siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of HAp and HAp-PEI
2.3. In Vitro Degradation of HAp-PEI
2.4. Cytotoxicity Assay
2.5. siRNA Loading and Transfection
2.5.1. siRNA Loading
2.5.2. siRNA-FAM Transfection
2.5.3. KRAS siRNA Transfection
2.6. Real-Time Quantitative PCR
2.7. Western Blot
2.8. In Vitro Anti-Pancreatic Tumor Cell Assay
2.9. Statistical Analysis
3. Results and Discussion
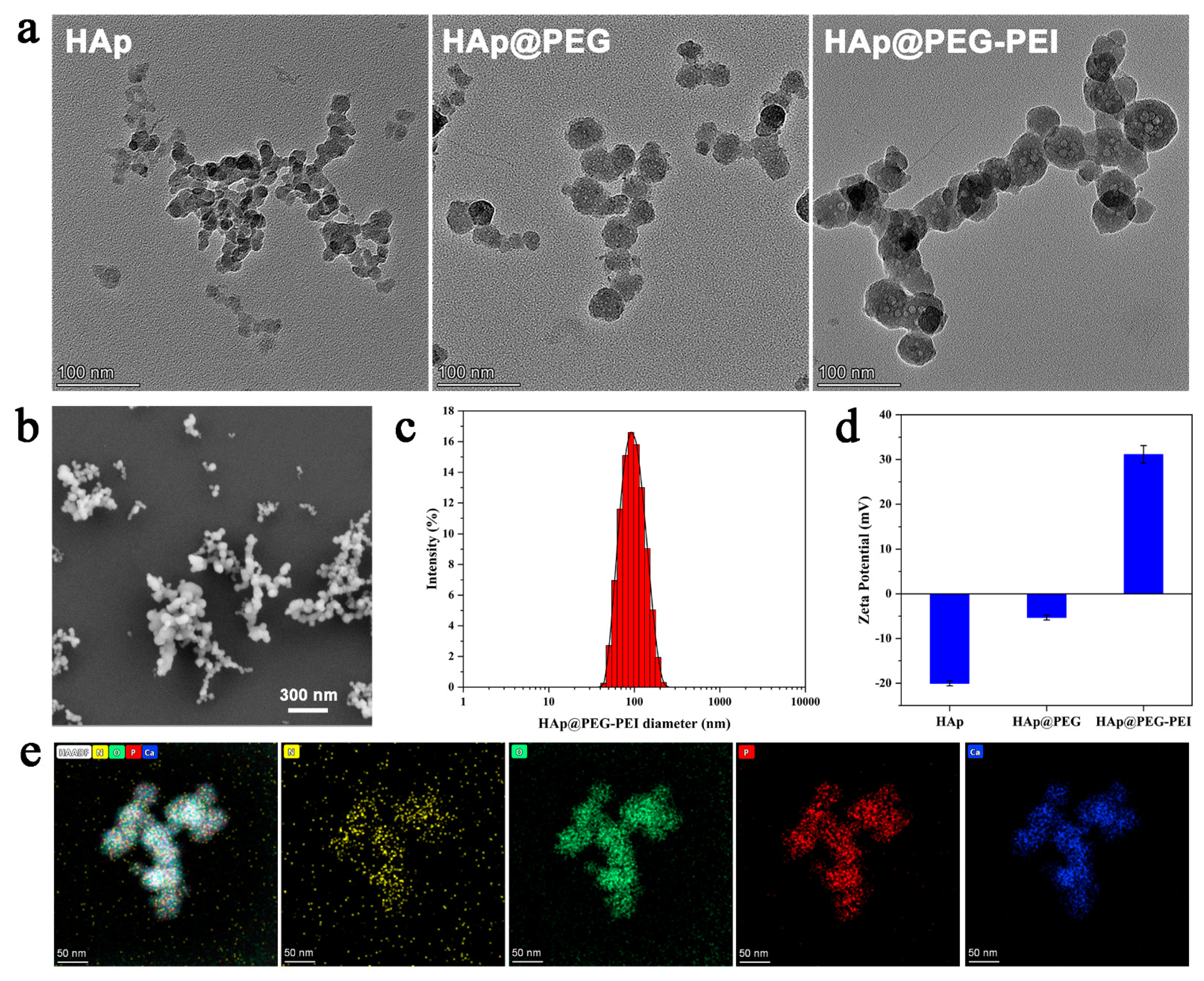
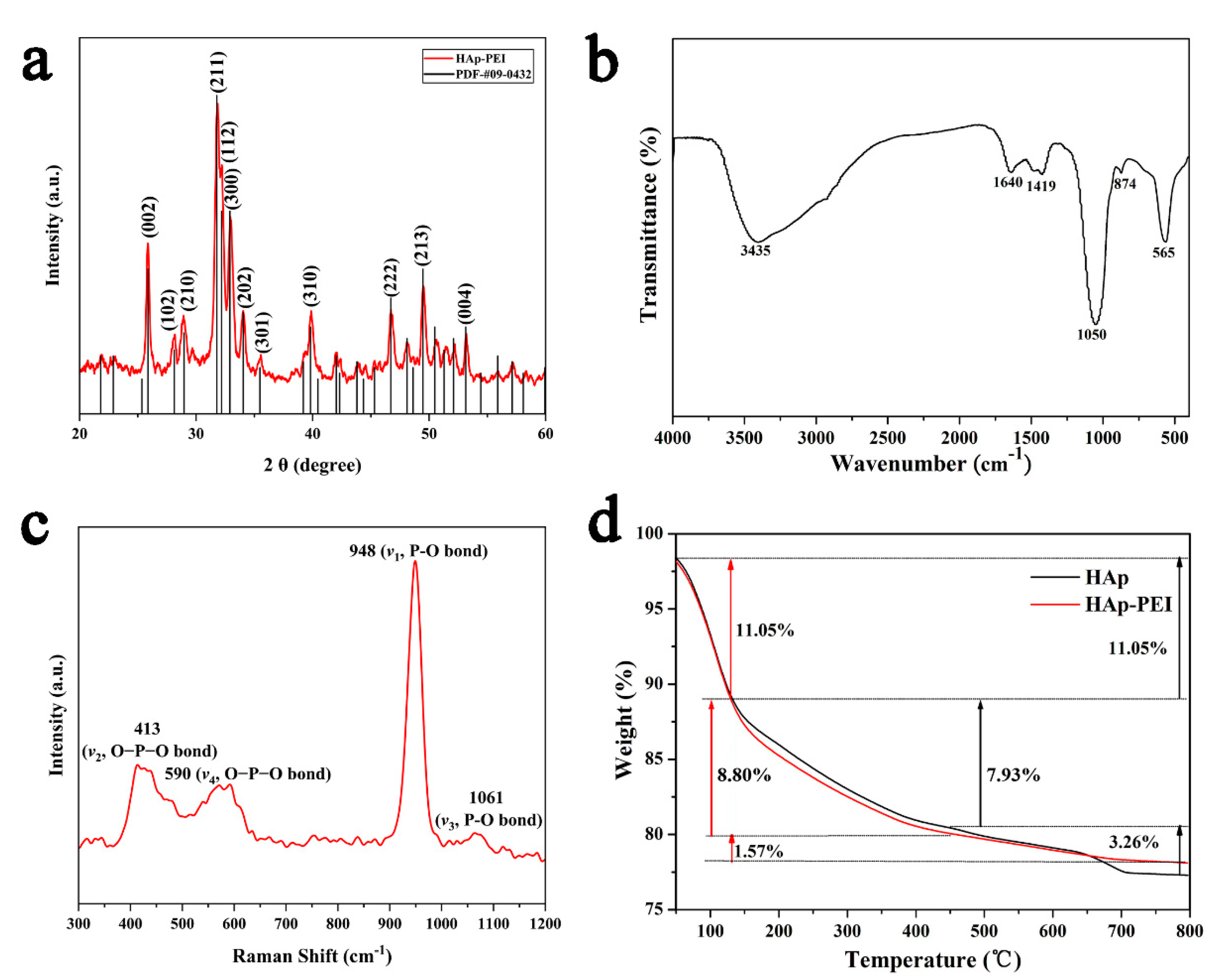
3.1. Characterization of HAp-PEI
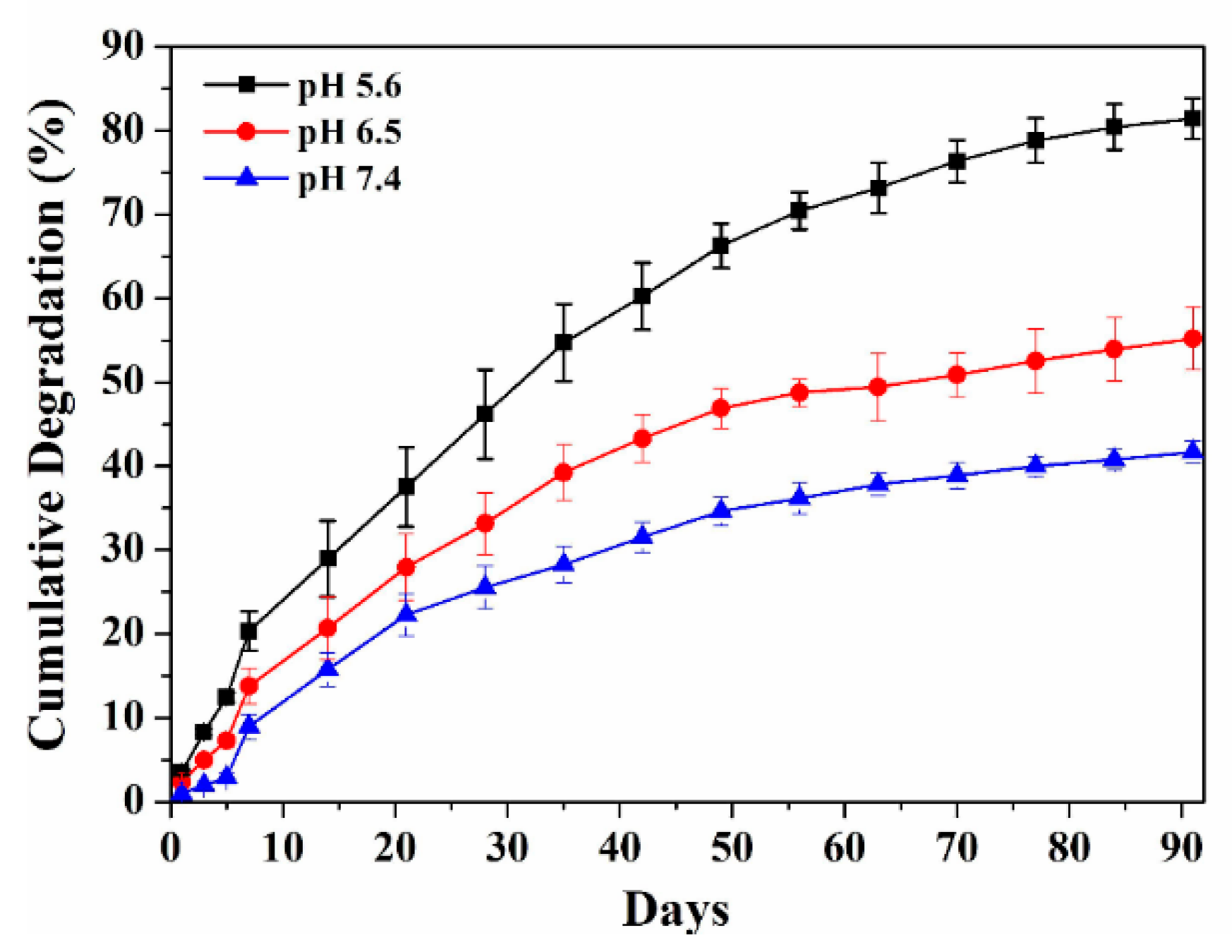
3.2. In Vitro Degradation of HAp-PEI
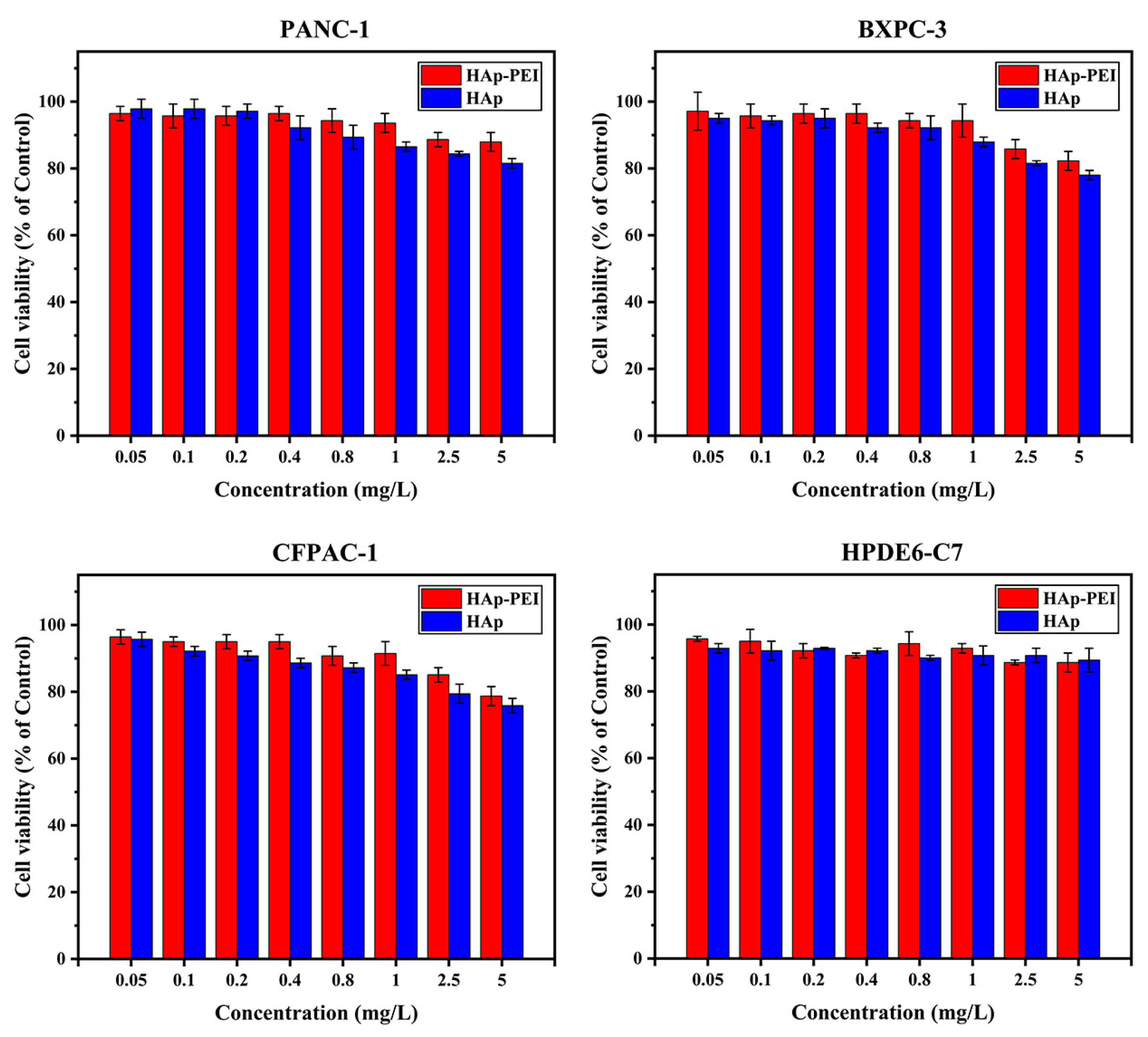
3.3. In Vitro Biocompatibility of HAp-PEI
3.4. siRNA Loading Capability
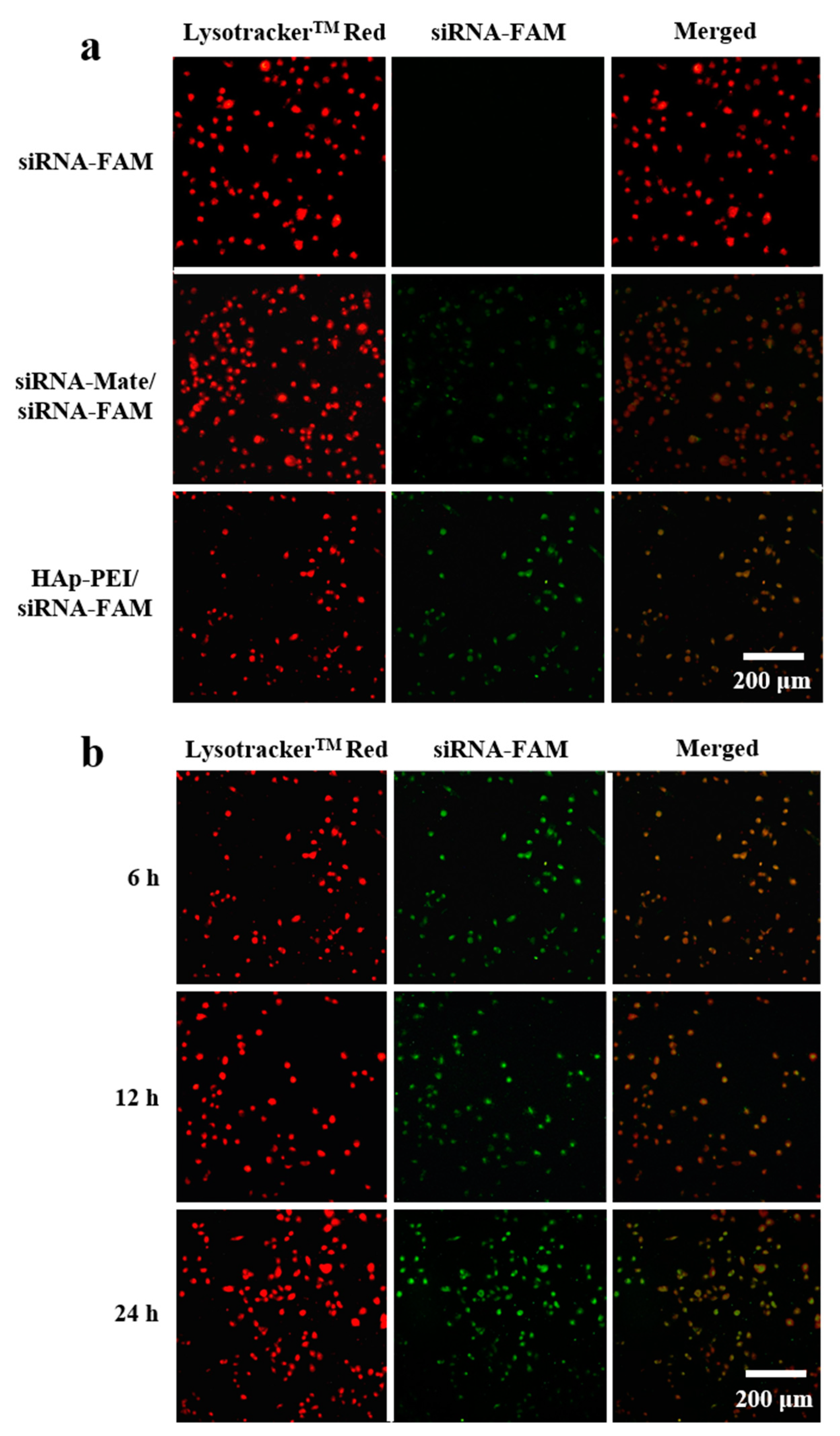
3.5. In Vitro Transfection of siRNA
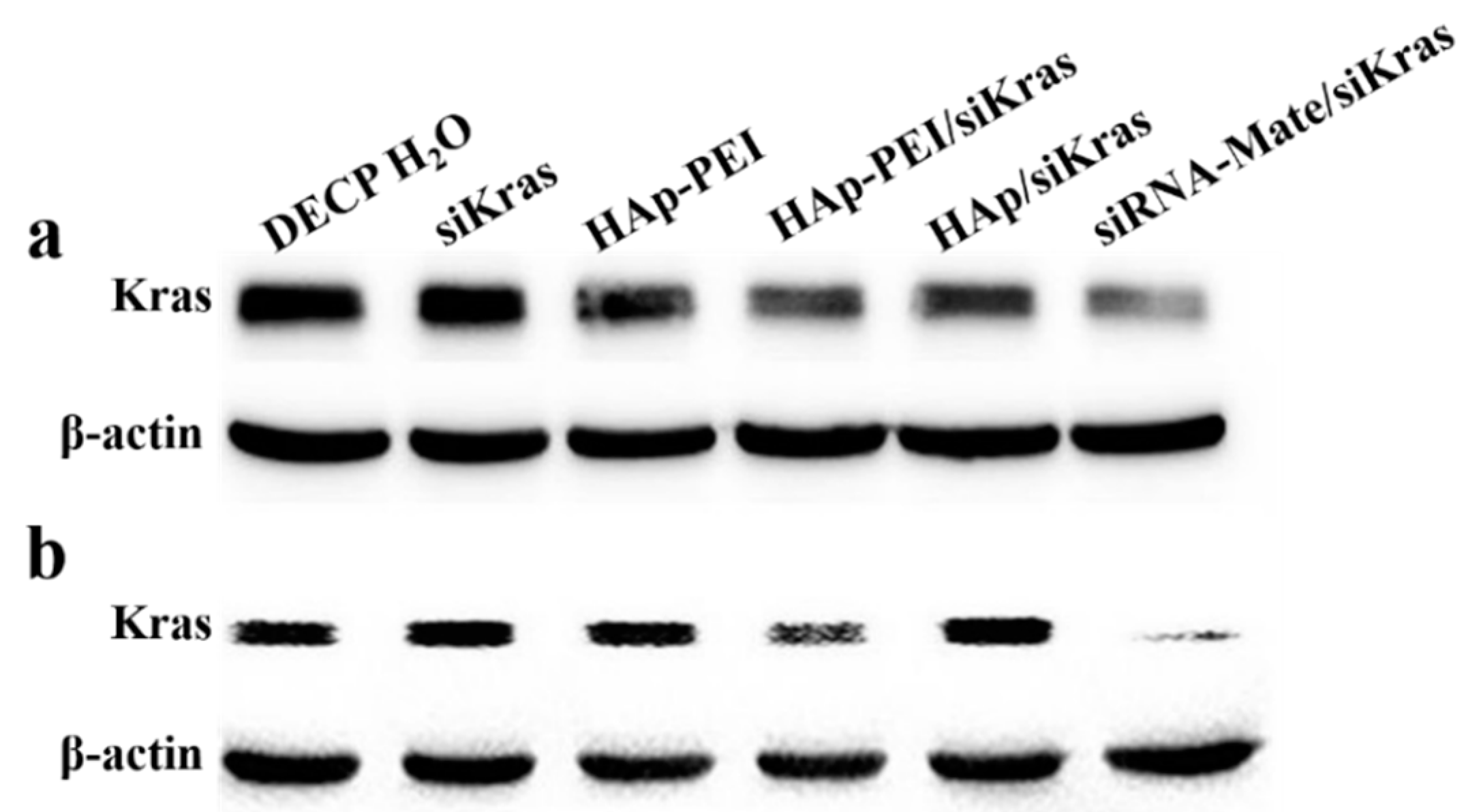
3.6. KRAS Gene Silencing Effect
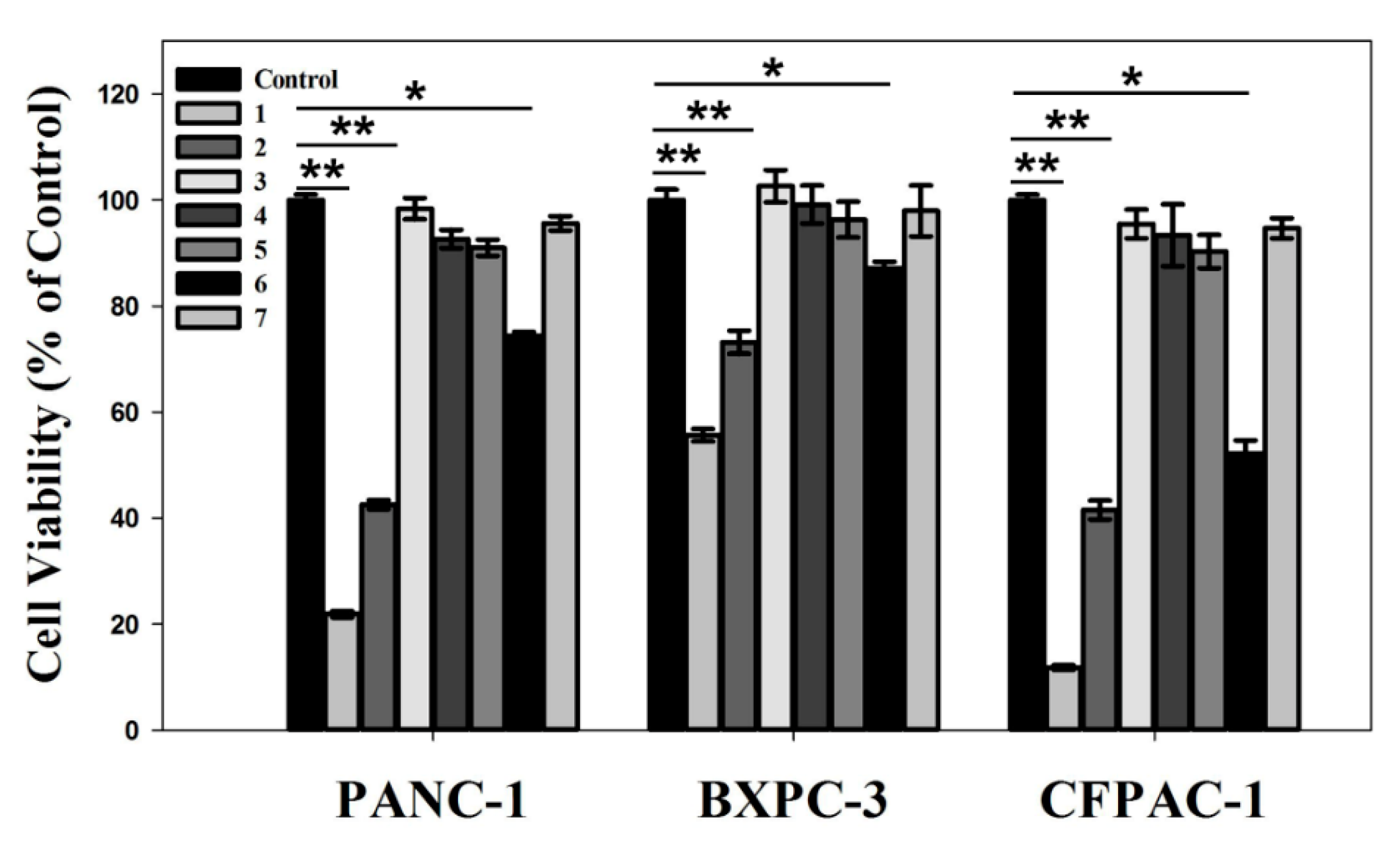
3.7. In Vitro Anti-Pancreatic Cancer Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sivakumar, S.; Bednarsch, J.; Wiltberger, G.; Kather, J.N.; Niehues, J.; de Vos-Geelen, J.; Valkenburg-van Iersel, L.; Kintsler, S.; Roeth, A. Nerve fibers in the tumor microenvironment in neurotropic cancer—pancreatic cancer and cholangiocarcinoma. Oncogene 2021, 40, 899–908. [Google Scholar] [CrossRef]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Qadan, M.; Ma, Y.; Visser, B.C.; Kunz, P.L.; Fisher, G.A.; Norton, J.A.; Poultsides, G.A. Reassessment of the current American Joint Committee on Cancer staging system for pancreatic neuroendocrine tumors. J. Am. Coll. Surg. 2014, 218, 188–195. [Google Scholar] [CrossRef]
- Haqq, J.; Howells, L.M.; Garcea, G.; Metcalfe, M.S.; Steward, W.P.; Dennison, A.R. Pancreatic stellate cells and pancreas cancer: Current perspectives and future strategies. Eur. J. Cancer 2014, 50, 2570–2582. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.-C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.-M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef]
- Che, Y.; Siprashvili, Z.; Kovalski, J.R.; Jiang, T.; Wozniak, G.; Elcavage, L.; Khavar, P.A. KRAS regulation by small non-coding RNAs and SNARE proteins. Nat. Commun. 2019, 10, 5118. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.; Rustgi, A.K. Mutant KRAS in the initiation of pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2005, 1756, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Bobbin, M.L.; Rossi, J.J. RNA interference (RNAi)-based therapeutics: Delivering on the promise? Annu. Rev. Pharmacol. Toxicol. 2016, 56, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutzer, J.N.; Ruzzene, M.; Guerra, B. Enhancing chemosensitivity to gemcitabine via RNA interference targeting the catalytic subunits of protein kinase CK2 in human pancreatic cancer cells. BMC Cancer 2010, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, K.; Guo, J.; Cheng, F.; Lv, J.; Jiang, W.; Lu, W.; Liu, J.; Pang, X.; Liu, M. Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat. Commun. 2016, 7, 11363. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.-T.; Xue, X.-H.; Dai, Z.-J.; Wang, X.-J.; Li, A.; Qin, Z.-Y. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J. Gastroenterol. 2006, 12, 2901–2907. [Google Scholar] [CrossRef]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154, 64–78. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Giger, E.V.; Castagner, B.; Räikkönen, J.; Mönkkönen, J.; Leroux, J.C. siRNA transfection with calcium phosphate nanoparticles stabilized with PEGylated chelators. Adv. Healthc. Mater. 2013, 2, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; He, B.; Mi, P. Calcium phosphate nanocarriers for drug delivery to tumors: Imaging, therapy and theranostics. Biomater. Sci. 2019, 7, 3942–3960. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Zhang, X. Synthesis and characterization of sintered Sr/Fe-modified hydroxyapatite bioceramics for bone tissue engineering applications. ACS Biomater. Sci. Eng. 2019, 6, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ruan, S.; Liu, A.; Kong, X.; Lee, I.S.; Chen, C. Laminin functionalized biomimetic apatite to regulate the adhesion and proliferation behaviors of neural stem cells. Int. J. Nanomed. 2018, 13, 6223–6233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Design. 2020, 197, 109269. [Google Scholar] [CrossRef]
- Gu, Y.H.; Yan, X.B.; Huang, D.; Han, R.; Wu, L.X. NR2B-siRNA mediated by hydroxyapatite nanoparticles relieves for Malin-induced pain of mice. Adv. Mater. Res. 2012, 343, 926–932. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Zhao, X.; Keen, L.; Kong, X. Calcium phosphate nanoparticles-based systems for siRNA delivery. Regen. Biomater. 2016, 3, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Bao, X.; Wang, H.; Zhu, S.; Liu, Z.; Chen, Q.; Ai, K.; Shi, B. TRPM2 promotes pancreatic cancer by PKC/MAPK pathway. Cell Death Dis. 2021, 12, 585. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Xu, S.; Shi, J.; Feng, D.; Yang, L.; Cao, S. Hollow hierarchical hydroxyapatite/Au/polyelectrolyte hybrid microparticles for multi-responsive drug delivery. J. Mater. Chem. B 2014, 2, 6500–6507. [Google Scholar] [CrossRef]
- Lai, W.; Chen, C.; Ren, X.; Lee, I.-S.; Jiang, G.; Kong, X. Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mater. Sci. Eng. C Mater. 2016, 62, 166–172. [Google Scholar] [CrossRef]
- Wilson, R.M.; Dowker, S.E.; Elliott, J.C. Rietveld refinements and spectroscopic structural studies of a Na-free carbonate apatite made by hydrolysis of monetite. Biomaterials 2006, 27, 4682–4692. [Google Scholar] [CrossRef]
- Feng, D.; Shi, J.; Wang, X.; Zhang, L.; Cao, S. Hollow hybrid hydroxyapatite microparticles with sustained and pH-responsive drug delivery properties. RSC Adv. 2013, 3, 24975–24982. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef] [PubMed]
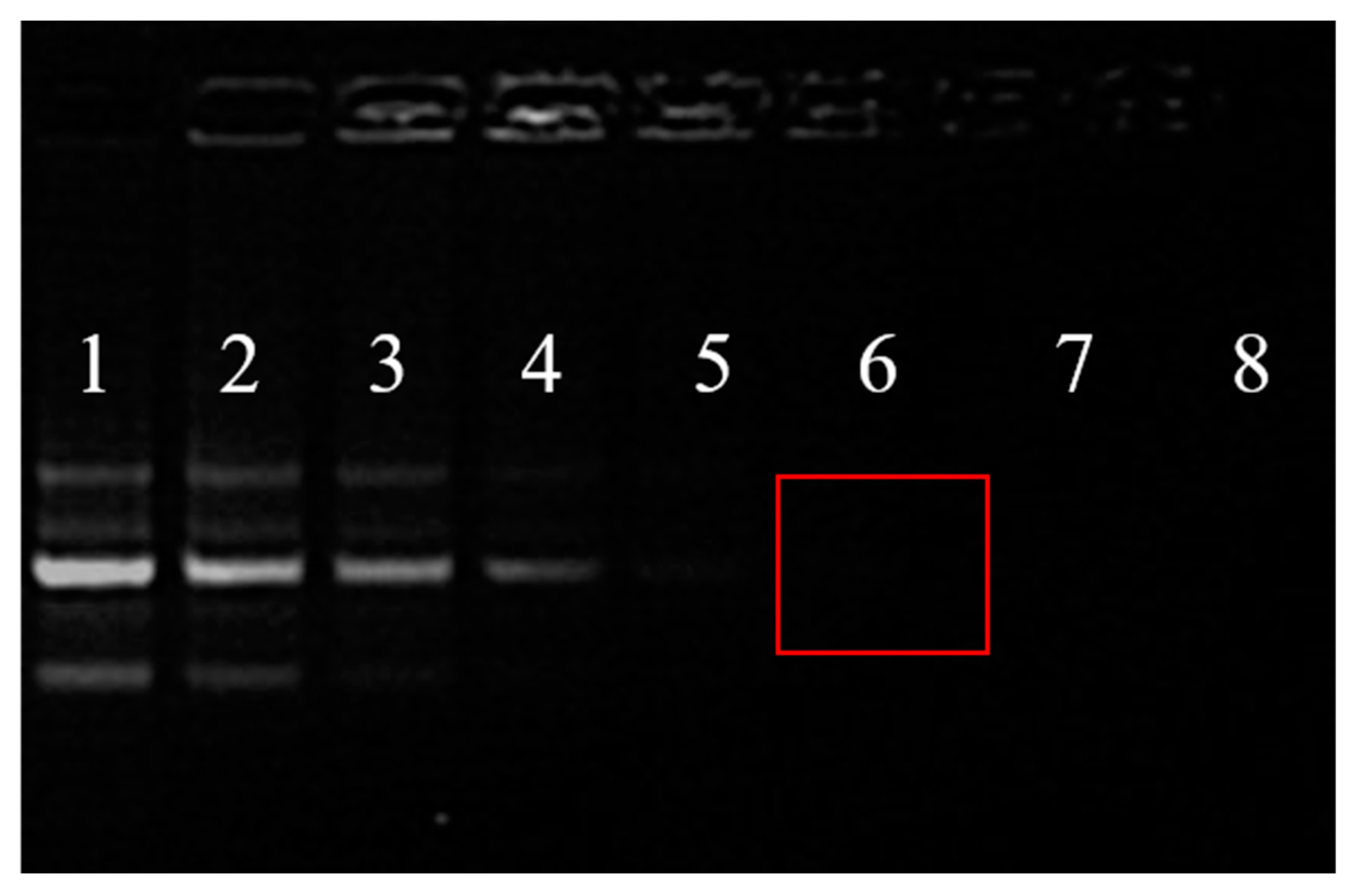

| Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| HAp-PEI/siRNA (w/w) | / | 1/1 | 2/1 | 4/1 | 6/1 | 8/1 | 10/1 | 12/1 |
| HAp-PEI (μg) | 0 | 2 | 4 | 8 | 12 | 16 | 20 | 24 |
| siRNA (μg) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| DEPC H2O | siKras | HAp-PEI | HAp-PEI/siKras | HAp/siKras | siRNA-Mate/siKras | |
|---|---|---|---|---|---|---|
| PANC-1 | 100% | 97.65% | 96.46% | 51.52% | 74.13% | 22.22% |
| CFPAC-1 | 100% | 106.15% | 104.03% | 67.04% | 105.10% | 32.87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Xu, X.; Iqbal, M.Z.; Zhao, Q.; Zhao, R.; Farheen, J.; Zhang, Q.; Zhang, P.; Kong, X. siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy. Pharmaceutics 2021, 13, 1428. https://doi.org/10.3390/pharmaceutics13091428
Luo D, Xu X, Iqbal MZ, Zhao Q, Zhao R, Farheen J, Zhang Q, Zhang P, Kong X. siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy. Pharmaceutics. 2021; 13(9):1428. https://doi.org/10.3390/pharmaceutics13091428
Chicago/Turabian StyleLuo, Dandan, Xiaochun Xu, M. Zubair Iqbal, Qingwei Zhao, Ruibo Zhao, Jabeen Farheen, Quan Zhang, Peiliang Zhang, and Xiangdong Kong. 2021. "siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy" Pharmaceutics 13, no. 9: 1428. https://doi.org/10.3390/pharmaceutics13091428
APA StyleLuo, D., Xu, X., Iqbal, M. Z., Zhao, Q., Zhao, R., Farheen, J., Zhang, Q., Zhang, P., & Kong, X. (2021). siRNA-Loaded Hydroxyapatite Nanoparticles for KRAS Gene Silencing in Anti-Pancreatic Cancer Therapy. Pharmaceutics, 13(9), 1428. https://doi.org/10.3390/pharmaceutics13091428







