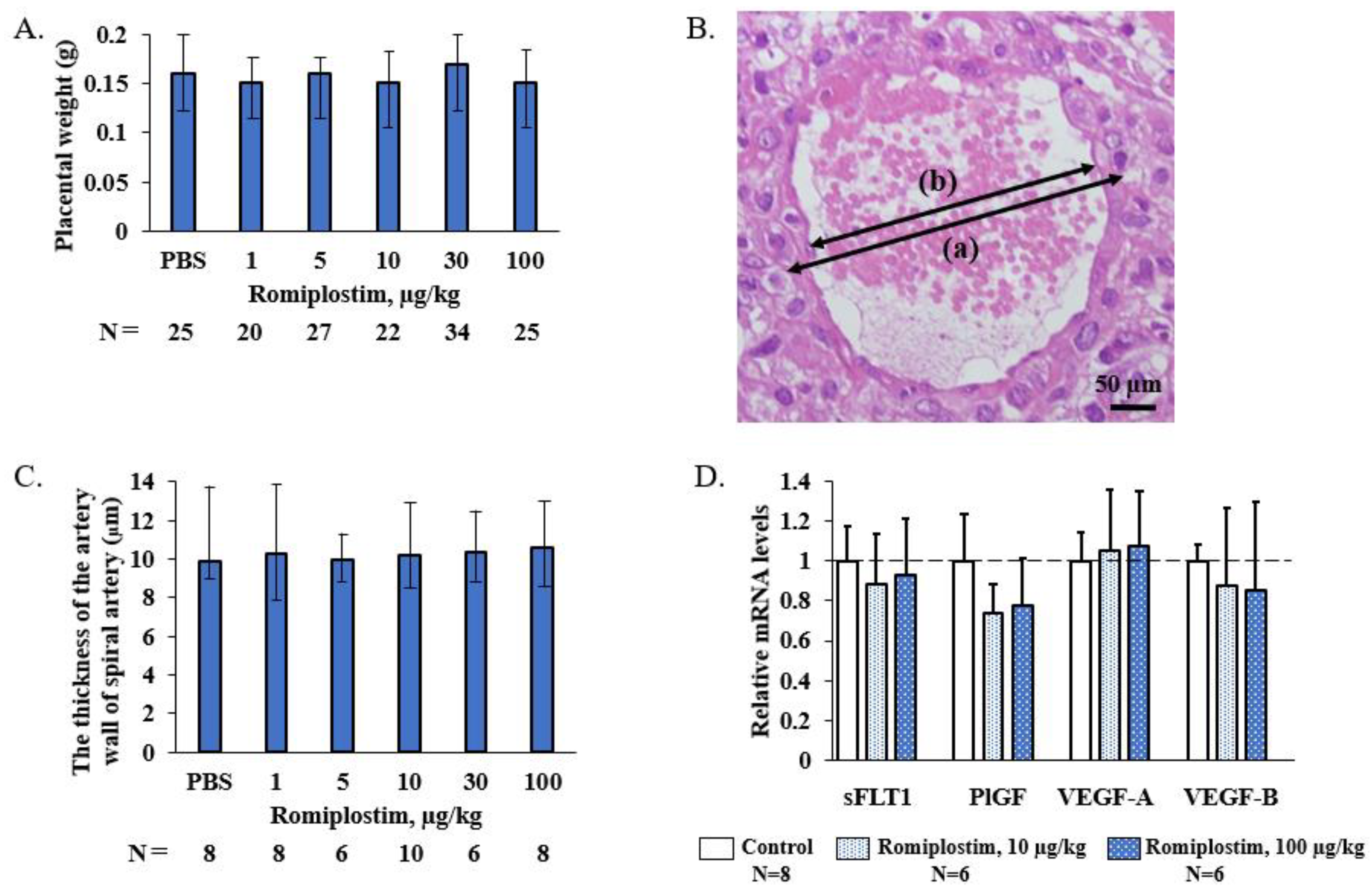
2.5. Measurement of the Vessel Wall of the Spiral Artery
2.6. RNA Extraction, cDNA Synthesis, and Quantitative PCR (qPCR)
2.7. Statistical Analysis
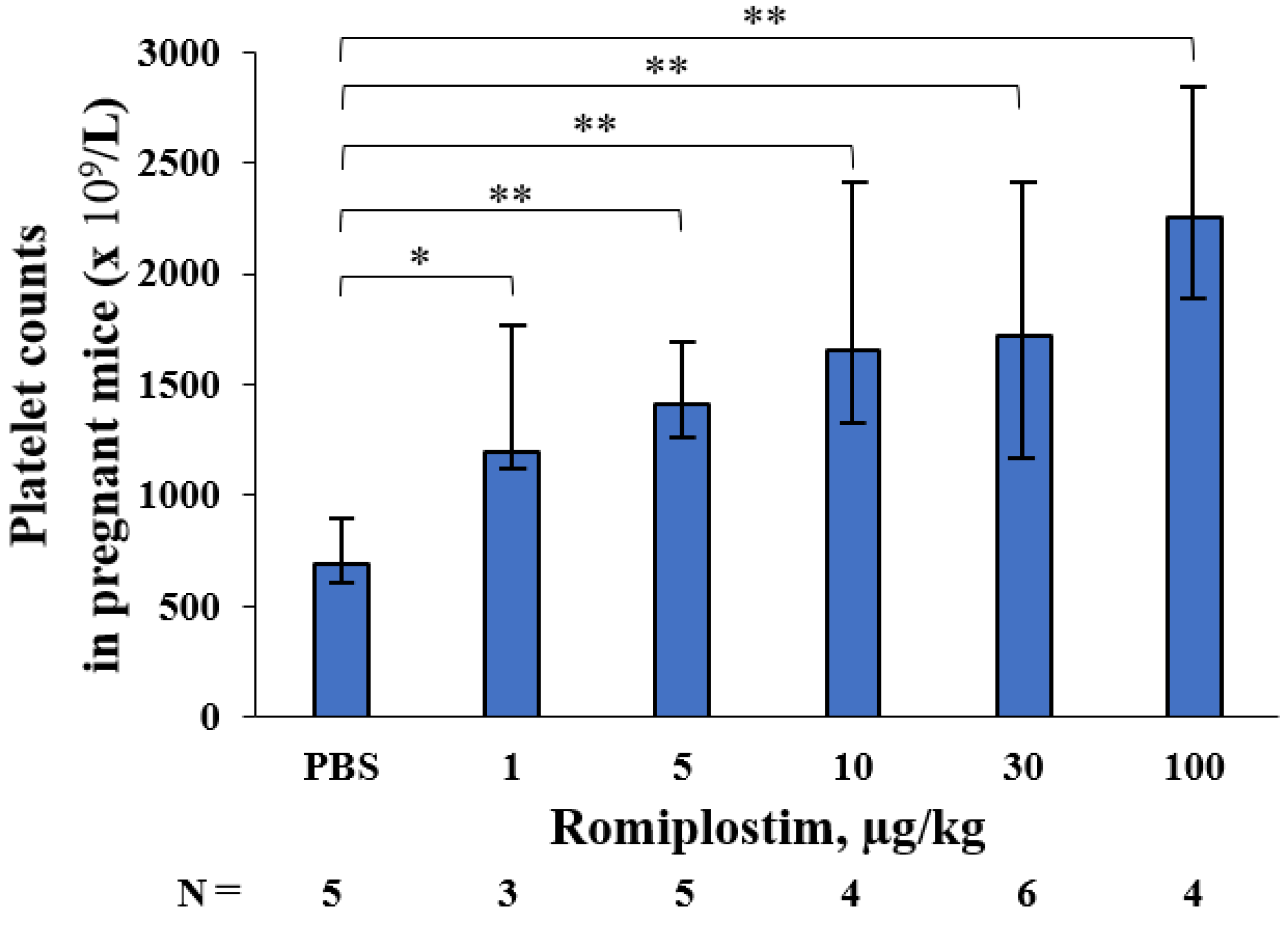
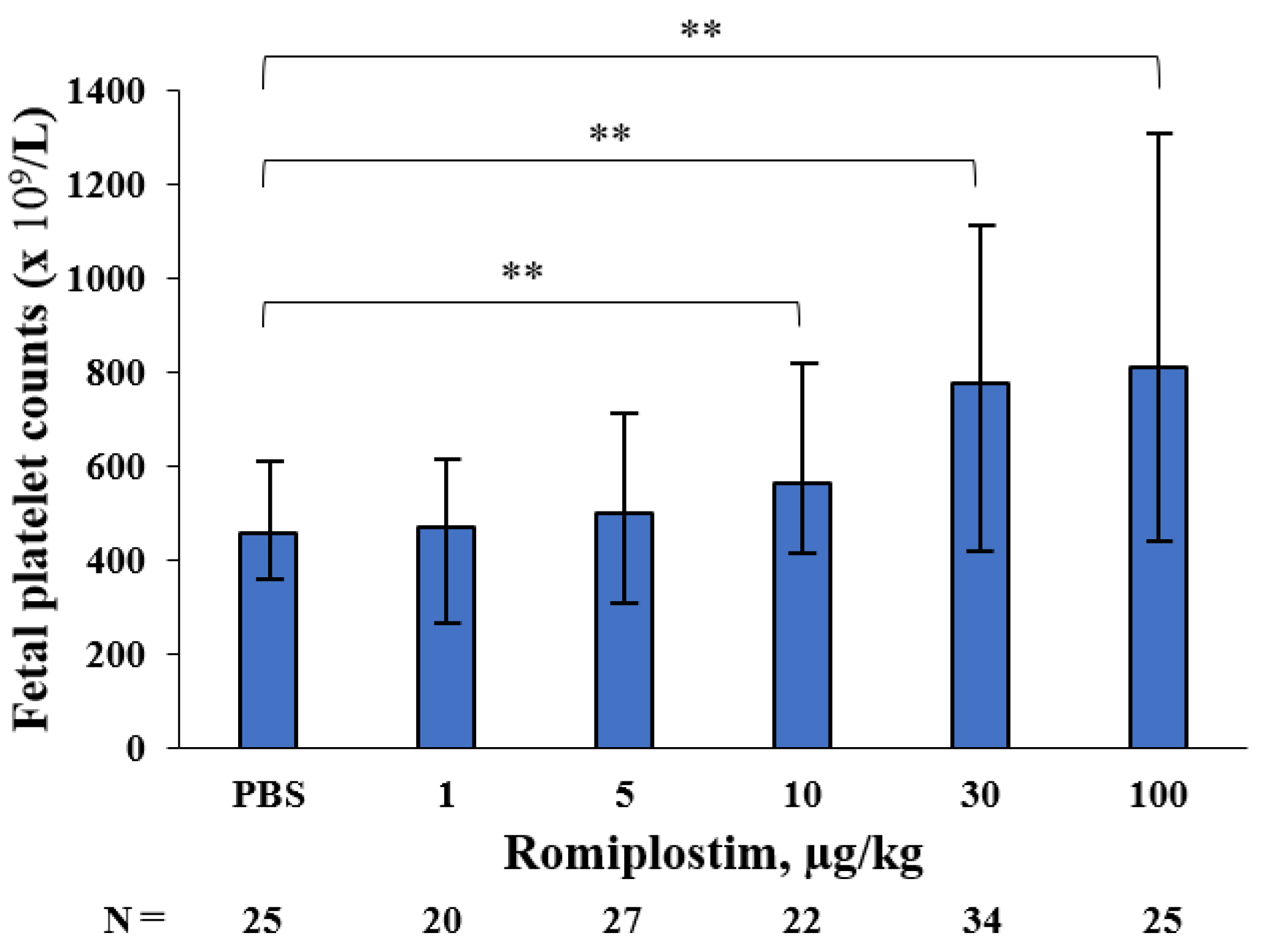
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambert, M.P.; Gernsheimer, T.B. Clinical updates in adult immune thrombocytopenia. Blood 2017, 129, 2829–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Care, A.; Pavord, S.; Knight, M.; Alfirevic, Z. Severe primary autoimmune thrombocytopenia in pregnancy: A national cohort study. BJOG 2018, 125, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Gondo, H.; Hamasaki, Y.; Nakayama, H.; Kondo, T.; Mitsuuchi, J.; Kawaga, Y.; Taniguchi, S.; Harada, M.; Niho, Y. Acute leukemia during pregnancy. Association with immune-mediated thrombocytopenia in mother and infant. Acta Haematol. 1990, 83, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hazel, C.; Swingler, R. Management of severe immune thrombocytopenia in pregnancy: A case study. Bjog-Int. J. Obstet. Gynaecol. 2021, 128, 74. [Google Scholar]
- Loustau, V.; Debouverie, O.; Canoui-Poitrine, F.; Baili, L.; Khellaf, M.; Touboul, C.; Languille, L.; Loustau, M.; Bierling, P.; Haddad, B.; et al. Effect of pregnancy on the course of immune thrombocytopenia: A retrospective study of 118 pregnancies in 82 women. Brit. J. Haematol. 2014, 166, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [Green Version]
- Piatek, C.I.; El-Hemaidi, I.; Feinstein, D.I.; Liebman, H.A.; Akhtari, M. Management of immune-mediated cytopenias in pregnancy. Autoimmun. Rev. 2015, 14, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Varghese, L.N.; Defour, J.P.; Pecquet, C.; Constantinescu, S.N. The Thrombopoietin Receptor: Structural Basis of Traffic and Activation by Ligand, Mutations, Agonists, and Mutated Calreticulin. Front. Endocrinol. 2017, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Howaidi, J.; AlRajhi, A.M.; Howaidi, A.; AlNajjar, F.H.; Tailor, I.K. Use of thrombopoietin receptor agonists in pregnancy. Hematol. Oncol. Stem Cell Ther. 2021, 5, 55–58. [Google Scholar]
- Agarwal, N.; Mangla, A. Thrombopoietin receptor agonist for treatment of immune thrombocytopenia in pregnancy: A narrative review. Ther. Adv. Hematol. 2021, 12, 20406207211001139. [Google Scholar] [CrossRef]
- Kapur, R.; Aslam, R.; Speck, E.R.; Rebetz, J.M.; Semple, J.W. Thrombopoietin receptor agonist (TPO-RA) treatment raises platelet counts and reduces anti-platelet antibody levels in mice with immune thrombocytopenia (ITP). Platelets 2020, 31, 399–402. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zhang, X.Q.; Huang, J.; Zeng, Y.; Liu, W.; Geng, C.; Li, K.W.; Yang, D.; Wu, S.F.; Wei, H.D.; et al. Relationships between Hematopoiesis and Hepatogenesis in the Midtrimester Fetal Liver Characterized by Dynamic Transcriptomic and Proteomic Profiles. PLoS ONE 2009, 4, e7641. [Google Scholar] [CrossRef] [Green Version]
- Guzin, K.; Tomruk, S.; Tuncay, Y.A.; Naki, M.; Sezginsoy, S.; Zemheri, E.; Yucel, N.; Kanadikirik, F. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch. Gynecol. Obstet. 2005, 272, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.D.; Barrette, V.F.; Gravel, J.; Carter, A.L.; Hatta, K.; Zhang, J.; Chen, Z.; Leno-Duran, E.; Bianco, J.; Leonard, S.; et al. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am. J. Reprod. Immunol. 2010, 63, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hiraga, J.; Hariyama, Y.; Takagi, Y.; Ohashi, H.; Kishigami, Y.; Oguchi, H.; Kagami, Y. A low birth weight infant with no malformations delivered by a primary immune thrombocytopenia patient treated with eltrombopag. Int. J. Hematol. 2018, 108, 109–111. [Google Scholar] [CrossRef]
- Erickson-Miller, C.L.; Delorme, E.; Tian, S.; Hopson, C.B.; Landis, A.J.; Valoret, E.I.; Sellers, T.S.; Rosen, J.; Miller, S.G.; Luengo, J.I.; et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells 2009, 27, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Mahevas, M.; Gerfaud-Valentin, M.; Moulis, G.; Terriou, L.; Audia, S.; Guenin, S.; Le Guenno, G.; Salles, G.; Lambotte, O.; Limal, N.; et al. Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood 2016, 128, 1625–1630. [Google Scholar] [CrossRef] [Green Version]
- McMillan, R.; Durette, C. Long-term outcomes in adults with chronic ITP after splenectomy failure. Blood 2004, 104, 956–960. [Google Scholar] [CrossRef]
- Frederiksen, H.; Maegbaek, M.L.; Norgaard, M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2014, 166, 260–267. [Google Scholar] [CrossRef]
- Zaja, F.; Barcellini, W.; Cantoni, S.; Carpenedo, M.; Caparrotti, G.; Carrai, V.; Di Renzo, N.; Santoro, C.; Di Nicola, M.; Veneri, D.; et al. Thrombopoietin receptor agonists for preparing adult patients with immune thrombocytopenia to splenectomy: Results of a retrospective, observational GIMEMA study. Am. J. Hematol. 2016, 91, E293–E295. [Google Scholar] [CrossRef] [Green Version]
- Provan, D.; Newland, A.C. Current Management of Primary Immune Thrombocytopenia. Adv. Ther. 2015, 32, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Rodeghiero, F.; Marranconi, E. Management of immune thrombocytopenia in woman: Current standards and special considerations. Expert Rev. Hematol. 2020, 13, 175–185. [Google Scholar] [CrossRef]
- Patil, A.S.; Dotters-Katz, S.K.; Metjian, A.D.; James, A.H.; Swamy, G.K. Use of a Thrombopoietin Mimetic for Chronic Immune Thrombocytopenic Purpura in Pregnancy. Obstet. Gynecol. 2013, 122, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.J.; Morton, M.R.; Svigos, J.; Ross, D.M.; Kane, S. Use of romiplostim in pregnancy for refractory idiopathic thrombocytopenic purpura: Two case reports with maternal and fetal outcomes and literature review. Obstet. Med. 2020, 13, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.; Nelson-Piercy, C.; Williamson, C.; Cooper, N.; Kesse-Adu, R.; Robinson, S. Refractory severe immune thrombocytopenia in a twin pregnancy. Obstet. Med. 2018, 11, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, M.; Karami, A.; Karami, N.; Masgareh, J.B. Romiplostim: Successful treatment of a pregnant woman with refractory immune thrombocytopenia a case report and literature review. Biomed. Res. Ther. 2018, 5, 2565–2571. [Google Scholar] [CrossRef]
- Alkaabi, J.K.; Alkindi, S.; Al Riyami, N.; Zia, F.; Balla, L.M.A.; Balla, S.M. Successful treatment of severe thrombocytopenia with romiplostim in a pregnant patient with systemic lupus erythematosus. Lupus 2012, 21, 1571–1574. [Google Scholar] [CrossRef]
- Decroocq, J.; Marcellin, L.; Le Ray, C.; Willems, L. Rescue Therapy with Romiplostim for Refractory Primary Immune Thrombocytopenia during Pregnancy. Obstet. Gynecol. 2014, 124, 481–483. [Google Scholar] [CrossRef]
- Maria, R.N.R.; Laura, R.L.; Angeles, P.B.; Laura, L.B. Use of Romiplostim during pregnancy as a rescue therapy in primary immune thrombocytopenia: Literature review and case description. Platelets 2020, 31, 403–406. [Google Scholar] [CrossRef] [PubMed]

| Romiplostim | Rate of Resorption (%) Median (Range) | Pups/Litter Median (Range) |
|---|---|---|
| PBS | 0 (0–16.7) | 11 (9–16) |
| 1 µg/kg | 0 (0–23.1) | 16 (10–16) |
| 5 µg/kg | 7.1 (0–14.3) | 13 (12–14) |
| 10 µg/kg | 7.7 (7.1–20.0) | 12 (12–13) |
| 30 µg/kg | 6.9 (0–9.1) | 13 (10–15) |
| 100 µg/kg | 8.7 (7.1–20.0) | 12 (10–16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakai, K.; Misugi, T.; Kitada, K.; Kurihara, Y.; Tahara, M.; Hamuro, A.; Nakano, A.; Koyama, M.; Kira, Y.; Tachibana, D. Effect of Thrombopoietin Receptor Agonist on Pregnant Mice. Pharmaceutics 2022, 14, 514. https://doi.org/10.3390/pharmaceutics14030514
Nakai K, Misugi T, Kitada K, Kurihara Y, Tahara M, Hamuro A, Nakano A, Koyama M, Kira Y, Tachibana D. Effect of Thrombopoietin Receptor Agonist on Pregnant Mice. Pharmaceutics. 2022; 14(3):514. https://doi.org/10.3390/pharmaceutics14030514
Chicago/Turabian StyleNakai, Kensaku, Takuya Misugi, Kohei Kitada, Yasushi Kurihara, Mie Tahara, Akihiro Hamuro, Akemi Nakano, Masayasu Koyama, Yukimi Kira, and Daisuke Tachibana. 2022. "Effect of Thrombopoietin Receptor Agonist on Pregnant Mice" Pharmaceutics 14, no. 3: 514. https://doi.org/10.3390/pharmaceutics14030514
APA StyleNakai, K., Misugi, T., Kitada, K., Kurihara, Y., Tahara, M., Hamuro, A., Nakano, A., Koyama, M., Kira, Y., & Tachibana, D. (2022). Effect of Thrombopoietin Receptor Agonist on Pregnant Mice. Pharmaceutics, 14(3), 514. https://doi.org/10.3390/pharmaceutics14030514






